- Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Gastrointestinal Cancer Center, Peking University Cancer Hospital & Institute, Beijing, China
Background: This study aimed to compare the clinical outcomes and patient benefits of uncut Roux-en-Y (URY) anastomosis and Billroth-II with Braun (BB) anastomosis after distal gastrectomy.
Methods: We retrospectively reviewed the data of patients who underwent URY or BB anastomosis after distal gastrectomy between March 2015 and December 2017. Clinical characteristics, survival data, postoperative recovery data, and long-term outcomes were recorded and compared between the two groups.
Results: A total of 231 patients were included, with 167 in the URY group and 64 in the BB group. Kaplan–Meier curves for overall survival showed no differences after propensity score matching (p = 0.488). Long-term postoperative quality of life evaluation also showed no significant differences. Compared to the BB group, patients in the URY group had a significantly shorter time to start a liquid diet after propensity score matching (67.6 h vs. 46.5 h, p = 0.003), and a lower occurrence of bile reflux on follow-up gastroscopy (p < 0.001).
Conclusion: The URY anastomosis appears to be a feasible method for digestive tract reconstruction after distal gastrectomy, resulting in less bile reflux and better postoperative recovery. However, there is no significant difference between URY and BB anastomosis in terms of overall survival and long-term quality of life.
Background
Since 1881, different techniques for gastrointestinal reconstruction have been invented and improved, including Billroth-I, Billroth-II, and Roux-en-Y anastomosis, which are used after distal gastrectomy. The Billroth-I anastomosis is considered to be the most physiologically appropriate, but only if tension-free anastomosis can be achieved between the remnant stomach and the duodenum. The Billroth-II anastomosis is more commonly used when there is a large proportion of the stomach removed, however, patients with the Billroth-II anastomosis suffer from some complications including dumping syndrome, input loop syndrome and bile reflux gastritis (1).
To alleviate the complications of Billroth-II, the Braun anastomosis, which brings together the input and output loops of the jejunum, has been added to the original procedure and has helped to reduce bile reflux to some extent (2). On the other hand, The Roux-en-Y anastomosis prevents bile reflux better than the Billroth-I and Billroth-II anastomoses and does not cause anastomotic tension, but it has its own problems. Studies in the late 1980s reported that more than 30% of patients who underwent the Roux-en-Y anastomosis experienced postoperative abdominal pain, fullness, nausea and even vomiting, which was defined as Roux-Y stasis syndrome (RSS) (3–6). As a solution, Stiegmann et al (7) first proposed the uncut Roux-en-Y (URY) anastomosis, which they believed would not only prevent bile and pancreatic reflux, but would also prevent RSS.
The clinical utility and potential patient benefits of URY and Billroth-II with Braun (BB) anastomosis after distal gastrectomy are still a subject of debate. A meta-analysis published in 2022 revealed that the incidence of reflux gastritis was significantly lower in the URY group than in the BB group, and URY group exhibited a shorter time to first passage of flatus or defecation and a shorter time to first solid diet than the BB group. This study concluded that URY anastomosis is a safe and effective method after distal gastrectomy, and it is better than BB anastomosis in terms of early postoperative recovery and low incidence of reflux gastritis (8).
Although URY anastomosis may have some benefits, evaluations of both procedures remain incomplete and long-term follow-up is lacking. Therefore, this study aimed to compare overall survival, postoperative recovery data, and long-term postoperative quality of life between patients who underwent URY and BB anastomosis after distal gastrectomy.
Methods
Study design
This was a real world, single-site, retrospective cohort study of patients who underwent URY or BB anastomosis after distal gastrectomy of gastric cancer. The study utilized a propensity score matching (PSM) method to minimize selection bias. The study population consisted of patients from Peking University Cancer Hospital who underwent an index distal gastric surgery with D2 Lymphadenectomy between March 2015 and December 2017. Patients who underwent multiple organ resections were excluded from the study. This research was approved by the Ethnical Committee of Peking University Cancer Hospital (2018YJZ56).
Surgical approaches
Billroth-II with Braun
The distal stomach was completely transected 5 cm away from the tumor using a 60 mm linear cutter closure, and the specimen was extracted. The jejunum was selected 25 cm distal to the Treitz ligament, and its lateral wall was cut and prepared for anastomosis. The distal greater curvature of the remnant stomach was cut, and the lateral anastomosis was performed by extending the 60 mm linear cutter closure into both arms of the lateral wall of the jejunum and the distal opening of the remnant stomach. The anastomosis was carefully assessed for tension and torsion, and the common opening was closed using a linear cutter closure. The lateral walls of the input loop, which was located 15 cm from the anastomosis, and the output loop, which was located 30 cm from the anastomosis, were cut. The two arms of the 60 mm linear cutter-closer were inserted to perform a lateral Braun anastomosis, and the common opening was closed using the linear cutter-closer. To reinforce the anastomosis and the gastric stump, interrupted sutures of Vecchio suture were applied to the serosal layer and muscular layer.
