- 1Department of Neurosurgery, Faculty of Medicine, Hacettepe University, Ankara, Turkey
- 2BTech Innovation, METU Technopark, Ankara, Turkey
- 3Department of Software Engineering, Cankaya University, Ankara, Turkey
- 4Department of Anatomy, Faculty of Medicine, Hacettepe University, Ankara, Turkey
Objective: Neurosurgical patient-specific 3D models have been shown to facilitate learning, enhance planning skills and improve surgical results. However, there is limited data on the objective validation of these models. Here, we aim to investigate their potential for improving the accuracy of surgical planning process of the neurosurgery residents and their usage as a surgical planning skill assessment tool.
Methods: A patient-specific 3D digital model of parasagittal meningioma case was constructed. Participants were invited to plan the incision and craniotomy first after the conventional planning session with MRI, and then with 3D model. A feedback survey was performed at the end of the session. Quantitative metrics were used to assess the performance of the participants in a double-blind fashion.
Results: A total of 38 neurosurgical residents and interns participated in this study. For estimated tumor projection on scalp, percent tumor coverage increased (66.4 ± 26.2%–77.2 ± 17.4%, p = 0.026), excess coverage decreased (2,232 ± 1,322 mm2–1,662 ± 956 mm2, p = 0.019); and craniotomy margin deviation from acceptable the standard was reduced (57.3 ± 24.0 mm–47.2 ± 19.8 mm, p = 0.024) after training with 3D model. For linear skin incision, deviation from tumor epicenter significantly reduced from 16.3 ± 9.6 mm–8.3 ± 7.9 mm after training with 3D model only in residents (p = 0.02). The participants scored realism, performance, usefulness, and practicality of the digital 3D models very highly.
Conclusion: This study provides evidence that patient-specific digital 3D models can be used as educational materials to objectively improve the surgical planning accuracy of neurosurgical residents and to quantitatively assess their surgical planning skills through various surgical scenarios.
Introduction
Neurosurgical training requires profound anatomical knowledge complemented with acquisition of visuospatial and fine motor skills (1–3). One of the main challenges in neurosurgical training is to comprehend the complex 3D relationships of pathological and anatomical structures, and apply this knowledge to surgical operations (3, 4). This requires dedication, effort, and practice as well as the use of various educational resources and tools to maximize learning experiences (1, 3, 5–10).
A wide spectrum of educational tools from cadaver dissections to virtual 3D models and simulators have been widely used to enhance training of neurosurgeons (11–13). Although generic models are useful to learn anatomy and normal or pathological variations, their potential to complement surgical planning skills for specific cases are limited since they do not reflect exact pathological anatomy of individual patients.
With the advent of neuroimaging and image processing technologies, patient-specific 3D models, either digital or printed, can now be produced and used for surgical planning simulations (14–17). Neurosurgical patient-specific 3D printed models have been shown to facilitate learning, enhance planning skills and improve surgical results in brain tumor, skull base and cerebrovascular surgeries (14–19). However, they have certain disadvantages such as need for special equipment and materials, higher costs, lengthy production process, lack of repetitive use, which limit their utility in every-day practice and training of neurosurgical residents (14, 18, 20, 21). On the other hand, high quality, multilayered digital 3D images or models can be easily produced with limited resources and adopted in the routine neurosurgical planning pipeline. Despite several studies published in recent years (22, 23), literature is scarce for the validation of these personalized-3D virtual models as both educational and assessment tools.
Materials and methods
Study design
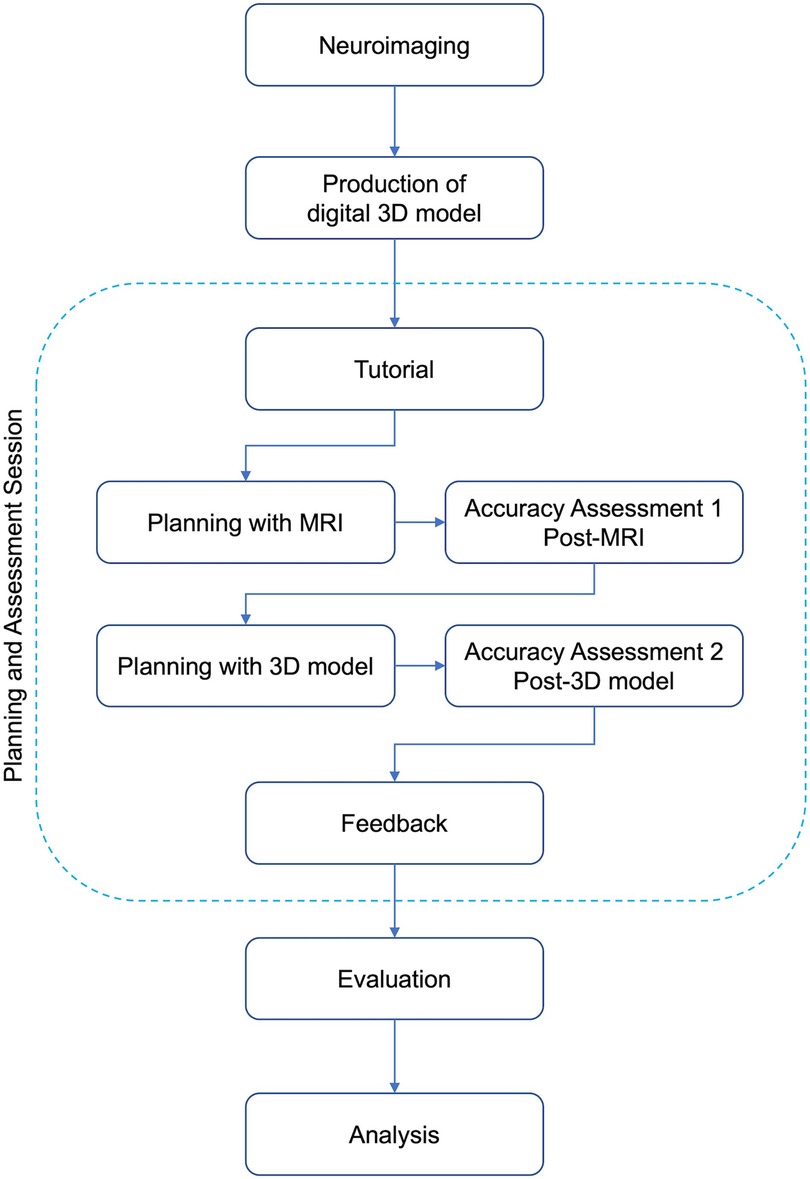
This study was conducted as a proof-of-concept study on current neurosurgical residents and interns (who completed one-month clinical rotation in neurosurgery) in one university hospital. The study was designed to demonstrate whether adding a brief practice with patient-specific digital 3D model to conventional surgical planning session with 2D images (axial, coronal and sagittal MRI) has any effect on the accuracy of surgical planning process of the residents. The design of the study is illustrated as a diagram in Figure 1. Briefly, the study included three phases: 1) preparation, (2) planning and assessment session, (3) evaluation and analysis.
