- 1Center of Excellence for Soil and Africa Research in Africa, College of Agriculture & Environmental Science, Mohammed VI Polytechnique University, Ben Guerir, Morocco
- 2Bioresources and Food Safety Laboratory, Faculty of Sciences and Technology Marrakech, Cadi-Ayyad University, Marrakech, Morocco
This study investigates the natural distribution of potentially toxic elements in western Ghana as affected by soil types (i.e., Acrisols and Ferralsols). Geo-accumulation indices (Igeo), Enrichment Factor (EF), and risk index (RI) were computed to evaluate soil pollution classes, while the Soil Quality Index (SQI) was calculated to assess soil quality’s effect on pollution hazard. The study revealed subtle differences in contamination patterns: Acrisols exhibited slightly elevated Igeo values for elements such as Se, Mo, Fe, and Ti, suggesting localized enrichment possibly linked to natural processes or minor external inputs. In contrast, Ferralsols showed moderate Igeo values for Cr and Ni, indicating some enrichment consistent with parent material characteristics. EF values for all elements in both soil types were below 2, classifying them as “depletion to minimal enrichment” and confirming that elemental concentrations are predominantly of geogenic origin rather than anthropogenic inputs. Principal Component Analysis (PCA) effectively distinguished the two soil types, with Acrisols associated with higher trace metal concentrations and greater organic matter content, while Ferralsols were influenced more by Al and Fe oxides. Heatmap analysis further highlighted distinct element clustering, with Cr, Ni, and Se more prominent in Ferralsols, and Mo, Ti, and other trace elements showing spatial variation in Acrisols. These findings underscore the influence of pedogenic processes and mineral weathering in shaping elemental distributions across soil types in tropical environments and support a soil-type-specific management approach to ensure environmental protection and sustainable land use. The Soil Quality Index indicated that Ferralsols (SQI range: –2.65 to 1.78) had slightly lower surface horizon quality, likely due to leaching, while Acrisols (SQI range: –2.84 to 3.89) showed higher quality in deeper horizons, reflecting better nutrient retention.
1 Introduction
Tropical ecosystems, spanning a wide range of eco-climatic conditions along the equatorial region, vary from intensely hot lowlands to snowcapped mountains and from regions with seasonal rainfall to areas with persistently humid climates (1). Among these, tropical wet evergreen forests such as those in Ghana’s rainforest regions are particularly significant due to their ecological importance, drawing considerable attention from ecologists and conservationists (2). Ferralsols, which are highly weathered soils, cover approximately 9.8 million hectares, representing 7.5% of the global land area and 25% of tropical lands, with the majority found in humid tropical regions (3). These soils play a crucial role in carbon storage, ecosystem support, and climate regulation, serving as vital resources for food production, timber, and medicinal plants (4). In Ghana, Ferrasols development is closely linked to the local climate and vegetation, particularly in the forest zone, where high organic matter accumulation enhances soil properties (5). Conversely, Acrisols, commonly found in Ghana’s coastal savanna zone, are prone to nutrient depletion especially nitrogen and phosphorus under continuous cultivation, making them more vulnerable to soil degradation (6). As a result, many land use systems in Sub-Saharan Africa are considered unsustainable due to persistent nutrient loss and negative nutrient balances (7).
In tropical soil, metal and metalloid behavior is strongly influenced by intense weathering, high rainfall, and the presence of iron (Fe) and aluminum (Al) oxides, which regulate their mobility and retention. These oxides play a key role in adsorbing trace elements such as chromium (Cr) and nickel (Ni), limiting their bioavailability. Manganese (Mn) and titanium (Ti) occur naturally in tropical soils, often reflecting the geochemical composition of the parent material. Essential micronutrients like copper (Cu) and zinc (Zn) can accumulate due to agricultural practices. In contrast, heavy metals such as lead (Pb) and cadmium (Cd), mainly introduced through anthropogenic activities, tend to be more mobile in acidic tropical soils, increasing environmental risks. The interactions between soil pH, organic matter, and clay content further influence metal retention and leaching, ultimately impacting soil fertility and contamination levels.
The mobility and bioavailability of metals and metalloids in terrestrial environments are largely dictated by their partitioning between solid and dissolved soil fractions. While significant advances have been made in soil health assessments, much of the research on critical metalloids has focused on temperate soils, primarily in Europe and North America (8). However, data on metal behavior in tropical soils remains limited, highlighting a crucial knowledge gap that must be addressed. Numerous studies have emphasized this scarcity of research on trace metal dynamics in tropical soils (9–16).
Given the risks associated with heavy metal contamination, ecological risk assessment in agricultural soils has gained increasing attention in recent years. Various approaches, such as the ecological risk index (IR) and the geoaccumulation index, are widely used to evaluate the impact of environmental factors on soil quality and measure the degree of contamination (17). These tools help identify areas at risk and assess the potential ecological hazards posed by metal pollution (18).
Western Ghana has been chosen as the study site due to its significance as a major agricultural hub, particularly for cocoa and oil palm cultivation where Acrisols and Ferralsols are widely distributed. According to Driessen et al. (19) and FAO (20) Acrisols correlate with several subgroups of Alfisols and Ultisols of the USDA system of classification. These are acidic, highly weathered soils (but less weathered than the Ferralsols) with accumulation of low activity clay in an argic subsurface horizon with low base saturation. They are found mainly on old land surfaces with hilly or undulating topography with wet monsoonal climate (19). Acrisols under a protective forest cover have a porous surface. Whereas Ferrosols are equivalent to the Oxisols of the USDA system, are leached and deeply weathered red soils of western Ghana. They occur typically in level to undulating land of Pleistocene age or older. They have stable microaggregates, good porosity, permeability and infiltration. The chemical fertility of these soils, whose clay fraction is dominated by low activity clays (kaolinte) and oxides of iron and aluminium, is poor for crop production (21). Soil pH is low, base saturation low and effective cation exchange capacity is only 3 to 4 cmol/kg soils. There is high retention by the soil colloids of applied phosphate leading to reduction in its immediate availability to crops. By and large, the agricultural environment in western Ghana is exigent. The land surface is generally very old, consist of sand and alluvial materials, and many of the materials of present-day soils have undergone both through anthropogenic and natural pedogenesis processes as the result, physical and chemical soil properties are inherently poor. Climate change and poor management aggravates the situation. Furthermore, there remains a considerable knowledge gap regarding the extent of metal accumulation in these soils and its potential impact on soil fertility and crop safety. Therefore, this study aims to assess and monitor soil quality in this vital agricultural zone, providing valuable insights for better soil management practices.
2 Materials and methods
2.1 Description of the study site
2.1.1 Study site location
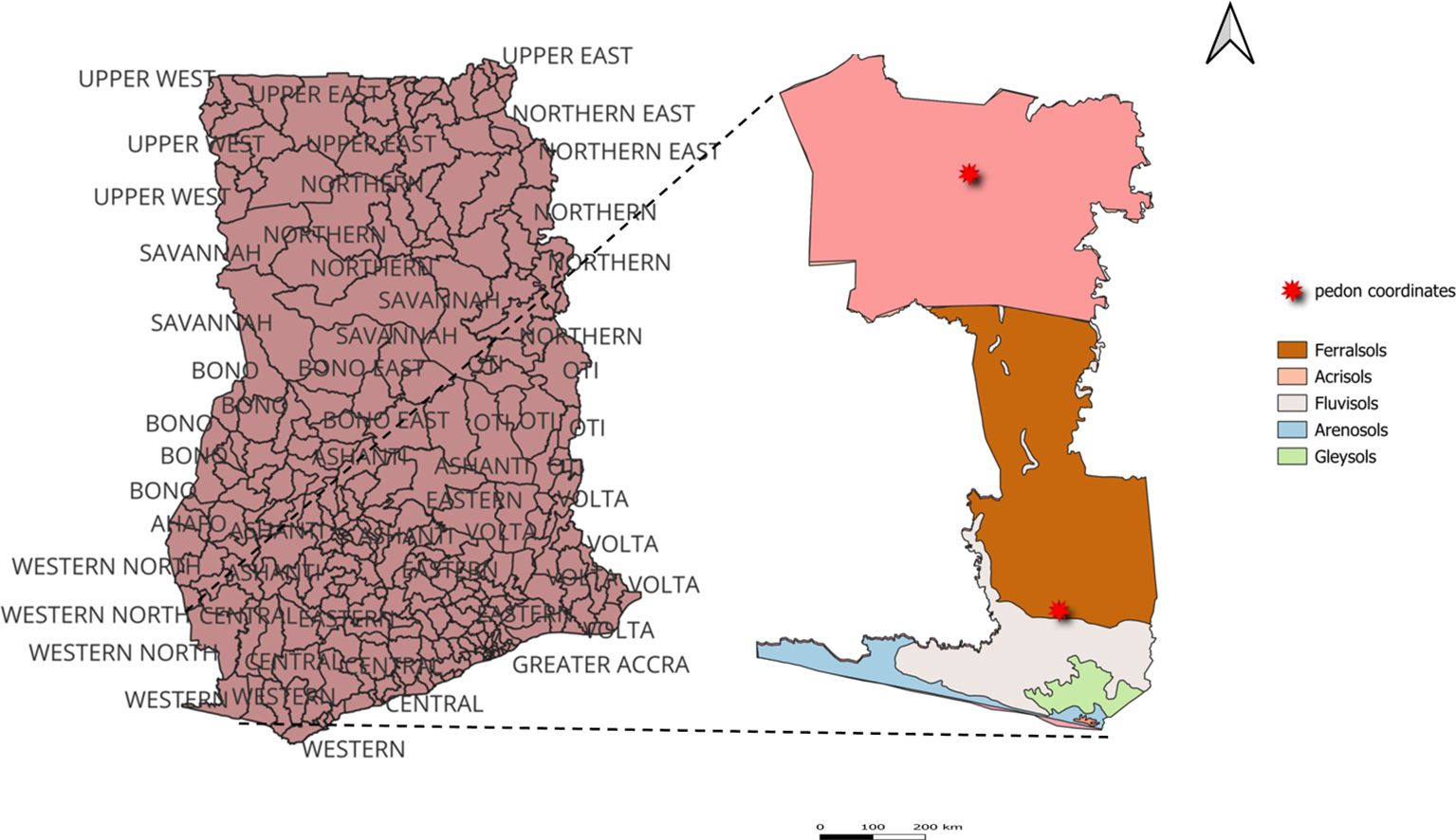
The study site is located in the western region of Ghana, specifically within the Aowin and Jomoro districts (See Figure 1). This area is endowed with dense forests primarily dominated by wet evergreen and Moist Semi-Deciduous Forests, which are typical of the humid tropical zone. These forests are characterized by high species diversity, closed canopies, and significant timber resources. Additionally, the region features riverine zones and coastal plains. The soil in this area are predominantly Ferralsols and Acrisols, supporting agricultural and commercial plantation activities with adequate soil management. Two soil pedons were selected for analysis, each representing different cropping systems. The first pedon, located in Ferrasols under an oil palm farm, is situated at coordinates N5°9’53.44853” and W2°37’53.41997”. This pedon consists of five soil horizons. The second pedon is the Acrisols, which is dominant in a cocoa farm, is positioned at N5°51’17.43514” and W2°46’23.70695”, and includes four distinct horizons.