Uncut Roux-en-Y
After completing the BB anastomosis, the proximal intestine of the input loop is closed using a 45 mm bladeless lumpectomy closure (ATS45NK) consisting of six rows of staples, positioned 3 cm proximal to the gastrointestinal anastomosis. In a minority of cases, TA30 linear closure or Vecchio suture closure was used as an alternative.
Data collection
An internal hospital database was used as the data source to select records that met the eligibility criteria. The database contains information on all admitted patients, including diagnoses, procedures, hospital costs, complications, and other relevant details. Patient demographics, procedural characteristics, type of reconstruction, and clinical characteristics (such as ASA score, diagnosis, surgical time, intraoperative bleeding, etc.) were collected. All data were retrospectively collected by administrative staff at the hospital and recorded in a separate database for data analysis.
General health status of preoperative patients, clinical TNM stage and complication classifications
We utilized the Eastern Cooperative Oncology Group Performance Status (ECOG-PS) to assess the preoperative general health status of patients (9). The initial TNM data was recorded based on the 7th edition of the American Joint Committee on Cancer (AJCC) staging system, and the study cohort was restaged according to the definitions of the 8th edition of the AJCC staging system (10, 11). Postoperative complications were graded using the Clavien-Dindo (CD) scoring system (12).
Nutrition assessment
The nutritional risk screening score 2002 (NRS2002), body mass index (BMI) and Onodera index were used to assess the patients’ nutritional status (13, 14).
Quality of life evaluation
The European Organization for Research and Treatment of Cancer (EORTC) Quality of life questionnaire (QLQ) core QLQ-C30 questionnaire with a gastric cancer-specific clinical assessment questionnaire QLQ-STO22 (Stomach module) was utilized in this study. These questionnaires were self-reported and self-assessed by the patients, and were administered both pre- and post-surgery. The obtained results were scored and normalized based on different dimensions such as functional, symptomatic, economic, and general health status. It should be noted that the Chinese version of the questionnaire has been translated and validated by Chinese scholars in the clinical setting of gastric cancer treatment (15).
Gastroscopy
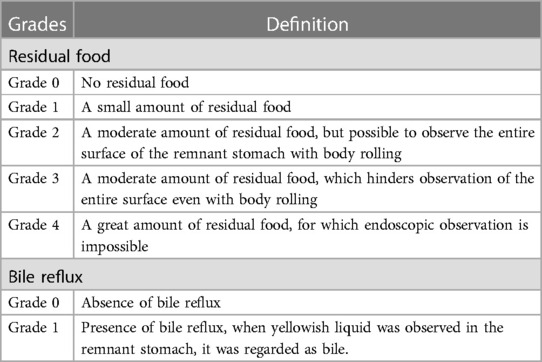
The patient underwent gastroscopy about 1 year after surgery. The amount of residual food and bile reflux were classified according to classifications proposed by Kubo et al. as shown in Table 1 (16).
Statistical analysis
All statistical analyses were performed using R Studio (version 4.2.2) and GraphPad Prism 9. Continuous variables were presented as mean ± standard deviation and tested with the independent t-test between groups if normally distributed, otherwise they were presented as median and interquartile range (IQR) and tested with the Mann–Whitney U test. Categorical variables were presented as frequency and percentage and tested with the chi-square test, Fisher's exact test, or Mann–Whitney U test. PSM was conducted using the R Studio and MatchIt package. The two groups of patients were matched 1:1 without putting back according to the nearest neighbor matching method with a caliper value of 0.02. Kaplan–Meier curves were used to compare differences in overall survival (OS) between the groups, and statistical significance was assessed using the log-rank test. All tests were bilateral, and p < 0.05 was considered statistically significant.