For the preparation phase, a brain tumor case was selected via search of our institutional database with the following criteria: (i) common pathology, (ii) good quality neuroimaging available, (iii) proximity to critical neurovascular structures, (iv) requirement for a relatively standard surgical planning.
Planning and assessment were performed for each neurosurgical resident separately in a face-to-face session using a structured methodology described below. Lastly, assessment of the performances was retrospectively conducted by using digital data collected during the accuracy assessment session.
Reconstruction of patient-specific 3D model
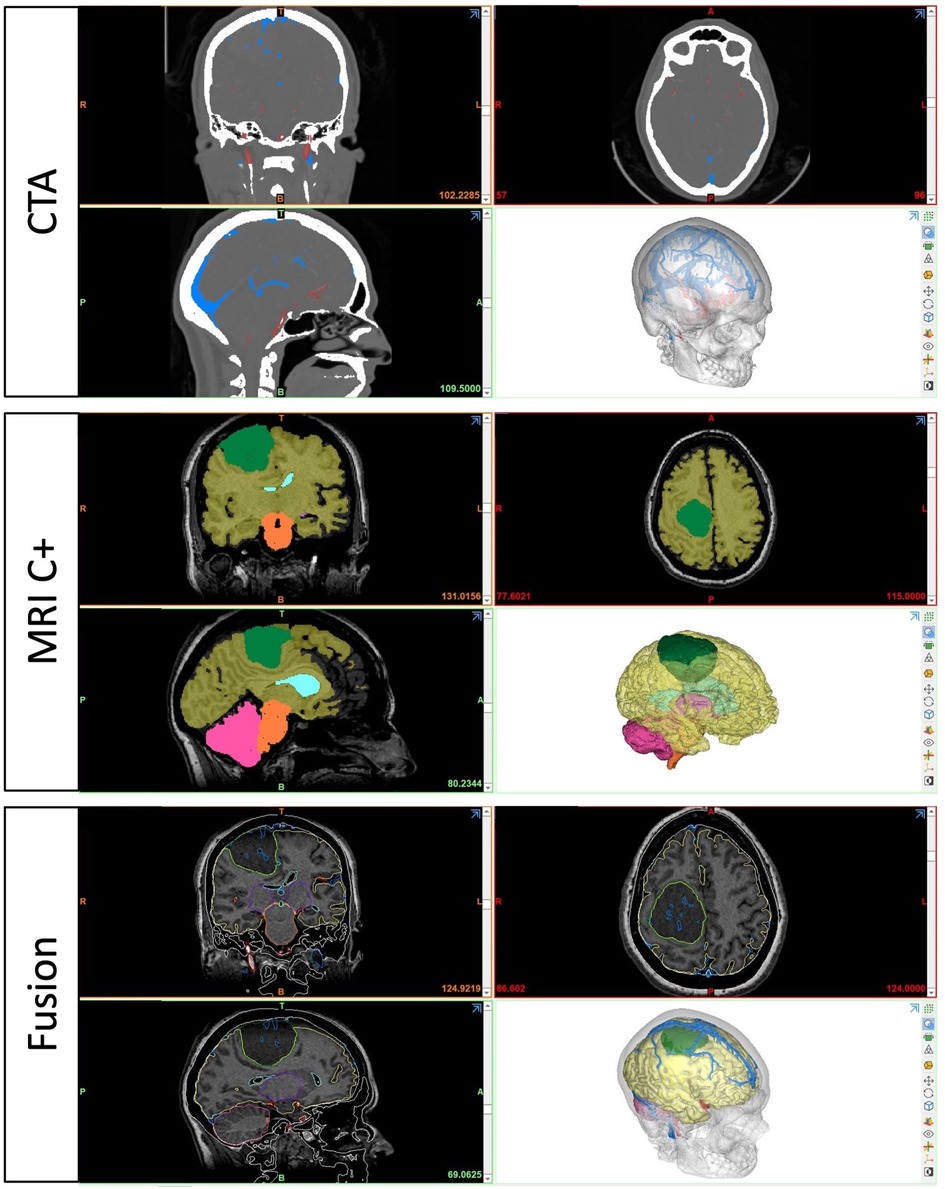
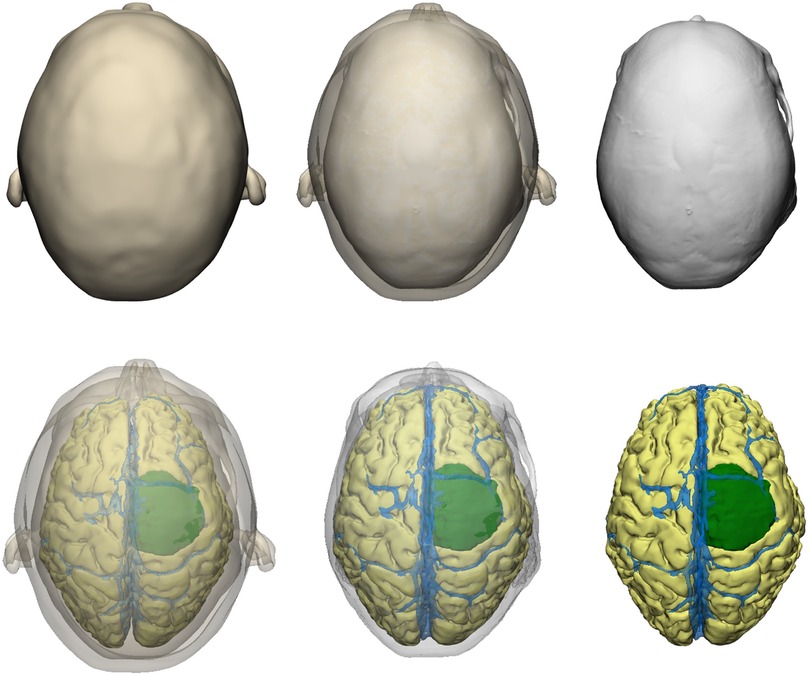
For the preparation phase, one brain tumor case (40-year-old female with large parasagittal/parafalcine meningioma) was selected for this study. Neuroimaging data including volumetric MRI and CT scans were used for the reconstruction of the patient-specific digital 3D model. All 3D planning and modeling studies were carried out with Mimics Innovation Suite 22.0 Software (Materialise, Leuven, Belgium). Briefly, DICOM files of magnetic resonance imaging (MRI) or computed tomography (CT) scans were imported into Mimics. 2-dimensional radiological images were visualized on axial, coronal and sagittal planes. Masking process was undertaken using Hounsfield unit (HU) values on 2D radiological images. Segmentation of various structures was done according to anatomical borders. Different imaging sequences were used for segmentation of different intracranial structures: CT angiography for bone and vessels, T1W images with contrast for brain, ventricles and tumor (Figure 2). All CT and MRI scans were merged and aligned with the Align Global Registration module. Surface-rendering technique was used to produce 3D models of different anatomical structures. Then, a design module (3-matic 14.0, Materialise, Leuven, Belgium) was used for fine-tuning and detailed modeling. This allowed us to simulate and rehearse surgical scenarios by freely rotating, positioning, trimming, and adjusting transparency of the model and its components virtually (Figure 3).