2.1.2 Study site characterization
The study area spans two ecological zones: the Coastal Rainforest Zone (CPh4) in the Jomoro district, where the first pedon is located, and the Cocoa Forest Zone (CF1) in the Aowin district, where the second pedon is situated. The altitudes range from 97 meters above sea level in Jomoro to 134 meters in Aowin.
The region experiences a humid tropical climate, with an average annual rainfall of 1,500–2,000 mm, primarily distributed between April and October. The dry season extends from November to March, with the heaviest rainfall typically occurring during the summer months. The mean maximum temperature is 32–34°C, recorded in March, while the mean minimum temperature is 22–24°C, observed in August. Relative humidity peaks at 80–90% during the wet season and declines to 60–70% in the dry season.
Agricultural activities dominate the land use in the region, with major crops including oil palm (Elaeis guineensis), cocoa (Theobroma cacao), maize (Zea mays), pineapple (Ananas comosus), and cassava (Manihot esculenta). These activities reflect the significant role of farming in the local economy.
2.2 Experimental design, soil sampling and preparation
2.2.1 Experimental design
The first pedon, classified as Ferralsols, was sampled from an oil palm (Elaeis oleifera) plantation managed under a monoculture system with no fertilizer application or agronomic interventions. This pedon included five distinct horizons: Horizon A (0–16 cm), Horizon B (16–35 cm), Horizon C (35–80 cm), Horizon D (80–120 cm), and Horizon R (120–200 cm). The second pedon, classified as Acrisols, was sampled from a cocoa (Theobroma cacao) farm intercropped with maize, reflecting a mixed cropping system. It comprised four horizons: Horizon A (1–50 cm), Horizon B (50–90 cm), Horizon C (90–120 cm), and Horizon D (120–180 cm).
The independent variables were horizon depth and land-use type (monoculture vs. intercropping), while the dependent variables included soil physical properties (e.g., texture), and chemical properties (e.g., pH, organic carbon, and nutrient, heavy metals concentrations). To ensure consistency, soil samples were systematically collected from the center of each horizon, following standardized protocols to minimize variability and maximize reproducibility. Triplicate sampling was conducted within each horizon to account for spatial heterogeneity.
2.2.2 Soil sampling and sample preparation
A total of 27 composite soil samples were collected, with three replicates taken from each horizon across the two representative soil profiles. Each pedon had four exposed sides, and soil from each horizon was sampled from all sides to ensure representativeness. Each composite sample was placed in a labeled bag. The samples then were air-dried, sieved to pass through 2mm to remove larger non-soil particles in preparation for soil analyses.
2.3 Soil physicochemical analysis
Exchangeable bases (Ca2+, Mg2+, K+, and Na+) were extracted with the stirring method using ammonium acetate following the NF X31-108V (2002) standard.The pH and electrical conductivity (EC) of soil were determined in 1:2 soil: water ratio (22). Organic carbon (OC) content was determined via the Loss on Ignition (LOI) method, involving sequential heating at 450°C for organic matter decomposition and 850°C for carbonate breakdown.Total carbon (C), nitrogen (N), and hydrogen (H) quantification, the Dumas combustion method was employed. Texture analysis, including sand, silt, and clay fractions, was performed using the hydrometer method.
2.4 Total elemental analysis
Soil samples weighing between 50 and100 milligrams were used for acid digestion. The digestion process involved a stepwise addition of acids: 2 mL of concentrated nitric acid (HNO₃), followed by 1.5 mL of hydrochloric acid (HCl), and finally, 0.5 mL of hydrofluoric acid (HF). The samples were then digested using a microwave digestion system to ensure complete dissolution of the soil matrix.
Elemental analysis was performed using an Agilent 5800 VDV ICP-OES. Data acquisition was conducted in both axial and radial modes, with three replicates measured over a 10-second integration time. To minimize interferences, polyatomic ion interferences were managed using Kinetic Energy Discrimination (KED) mode with helium as the collision gas. Calibration was performed using multi-element standard solutions (CCS4, CCS5, CCS6 from Inorganic Ventures, USA) prepared in 2% HNO₃. Quality control was ensured through the use of certified reference materials, including EnviroMAT Worn Water EUB and EnviroMAT Subterranean Water ES-H-2 (SCP Sciences, France), as well as SRLS-6, a reference material composed of riverine water for trace metal analysis (National Research Council Canada). Reagents used included ultrapure water (18.2 MΩ·cm at 25°C, Milli-Q Reference, Millipore) and high-purity acids (HNO₃ 69%, HCl 37%, TMA-VWR International).
2.5 Data analyses
2.5.1 Weighted mean, trend and specific range
Weighted mean (W), trend (T) and specific range (R) were employed to evaluate the status of metal contaminants in Acrisols and Ferralsols for each profile samples as described by (23).
The weight mean (W) concentration for a profile was obtained by calculating using Equation 1:
Where W= weighted mean, c = concentration of elements in the layer; d = thickness of layer and P = depth of profile
Trend (T) was calculated using Equation 2 to acquire some information on any change in concentration with depth.
Where (T) denotes the trend, W the weighted mean concentration and (S) the concentration in the surface layer.
The variableness of the contaminants was studied by calculating the specific range (R) using Equation 3.
Where, R is the specific range, H is the highest and L is the lowest observed concentrations in the profile, and W is the weighted mean.
2.5.2 Quantification of soil contamination level
Although several methods have been developed to measure status of heavy metal pollution, in this study geo-accumulation index, contaminant factor, enrichment factor representing the single group indices whereas multielement pollution load index (PLI) was employed from the total complex indices. Brief description of each index is provided in below:
2.5.3 Index of geo-accumulation
An index of geo-accumulation (Igeo) was used to determine metal contamination level in soil samples by comparing the current concentrations with background values by calculating using Equation 4 as described in Banat et al. (24),
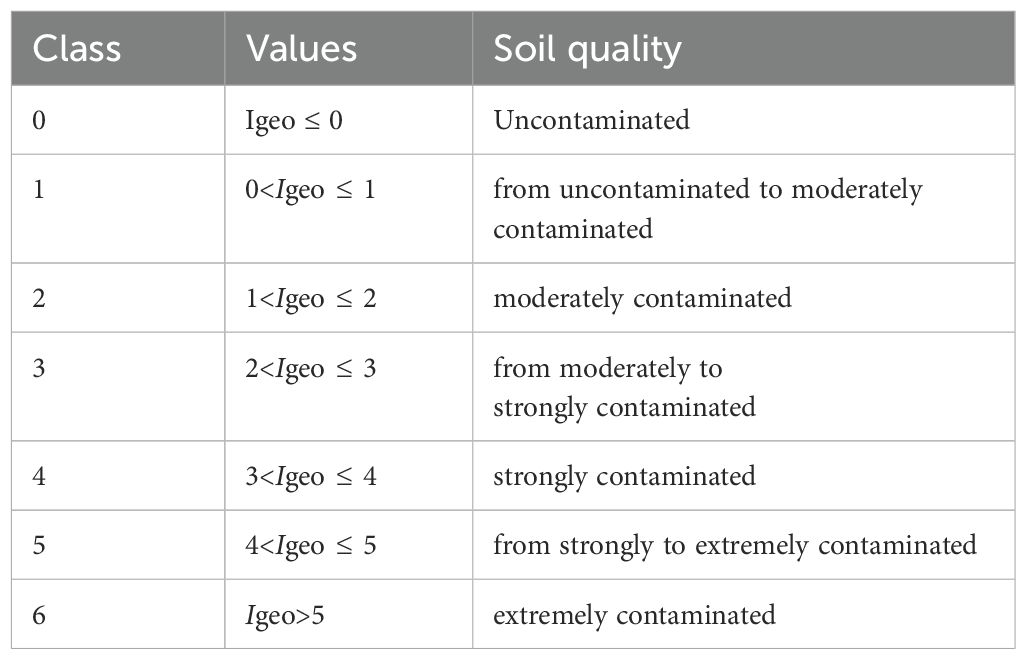
where Ci is the measured concentration of the examined metal i in the soil sample, and Bn is the geochemical background concentration or reference value of the metal i. Factor 1.5 is used because of possible variations in background values for a given metal in the environment as well as very small anthropogenic influences. The geo-accumulation index (Igeo) was distinguished into six classes by Buccolieri (25) (Table 1).
2.5.4 Enrichment factor
An element enrichment factor (EF) was calculated to speculate on the origin of elements in the soil samples as described by Reimann and de Caritat, (2005). The formula used to calculate is in Equation 5.
where Ci is the content of element i in the soil samples, and Cie is content of immobile element in the soil sample. So (Ci/Cie) S is the heavy metal to immobile element ratio in the soil samples, and (Ci/Cie)RS is the heavy metal to immobile element ratio in the selected reference sample (26). The immobile element selected for this study is Fe (27). According to (28), five contamination categories were used to identify sources of contaminants. These are of the enrichment factor: EF<2, depletion to mineral enrichment; 2≤EF<5, moderate enrichment; 5≤EF<20, significant enrichment; 20≤EF<40, very high enrichment; and EF>40, extremely high enrichment.
2.5.5 Assessment of soil metals risk
Risk Index (RI) was used to assess the degree of heavy metal pollution in soils according to the toxicity of metals and the response of the environment (29). RI values for soil samples were computed stepwise, viz, the first step was the calculation of the risk quotient (Q) for each analysed metal that exceeded the limit risk values. In the second step, the RI was determined from the summation QERI as expressed in Equation 6.
Where:
Aci: concentration of metal i in the soil sample,
Rci: reference (background) concentration of metal i,
QERI: risk quotient for metal i, and
RI: Risk Index calculated as the sum of all QERI values.
The RI values were rated as low risk <30; moderate: 30<RI<60; considerable risk: 60<RI<120; high risk: >120 < very high risk.
2.5.6 Soil quality index calculations
To assess soil quality variations across horizons, SQI was computed using principal component analysis (PCA). Because numeric values of the soil indicators are on very different scales of magnitude and even on different units, standardization was employed to make sure that comparability across variables are possible (30). Principal component loadings of each soil type were used to derive the SQI using Equation 7.
2.6 Statistical data analyses
Statistical analyses were conducted to examine relationships among elemental concentrations and soil properties. An independent t-test was used to correlate the results of soil indices. Pearson’s correlation coefficients were calculated to assess the strength and direction of these relationships, with statistical significance set at p < 0.05. A heatmap was generated in R using the ggplot2 and reshape2 packages to visually represent the correlations. Furthermore, PCA was performed using the FactoMineR and factoextra packages in R to identify patterns and reduce data dimensionality. To ensure comparability across variables, the dataset was standardized using z-scale transformation prior to PCA.