Results
Patient characteristics
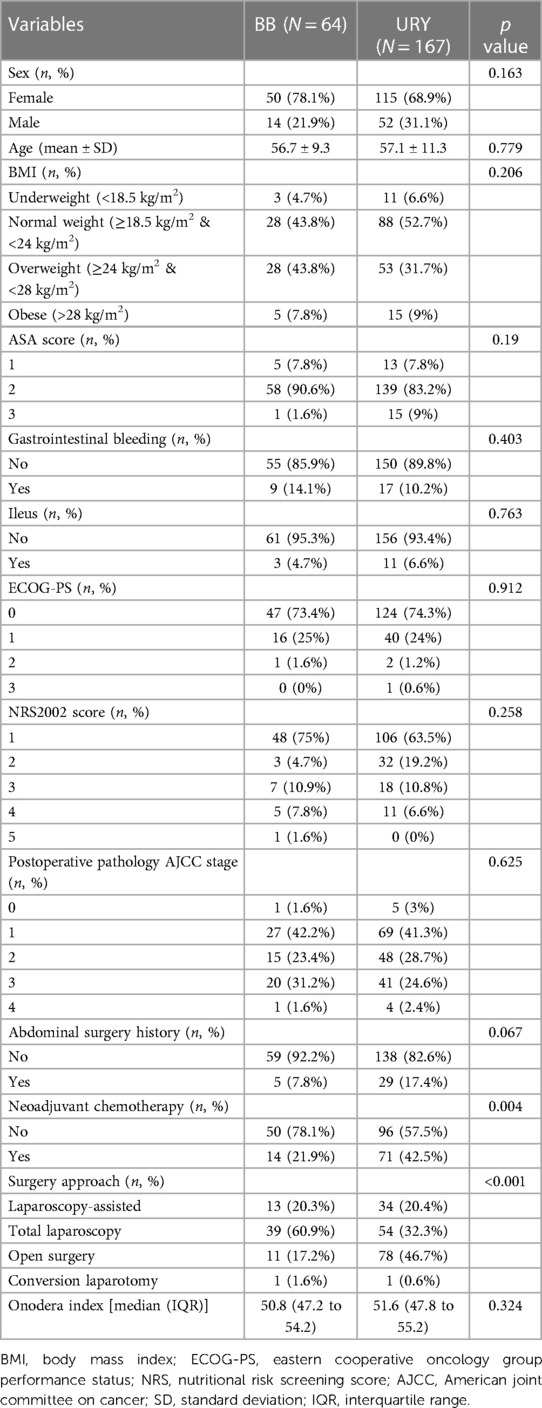
A total of 231 patients were included in this study, with 167 patients in the URY group and 64 patients in the BB group. A significant difference was observed between the two groups in terms of neoadjuvant chemotherapy (p = 0.004) and surgery approach (p < 0.001). Other demographic and clinicopathologic characteristics including sex, age, BMI, ASA score, gastrointestinal bleeding, ileus, ECOG-PS, NRS2022 score, postoperative pathology AJCC stage, abdominal surgery history, Onodera index were comparable between the two groups. The detailed demographic and clinicopathologic characteristics of the two groups are presented in Table 2.
PSM model construction
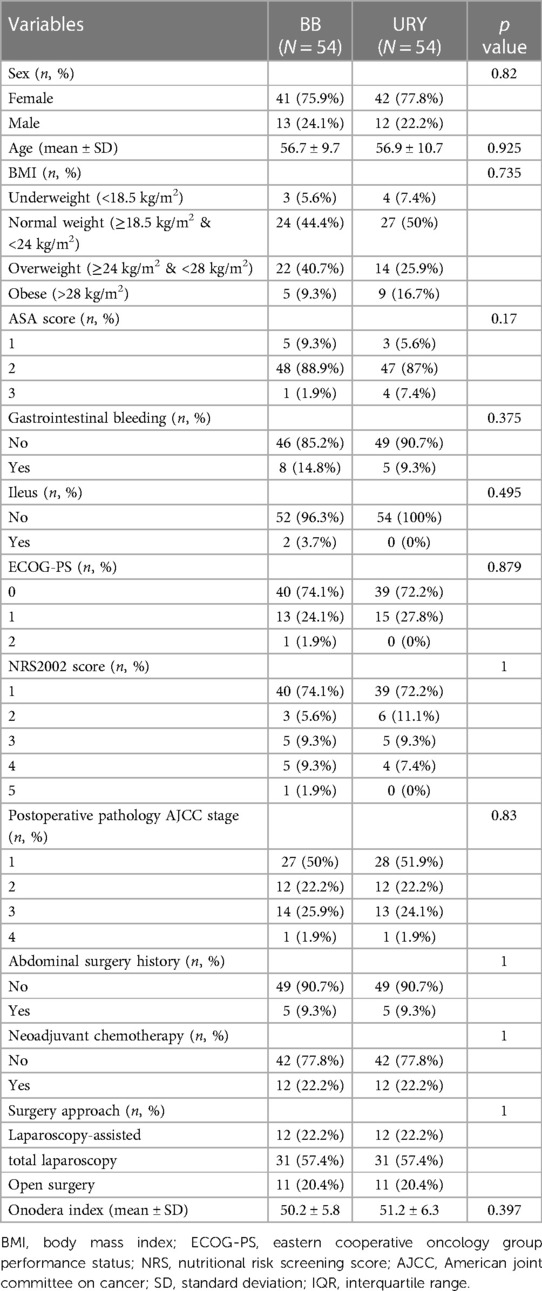
Examination of the baseline characteristics revealed a significant difference in neoadjuvant chemotherapy and surgery approach between the groups. PSM was used to minimize the impact of latent selective bias. The final matching variables included neoadjuvant chemotherapy, surgery approach, abdominal surgery history, sex, and postoperative pathology AJCC stage. After PSM, a total of 108 patients were successfully matched, with 54 in each group, and no statistically significant differences were observed in the baseline characteristics between the two groups (all p > 0.05) (Table 3).
