- 1Department of Pain, Beijing Jishuitan Hospital, Capital Medical University, Beijing, China
- 2Department of Anesthesiology, Beijing Jishuitan Hospital, Capital Medical University, Beijing, China
- 3Department of Anesthesiology, Beijing Shijitan Hospital, Capital Medical University, Beijing, China
Objective: To investigate the effect of single femoral nerve block (SFNB) with 0.2% ropivacaine 50 ml on postoperative pain and muscle strength in elderly patients undergoing knee replacement.
Methods: Ninety-four patients scheduled for primary total knee arthroplasty (TKA) were randomized into two groups. The patients in the SFNB group received SFNB with 50 ml 0.2% ropivacaine (n = 48), while the patients in the continuous femoral nerve block (CFNB) group (n = 46) received CFNB with an initial load of 20 ml 0.5% ropivacaine and a continuous injection of 0.2% ropivacaine at a rate of 5 ml/h. After the surgery, all patients were administered patient-controlled intravenous analgesia. The primary outcome was the visual analogue scale (VAS) score at 24 h postoperatively. The secondary outcomes included: (a) Pain scores at 2 h, 6 h, 12 h, 48 h, and 72 h after surgery, and the total dosage of celecoxib; (b) Muscle strength of the quadriceps at 2 h, 6 h, 12 h, 24 h, 48 h, and 72 h postoperatively; (c) Range of motion (ROM) at 24 h, 72 h, and 1 week after surgery; (d) American Knee Society knee score (AKS) at 1 week postoperatively; (e) Related complications (e.g., nausea and vomiting), and length of hospitalization; (f) General Comfort Questionnaire (GCQ) score at 72 h postoperatively.
Results: (a) There were no statistically significant differences in VAS scores (p > 0.05) or the total dosage of celecoxib (p > 0.05) between the two groups at various time points; (b) The muscle strength scores in the SFNB group were higher than those in the CFNB group (p < 0.05) at 6 h, 12 h, and 24 h postoperatively; (c) Knee ROM in the SFNB group was better than in the CFNB group (p < 0.05); (d) There were no significant differences in adverse events between the two groups (p > 0.05); (e) The physiological and psychological scores on the GCQ in the SFNB group were higher than those in the CFNB group (p < 0.05).
Conclusion: SFNB, with 0.2% ropivacaine 50 ml provides effective pain relief, and improves patient comfort after surgery, without increasing adverse effects. SFNB is a safe and convenient option for postoperative pain management following knee surgery.
1 Introduction
Total knee arthroplasty (TKA) is highly effective for treating arthritis, significantly improving knee function (1). Effective postoperative pain management is crucial for rapid recovery (2).
Continuous femoral nerve block (CFNB) is considered the “gold standard” for postoperative pain control after TKA, reducing opioid use (3, 4). However, it can delay quadriceps strength recovery and cause complications like catheter prolapse or increased discomfort in elderly patients. Thus, exploring a simpler analgesic protocol is clinically important.
Recently, nerve blocks using high-volume, low-concentration anesthetics have gained attention, as they reduce motor block while providing long-lasting analgesia (5, 6). This study aimed to compare the analgesic duration and effectiveness of single femoral nerve block (SFNB) with 0.2% ropivacaine 50 ml vs. CFNB with 0.2% ropivacaine through a randomized controlled trial, to improve pain management strategies.
2 Method
2.1 General information
This study was approved by the Medical Ethics Committee of Beijing Jishuitan Hospital and registered with the China Clinical Trial Center (number: ChiCTR2100047747). All patients and their families signed informed consent forms prior to the trial. A total of 100 patients (57 males and 43 females, aged 65–90 years) undergoing TKA from June 2021 to October 2022 were selected and randomly divided into two groups: the SFNB group and the CFNB group.
2.2 Subjects
Inclusion criteria:
(1) Age >65 years,
(2) ASA Grades (American Society of Anesthesiologists Grades) ≤Grade III,
(3) Undergoing knee replacement with spinal anesthesia, and the operation time was less than 3 h.