3 Results and discussion
3.1 Statistical evaluation of metal contaminants in Acrisols and Ferralsols
The comparative analysis of nutrient and trace element concentrations in Acrisols and Ferralsols highlights distinct patterns of distribution, variability, and trends, which are reflective of their contrasting pedogenic processes (Table 2).
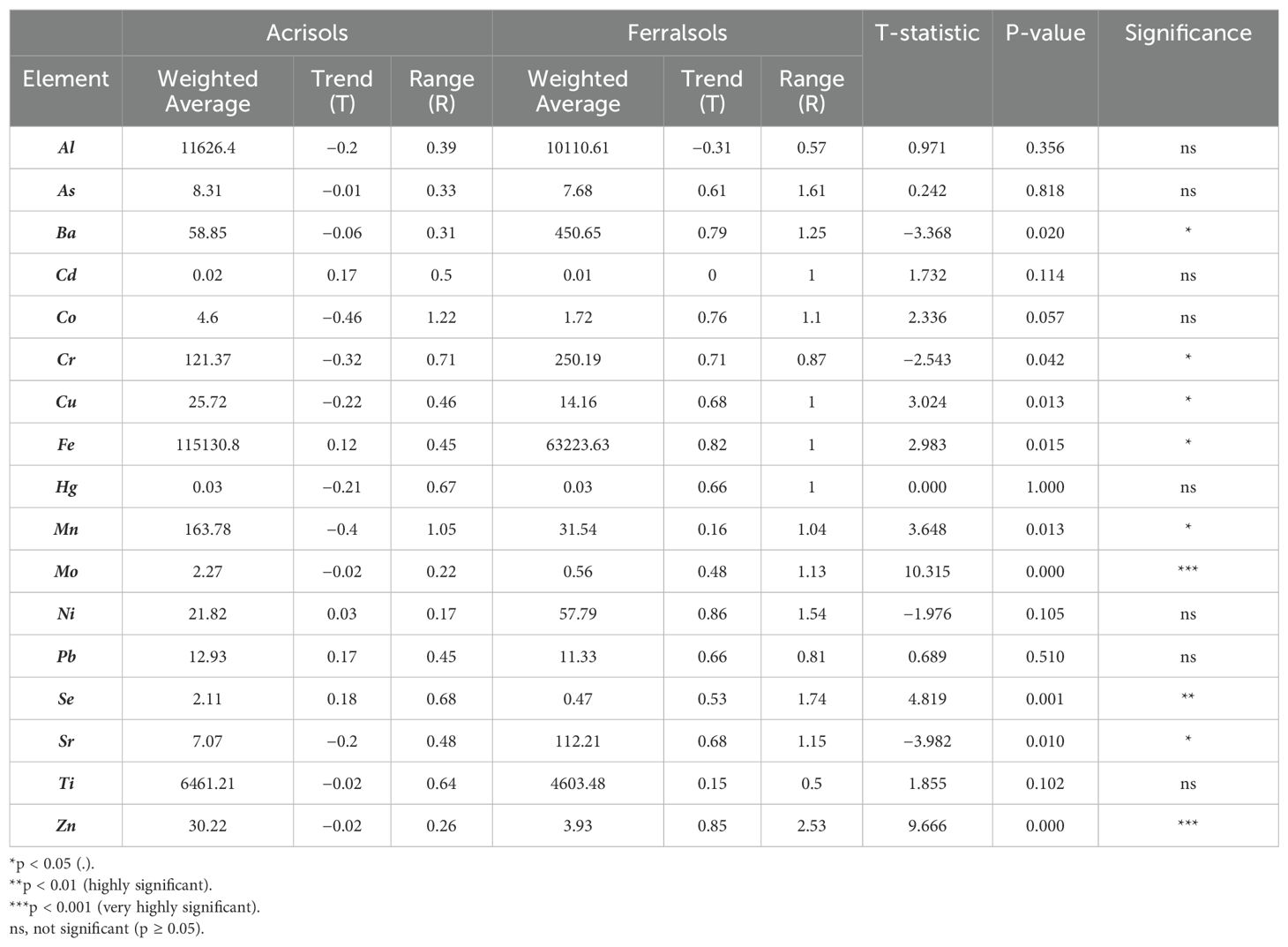
Table 2. Elemental concentrations and statistical comparison between Acrisols and Ferralsols: weighted averages, trends, range, t-Statistics, p-values, and significance.
Acrisols exhibit a weighted average Al concentration of 11,626.4 mg/kg, with a negative trend (T = −0.2) and a narrow range (R = 0.39), indicating progressive leaching and depletion. In contrast, Ferralsols show a slightly lower Al concentration (10,110.61 mg/kg) but greater variability (R = 0.57) and a more pronounced negative trend (T = −0.31). Fe is the most abundant element in both soils, with Acrisols displaying a significantly higher concentration (115,130.8 mg/kg) than Ferralsols (63,223.63 mg/kg). However, the positive trend in Ferralsols (T = 0.82) suggests ongoing Fe enrichment, likely due to sesquioxide accumulation, whereas Acrisols exhibit a weaker trend (T = 0.12), indicating less translocation (31). The dominance of Fe and Al in these soils reflects intense tropical weathering, leading to Fe oxide accumulation and aluminosilicate formation. Zn is present in moderate amounts, while trace elements like As, Cd, and Co appear in lower concentrations. The similarity in weighted averages between Acrisols and Ferralsols suggests that both soils undergo comparable pedogenic processes, shaped by tropical climatic conditions and parent material influences.
Elemental distribution patterns reveal that highly symmetrical elements such as Al, Cd, Mo, and Ti exhibit low T and R values across both soils, indicating a uniform distribution. For instance, Cd in Acrisols has a T value of 0.17 and R of 0.50, whereas in Ferralsols, T remains at 0 and R at 1. Conversely, asymmetrical elements such as Fe, Ba, and Ni display higher T and R values, particularly in Ferralsols, signifying greater variability. Ba in Ferralsols (T = 0.79, R = 1.25) contrasts sharply with its lower values in Acrisols (T = −0.06, R = 0.31). Moderate symmetrical elements like Zn and As exhibit intermediate T and R values, suggesting localized variations in distribution. These patterns reflect stable pedogenic processes for some elements, while others indicate more dynamic redistribution through leaching and mineral dissolution (32).
Ferralsols contain significantly higher concentrations of Ba (450.65 mg/kg), Fe, Sr, and Cr, with Sr showing a highly significant difference (p < 0.01). Acrisols have higher concentrations of Zn (30.22 mg/kg), Cu, Mn, and Mo, with Mo and Zn showing very highly significant differences (p < 0.001). Acrisols also exhibit greater variability in Co (4.6 mg/kg, T = −0.46, R = 1.22) compared to Ferralsols (1.72 mg/kg, T = 0.76, R = 1.1) The higher trace metal content in Ferralsols may be attributed to parent material differences, as soils derived from mafic crystalline rocks generally contain elevated trace metal concentrations. In sedimentary soils, trace metal levels are strongly influenced by sediment type (33).
While tropical rainforest conditions tend to homogenize soil mineralogy, mainly leaving quartz, kaolinite, gibbsite, goethite, and hematite, variations in trace metal concentrations can still be linked to parent rock composition (33, 34).
Based on the t-test, several elements show significant differences between Acrisols and Ferralsols. Ferralsols have significantly higher concentrations of Ba, Cr, and Sr (p < 0.05), while Acrisols contain significantly higher levels of Cu, Fe, Mn, Mo, Zn, and Se, with Mo, Zn, and Se showing highly significant differences (p < 0.01). Elements such as Al, As, Cd, Co, Hg, Ni, Pb, and Ti do not show significant variation between the two soil types (p > 0.05). These differences can be explained by the distinct mineralogical and geochemical characteristics of the soils, Ferralsols, often formed on mafic parent material, tend to accumulate Ba, Cr, and Sr due to their association with resistant minerals and specific geochemical weathering patterns (35). Meanwhile, Acrisols, which typically develop under more intense weathering and leaching, can retain higher levels of trace metals such as Cu, Fe, Mn, Mo, Zn, and Se through strong adsorption to sesquioxides and organic matter (36). Elements showing no significant difference are likely more uniformly distributed in the parent material or have geochemical behaviors that lead to stable baseline concentrations across soil types.
3.2 Soil pollution hazard assessment using geo-accumulation indices
Some studies have been carried out to determine the long-term behavior of chemical elements in soils (37, 38) However, many questions remain in tropical soil about the mobility of trace metals, probably caused by the scarcity of long-term studies in these regions. The results presented in the previous section did not provide sufficient information on vertical viability. Here, the geochemical features along the soil profiles allow a much more accurate analysis. The comparison of elemental concentrations and geo-accumulation indices (Igeo) between Acrisols and Ferralsols highlights distinct patterns of natural enrichment and potential contamination across the two soil types (Table 3).
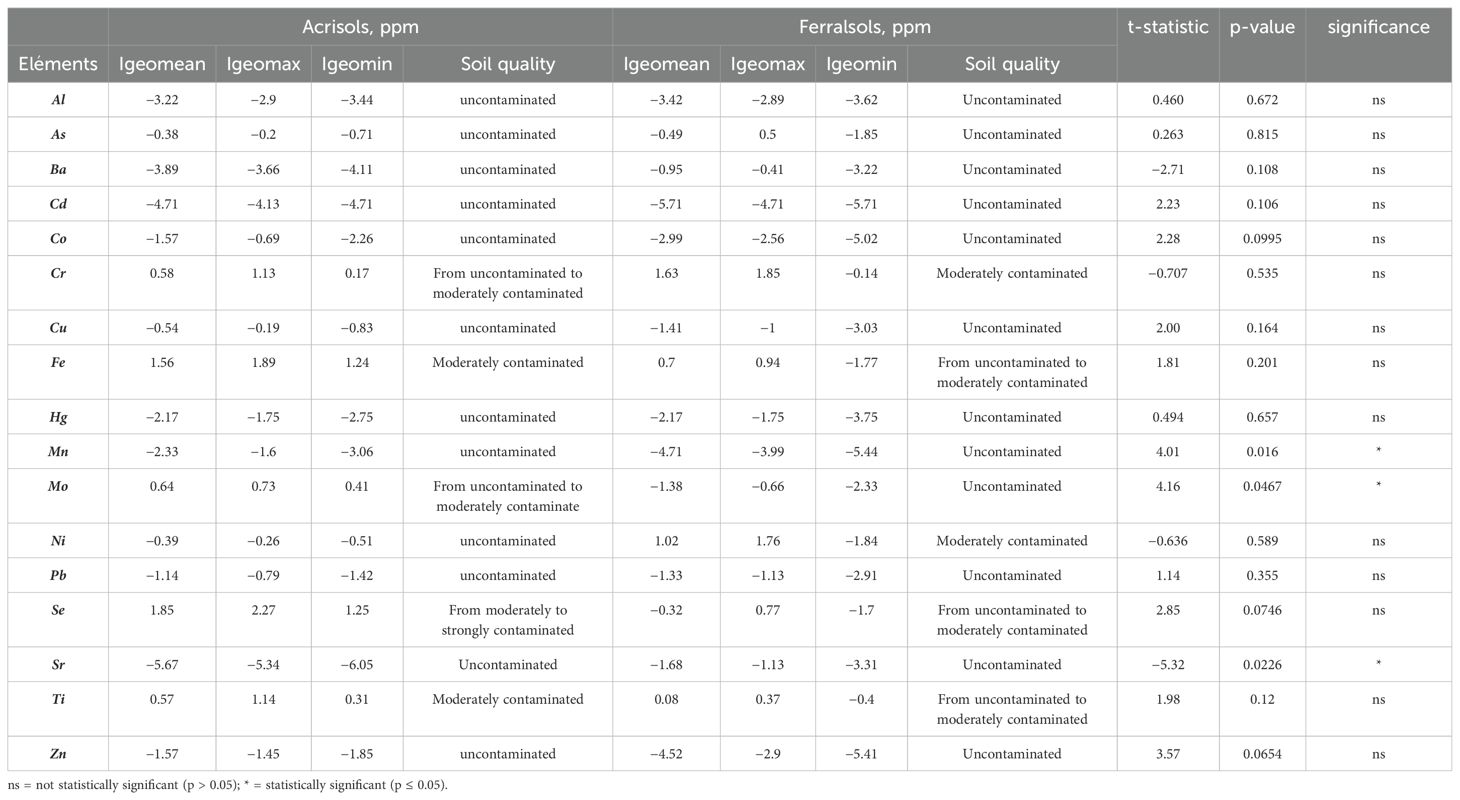
Table 3. Geo-accumulation (Igeo) index classes for potentially toxic elements in Acrisols and Ferralsols.