Postoperative recovery data after PSM
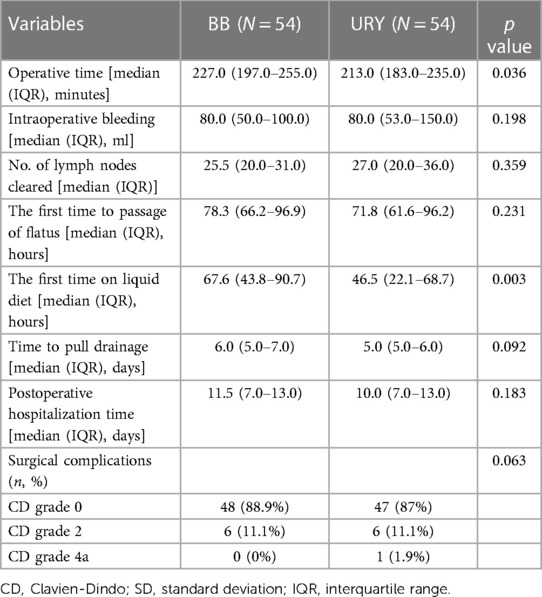
Compared to the BB group, the URY group had significantly shorter time to start a liquid diet (median (IQR), 46.5 (22.1–68.7) hours vs. 67.6 (43.8–90.7) hours, p = 0.003), and shorter operative time (median (IQR), 213.0 (183.0–235.0) minutes vs. 227.0 (197.0–255.0) minutes, p = 0.036). There were no significant differences between the two groups in intraoperative bleeding, No. of lymph nodes cleared, the first time to passage of flatus, time to pull drainage, postoperative hospitalization time, or surgical complications (Table 4).
Overall survival
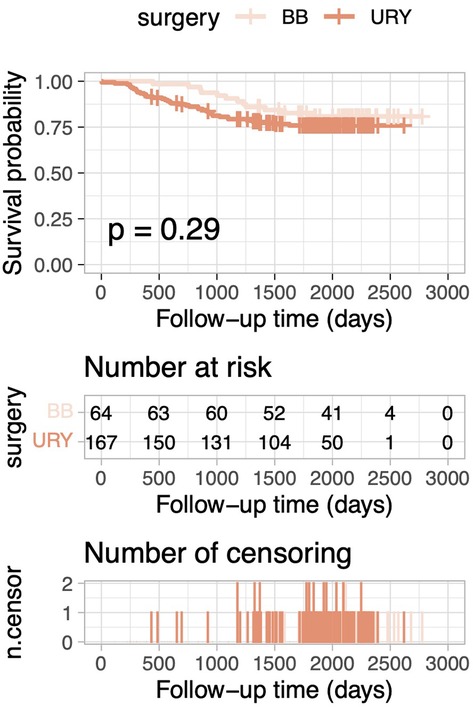
After PSM, the 5-year OS rate was 84.6% in the URY group and 88.8% in the BB group. Kaplan–Meier curves for OS demonstrated no statistically significant difference between the two groups (log-rank test, p = 0.488; Figure 1). Similarly, for the overall cohort before PSM, Kaplan–Meier curves for OS also indicated no statistically significant differences (log-rank test, p = 0.287; Figure 2).