Exclusion criteria:
(1) Refusal to participate in the study,
(2) Patients with abnormal coagulation function or those recently taking anticoagulant/antiplatelet drugs,
(3) Patients with schizophrenia, epilepsy, Parkinson's disease, or myasthenia gravis,
(4) Inability to communicate due to coma, severe dementia, or speech disorder,
(5) Recent history of craniocerebral injury, neurosurgery, or spinal surgery,
(6) Patients with sick sinus syndrome, severe sinus bradycardia (heart rate <50 beats/min), or severe atrioventricular block without pacemaker implantation,
(7) Severe abnormal liver function (Child-Pugh grade C),
(8) Severe renal dysfunction (preoperative dialysis),
(9) ASA Grade IV and above,
(10) Patients with skin rupture, infection, vasculitis, or local surgical needs in the groin area.
(11) Patients with chronic pain (VAS score >6), including severe joints pain, low back pain, and tumor pain.
Rejection criteria:
(1) Failed spinal anesthesia,
(2) Patients who experienced severe hypotension, severe allergic reactions, or toxic reactions to local anesthetic drugs during surgery,
(3) Patients who developed severe delirium during or after surgery.
2.3 Anesthesia method
In the operating room, all patients underwent continuous monitoring of ECG, oxygen saturation, and blood pressure. A peripheral intravenous route was established before surgery, and 5 ml/kg/h of Ringer's lactate solution was infused intravenously. Oxygen was administered at 3–5 L/min via a mask. The radial artery was punctured and catheterized under local anesthesia with 1% lidocaine to monitor invasive arterial pressure. Subarachnoid block was routinely performed in both groups. This block was administered at the L3–4 lumbar space using 0.2% ropivacaine, 3.0 ml. The degree of sensory block (assessed by a temperature test) was evaluated by anesthesiologists not involved in the study, and the anesthesia plane was adjusted to the T10 level. Intraoperatively, systolic blood pressure (SBP) was maintained at no less than 80% of the baseline level. Atropine and norepinephrine were prepared to manage bradycardia (heart rate <50 bpm or below 80% of baseline) and hypotension (SBP <90 mmHg or below 80% of baseline SBP).
Management of adverse events during the operation: (1) Norepinephrine was continuously infused at an initial dose of 8–12 μg/min to prevent and treat hypotension (SBP <80% of baseline); (2) Ondansetron 8 mg was administered to prevent and treat nausea and vomiting; (3) Atropine 0.25 mg was used to prevent and treat bradycardia (heart rate <50 beats/min); (4) Patients experiencing allergic reactions were treated with 80 mg methylprednisolone. The internal environment was adjusted based on blood gas analysis results.
At the end of the procedure, all patients underwent ultrasound-guided femoral nerve block and were randomly divided into two groups: SFNB and CFNB.
Upon entering the room, patients were positioned supine for the ultrasound-guided sciatic nerve block. The ultrasound probe was placed horizontally, 2 cm below the inguinal ligament, with its long axis perpendicular to the longitudinal axis of the thigh. The femoral vein and femoral artery were clearly visualized, with the sciatic nerve arranged from the inside to the outside of the inguinal ligament.
A 20G venous catheter needle was inserted through the sartorius muscle to reach the femoral nerve, located on the surface of the iliopsoas muscle. A small amount of 0.9% sodium chloride solution was injected to observe the diffusion.
In the SFNB group, patients received 50 ml of 0.2% ropivacaine (Registration number: H20140763, AstraZeneca AB Sweden, 10 ml:100 mg) injected around the femoral nerve. In the CFNB group, patients were initially injected with 20 ml of 0.5% ropivacaine around the femoral nerve, followed by the placement and fixation of a catheter for postoperative self-controlled analgesia. The analgesic pump contained 250 ml of 0.2% ropivacaine, with a background dose of 5 ml, a bolus of 5 ml, and a lock-out time of 30 min.
At the end of the surgery, all patients were transferred to the surgical intensive care unit (SCIU). If postoperative pain exceeded 4 (VAS score >4), 200 mg of celecoxib was administered orally.
2.4 Observation indicators
2.4.1 Primary outcome
The visual analogue scale (VAS) was used to assess pain (both at rest and during exercise) 24 h after surgery. The VAS score ranges from 0 to 10 points, with higher scores indicating more severe pain.
2.4.2 Secondary outcome
① VAS (at rest and during exercise) was measured at 2 h, 6 h, 12 h, 48 h, and 72 h post-surgery, along with the dosage of remedial analgesic celecoxib.