The contrasting contamination profiles observed between Acrisols and Ferralsols are shaped by both their inherent mineralogical composition and anthropogenic influences. Acrisols generally exhibit higher contamination levels for most trace elements, as reflected by their less negative or even positive Igeo values, suggesting significant deviation from natural geochemical baselines. For instance, (Al) in Acrisols has a weighted mean concentration of 11,626.4 ppm with an Igeo mean of (−3.22), while (Cd), although present at a very low concentration of 0.02 ppm, has a higher Igeo mean (−4.71) than in Ferralsols, indicating relatively greater enrichment. These patterns likely stem from the acidic, siliciclastic nature of Acrisols’ parent materials, such as granite or gneiss which typically have lower native metal contents and reduced capacity for trace metal retention.
In contrast, Ferralsols tend to display more negative Igeo values, indicative of lower contamination levels and concentrations closer to natural background thresholds. However, certain exceptions are notable. (Cr) in Ferralsols reaches a mean of 250.19 ppm (Igeo = 1.63), surpassing levels found in Acrisols (121.37 ppm; Igeo = 0.58), and nickel (Ni) also shows substantial enrichment (57.79 ppm; Igeo = 1.02 vs. 21.82 ppm; Igeo = −0.39 in Acrisols). These enrichments may be attributed to the mineralogical origin of Ferralsols, which often develop from mafic or ultramafic rocks such as basalt or amphibolite. Such parent materials contain Fe and Mn-oxides like goethite, hematite, and lithiophorite, which have high sorption capacities and can strongly retain trace metals (39).
Beyond lithological factors, anthropogenic activities also contribute to differential contamination. Agricultural practices, particularly fertilizer and pesticide application are known to introduce metals such as Zn, Cu, and Mo into the soil (40, 41). This may explain the comparatively elevated Mo concentrations in Acrisols (2.25 ppm; Igeo = −1.52). Additionally, small scale mining and industrial emissions, common in parts of Western Ghana, further exacerbate contamination, particularly for elements like Pb, Cd, and Hg. Acrisols, being more weathered and having lower oxide buffering capacities, may be especially vulnerable to these inputs (42).
T-test analyses confirm statistically significant differences between soil types for Mn (p = 0.016), Mo (p = 0.0467), and Sr (p = 0.0226). Mn and Sr are more abundant in Ferralsols, consistent with their mineral origin and higher retention capacity, while Mo enrichment in Acrisols likely reflects anthropogenic influence.
Although t-test analyses revealed no statistically significant differences (p > 0.05) for most elements between Acrisols and Ferralsols (e.g., Al, As, Cd, Co, Cu, Fe, Fe, Hg, Ni, Pb, Se, Ti), this suggests that the two soil types share similar geochemical backgrounds and contamination levels for these trace elements. Consequently, variability in elemental concentrations is likely influenced more by localized environmental factors or soil profile heterogeneity than by soil classification per se. These findings emphasize the need for integrated soil management approaches that consider a suite of physicochemical properties alongside site-specific conditions to accurately assess contamination risks and support sustainable land-use practices in tropical regions.
3.3 Sources of potentially toxic elements
EF and QRET were used to assess the potential sources and ecological risks of elements in Acrisols and Ferralsols (32). As shown in Table 4, all EF values for both soil types are below 2, classifying them within the “depletion to minimal enrichment” category as defined by (28). This indicates that the elements examined are primarily of natural origin, with no significant anthropogenic contributions.
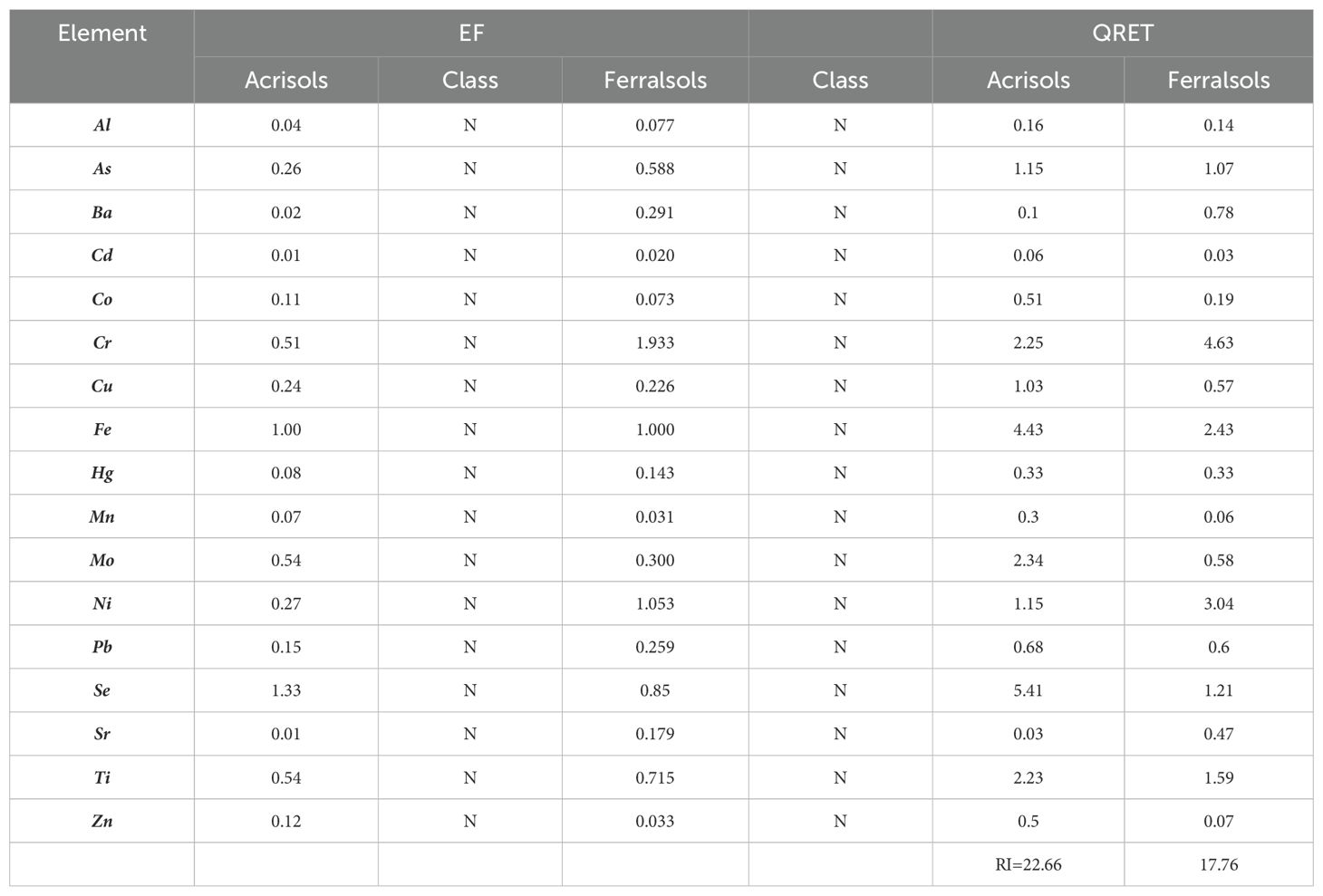
Table 4. Classification and comparative analysis of elemental enrichment factors (EF) and quality risk evaluation (QRET) in Acrisols and Ferralsols.
Selenium (Se) exhibits EF values of 1.33 in Acrisols and 0.85 in Ferralsols, both consistent with natural background levels. Although Se can be influenced by environmental factors such as wet atmospheric deposition (43–45), the current data do not support anthropogenic enrichment in the study area (46). further highlight that Se concentrations tend to be higher in regions receiving over 700 mm of annual rainfall, which may explain some of the observed variability.
Despite the uniformly low EF values, QRET analysis highlights Se (QRET = 5.41), Mo (2.34), and Cr (2.25) as predominant contributors to ecological risk in Acrisols, while Cr (4.63) and Ni (3.04) lead risk scores in Ferralsols. These elevated QRET values reflect inherent geochemical characteristics rather than external contamination. Cr and Ni- enrichments in Ferralsols are primarily lithogenic: these soils form on mafic to ultramafic parent materials (e.g. peridotite, serpentinite) rich in Fe–Mg minerals and iron oxides, which naturally concentrate Cr and Ni through weathering and oxide formation (47). Iron oxides such as goethite and hematite possess high sorptive capacity, immobilizing trace metals even under tropical conditions. Se and Mo accumulation in Acrisols, though also tied to parent geology, may be further enhanced by agricultural inputs, particularly phosphate fertilizers known to supply Mo and Se to soils, where their ecotoxicological weight elevates risk potential despite low enrichment factors (48).
Despite their classification as geogenic based on EF values, potential anthropogenic contributions, especially from agriculture or nearby mining activities, cannot be fully excluded and may contribute locally to the observed elemental load. The overall Risk Index (RI) indicates a low ecological risk for both soil types, with Acrisols (RI = 22.66) slightly higher than Ferralsols (RI = 17.76). This variation is mainly attributed to naturally elevated concentrations of certain elements rather than anthropogenic contamination. These results highlight the necessity of differentiating between inherent geochemical background levels and pollution sources when assessing soil quality and guiding land management strategies.