Planning and assessment session
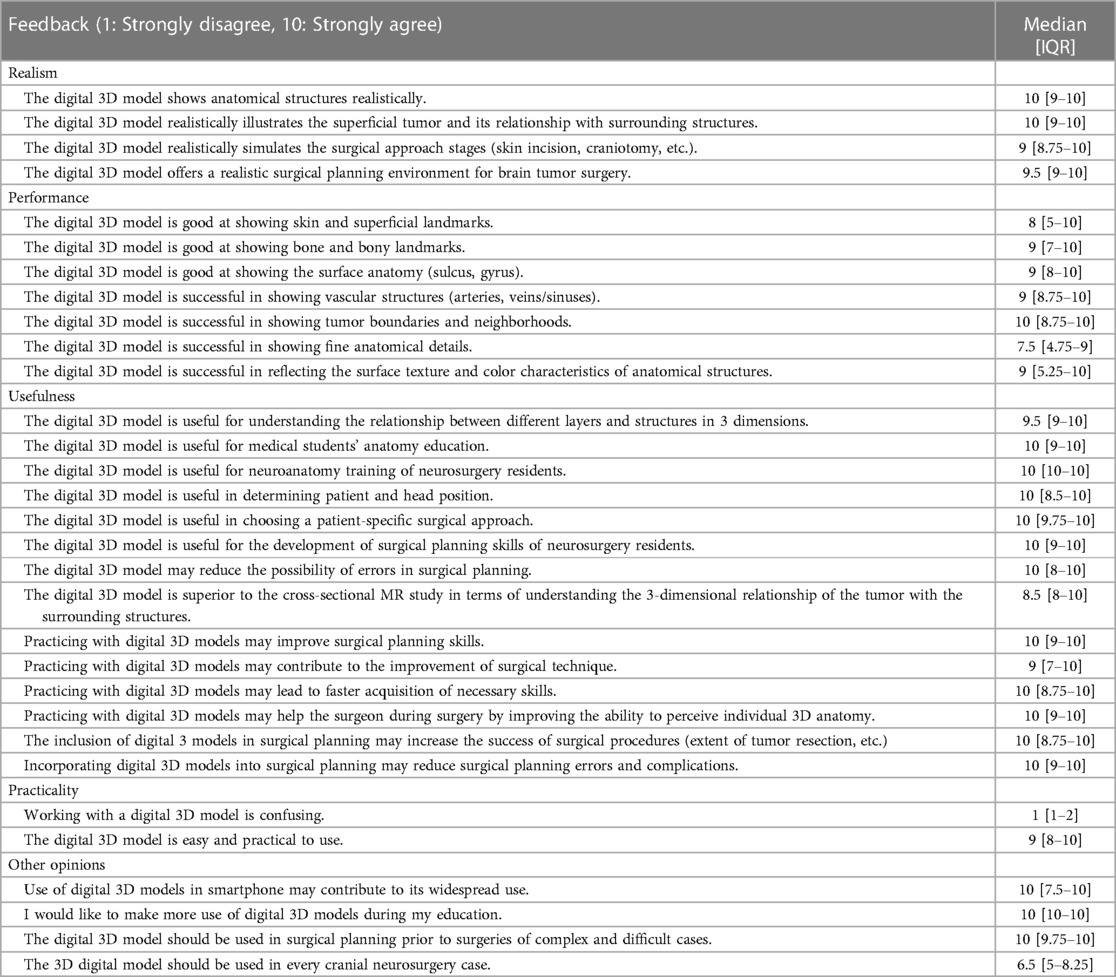
A dedicated planning and assessment session in a quiet room was designed for this study. Two computers were used for the session, one for studying standard MRI as well as demographic data collection, assessment questions, and feedback, whereas the other was used for 3D model visualization and surgical planning assessment (Figure 4A). Participants followed the instructions for planning and assessment procedures with the guidance of an instructor who was not involved in the study design, assessment, and analysis (first blinding). After receiving a brief tutorial about the session and filling in the demographic and baseline information, the participant had 5 min to study the MRI examination (all 2D images at three planes) of the patient (planning with MRI). After that, they were asked to answer questions about the case (basic concepts, anatomy, radiology, surgical technique etc.). After these assessment questions, they were invited to perform virtual planning on the 3D model. For this accuracy assessment session, only opaque digital skin and bone models were used without exposing underlying tumor, brain, or intracranial vessels. Surgical planning steps included the drawing of (i) tumor projection on skin (painted area) (Figure 4B), (ii) linear and U-shaped incision (Figures 4C,D), (ii) burr holes and craniotomy borders (Figures 4E,F). Planning of each surgical step on the digital 3D model was individually recorded after each assessment session for each participant. After the Accuracy Assessment-1 (post-planning with MRI), the participant had a 5-min planning session studying the patient-specific 3D model. For planning with the 3D model, participants were free to use the 3D model to its full extent with all available viewer options (transparency, trimming etc.). After the second planning session, the participant underwent the same assessment again (questions, drawings) to better understand if there is an improvement in their surgical planning strategies. This assessment for the post-planning with the 3D model was regarded and recorded as the Accuracy Assessment-2. After all planning and assessment procedures were completed, participants were asked to provide their opinions (feedback) about the given statements by using a Likert-scale from 1 (strongly disagree) to 10 (strongly agree). The statements were about the realism, utility, practicality, and future potential of the digital 3D models (Table 1). All data were collected electronically using Google Forms. Surgical planning assessment data were saved as Mimics planning files.

Figure 4. Planning and assessment session. (A) Photograph of the study setup. Drawings of (B) anticipated tumor projection on scalp, (C) linear incision, (D) U-shape incision, (E) burr holes and (F) craniotomy on two Accuracy Assessment layers (skin and bone) of the model.