② Quadriceps muscle strength was evaluated using the manual muscle test (MMT) at 2 h, 6 h, 12 h, 24 h, 48 h, and 72 h after surgery (7). The MMT score ranges from 0 to 5, where: 5 indicates normal resistance to gravity and external force, 4 indicates resistance to gravity and partial resistance, 3 indicates resistance to gravity but not to additional resistance, 2 indicates no resistance to gravity, but with full joint movement, 1 indicates muscle contraction without joint movement, 0 indicates complete paralysis with no muscle contraction.
③ Postoperative rehabilitation was assessed by measuring the range of knee joint motion (ROM) at 24 h, 72 h, and 1 week after surgery (8). The American Knee Society Knee Score (AKS) was used to evaluate functional status 1 week postoperatively. The AKS consists of two components: The knee score includes pain (50 points), ROM (25 points), and stability (25 points), with points deducted for knee flexion and extension contracture. The functional score evaluates walking ability (50 points) and stair climbing ability (50 points), with deductions made for functional impairments.
④ Complications occurring during hospitalization were recorded, including nausea, vomiting, headache, vertigo, fever, hypotension, bradycardia, infections (urinary system, surgical incision, lung, etc.), deep vein thrombosis of the lower limbs, urinary retention, pruritus, catheter prolapse, and the length of hospital stay.
⑤ The General Comfort Questionnaire (GCQ) was used to assess patient comfort 72 h after surgery (9). The scale includes 28 items across four dimensions: physiological, mental, social-cultural, and environmental. A Likert scale from 1 to 4 was used for scoring, with higher scores indicating greater comfort.
2.5 Randomization process and allocation concealment
All patients were assigned numbers, and random numbers were generated using SPSS 25.0 (SPSS Inc., Chicago, IL, USA) statistical software. This study is single-blinded. To minimize evaluation bias, assessors responsible for screening and outcome assessment will be blinded to group assignments. The anesthesiologist will be aware of each patient's group but will remain isolated from the research results. Patients will not be informed of their group allocation. Randomization will be conducted using a computer-generated blocked randomization sequence. A nurse will generate the allocation sequence and prepare sealed, numbered envelopes. The anesthesiologist will open each envelope only when the patient enters the operating room.
2.6 Statistical analysis
SPSS 25.0 (SPSS Inc., Chicago, IL, USA) statistical software was used for data analysis. Measurement data were expressed as mean ± standard deviation (SD), and non-normally distributed data were expressed as median [interquartile range] [Median, IQR (Q25–Q75)]. The independent sample t-test was applied to compare normally distributed data between groups. Repeated measures analysis of variance was used to compare normally distributed data across different time points between the two groups, with the Bonferroni test applied for pairwise comparisons at each time point. For non-normally distributed data, a generalized estimating equation (GEE) model was used to compare differences in relevant indicators between the two groups at different time points and to clarify the interaction between time and grouping. Cross-group comparisons of adverse event incidence were conducted using the Chi-square (χ2) test. The p value of <0.05 was considered statistically significant.
2.7 Sample size evaluation
The PASS 15.0 software package was used to determine the sample size. The significance level (α) was set at 0.05, and the power (β) at 0.2. The reduction in VAS score at 24 h post-surgery was chosen as the primary outcome. Based on previous studies and reports, the difference in VAS score reduction between the SFNB and CFNB groups at 24 h post-surgery was −0.5. The non-inferiority margin (δ) was set at 1, and a non-inferiority test was applied. The sample size was calculated to be equal for both the SFNB and CFNB groups, with a SD of 0.9 and 0.7, respectively. The required sample size was determined to be 42. Accounting for a follow-up loss rate of less than 10%, the final sample size was set at 50 per group, with a total of 100 subjects.
3 Results
3.1 Basic information
A total of 94 patients successfully completed the study. In the SFNB group, 1 patient developed severe delirium postoperatively, and 1 case was converted to general anesthesia due to spinal anesthesia failure. Both were excluded, leaving 48 patients who completed the study. In the CFNB group, 2 patients required general anesthesia, 1 patient developed delirium, and 1 patient experienced catheter prolapse on the first postoperative day. These patients were also excluded, leaving 46 patients for final analysis (Figure 1).