Although EF values suggest predominantly geogenic origins for Cr, Ni, and Pb, it remains essential to compare these concentrations against established environmental and toxicological benchmarks. In Ferralsols, the Cr concentration (250.19 mg/kg) exceeds the U.S. EPA residential soil screening level of 100 mg/kg, while Ni (57.79 mg/kg) slightly surpasses the recommended threshold of 50 mg/kg (49). Pb concentrations (maximum 35.33 mg/kg) remain well below the threshold of 400 mg/kg for residential soils (49) and the FAO/WHO permissible limit of 100 mg/kg for agricultural soils (50).These findings suggest a moderate ecological concern for Cr and Ni in Ferralsols, while Pb poses minimal risk. As such, localized geochemical monitoring remains advisable to support sustainable land management.
3.4 Potentially toxic elements as affected by the Soil quality index
To ensure a reliable Soil Quality Index (SQI) for Acrisols and Ferralsols under oil palm and cocoa cultivation, multiple soil parameters reflecting fertility, structure, and nutrient status were evaluated. These included pH, exchangeable cations (Ca2+, Mg2+, K+, Na+), organic carbon, and soil texture. Soils exhibited acidic pH values (4.8–5.9), consistent with Dabin’s classification of low-fertility soils (pH 4.75–5.1) (51) (Table 5). Exchangeable Ca2+ was the dominant cation but declined with depth due to leaching, while Mg2+, K+, and Na+ remained relatively stable. Organic carbon concentrations were highest in the surface layers and decreased sharply with depth, reflecting limited organic matter inputs. Texturally, Acrisols ranged from sandy clay to sandy clay loam, whereas Ferralsols exhibited greater variation, including loam and sandy loam. In both soils, clay content increased with depth, indicating processes of clay illuviation.
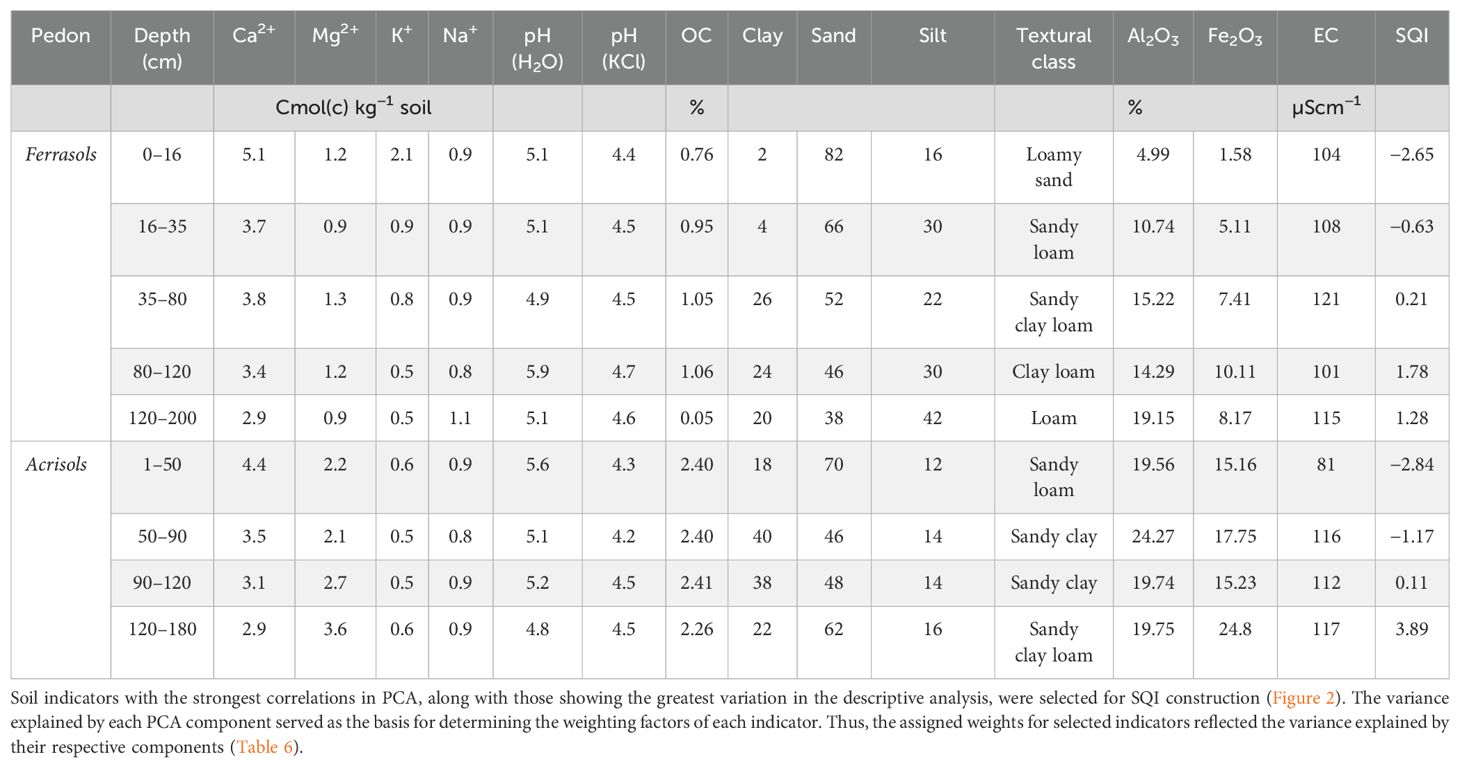
Table 5. Chemical and granulometric properties of the studied soils with corresponding SQI for each horizon.
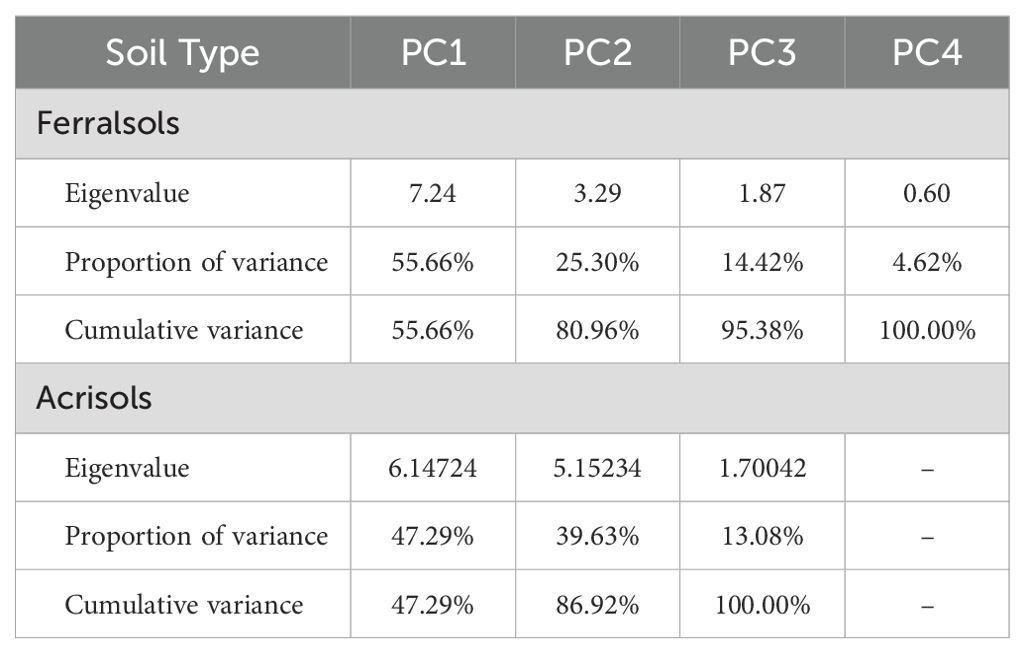
SQI was computed using 13 selected soil attributes (Table 5). For Ferralsols, the first principal component (PC1), which accounted for the highest variance (55.66%) (Figure 2A), was used in the SQI calculation. In Acrisols, both PC1 (47.29%) and PC2 (39.63%) were included, as together they explained the majority of variability in the dataset (Table 6, Figure 2B).
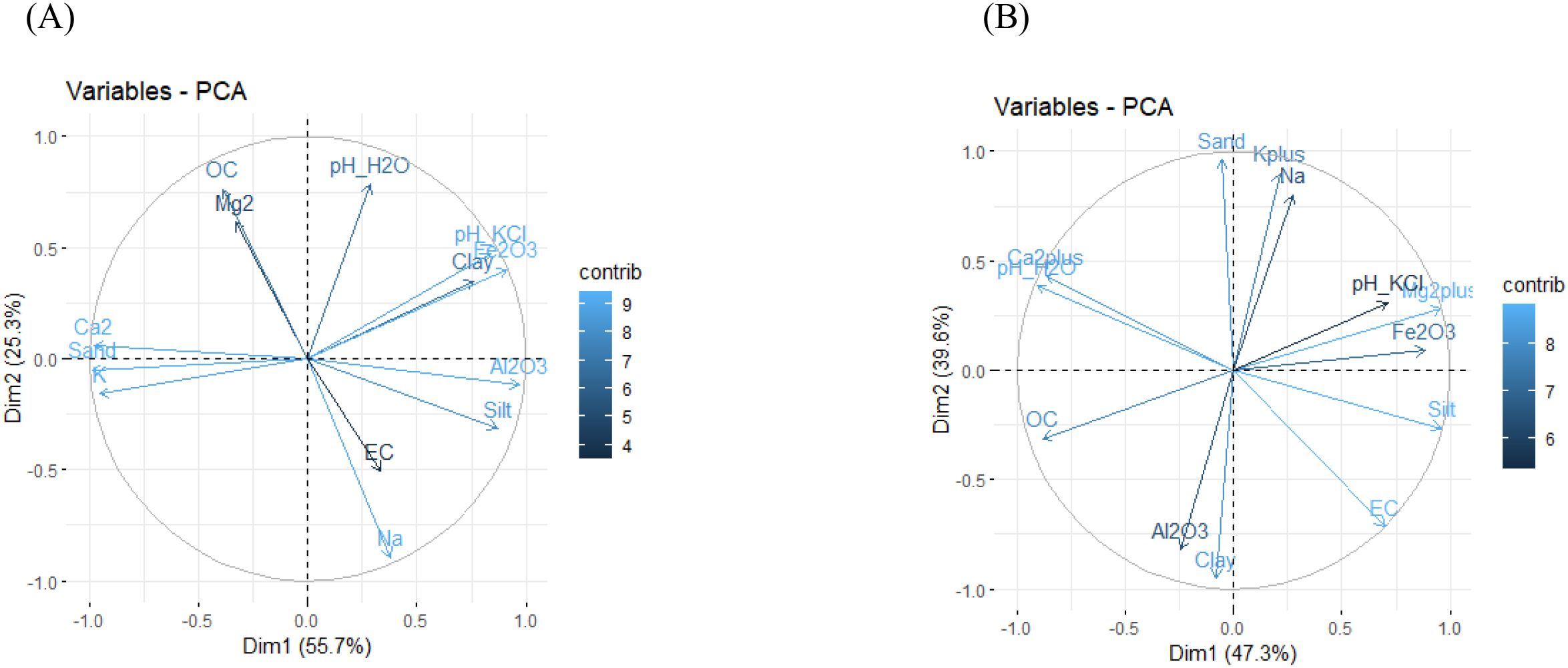
Figure 2. PCA of Ferralsols (A) and Acrisols (B), with the dimensions expressed as percentages used for SQI calculations.