Long-term postoperative quality of life evaluation between the BB group and the URY group
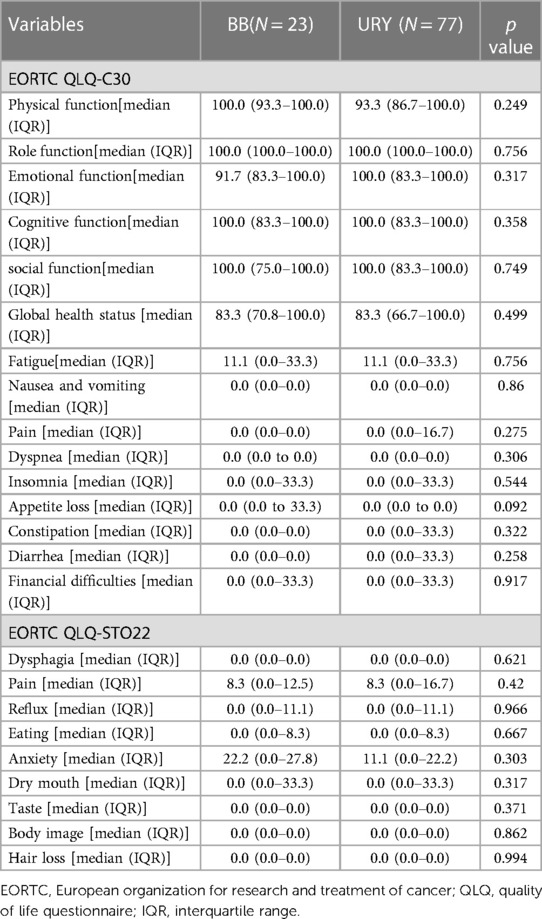
Of all the 231 patients in the overall cohort, 100 patients were followed up after 6 months post-surgery, consisting of 77 patients in the URY group and 23 patients in the BB group. The median follow-up time was 710 days. No statistically significant differences were observed between the two groups (Table 5). The EORTC QLQ-C30 comprises of 30 items including five function scales (physical, role, cognitive, emotional, social), three symptom scales (fatigue, pain, nausea and vomiting), a global health status and QOL scale, and single items (dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties). Each scale or item is transformed into a score ranging from 0 to 100, with higher scores indicating better global health status or functional status, or worse symptom status. The EORTC QLQ-STO22 consists of 22 items, including five scales (dysphagia, chest and abdominal pain, reflux, eating restrictions, anxieties) and four individual items (dry mouth, body image, taste problems, hair loss) that reflecting disease symptoms, treatment side effects, and emotional issues specific to gastric cancer. Higher scores on this questionnaire indicate greater symptomatic problems (17, 18).
Gastroscopy results
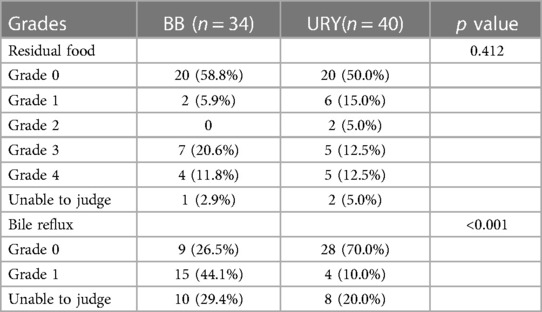
Of all the 231 patients, 74 underwent gastroscopy approximately a year post-surgery, with 40 in the URY group and 34 in the BB group. The median time of follow-up was 12 months. Comparing the two groups, the URY group had a significantly lower incidence of bile reflux (p < 0.001), while there was no significant difference between the groups in terms of residual food. (Table 6).
Discussion
Currently, various techniques are available for reconstructing the digestive tract after distal gastrectomy, including Billroth-I, Billroth-II, Billroth-II with Braun, Roux-en-Y, and uncut Roux-en-Y. Nevertheless, the choice of reconstruction methods after distal gastrectomy is still controversial. Surgeons continue to search for the most effective reconstruction method, with the aim of minimizing postoperative complications and ensuring better postoperative quality of life.