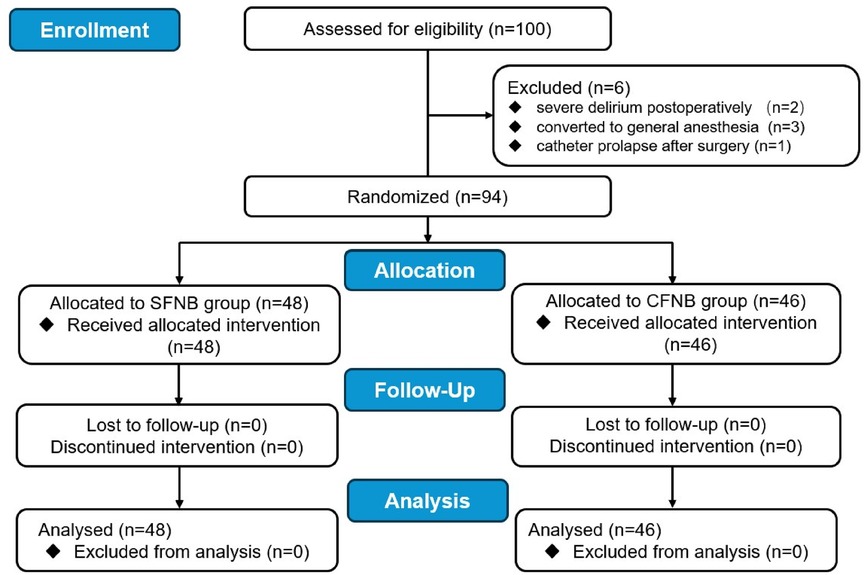
Figure 1. Flow diagram of this study. SFNB, single femoral nerve block; CFNB, Continuous femoral nerve block; TKA, total knee arthroplasty.
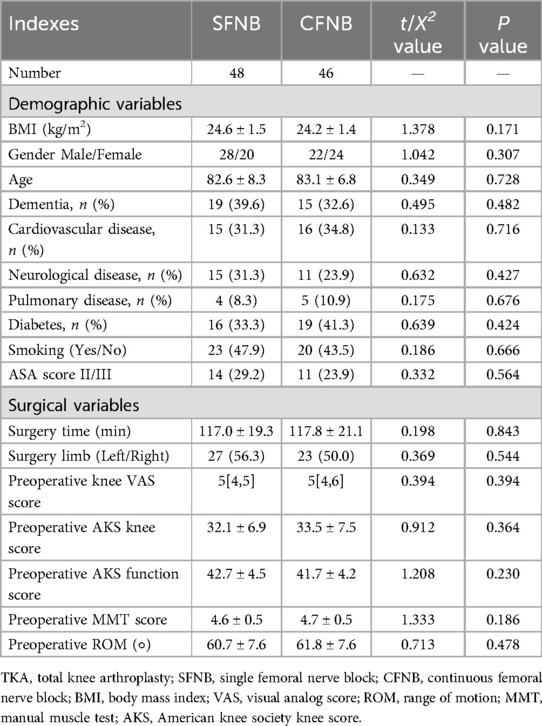
There were no statistically significant differences between the two groups in terms of age, gender distribution, body mass index, ASA grading, previous medical history (cardiovascular disease, cerebrovascular disease, pulmonary disease, diabetes, smoking history), left or right knee surgery, or surgery time (p > 0.05).
Besides, the two groups were comparable with no significant differences in surgery limb, preoperative knee VAS score, AKS knee score and function score, MMT score, as well as ROM (p > 0.05), as shown in Table 1.