In Ferralsols, the surface horizon (0–16 cm) exhibited the lowest SQI (−2.65), likely due to leaching and nutrient depletion. The middle horizons (35–80 cm and 80–120 cm) showed improved SQI values (−0.63 and 0.21, respectively), reflecting moderate nutrient retention, while the deeper horizon (120–200 cm) has the highest SQI value (1.78). In Acrisols, the top horizon (1–50 cm) had the lowest SQI (−2.84), suggesting severe nutrient leaching. However, the middle horizons (50–90 cm and 90–120 cm) displayed the higher SQI values (−1.17 and 0.11, respectively), indicating optimal nutrient retention and soil quality. The deepest horizon (120–180 cm) exhibited a higher SQI (3.89), consistent with the ferrasols pedon, showcasing a better fertility at greater depths.
In Ferralsols, SQI values range from −2.65 to 1.78, with mostly negative values indicating poor soil quality due to low organic carbon (OC), and clay content. This low organic carbon content can be attributed to the low amount of organic materials applied to the soil and to the complete removal of the biomass in the field, as it was observed by Pansu (52). The increase at 80–120 cm (SQI = 1.78) can be linked to improved cation retention at this depth, but the overall trend suggests nutrient depletion due to intense weathering and leaching.
Acrisols, in contrast, exhibit a wider SQI range (−2.84 to 3.89), with the surface horizon showing the lowest value (−2.84), likely due to high leaching, acidic pH, and relatively lower clay content. However, deeper layers (50–120 cm) show positive SQI values,which can be attributed to higher Mg2+ and clay content, enhancing nutrient retention and soil structure (53). The deeper horizon (120–180 cm) records a very positive SQI (3.89), possibly due to increased biological activity. Given that the soils of tropical regions are highly degraded, the quantities of exchangeable cations are limiting factors in agricultural productivity (54). As a result of this, the acidification of soil upper layers is observed (55). On the other hand, Lal & Stewart (56) suggest that continuous and intensive cultivation practices can explain the deterioration of the soil aggregates, combined with the low return of plant biomass to the soil in cultivated land. These SQI trends align with the advanced weathering and pedogenic characteristics of tropical soils, where surface horizons are often nutrient-depleted due to leaching, while subsurface horizons may exhibit some degree of nutrient accumulation, particularly in Acrisols.
A t-test was performed to assess whether the Soil Quality Index (SQI) differs significantly between Ferralsols and Acrisols. The mean SQI values were nearly identical for Ferralsols (−0.0020) and Acrisols (−0.0025), with variances of 3.07 and 8.19, respectively. The t-statistics (−0.0003) and degrees of freedom (4.75) indicate a reliable estimate, while the high p-value (0.9998) and the 95% confidence interval ([−4.26, 4.26]), which includes zero; confirm there is no statistically significant difference in SQI between the two soil types. Thus, any observed variation is likely due to chance.
3.5 Correlation of soil properties and potentially toxic elements
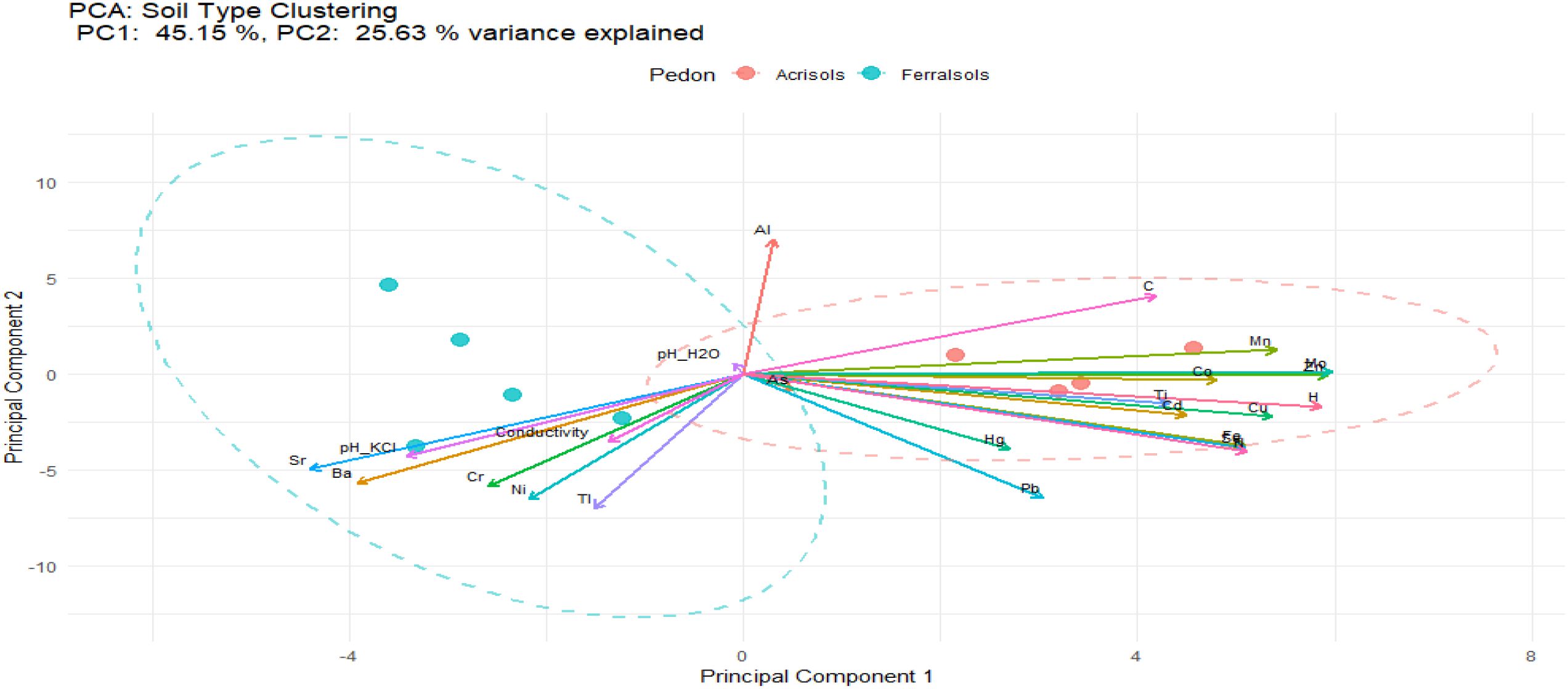
Principal component analysis (PCA) provides a clear differentiation between Acrisols and Ferralsols based on their elemental compositions and soil properties (Figure 3). The first two principal components (PC1 and PC2) represent 70.78% of the total variance, with PC1 accounting for 45.15% and PC2 contributing 25.63%. This spatial distinction suggests that the two soil types have distinct compositional profiles.
For Acrisols, the clustering of elements such as Ti, Co, Cd, Hg, Pb, Fe, Mn, Zn, Mo, Se, and total C, H, and N within the ellipse indicates that these parameters strongly contribute to their characterization. The similar lengths of their arrows suggest comparable levels of influence on PC1 and PC2. In contrast, Ferralsols are characterized by elements like Sr, Ba, Ti, Ni, Cr, and soil properties such as pH (KCl), conductivity, and CEC. The arrows for Sr, Ba, and pH (KCl) are the longest in this cluster, highlighting their dominant role in defining the variability within Ferralsols. The large spread of the Ferralsols ellipse along the x-axis implies greater variability in their elemental and physicochemical composition compared to the Acrisols. The unique positioning and long upward arrow of Al distinguishes it as a critical variable contributing to the separation along PC2. This implies that Al concentrations vary significantly between the two soil types and play a pivotal role in distinguishing their profiles.
The differentiation highlighted by the PCA between the soil types reflects the distinct geochemical and pedological processes influenced by their environmental conditions and parent materials. For Acrisols, the enrichment in trace elements like Ti, Co, Cd, Hg, Pb, Fe, Mn, Zn, Mo, and Se can be attributed to the weathering of parent rocks rich in these trace elements, limited leaching due to relatively lower pH or water movement, or anthropogenic inputs. The clustering of organic components, including total carbon C, H, and N, could indicate higher organic matter content or organic-mineral associations typical of Acrisols, which often form in areas with moderate weathering and vegetation cover.
In contrast, Ferralsols, characterized by higher variability in properties like Sr, Ba, pH (KCl), conductivity, and CEC, reflect their formation under intense weathering conditions common in tropical regions. These soils are often derived from deeply weathered parent material such as basalt or granites, leading to the accumulation of resistant minerals like aluminum oxides and iron oxides. The dominance of Ba and Sr may suggest the retention of these elements in secondary minerals or the influence of specific rock types, while the strong influence of pH (KCl) and CEC reflects the soil’s physicochemical properties influenced by clay mineralogy, organic matter interactions, and nutrient exchange dynamics. The prominent role of Al, as indicated by its distinct and long arrow, suggests that Al may be a significant marker of Ferralsols formation, likely due to the dominance of kaolinite and gibbsite, which are common in these soils (57).
3.6 Pearson correlation analysis
The Pearson correlation matrix between elements, indices and soil attributes is presented in (Figure 4), in the form of a matrix heatmap. The tricolor heatmap shows dark red boxes with strong positive, dark blue boxes with strong negative, and white boxes with intermediate correlation.