Several studies have compared these reconstructing methods. Li et al. conducted a systematic review which revealed that URY anastomosis significantly reduce the rate of reflux gastritis after distal gastrectomy among Billroth-I, Billroth-II, Billroth-II with Braun, and Roux-en-Y anastomosis. Besides, URY tended to be a more favorable method due to its operative simplicity, safety, and some other reasons (19). A meta-analysis compared laparoscopic URY and BB anastomosis after distal gastrectomy which found that URY anastomosis is better than BB in terms of early postoperative recovery and low incidence of reflux gastritis (8). Wang et al. reported that none of the patients in the URY group experienced bile reflux on gastroscopy or upper gastrointestinal contrast examination at 3 months post-surgery, whereas 29.03% of patients in the BB group experienced bile reflux (p < 0.0001). And the bile reflux at 6 months after surgery was also significantly more frequent in the BB group than in the URY group (20). In the present study, we collected the gastroscopy results of the overall cohort, with 74 patients undergoing this examination approximately one year after surgery. The results demonstrated significantly less bile reflux occurring in the URY group than in the BB group (p < 0.001), which is consistent with findings from previous literature. Regarding the postoperative recovery data, the first time to start a liquid diet in URY group patients was significantly earlier than that in BB group patients (p = 0.001), indicating that the gastrointestinal function of URY group patients may recover faster and meet the clinical criteria for liquid diet earlier, which is similar with previous studies. Additionally, other postoperative data, such as intraoperative bleeding and surgical complications, did not differ significantly between the URY and BB groups, which may suggest that both procedures are equally safe. Furthermore, studies have shown that the Biofragmentable Anastomotic Ring (BAR) is a safe and time-efficient method for performing Roux-en-Y jejunojejunostomy in gastric cancer surgery (21). Exploring different anastomotic techniques, such as BAR, may be a new way to improve the safety and quality of gastric cancer surgery.
To our knowledge, our study is the first to report on the comparison of OS between the URY and BB groups following distal gastrectomy. The results showed no significant difference between these two groups. Additionally, our study was unique in its reporting of long-term postoperative quality of life. We found no significant differences between the URY group and BB group in any of the measured variables, including reflux symptoms. This may be due to several reasons. Firstly, bile reflux in the remnant stomach does not necessarily correlate with reflux symptoms in patients who have undergone distal gastrectomy, as the cardia structure remains intact and should theoretically provide a normal anti-reflux effect. Secondly, the sample size was relatively small and the follow-up data for the post-operative quality of life questionnaire was lost to a greater extent. Furthermore, we used the BB anastomosis for comparison, which adds a Braun anastomosis to the traditional Billroth-II procedure, allowing bile and pancreatic juice to be diverted from the proximal to the distal jejunum through the Braun anastomosis, thus reducing the amount of refluxed bile (2). Some studies suggest a possible association between bile reflux and gastric stump cancer or reflux symptom, but no difference shown in our study between URY group and BB group (22, 23).
In our study, there were several significant differences observed between the two groups before PSM. A higher percentage of patients received neoadjuvant chemotherapy in the BB group than URY group, and more total laparoscopy approach was used in the BB group while more open surgeries in the URY group. The above factors may affect the outcomes of the surgery. Therefore, in the PSM analysis, included neoadjuvant chemotherapy, surgery approach, abdominal surgery history, sex, and postoperative pathology AJCC stage as matching variables. After 1:1pairing, no statistically significant differences were observed between the two groups, which enhanced the comparability of the study groups. For the long-term postoperative quality of life evaluation and gastroscopy follow-up results, the data were not sufficient to utilize the PSM model. The collection of follow-up data was conducted during patients’ post-discharge outpatient visits at our hospital. However, as many of our patients came from different provinces, they received post-discharge treatment in hospitals within their own provinces. Therefore, comprehensive data on long-term postoperative quality of life and gastroscopy follow-up were not available for analysis using PSM model.
With respect to postoperative complications, the total incidence of complications was 14.72% (34/231). No significant difference was found in postoperative complications between the URY group and the BB group after PSM. Among the 167 URY patients, 16 cases (9.6%) of CD grade 2 or lower complications, 6 cases (3.6%) of CD grade 3–4 complications, and 1 case (0.6%) of perioperative death due to myocardial infarction were observed. There was 1 case (0.6%) of anastomotic leakage related to the anastomotic site and 1 case (0.6%) of diarrhea. Wang et al (20) conducted a randomized controlled trail (RCT) study comparing the postoperative complications between the URY group and the BB group, which showed no significant difference (URY vs. BB, 4.84% vs. 6.45%, p = 0.70). Uyama et al (24) retrospectively analyzed 42 patients who underwent laparoscopy-assisted URY anastomosis and reported an overall postoperative complications rate of 4.8%. Yang et al (25) reported an overall postoperative complications rate of 7.6% in 79 cases of laparoscopy-assisted URY anastomosis in an RCT study. A multicenter prospective cohort study conducted in China reported an overall postoperative complication rate of 18.14% (412/2271) for gastric surgeries (26). Our study’s results were comparable to the former studies. Although the postoperative complication rate in our study was marginally higher than some studies, this discrepancy could potentially be attributed to differences in the recognition and registration of postoperative complications of gastric cancer in different centers.