3.2 Comparison of postoperative VAS at different time points
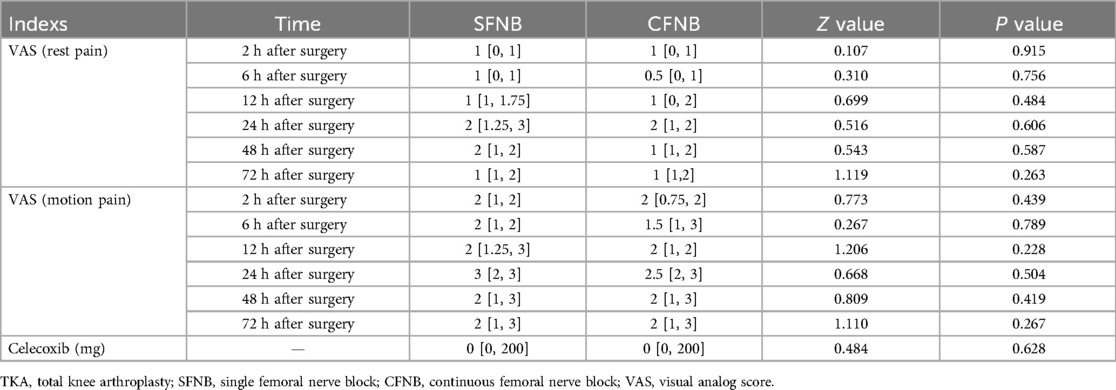
The VAS was used to evaluate pain at rest and during movement at different time points. The results indicated that both SFNB and CFNB provided effective postoperative analgesia. There were no significant differences in VAS scores for both resting and activity-related pain between the two groups at 2 h, 6 h, 12 h, 24 h, 48 h, and 72 h postoperatively (p > 0.05). Within each group, the results showed that the analgesic effect of both SFNB and CFNB lasted for 72 h post-surgery. Additionally, there was no significant difference in the total dose of celecoxib taken between the two groups after surgery (p > 0.05). Considering the interaction between time and groups on the results, we used the generalized estimating equation (GEE) model to further compare differences in VAS (rest pain and motion pain) between the two groups at different time points. The effect significance tests showed that the interaction between time and groups on VAS (rest pain) and VAS (motion pain) were not statistically significant (Wald Chi-Square rest pain = 1.369, Prest pain = 0.928 > 0.05; Wald Chi-Square motion pain = 0.466, Pmotion pain = 0.993 > 0.05), which proved that there were no significant interaction on the VAS results between time and groups, as shown in Table 2.
3.3 Comparison of quadriceps muscle strength and knee function
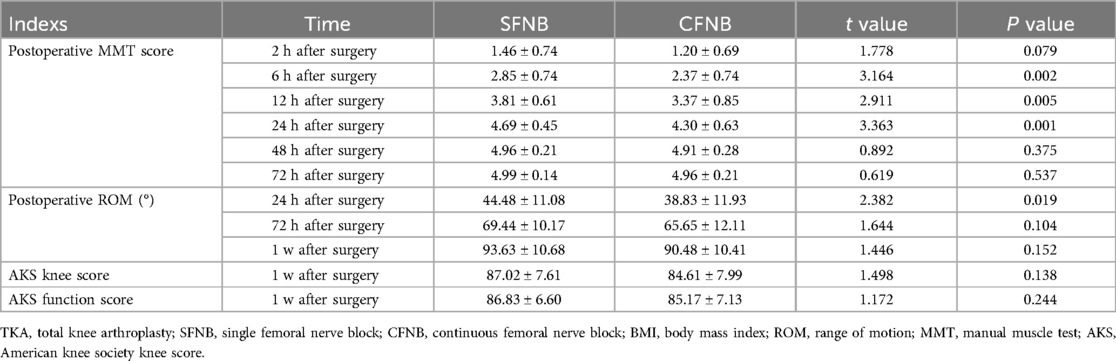
The results demonstrated that quadriceps muscle strength in both groups began to recover gradually starting 2 h after surgery. Between-group comparisons showed that the MMT scores in the SFNB group were significantly higher than those in the CFNB group (p < 0.05) at 6 h, 12 h, and 24 h post-surgery. Similarly, the knee ROM in the SFNB group was significantly better than in the CFNB group at 24 h post-surgery. There were no significant differences in muscle strength between the two groups 2 days postoperatively (p > 0.05), nor were there significant differences in knee ROM and AKS scores at 3 days (p > 0.05) and 1 week after surgery (p > 0.05). We also used the GEE model to compare differences in MMT score between the two groups at different time points. The effect significance test showed that there were significant differences between multiple time measurements (Wald Chi-SquareMMT score = 18.759, PMMT score = 0.002 < 0.05). We further compared the difference of MMT score at six times by correcting the interference of time factors. The results showed that there were no significant differences on the MMT score between the two groups at the time of 2 h, 48 h and 72 h after surgery (P2h after surgery = 0.072 > 0.05; P48h after surgery = 0.371 > 0.05; P72h after surgery = 0.535 > 0.05); while the MTT score of the SFNB group were all significantly higher than those of the CFNB group at the other three times. (P6h after surgery = 0.001 < 0.05; P12h after surgery = 0.003 < 0.05; P24h after surgery = 0.001 < 0.05), as shown in Table 3.