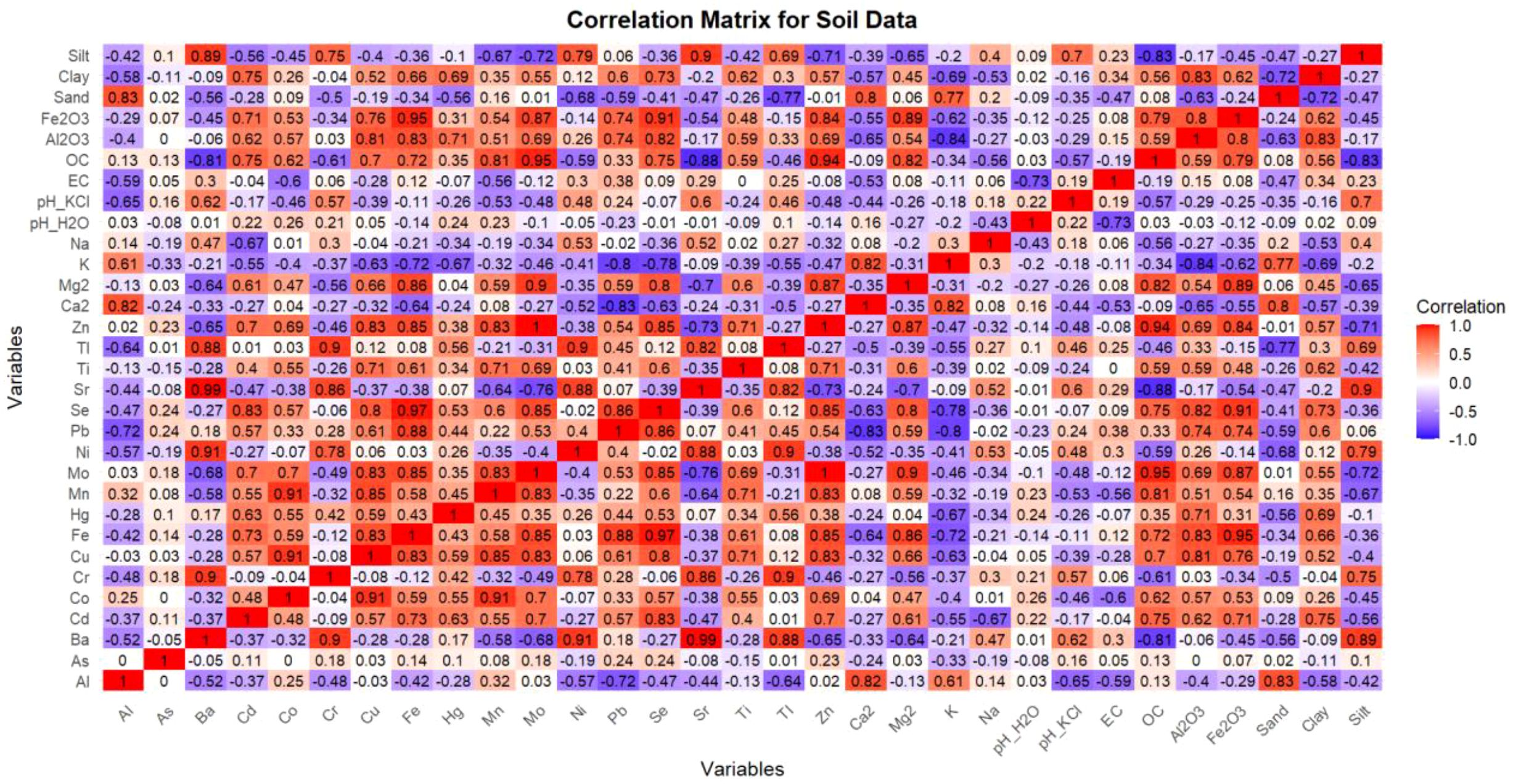
Figure 4. Heatmap of Pearson’s correlation coefficients between soil attributes, elements-trace metals for the two soil pedons. Deep blue colors reflect negative positive correlations, whereas deep red colors reflect strong positive correlations.
The revised Pearson correlation heatmap highlights significant associations between elemental concentrations and key soil physicochemical properties, offering insights into the mechanisms controlling trace metal behavior in Acrisols and Ferralsols. Strong positive correlations were observed between Fe2O3 and several trace metals, including Cd (r = 0.71), Co (r = 0.53), Cu (r = 0.76), Pb (r = 0.74), Se (r = 0.91), and Zn (r = 0.84), underscoring the critical role of iron oxides in the adsorption and immobilization of potentially toxic elements. These associations reflect the high affinity of iron oxides for metal binding, which helps reduce metal mobility and bioavailability in tropical soils.
Similarly, Al2O3 displayed strong correlations with metals such as Cd (r = 0.62), Co (r = 0.57), Cu (r = 0.81), Fe (r = 0.83), Hg (r = 0.71), Mo (r = 0.69), and Zn (r = 0.76), suggesting that aluminum oxides also contribute significantly to trace element retention. The high surface area and reactive hydroxyl groups of Al2O3 likely facilitate the formation of inner-sphere complexes with metal ions, enhancing their sorption and stabilization.
Organic carbon (OC) showed strong correlations with Fe2O3 (r = 0.79), Al2O3 (r = 0.78), and Mg2+ (r = 0.80), indicating the synergistic role of soil organic matter and oxides in metal complexation. These interactions may promote organo-mineral associations that further stabilize trace metals. Additionally, clay content correlated moderately with both Fe2O3 (r = 0.62) and OC (r = 0.60), suggesting that fine-textured soils enhance the retention capacity for trace elements due to increased surface area and cation exchange potential.
Exchangeable bases such as Ca2+ and Mg2+ also demonstrated notable correlations with elements including Zn, Cu, Se, and K, reflecting their involvement in metal mobility and nutrient dynamics. Ca2+, in particular, showed strong positive correlations with Al (r = 0.82), K (r = 0.82), and sand content (r = 0.80), suggesting its role in ion exchange and soil structural processes.
Overall, the correlation analysis underscores that trace element distribution in these soils is predominantly governed by intrinsic factors such as oxide content, organic matter, and texture, rather than contamination indices. These findings reinforce the need to assess geochemical interactions using independent variables for more robust interpretations.
4 Conclusion
This study presents a detailed comparative analysis of the geochemical behavior, contamination potential, and soil quality of Acrisols and Ferralsols within a tropical environment. Distinct elemental distribution patterns were identified, with Ferralsols exhibiting elevated concentrations of Ba, Zn, Cu, and Fe, accompanied by lower (SQI) values in surface horizons (–2.65 to 1.78), likely reflecting enhanced leaching and organic matter depletion. Conversely, Acrisols demonstrated higher nutrient retention, particularly in subsurface horizons (SQI: –2.84 to 3.89), and showed moderate enrichment of elements such as Se, Mo, Fe, and Ti. Pearson correlation analysis, revealed significant positive relationships between heavy metal concentrations and soil physicochemical properties, including organic carbon, clay content, (Al2O3), and (Fe2O3). These correlations confirm the pivotal role of mineralogy and soil texture in modulating metal mobility and retention. The evidence indicates that Ferralsols are more susceptible to nutrient depletion and potential quality degradation due to their susceptibility to intense weathering and leaching, despite their higher oxide content, which influences metal retention. In contrast, Acrisols demonstrate greater resilience through improved nutrient conservation and buffering capacity but may be prone to moderate accumulation of specific trace elements. From a land management perspective, these findings suggest that Acrisols possess a comparatively higher agricultural sustainability potential, contingent upon regular monitoring of trace metal accumulation, while Ferralsols necessitate targeted soil conservation measures, including organic amendments and erosion control. Tailored management strategies that consider these soil-specific geochemical dynamics are essential to optimize crop productivity and minimize ecological risks in tropical agroecosystems.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MM: Validation, Methodology, Formal Analysis, Data curation, Writing – review & editing, Conceptualization, Software, Writing – original draft, Investigation. AB: Validation, Supervision, Writing – review & editing. FK: Writing – review & editing, Supervision, Resources, Project administration, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We gratefully acknowledge the OCP Nutricrop group for financial assistance to undertake the research project. We are also grateful the CESFRA Laboratory, the Mohammed VI Polytechnic University for their assistance during samples analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dickinson G and Murphy K. ECOSYSTEMS. In: Routledge introd to environ ser. London (UK), New York, USA: Routledge (2007). p. 1–23.
2. Corlett RT, Primack RB, and Schwarz E. Tropical rainforest conservation : A global perspective. Oxford, UK: Trop For community Ecol (2006) p. 442–57.
3. Dwomo O and Dedzoe C. Oxisol (Ferralsol) development in two agro-ecological zones of Ghana: A preliminary evaluation of some profiles. J Sci Technol. (2010) 30:102–11. doi: 10.4314/just.v30i2.60538
4. Beinroth FH. Geomorphic relationships of oxisols and ultisols on kauai, hawaii. Soil Sci Soc Am. (1973) 38:128–31. doi: 10.2136/sssaj1974.03615995003800010039x
5. von Uexküll HR and Mutert E. Global extent, development and economic impact of acid soils. Plant Soil. (1995) 171:1–15. doi: 10.1007/BF00009558
6. Alimonti A, Petrucci F, Krachler M, Bocca B, and Caroli S. Reference values for chromium, nickel and vanadium in urine of youngsters from the urban area of Rome. J Environ Monit. (2000) 2:351–4. doi: 10.1039/b001616k
7. Smaling EMA, Fresco LO, and De Jager A. Classifying, monitoring and improving soil nutrient stocks and flows in african agriculture. Ambio. (1996) 25:492–6.
8. Tipping E, Rieuwerts J, Pan G, Ashmore MR, Lofts S, Hill MTR, et al. The solid-solution partitioning of heavy metals (Cu, Zn, Cd, Pb) in upland soils of England and Wales. Environ pollut. (2003) 125:213–25. doi: 10.1016/S0269-7491(03)00058-7
9. Kookana RS and Naidu R. Effect of soil solution composition on cadmium transport through variable charge soils. Geoderma. (1998) 84:211–23. doi: 10.1016/S0016-7061(97)00131-6
10. Onyatta JO and Huang PM. Chemical speciation and bioavailability index of cadmium for selected tropical soils in Kenya. Geoderma. (1999) 93:87–101. doi: 10.1016/S0016-7061(99)00002-6
11. Mbila MO, Thompson ML, Mbagwu JSC, and Laird DA. Distribution and movement of sludge-derived trace metals in selected Nigerian soils. J Environ Qual. (2001) 30:1667–74. doi: 10.2134/jeq2001.3051667x
12. Appel C and Ma L. Concentration, pH, and surface charge effects on cadmium and lead sorption in three tropical soils. J Environ Qual. (2002) 31:581–9. doi: 10.2134/jeq2002.5810
13. Rosolen V, Herpin U, Fränzle S, Breulmann G, de Camargo PB, Paganini WS, et al. Land application of wastewater in Brazil - A scientific challenge: Chemical characterization of soil at Populina, São Paulo State. J Soils Sediments. (2005) 5:112–20. doi: 10.1065/jss2005.05.137
14. Bertoncini EI, Mattiazzo ME, and Rossetto R. Sugarcane yield and heavy metal availability in two biosolid-amended oxisols. J Plant Nutr. (2004) 27:1243–60. doi: 10.1081/PLN-120038546
15. De Alcântara MAK and De Camargo OA. Chromium movement in columns of two highly weathered soils. Commun Soil Sci Plant Anal. (2004) 35:599–613. doi: 10.1081/CSS-120030346
16. Udom BE, Mbagwu JSC, Adesodun JK, and Agbim NN. Distributions of zinc, copper, cadmium and lead in a tropical ultisol after long-term disposal of sewage sludge. Environ Int. (2004) 30:467–70. doi: 10.1016/j.envint.2003.09.004
17. Mugoša B, Ðuroviā D, Nedović-Vuković M, Barjaktarović-Labović S, and Vrvić M. Assessment of ecological risk of heavy metal contamination in coastal municipalities of Montenegro. Int J Environ Res Public Health. (2016) 13:403. doi: 10.3390/ijerph13040393
18. Fairbrother A, Wenstel R, Sappington K, and Wood W. Framework for metals risk assessment. Ecotoxicol Environ Saf. (2007) 68:145–227. doi: 10.1016/j.ecoenv.2007.03.015
19. Driessen P, Deckers J, and Spaargaren O. Lecture notes on the major soil of the world (2001). Available online at: http://www.fao.org/documents/show_cdr.asp?url_file=/DOCREP/003/Y1899E/y1899e06.htm (Accessed July 10, 2025).