Assessment methodology
We aimed to assess the patient-specific radiological anatomy knowledge and surgical planning skills using the answers and drawings (i.e., surgical plans). The whole assessment was done by two neurosurgeons who were blind to identity and baseline information about the participant as well as the order of assessment (second blinding). The following parameters were measured and analyzed: (i) tumor projection on skin (percent tumor coverage, excess coverage), (ii) linear incision: deviation from center of tumor (mm), (iii) accuracy of U-incision (assessed by points from 0-the least accurate to 4-the most accurate), (iv) accuracy of craniotomy borders (assessed by points from 0-the least accurate to 4-the most accurate) (Figure 5). To calculate craniotomy score, each edge of craniotomy was scored 1 if it was within 5 mm–15 mm of tumor border (if three edges were within these limits, composite craniotomy score was 3). A similar approach was adopted for U-shape incision, the base of skin flap got an extra point if an imaginary line connecting two corners was within the limits. We also calculated the sum of deviation of 4 edges (margins) from the acceptable standard craniotomy borders (craniotomy margin deviation) as a more robust alternative to craniotomy score. For tumor projection, percent tumor coverage was calculated as covered (overlapping) tumor area/total calculated tumor area (2,717 mm2), whereas excess coverage was measured as the portion of painted area (as drawn by the participant) which did not match the underlying tumor (mm2).
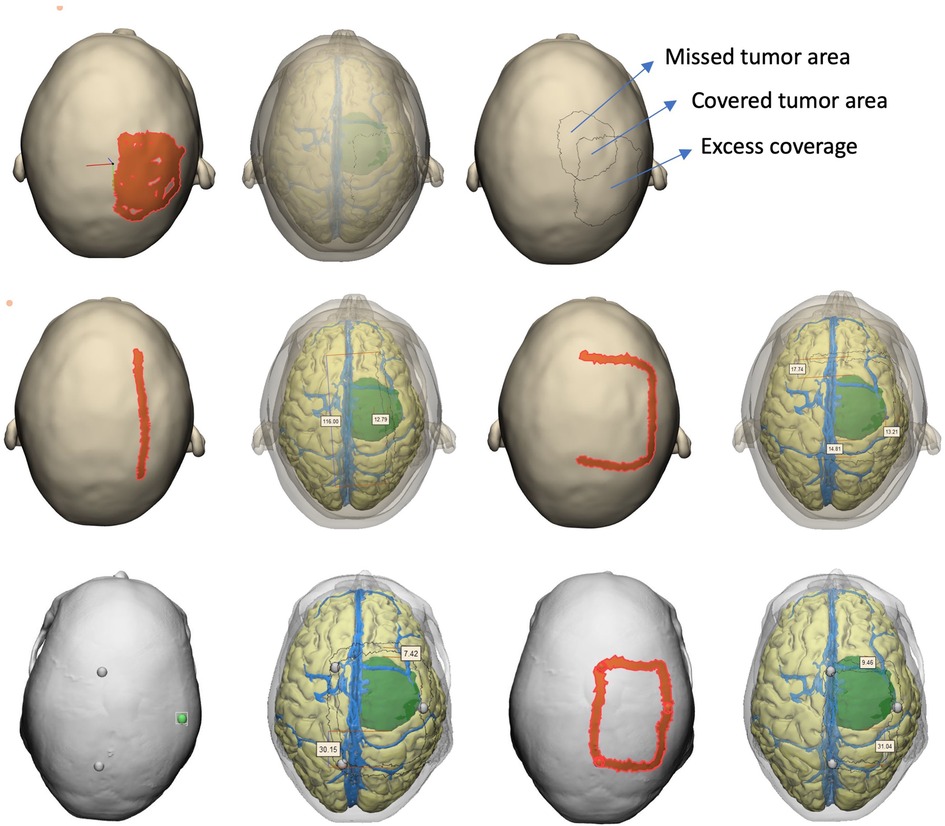
Figure 5. Assessment methodology. After saving each participant's drawings in digital format, assessors used the full model with transparency option to objectively measure the accuracy of drawings with reference to underlying tumor.
Statistical analysis
All statistical analyses were performed using the Prism 9 software (GraphPad Software, LLC., 2021). Descriptive statistics were presented using mean and standard deviation for parametric, median [interquartile range] for the non-parametric, count (percentage) for categorical variables. Paired t-test and Wilcoxon matched-pairs signed rank test were used to test whether there was a difference between measurements in Accuracy Assessment-1 (post-planning with MRI) and Accuracy Assessment-2 (post-planning with 3D model), for parametric and non-parametric variables, respectively. P value less than 0.05 was considered statistically significant.
Results
Baseline characteristics of participants
A total of 38 participants (14 neurosurgical residents, 24 interns) were included in this study. Mean age of residents was 29.7 ± 3.2 years, whereas it was 23.8 ± 1.2 for interns (6th year medical students). Four residents were in the first year (28.6%), two in the second year (14.3%), four in the third year (28.6%), three in the fourth year (21.4%), and two in the fifth year (14.3%) of residency program. Only two residents (14.3%) had prior experience with 3D modeling/3D printing/VR/AR.
Surgical planning skills
We compared the performance of participants on Accuracy Assessment-1 and Accuracy Assessment-2. For estimated tumor projection on scalp, percent tumor coverage increased (66.4 ± 26.2%–77.2 ± 17.4%, p = 0.026), and excess coverage decreased (2,232 ± 1,322 mm2–1,662 ± 956 mm2, p = 0.019) despite no significant change in painted area (4,036 ± 1,611 mm2–3,760 ± 1,081 mm2, p = 0.272) between Accuracy Assessment-1 and Accuracy Assessment-2 (Figure 6). For linear skin incision, deviation from tumor epicenter significantly reduced from 16.3 ± 9.6 mm to 8.3 ± 7.9 mm after training/planning with 3D model only in residents (p = 0.02) but not in interns. However, U-shape incision (1 [0–2] vs. 1 [0–2], p = 0.147) and craniotomy scores (2 [1–3] vs. 2 [1–3], p = 0.282) did not change significantly between two assessments. On the other hand, sum of craniotomy margin deviations from an acceptable standard decreased significantly after training with 3D model (57.3 ± 24.0 mm–47.2 ± 19.8 mm, p = 0.024) (Figure 6).