The limitations of this study included: firstly, the sample size of BB group was relatively small. Although PSM was utilized to reduce bias, it was not possible to completely eliminate all confounding factors. Secondly, the scarcity of follow-up data on quality of life may have masked some of the actual differences between the two groups. Thirdly, short-term quality of life data was not collected due to the initial oversight regarding the significance of patients’ quality of life in the early years.
Conclusion
The URY anastomosis appears to be a feasible method for digestive tract reconstruction after distal gastrectomy, resulting in less bile reflux and better postoperative recovery. However, there is no significant difference between URY and BB anastomosis in terms of overall survival and long-term quality of life for patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Peking University Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TW: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – original draft. ZW: Conceptualization, Data curation, Funding acquisition, Methodology, Supervision, Writing – review & editing. YC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. YL: Data curation, Project administration, Resources, Supervision, Writing – original draft. FP: Data curation, Resources, Supervision, Writing – original draft. FS: Conceptualization, Data curation, Resources, Supervision, Writing – review & editing. ZL: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing. JJ: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Joint Fund of National Natural Science Foundation of China (U20A20371 to JJ); Clinical Medicine Plus X-Young Scholars Project, Peking University, the Fundamental Research Funds for the Central Universities, and the Science Foundation of Peking University Cancer Hospital (to ZW); Incubating Program of Haidian District (HP2023-19-503001 to ZW).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Piessen G, Triboulet JP, Mariette C. Reconstruction after gastrectomy: which technique is best? J Visc Surg. (2010) 147(5):e273–83. doi: 10.1016/j.jviscsurg.2010.09.004
2. Vogel SB, Drane WE, Woodward ER. Clinical and radionuclide evaluation of bile diversion by braun enteroenterostomy: prevention and treatment of alkaline reflux gastritis. An alternative to Roux-en-Y diversion. Ann Surg. (1994) 219(5):458–65. discussion 65–6. doi: 10.1097/00000658-199405000-00003
3. Britton JP, Johnston D, Ward DC, Axon AT, Barker MC. Gastric emptying and clinical outcome after Roux-en-Y diversion. Br J Surg. (1987) 74(10):900–4. doi: 10.1002/bjs.1800741010
4. van der Mijle HC, Kleibeuker JH, Limburg AJ, Bleichrodt RP, Beekhuis H, van Schilfgaarde R. Manometric and scintigraphic studies of the relation between motility disturbances in the roux limb and the Roux-en-Y syndrome. Am J Surg. (1993) 166(1):11–7. doi: 10.1016/S0002-9610(05)80574-4
5. Gustavsson S, Ilstrup DM, Morrison P, Kelly KA. Roux-Y stasis syndrome after gastrectomy. Am J Surg. (1988) 155(3):490–4. doi: 10.1016/S0002-9610(88)80120-X
6. Calomino N, Malerba M, Tanzini G. Total gastrectomy and quality of life. Minerva Chir. (1998) 53(3):135–40.9617108
7. Van Stiegmann G, Goff JS. An alternative to Roux-en-Y for treatment of bile reflux gastritis. Surg Gynecol Obstet. (1988) 166(1):69–70. doi: 10.1002/pd.1970080113
8. Jiao YJ, Lu TT, Liu DM, Xiang X, Wang LL, Ma SX, et al. Comparison between laparoscopic uncut Roux-en-Y and Billroth II with Braun anastomosis after distal gastrectomy: a meta-analysis. World J Gastrointest Surg. (2022) 14(6):594–610. doi: 10.4240/wjgs.v14.i6.594
9. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. (1982) 5(6):649–55. doi: 10.1097/00000421-198212000-00014
10. Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz A, Greene F. AJCC Cancer Staging Manual. New York: Springer (2010).
11. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. (2017) 67(2):93–9. doi: 10.3322/caac.21388
12. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
13. Kondrup J, Rasmussen HH, Hamberg O, Stanga Z, Ad Hoc EWG. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. (2003) 22(3):321–36. doi: 10.1016/S0261-5614(02)00214-5
14. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. (1984) 85(9):1001–5.6438478
15. Liu W, Yang G, Rui Y, Lin Y, Wang S, Zhang L. The validation of EORTC QLQ-STO22 scale in patients with gastric cancer in China. Cancer Res Clin. (2016):595–9. doi: 10.3760/cma.j.issn.1006-9801.2016.09.005
16. Kubo M, Sasako M, Gotoda T, Ono H, Fujishiro M, Saito D, et al. Endoscopic evaluation of the remnant stomach after gastrectomy: proposal for a new classification. Gastric Cancer. (2002) 5(2):83–9. doi: 10.1007/s101200200014
17. Kobayashi D, Kodera Y, Fujiwara M, Koike M, Nakayama G, Nakao A. Assessment of quality of life after gastrectomy using EORTC QLQ-C30 and STO22. World J Surg. (2011) 35(2):357–64. doi: 10.1007/s00268-010-0860-2
18. Fayers P, Bottomley A. Quality of life research within the EORTC—the EORTC QLQ-C30. Eur J Cancer. (2002) 38:125–33. doi: 10.1016/S0959-8049(01)00448-8
19. Li Y, Wang Q, Yang KL, Wang J, Jiang KW, Ye YJ. Uncut Roux-en-Y might reduce the rate of reflux gastritis after radical distal gastrectomy: an evidence mapping from a systematic review. Int J Surg. (2022) 97:106184. doi: 10.1016/j.ijsu.2021.106184
20. Wang J, Wang Q, Dong J, Yang K, Ji S, Fan Y, et al. Total laparoscopic uncut Roux-en-Y for radical distal gastrectomy: an interim analysis of a randomized, controlled, clinical trial. Ann Surg Oncol. (2021) 28(1):90–6. doi: 10.1245/s10434-020-08710-4
21. Marano L, Braccio B, Schettino M, Izzo G, Cosenza A, Grassia M, et al. Sutureless jejuno-jejunal anastomosis in gastric cancer patients: a comparison with handsewn procedure in a single institute. BMC Surg. (2012) 12(Suppl 1):S27. doi: 10.1186/1471-2482-12-S1-S27
22. Kondo K. Duodenogastric reflux and gastric stump carcinoma. Gastric Cancer. (2002) 5(1):16–22. doi: 10.1007/s101200200002
23. Park JY, Kim YJ. Uncut Roux-en-Y reconstruction after laparoscopic distal gastrectomy can be a favorable method in terms of gastritis, bile reflux, and gastric residue. J Gastric Cancer. (2014) 14(4):229–37. doi: 10.5230/jgc.2014.14.4.229
24. Uyama I, Sakurai Y, Komori Y, Nakamura Y, Syoji M, Tonomura S, et al. Laparoscopy-assisted uncut Roux-en-Y operation after distal gastrectomy for gastric cancer. Gastric Cancer. (2005) 8(4):253–7. doi: 10.1007/s10120-005-0344-5
25. Yang D, He L, Tong WH, Jia ZF, Su TR, Wang Q. Randomized controlled trial of uncut Roux-en-Y vs Billroth II reconstruction after distal gastrectomy for gastric cancer: which technique is better for avoiding biliary reflux and gastritis? World J Gastroenterol. (2017) 23(34):6350–6. doi: 10.3748/wjg.v23.i34.6350
Keywords: gastric cancer, gastrectomy, reconstructive surgical procedure, anastomosis, quality of life
Citation: Wei T, Wu Z, Chen Y, Li Y, Pang F, Shan F, Li Z and Ji J (2024) Comparison of uncut Roux-en-Y anastomosis and Billroth-II with Braun anastomosis after distal gastrectomy. Front. Surg. 11:1390876. doi: 10.3389/fsurg.2024.1390876
Received: 24 February 2024; Accepted: 20 March 2024;
Published: 28 March 2024.
Edited by:
Luigi Marano, Academy of Applied Medical and Social Sciences, PolandReviewed by:
Natale Calomino, University of Siena, ItalyShisong Zhang, Children’s Hospital Affiliated to Shandong University, China
© 2024 Wei, Wu, Chen, Li, Pang, Shan, Li and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiafu Ji amlqaWFmdUBoc2MucGt1LmVkdS5jbg==
†These authors have contributed equally to this work
Abbreviations URY, uncut Roux-en-Y; BB, Billroth-II with Braun; PSM, propensity score matching; ASA, American society of anesthesiologists; ECOG-PS, eastern cooperative oncology group performance status; AJCC, American joint committee on cancer; CD, Clavien-Dindo; NRS2002, nutritional risk screening score 2002; BMI, body mass index; EORTC, European organization for research and treatment of cancer; QLQ, quality of life questionnaire; IQR, interquartile range; OS, overall survival; RCT, randomized controlled trail; SD, standard deviation.
 Tianxiao Wei
Tianxiao Wei Zhouqiao Wu†
Zhouqiao Wu† Ziyu Li
Ziyu Li Jiafu Ji
Jiafu Ji