20. FAO. World reference base for soil resources 2006: A framework for international classification, correlation and communication. World Soil Resources Reports No. 103. Rome, Italy: Food and Agriculture Organization of the United Nations (2006)
21. Ganatsios HP, Tsioras PA, Papaioannou AG, and Blinn CR. Short term impacts of harvesting operations on soil chemical properties in a mediterranean oak ecosystem. Croat J For Eng. (2021) 42:463–76. doi: 10.5552/crojfe.2021.1100
22. Thomas GW. Chapter 16. Methods soil anal. In: Part 3. SSSA book series no. 5. Madison, WI, USA: Soil Science Society of America (SSSA) (1996). p. 107–15.
23. Oertel A and Giles J. Trace element contents of some Queensland soils. Soil Res. (1963) 1:215–22. doi: 10.1071/SR9630215
24. Banat KM, Howari FM, and Al-Hamad AA. Heavy metals in urban soils of central Jordan: should we worry about their environmental risks? Env. Res.. (2005) 97:258–73. doi: 10.1016/j.envres.2004.04.002
25. Buccolieri A, Buccolieri G, Cardellicchio N, Dell’Atti A, Di Leo A, and Maci A. Heavy metals in marine sediments of Taranto Gulf (Ionian Sea, Southern Italy). Mar Chem. (2006) 99:227–35. doi: 10.1016/j.marchem.2005.09.009
26. Zhang J and Liu CL. Riverine composition and estuarine geochemistry of particulate metals in China weathering features, anthropogenic impact and chemical fluxes. Estuar. Coast. Shelf Sci. (2002) 54:1051–70. doi: 10.1006/ecss.2001.0879
27. Blaser P, Zimmermann S, Luster J, and Shotyk W. Critical examination of trace‑element enrichments and depletions in soils: As, Cr, Cu, Ni, Pb and Zn in Swiss forest soils. Sci. Total Environ. (2000) 249:257–80. doi: 10.1016/S0048-9697(99)00522-7
28. Sutherland RA, Tolosa CA, Tack FMG, and Verloo MG. Characterization of selected element concentrations and enrichment ratios in background and anthropogenically impacted roadside areas. Arch Environ Contam Toxicol. (2000) 38:428–38. doi: 10.1007/s002440010057
29. Hakanson L. An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. (1980) 14:975–1001. doi: 10.1016/0043-1354(80)90143-8
30. Mukherjee A and Lal R. Comparison of soil quality index using three methods. PloS One. (2014) 9. doi: 10.1371/journal.pone.0105981
31. De Matos AT, Fontes MPF, Da Costa LM, and Martinez MA. Mobility of heavy metals as related to soil chemical and mineralogical characteristics of Brazilian soils. Environ pollut. (2001) 111:429–35. doi: 10.1016/S0269-7491(00)00088-9
32. Barreto Mascarenhas R, Bomfim de Jesus T, Gloaguen TV, Del’Arco Vinhas Costa O, and Wyzykowski J. Quality reference values for trace metals in Podzols, Ferralsols, and Acrisols of Brazilian Atlantic rainforest. Catena. (2022) 210:105879. doi: 10.1016/j.catena.2021.105879
33. Gloaguen TV and Passe JJ. Importance of lithology in defining natural background concentrations of Cr, Cu, Ni, Pb and Zn in sedimentary soils, northeastern Brazil. Chemosphere. (2017) 186:31–42. doi: 10.1016/j.chemosphere.2017.07.134
34. Caires SMDE. Determinação dos teores naturais de metais pesados em solos do estado de minas gerais como subsídio ao estabelecimento de valores de referência de qualidade. Belo Horizonte, Brazil: Fundação Estadual do Meio Ambiente (FEAM) (2009). pp. 1–270.
35. Deckers J, Lelis Leal de Souza JJ, Mantel S, Neil-yohan M, Obame RM, and Vancampenhout K. WRB documentation centre FERRALSOLS : lecture notes. Leuven, Belgium: WRB Documentation Centre (2024). pp. 1–16. pp. 1–16.
36. Batjes NH. Soil parameter estimates for the soil types of the world for use in global and regional modelling. Soil Use Manag. (2002) 18:232–5. doi: 10.1111/j.1475-2743.2002.tb00244.x
37. Fujikawa Y, Fukui M, and Kudo A. Vertical distributions of trace metals in natural soil horizons from Japan. Part 1. Effect of soil types. Water Air Soil pollut. (2000) 124:1–21. doi: 10.1023/A:1005120204500
38. Palumbo B, Angelone M, Bellanca A, Dazzi C, Hauser S, Neri R, et al. Influence of inheritance and pedogenesis on heavy metal distribution in soils of Sicily, Italy. Geoderma. (2000) 95:247–66. doi: 10.1016/S0016-7061(99)00090-7
39. Fontes MPF and Weed SB. Iron oxides in selected Brazilian oxisols: I. Mineralogy. Soil Sci Soc Am J. (1991) 55:1143–9. doi: 10.2136/sssaj1991.03615995005500040040x
40. Alloway BJ, Jackson AP, and Morgan H. The accumulation of cadmium by vegetables grown on soils contaminated from a variety of sources. Sci Total Environ. (1990) 91:223–36. doi: 10.1016/0048-9697(90)90300-J
41. Kabata-Pendias A. Behavioural properties of trace metals in soils. Appl Geochemistry. (1993) 8:3–9. doi: 10.1016/S0883-2927(09)80002-4
42. Bansah KJ and Addo WK. Phytoremediation potential of plants grown on reclaimed spoil lands. Ghana Min J. (2016) 16:68. doi: 10.4314/gmj.v16i1.8
43. Suess E, Aemisegger F, Sonke JE, Sprenger M, Wernli H, and Winkel LHE. Marine versus Continental Sources of Iodine and Selenium in Rainfall at Two European High-Altitude Locations. Environ Sci Technol. (2019) 53:1905–17. doi: 10.1021/acs.est.8b05533
44. Pearson C, Howard D, Moore C, and Obrist D. Mercury and trace metal wet deposition across five stations in Alaska: Controlling factors, spatial patterns, and source regions. Atmos Chem Phys. (2019) 19:6913–29. doi: 10.5194/acp-19-6913-2019
45. Uchiyama R, Okochi H, Ogata H, Katsumi N, and Nakano T. Characteristics of trace metal concentration and stable isotopic composition of hydrogen and oxygen in “urban-induced heavy rainfall” in downtown Tokyo, Japan; The implication of mineral/dust particles on the formation of summer heavy rainfall. Atmos Res. (2019) 217:73–80. doi: 10.1016/j.atmosres.2018.10.017
46. Borges CS, Weindorf DC, Nascimento DC, Curi N, Guilherme LRG, Carvalho GS, et al. Comparison of portable X-ray fluorescence spectrometry and laboratory-based methods to assess the soil elemental composition: Applications for wetland soils. Environ Technol Innov. (2020) 19:100826. doi: 10.1016/j.eti.2020.100826
47. Siebecker MG, Chaney RL, and Sparks DL. Natural speciation of nickel at the micrometer scale in serpentine (ultramafic) topsoils using microfocused X-ray fluorescence, diffraction, and absorption. Geochem Trans. (2018) 19:1–16. doi: 10.1186/s12932-018-0059-2
48. Quantin C, Ettler V, Garnier J, and Šebek O. Sources and extractibility of chromium and nickel in soil profiles developed on Czech serpentinites. Comptes Rendus - Geosci. (2008) 340:872–82. doi: 10.1016/j.crte.2008.07.013
49. USEPA. Supplemental guidance for developing soil screening levels for superfund sites. Office of solid waste and emergency response (OSWER). Washington, DC: United States Environ Prot Agency (2002) p. 1–187. Available online at: https://nepis.epa.gov/Exe/ZyPDF.cgi/91003IJK.PDF?Dockey=91003IJK.PDF (Accessed July 10, 2025).
50. Commission CA. Report of the 33rd session of the codex committee on food additives and contaminants. Codex alimentarius commission. The Hague, The Netherlands: Joint FAO/WHO Food Standards Programme (2001) p. 2–7.
51. Dabin B. Les facteurs de la fertilité des sols des régions tropicales en culture irriguée. Paris, France: Bull Assoc française d’Etude du Sol (1961) p. 108–30.
52. Pansu J, Giguet-Covex C, Ficetola GF, Gielly L, Boyer F, Zinger L, et al. Reconstructing long-term human impacts on plant communities: An ecological approach based on lake sediment DNA. Mol Ecol. (2015) 24:1485–98. doi: 10.1111/mec.13136
53. Gelman F, Binstock R, and Halicz L. Application of the Walkley-Black titration for the organic carbon quantification in organic rich sedimentary rocks. Fuel. (2012) 96:608–10. doi: 10.1016/j.fuel.2011.12.053
54. Pulido-Moncada M, Ball BC, Gabriels D, Lobo D, and Cornelis WM. Evaluation of soil physical quality index S for some tropical and temperate medium-textured soils. Soil Sci Soc Am J. (2015) 79:9–19. doi: 10.2136/sssaj2014.06.0259
55. Rangel-Peraza JG, Padilla-Gasca E, López-Corrales R, Medina JR, Bustos-Terrones Y, Amabilis-Sosa LE, et al. Robust soil quality index for tropical soils influenced by agricultural activities. J Agric Chem Environ. (2017) 06:199–221. doi: 10.4236/jacen.2017.64014
56. Lal R and Stewart BA. Food security and soil quality Vol. 11. Boca Raton: CRC Press (2010) p. 1–14.
Keywords: metals, tropical soils, soil properties, sustainable agriculture, soil fertility
Citation: El Mellouki M, Boularbah A and Kebede F (2025) Quantitative evaluation of potentially toxic elements and associated risks in Acrisols and Ferralsols of western Ghana. Front. Soil Sci. 5:1638448. doi: 10.3389/fsoil.2025.1638448
Received: 30 May 2025; Accepted: 01 July 2025;
Published: 23 July 2025.
Edited by:
Naser A. Anjum, Aligarh Muslim University, IndiaReviewed by:
Anna Grobelak, Częstochowa University of Technology, PolandLinda Osei, University of Mines and Technology, Ghana
Copyright © 2025 El Mellouki, Boularbah and Kebede. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meryem El Mellouki, TWVyeWVtLmVsbWVsbG91a2lAdW02cC5tYQ==
 Meryem El Mellouki
Meryem El Mellouki Ali Boularbah
Ali Boularbah Fassil Kebede
Fassil Kebede