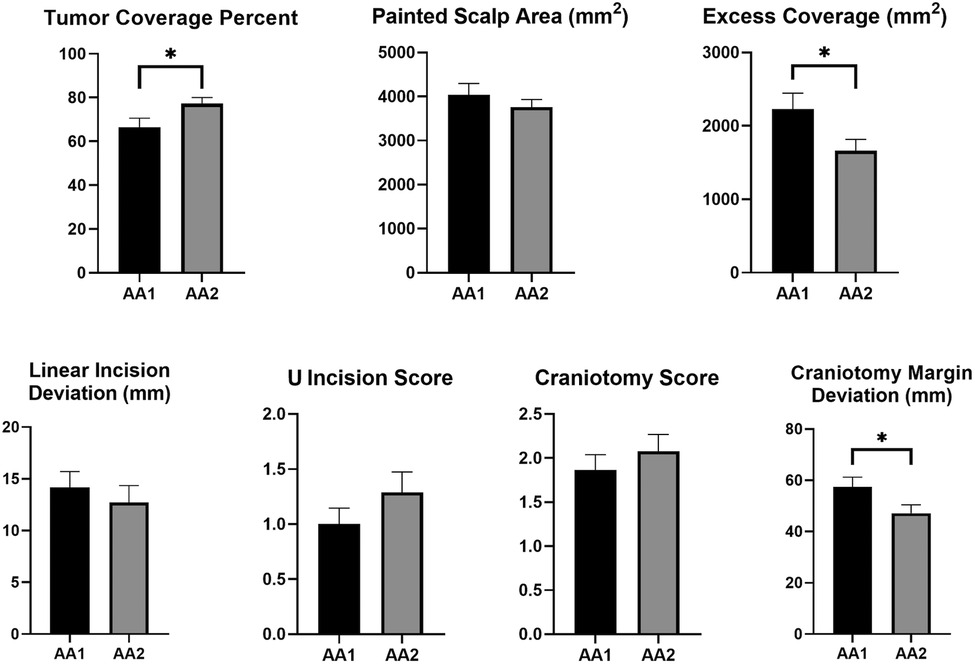
Figure 6. Change of the performance metrics between two assessment sessions. Accuracy Assessment-1 (AA-1): Post-MRI assessment, Accuracy Assessment-2 (AA-2): Post-3D model assessment. * represents significant difference at p < 0.05.
Qualitative evaluation of digital 3d models
The participants scored realism, performance, usefulness, and practicality of the digital 3D models very highly (Table 1). The only aspect that they scored relatively lower was the demonstration of fine anatomical details [median 7.5 (4.75–9)]. Also, although the residents strongly agree that these models should be used for complex cases [median 10 (9.75–10)], they think that the 3D models are probably not necessary for every neurosurgical case [median 6.5 (5–8.25)].
Discussion
Neurosurgical practice and education have seen tremendous change due to recent advances in medical, imaging and education technologies (1–3, 24). Coronavirus pandemic has further accelerated the digital transformation of our profession and the way it is thought and learnt (25). Hence, there is an everlasting need for more effective, realistic, and practical educational tools and methods to train the next generation of neurosurgeons (1–3). Despite the explosion of such tools and materials, objective validation is still lacking for many of them. In this proof-of-concept study, we validated the personalized-3D digital models as easily accessible, yet effective neurosurgical planning tools to improve the understanding of the 3D patient-specific anatomy and thus the accuracy of surgical planning for the first time in the literature.
First, we have showed that patient-specific digital 3D models are perceived as realistic, useful, and practical tools for education and surgical planning by neurosurgery residents (face validity). Second, we have demonstrated that their use as an assessment tool is also feasible, reliable and capable of generating numerous performance metrics for assessment of neurosurgical planning skills. Third, our study design allowed us to reveal that brief training with 3D model after a planning session with conventional 2D MRI slices provided an extra benefit in accurately predicting tumor projection on scalp and incision planning.
There are many studies evaluating the use of 3D digital and physical models as novel educational and objective assessment tools in the literature (14, 15, 19–21, 26). 3D printing has numerous advantages and applications, such as gaining better insight into patient-specific anatomy, better pre-operative planning, mock simulated surgeries, simulation-based training and education, development of surgical guides and other tools, patient-specific implants, bioprinted organs or structures, and counseling of patients (27). Numerous studies examined the efficacy of 3D-printed models on surgical planning, resident and patient education using quantitative and qualitative metrics (14, 15, 19–21, 26). Although it became widely accessible and used in recent years, patient-specific 3D-printing for intracranial pathologies are not routinely used in everyday practice largely due to their cost and production times. The incorporation of 3D printing technology in medical education and practice heralds a paradigm shift, offering unprecedented opportunities for innovation and advancement. Beyond traditional educational methodologies, 3D models empower learners to engage in hands-on experiences, fostering deeper understanding and retention of complex anatomical concepts. Moreover, the interactive nature of 3D models facilitates collaborative learning environments, where students and practitioners can explore anatomical variations and pathological conditions in real-time. As the technology continues to evolve, there is growing interest in harnessing artificial intelligence algorithms to automate the generation of patient-specific 3D models, streamlining the production process and enhancing accessibility. Despite the current challenges, the ongoing refinement of 3D printing techniques and the increasing affordability of equipment hold promise for the widespread adoption of patient-specific 3D printing in neurosurgical practice soon. It is inevitable that systems that support long learning curves, are patient-specific, and are used as standards in resident education, will be developed in the near future.
Virtual simulation-based training is also increasingly being used for assessment and training of psychomotor skills involved in many fields of medicine (28–31). A digital 3D model offers advantages over digital 2D or physical models in interactivity, perspective, access, and cost, either as a stand-alone learning asset or as part of a larger digital learning object (27). Recently, Ros et al. conducted a prospective randomized controlled study of 173 medical students to assess whether immersive virtual reality (VR) technique leads to improvement in learning the technique of external ventricular drainage. They showed that VR group demonstrated significantly better short-term results than the control group (P = 0.01). The same trend was seen at six months (32).
The application of artificial intelligence and machine learning technologies has also provided new methodologies to utilize large amounts of data for educational purposes. Mirchi et al. aimed to introduce a new framework using explainable artificial intelligence for simulation-based training in surgery and validate the framework by creating the Virtual Operative Assistant, an automated educational feedback platform with the contribution of twenty-eight skilled participants (14 staff neurosurgeons, 4 fellows, 10 PGY 4–6 residents) and 22 novice participants (10 PGY 1–3 residents, 12 medical students). Participants performed a virtual reality subpial brain tumor resection task on the NeuroVR simulator using a simulated ultrasonic aspirator and bipolar. The Virtual Operative Assistant successfully classified skilled and novice participants using 4 metrics with an accuracy, specificity, and sensitivity of 92, 82% and 100%, respectively (33).
Recent advances in imaging, image-processing and computer vision technologies provided various tools and software solutions to easily produce personalized or patient-specific 3D digital models with high resolution and quality (34–36). Their incorporation into daily neurosurgical practice provides vast opportunities for surgical planning, intraoperative guidance, and education. They can be combined or complemented with other technologies to enhance their potential as educational or surgical tools and simulators (37–40). Dho et al. recently established a 3D-printed brain tumor model production system, and their validation study showed significant superiority of the 3D-printed models in surgical planning regarding surgical posture (p = 0.0147) and craniotomy design (p = 0.0072) compared to conventional magnetic resonance images. They noticed that the benefit was more pronounced for neurosurgeons with less experience (14).
The prospects for application of digital 3D models, virtual reality and 3D printing in neurosurgical education are extensive (41). As the technologies advance, it is likely that current issues such as cost, and accuracy will be addressed and become less significant (20, 26). However, currently 3D printed models have certain limitations that prevent their frequent use. The requirement for specific printers and various materials, particularly for soft-tissue printing, makes the process expensive and lengthy. The success of 3D printed models also rely on the quality of digital 3D models created in a format recognized by a 3D printer. Rendering and segmentation of medical imaging data to produce patient-specific digital 3D models may also require special software and expert skills. Time and effort spent by an engineer, technician, radiologist, anatomist or biomedical illustrator to segment or create a digital 3D model add to the costs (42). However, with the advent of digital technologies and open-source software, these processes become more accessible, easier, faster and cheaper. We envision that the costs will diminish considerably in near future so that any neurosurgeon can use these patient-specific 3D digital models in everyday clinical practice. Therefore, there is a huge potential for their use in training, surgical planning, and educational assessment (43–45). This potential should be further explored by neurosurgeons, educators, trainees and professional organizations.
One of the main challenges in assessing the utility of virtual educational tools is the need of an objective evaluation method for performance or learning (46, 47). Most virtual simulators designed for neurosurgical education depend solely on the appraisal of resident/trainees' subjective judgements and lack the objective, measurable and repeatable parameters or metrics necessary to validate the usefulness of the system (33, 47). Presented is the first study in the literature to assess the effects of incorporating a patient-specific 3D virtual model for educational purposes, on incision and craniotomy planning skills of neurosurgery residents. Our study design enabled us to monitor the improvements in accuracy of linear incisions and corresponding craniotomies made by residents in a blinded fashion. They were asked to draw perceived tumor projection, a linear and a horseshoe (U-shaped) incision on scalp layer of the 3D model, and corresponding craniotomy on bone layer of the 3D model. Evaluation methodology was simple, yet objective, quantitative, measurable and reproducible. The literature consists of both more complex simulation scenarios such as skull base tumors or aneurysms clipping and simpler ones such as external ventricular drain placement. The assessment methodology used in our study also showed that numerical parameters such as distance, area, percent area provide more powerful and accurate metrics than rough scoring measures such as those used in the U-incision or craniotomy scores in the current study. Also, the study design allowed a fair comparison of the use of 3D model with conventional neurosurgical planning (i.e., examining 2D MRI sections). We did not elect to compare two techniques, rather evaluated the utility of 3D model as a complementary tool as in clinical practice. Indeed, brief training with 3D model after conventional planning led to significant improvements in performance metrics.
Despite its several strengths, the current study has also some limitations. First of all, this is proof-of-concept study and therefore focused on only one surgical case and a handful of performance metrics. It's important to note that the evaluation of performance metrics in this study was constrained by the necessity to manage session duration effectively. Since session time is important for such performance assessment studies, we had to limit our study to only one case and a few metrics as the session duration was already over 40 min (tutorial, collection of demographic data, practice with MRI scan, first assessment, short break, practice with 3D model, second assessment, feedback) even for a single case. Second, we had a relatively small number of neurosurgical residents and interns from one institution. Third, we used the same digital model, but with only skin and bone layers, for assessment. This may have artificially increased the chance of improvement with the use of 3D model as a planning tool over conventional planning with MRI. Alternatively, we may have used real surgical scenario in the OR, or a physical anatomical head model. Nevertheless, we believe that using the same model also enabled us to perform more precise and objective measurements specific to the individual pathological anatomy. Also, its fully digital nature enables repetitive use, multiple recordings, and retrieval of recorded planning data. Future studies with larger cohorts including experts and trainees at different levels, and with variety of surgical scenarios (e.g., deep brain tumors, skull base and vascular pathologies) and performance metrics (precision, depth perception, time, number of errors, appreciation of 3D relationships etc.) can further explore the potential of personalized digital 3D models (i.e., content, construct and criterion validity) and establish the optimal methodology to use their full potential in neurosurgical training and practice.
Conclusion
Digital 3D images, models, and simulators are useful for developing surgical planning skill set. However, their standardization and validation are equally important for their incorporation into neurosurgical curriculum. Here, we showed that readily accessible patient-specific digital 3D models can be effectively used both as educational and assessment tools by evaluating a trainee's perception of intracranial lesions through their imagination of tumor projection on scalp and decisions such as skin incision and craniotomy placement. This tool has many advantages including no or minimal cost, numerous virtual simulation options, repetitive use, archiving possibility, objective measurements, suitability for remote or distant assessment and analysis. They can be incorporated into standard surgical planning workflow of residents, and also be used as a skills assessment tool in local programs as well as national or international board examinations. Therefore, neurosurgery education can benefit from these tools and methodological approach described in this study.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
SH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. MG: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. BB: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft. OT: Conceptualization, Formal Analysis, Methodology, Software, Visualization, Writing – review & editing. II: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. NC: Software, Supervision, Writing – review & editing. IT: Conceptualization, Data curation, Formal Analysis, Supervision, Writing – review & editing. MB: Conceptualization, Data curation, Formal Analysis, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study has been supported by the Hacettepe University Scientific Research Projects Coordination Unit with a research infrastructure development grant (Project ID 19764).
Conflict of interest
The authors declare the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Harrop J, Lobel DA, Bendok B, Sharan A, Rezai AR. Developing a neurosurgical simulation-based educational curriculum: an overview. Neurosurgery. (2013) 73:S25–S9. doi: 10.1227/NEU.0000000000000101
2. Selden NR, Origitano TC, Hadjipanayis C, Byrne R. Model-based simulation for early neurosurgical learners. Neurosurgery. (2013) 73:S15–24. doi: 10.1227/NEU.0000000000000058
3. Tomlinson SB, Hendricks BK, Cohen-Gadol A. Immersive three-dimensional modeling and virtual reality for enhanced visualization of operative neurosurgical anatomy. World Neurosurg. (2019) 131:313–20. doi: 10.1016/j.wneu.2019.06.081
4. Kockro RA, Stadie A, Schwandt E, Reisch R, Charalampaki C, Ng I, et al. A collaborative virtual reality environment for neurosurgical planning and training. Oper Neurosurg. (2007) 61(suppl_5):ONSE379–91. doi: 10.1227/01.neu.0000303997.12645.26
5. Cagiltay NE, Ozcelik E, Isikay I, Hanalioglu S, Suslu AE, Yucel T, et al. The effect of training, used-hand, and experience on endoscopic surgery skills in an educational computer-based simulation environment (ECE) for endoneurosurgery training. Surg Innov. (2019) 26(6):725–37. doi: 10.1177/1553350619861563
6. Cikla U, Sahin B, Hanalioglu S, Ahmed AS, Niemann D, Baskaya MK. A novel, low-cost, reusable, high-fidelity neurosurgical training simulator for cerebrovascular bypass surgery. J Neurosurg. (2018) 130(5):1663–71. doi: 10.3171/2017.11.JNS17318
7. Waran V, Narayanan V, Karuppiah R, Pancharatnam D, Chandran H, Raman R, et al. Injecting realism in surgical training—initial simulation experience with custom 3D models. J Surg Educ. (2014) 71(2):193–7. doi: 10.1016/j.jsurg.2013.08.010
8. Zammar SG, El Tecle NE, El Ahmadieh TY, Adelson PD, Veznedaroglu E, Surdell DL, et al. Impact of a vascular neurosurgery simulation-based course on cognitive knowledge and technical skills in European neurosurgical trainees. World Neurosurg. (2015) 84(2):197–201. doi: 10.1016/j.wneu.2014.12.001
9. Sahin B, Aydin SO, Yilmaz MO, Saygi T, Hanalioglu S, Akyoldas G, et al. Contralateral vs. ipsilateral approach to superior hypophyseal artery aneurysms: an anatomical study and morphometric analysis. Front Surg. (2022) 9:915310. doi: 10.3389/fsurg.2022.915310
10. Hanalioglu S, Gurses ME, Mignucci-Jiménez G, González-Romo NI, Winkler EA, Preul MC, et al. Infragalenic triangle as a gateway to dorsal midbrain and posteromedial thalamic lesions: descriptive and quantitative analysis of microsurgical anatomy. J Neurosurg. (2023) 140(3):866–79. doi: 10.3171/2023.6.JNS222871
11. Cagiltay NE, Ozcelik E, Sengul G, Berker M. Construct and face validity of the educational computer-based environment (ECE) assessment scenarios for basic endoneurosurgery skills. Surg Endosc. (2017) 31:4485–95. doi: 10.1007/s00464-017-5502-4
12. Delorme S, Laroche D, DiRaddo R, Del Maestro RF. Neurotouch: a physics-based virtual simulator for cranial microneurosurgery training. Oper Neurosurg. (2012) 71:ons32–42. doi: 10.1227/NEU.0b013e318249c744
13. Gurses ME, Gungor A, Hanalioglu S, Yaltirik CK, Postuk HC, Berker M, et al. Qlone®: a simple method to create 360-degree photogrammetry-based 3-dimensional model of cadaveric specimens. Oper Neurosurg. (2021) 21(6):E488–E93. doi: 10.1093/ons/opab355
14. Dho Y-S, Lee D, Ha T, Ji SY, Kim KM, Kang H, et al. Clinical application of patient-specific 3D printing brain tumor model production system for neurosurgery. Sci Rep. (2021) 11(1):7005. doi: 10.1038/s41598-021-86546-y
15. Panesar SS, Magnetta M, Mukherjee D, Abhinav K, Branstetter BF, Gardner PA, et al. Patient-specific 3-dimensionally printed models for neurosurgical planning and education. Neurosurg Focus. (2019) 47(6):E12. doi: 10.3171/2019.9.FOCUS19511
16. Randazzo M, Pisapia JM, Singh N, Thawani JP. 3D Printing in neurosurgery: a systematic review. Surg Neurol Int. (2016) 7(Suppl 33):S801–9. doi: 10.4103/2152-7806.194059
17. Vakharia VN, Vakharia NN, Hill CS. Review of 3-dimensional printing on cranial neurosurgery simulation training. World Neurosurg. (2016) 88:188–98. doi: 10.1016/j.wneu.2015.12.031
18. Colaguori F, Marin-Mera M, McDonnell M, Martínez J, Valero-Moreno F, Damon A, et al. Three-dimensionally printed surgical simulation tool for brain mapping training and preoperative planning. Oper Neurosurg. (2021) 21(6):523–32. doi: 10.1093/ons/opab331
19. Lan Q, Zhu Q, Xu L, Xu T. Application of 3D-printed craniocerebral model in simulated surgery for complex intracranial lesions. World Neurosurg. (2020) 134:e761–e70. doi: 10.1016/j.wneu.2019.10.191
20. Błaszczyk M, Jabbar R, Szmyd B, Radek M. 3D printing of rapid, low-cost and patient-specific models of brain vasculature for use in preoperative planning in clipping of intracranial aneurysms. J Clin Med. (2021) 10(6):1201. doi: 10.3390/jcm10061201
21. McGuire LS, Fuentes A, Alaraj A. Three-dimensional modeling in training, simulation, and surgical planning in open vascular and endovascular neurosurgery: a systematic review of the literature. World Neurosurg. (2021) 154:53–63. doi: 10.1016/j.wneu.2021.07.057
22. Fiani B, De Stefano F, Kondilis A, Covarrubias C, Reier L, Sarhadi K. Virtual reality in neurosurgery: “can you see it?”–a review of the current applications and future potential. World Neurosurg. (2020) 141:291–8. doi: 10.1016/j.wneu.2020.06.066
23. Sugiyama T, Clapp T, Nelson J, Eitel C, Motegi H, Nakayama N, et al. Immersive 3-dimensional virtual reality modeling for case-specific presurgical discussions in cerebrovascular neurosurgery. Oper Neurosurg. (2021) 20(3):289–99. doi: 10.1093/ons/opaa335
24. Gurses ME, Gonzalez-Romo NI, Xu Y, Mignucci-Jiménez G, Hanalioglu S, Chang JE, et al. Interactive microsurgical anatomy education using photogrammetry 3D models and an augmented reality cube. J Neurosurg. (2024) 1(aop):1–10. doi: 10.3171/2023.10.JNS23516
25. Sahin B, Hanalioglu S. The continuing impact of coronavirus disease 2019 on neurosurgical training at the 1-year mark: results of a nationwide survey of neurosurgery residents in Turkey. World Neurosurg. (2021) 151:e857–e70. doi: 10.1016/j.wneu.2021.04.137
26. Damon A, Clifton W, Valero-Moreno F, Quinones-Hinojosa A. Cost-effective method for 3-dimensional printing dynamic multiobject and patient-specific brain tumor models. World Neurosurg. (2020) 140:173–9. doi: 10.1016/j.wneu.2020.04.184
27. Meyer-Szary J, Luis MS, Mikulski S, Patel A, Schulz F, Tretiakow D, et al. The role of 3D printing in planning complex medical procedures and training of medical professionals—cross-sectional multispecialty review. Int J Environ Res Public Health. (2022) 19(6):3331. doi: 10.3390/ijerph19063331
28. Gélinas-Phaneuf N, Choudhury N, Al-Habib AR, Cabral A, Nadeau E, Mora V, et al. Assessing performance in brain tumor resection using a novel virtual reality simulator. Int J Comput Assist Radiol Surg. (2014) 9:1–9. doi: 10.1007/s11548-013-0905-8
29. McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. (2011) 86(6):706–11. doi: 10.1097/ACM.0b013e318217e119
30. Seymour NE, Gallagher AG, Roman SA, O’brien MK, Bansal VK, Andersen DK, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. (2002) 236(4):458. doi: 10.1097/00000658-200210000-00008
31. Stefanidis D, Scerbo MW, Montero PN, Acker CE, Smith WD. Simulator training to automaticity leads to improved skill transfer compared with traditional proficiency-based training: a randomized controlled trial. Ann Surg. (2012) 255(1):30–7. doi: 10.1097/SLA.0b013e318220ef31
32. Ros M, Debien B, Cyteval C, Molinari N, Gatto F, Lonjon N. Applying an immersive tutorial in virtual reality to learning a new technique. Neurochirurgie. (2020) 66(4):212–8. doi: 10.1016/j.neuchi.2020.05.006
33. Mirchi N, Bissonnette V, Yilmaz R, Ledwos N, Winkler-Schwartz A, Del Maestro RF. The virtual operative assistant: an explainable artificial intelligence tool for simulation-based training in surgery and medicine. PLoS One. (2020) 15(2):e0229596. doi: 10.1371/journal.pone.0229596
34. Martín-Noguerol T, Paulano-Godino F, Riascos RF, Calabia-Del-Campo J, Márquez-Rivas J, Luna A. Hybrid computed tomography and magnetic resonance imaging 3D printed models for neurosurgery planning. Ann Transl Med. (2019) 7(22):684. doi: 10.21037/atm.2019.10.109
35. Sato M, Tateishi K, Murata H, Kin T, Suenaga J, Takase H, et al. Three-dimensional multimodality fusion imaging as an educational and planning tool for deep-seated meningiomas. Br J Neurosurg. (2018) 32(5):509–15. doi: 10.1080/02688697.2018.1485877
36. Shui W, Zhou M, Chen S, Pan Z, Deng Q, Yao Y, et al. The production of digital and printed resources from multiple modalities using visualization and three-dimensional printing techniques. Int J Comput Assist Radiol Surg. (2017) 12(1):13–23. doi: 10.1007/s11548-016-1461-9
37. Perin A, Carone G, Rui CB, Raspagliesi L, Fanizzi C, Galbiati TF, et al. The “STARS–CT-MADE” study: advanced rehearsal and intraoperative navigation for skull base tumors. World Neurosurg. (2021) 154:e19–28. doi: 10.1016/j.wneu.2021.06.058
38. Mazur T, Mansour TR, Mugge L, Medhkour A. Virtual reality–based simulators for cranial tumor surgery: a systematic review. World Neurosurg. (2018) 110:414–22. doi: 10.1016/j.wneu.2017.11.132
39. Gurses ME, Gungor A, Gökalp E, Hanalioglu S, Okumus SYK, Tatar I, et al. Three-dimensional modeling and augmented and virtual reality simulations of the white matter anatomy of the cerebrum. Oper Neurosurg. (2022) 23(5):355–66. doi: 10.1227/ons.0000000000000361
40. Gurses ME, Gungor A, Rahmanov S, Gökalp E, Hanalioglu S, Berker M, et al. Three-dimensional modeling and augmented reality and virtual reality simulation of fiber dissection of the cerebellum and brainstem. Oper Neurosurg. (2022) 23(5):345–54. doi: 10.1227/ons.0000000000000358
41. Gonzalez-Romo NI, Hanalioglu S, Mignucci-Jiménez G, Abramov I, Xu Y, Preul MC. Anatomic depth estimation and 3-dimensional reconstruction of microsurgical anatomy using monoscopic high-definition photogrammetry and machine learning. Oper Neurosurg. (2023) 24(4):432–44. doi: 10.1227/ons.0000000000000544
42. Fredieu JR, Kerbo J, Herron M, Klatte R, Cooke M. Anatomical models: a digital revolution. Med Sci Educ. (2015) 25:183–94. doi: 10.1007/s40670-015-0115-9
43. Gurses ME, Hanalioglu S, Mignucci-Jiménez G, Gökalp E, Gonzalez-Romo NI, Gungor A, et al. Three-dimensional modeling and extended reality simulations of the cross-sectional anatomy of the cerebrum, cerebellum, and brainstem. Oper Neurosurg. (2023) 25(1):3–10. doi: 10.1227/ons.0000000000000703
44. Gonzalez-Romo NI, Mignucci-Jiménez G, Hanalioglu S, Gurses ME, Bahadir S, Xu Y, et al. Virtual neurosurgery anatomy laboratory: a collaborative and remote education experience in the metaverse. Surg Neurol Int. (2023) 14:90. doi: 10.25259/SNI_162_2023
45. Hanalioglu S, Romo NG, Mignucci-Jiménez G, Tunc O, Gurses ME, Abramov I, et al. Development and validation of a novel methodological pipeline to integrate neuroimaging and photogrammetry for immersive 3D cadaveric neurosurgical simulation. Front Surg. (2022) 9:878378. doi: 10.3389/fsurg.2022.878378
46. Aoun SG, El Ahmadieh TY, El Tecle NE, Daou MR, Adel JG, Park CS, et al. A pilot study to assess the construct and face validity of the northwestern objective microanastomosis assessment tool. J Neurosurg. (2015) 123(1):103–9. doi: 10.3171/2014.12.JNS131814
Keywords: 3D model, surgical planning, simulation, assessment, education, brain tumor
Citation: Hanalioglu S, Gurses ME, Baylarov B, Tunc O, Isikay I, Cagiltay NE, Tatar I and Berker M (2024) Quantitative assessment and objective improvement of the accuracy of neurosurgical planning through digital patient-specific 3D models. Front. Surg. 11:1386091. doi: 10.3389/fsurg.2024.1386091
Received: 14 February 2024; Accepted: 8 April 2024;
Published: 24 April 2024.
Edited by:
Cesare Zoia, San Matteo Hospital Foundation (IRCCS), ItalyReviewed by:
Fabio Cofano, University of Turin, ItalyHani J. Marcus, University College London, United Kingdom
Jia-En Danny Chen, University College London, United Kingdom, in collaboration with reviewer [HJM]
© 2024 Hanalioglu, Gurses, Baylarov, Tunc, Isikay, Cagiltay, Tatar and Berker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sahin Hanalioglu aGFuYWxpb2dsdUBoYWNldHRlcGUuZWR1LnRy; c2FoaW5oYW5hbGlvZ2x1QGdtYWlsLmNvbQ==
†Present Addresses: Muhammet Enes Gurses, Department of Neurological Surgery, Leonard M. Miller School of Medicine, University of Miami, Coral Gables, FL, United States
 Sahin Hanalioglu
Sahin Hanalioglu Muhammet Enes Gurses
Muhammet Enes Gurses Baylar Baylarov
Baylar Baylarov Osman Tunc2
Osman Tunc2 Nergiz Ercil Cagiltay
Nergiz Ercil Cagiltay