3.4 Comparison of complications during hospitalization
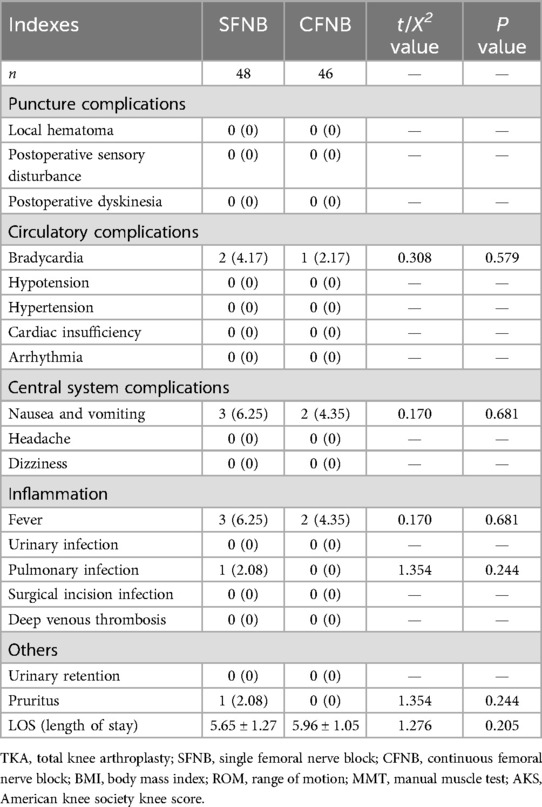
The complications contained four aspects, including puncture complications, circulatory complications, inflammation, central system complications, and so on. The results showed that, in the SFNB group, 3 patients experienced nausea and vomiting, 1 had vertigo, 2 developed bradycardia, 3 had fevers, and 1 patient had a pulmonary infection; all complications were resolved successfully. In the CFNB group, nausea and vomiting occurred in 2 patients, bradycardia in 1 patient, and fever in 2 patients. There were no significant differences in complications or LOS between the two groups (p > 0.05), as shown in Table 4.
3.5 Comparison of the GCQ scores in the two groups
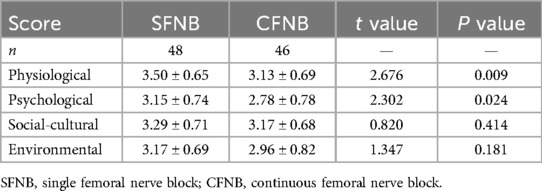
As shown in Table 5, the physiological and psychological assessment scores of the GCQ in the SFNB group were significantly higher than those in the CFNB group (p < 0.05). However, there were no significant differences in the social-cultural and environmental scores between the two groups (p > 0.05). In the CFNB group, two-thirds of the patients reported psychological and physical discomfort during daily activities or rehabilitation exercises, due to the presence of the catheter, seepage from the PCA, and pruritus caused by the adhesive tape.
4 Discussion
In recent years, the incidence of knee joint diseases has risen, and TKA surgery has been widely adopted (10). Knee replacement is commonly used to alleviate pain caused by severe knee function degradation, correct deformities, and improve quality of life (11). However, postoperative pain often hinders early joint rehabilitation, slowing knee recovery. This highlights the need for an effective analgesic method for such surgeries.
Patient-controlled intravenous analgesia (PCIA) is frequently used in clinical practice; however, it can cause sedation along with pain relief (12). Given that most TKA patients are elderly, sedation may reduce alertness, impeding early postoperative training (13). Research has shown that the femoral nerve primarily controls sensation in the anterior thigh and the knee through its branches (14). Therefore, ultrasound-guided femoral nerve block (FNB) can be an effective multimodal analgesic approach (15). CFNB provides strong analgesia and is considered the “gold standard” for postoperative pain management in TKA patients (16, 17). However, CFNB can impair muscle strength and delay early postoperative training. Additionally, femoral nerve catheterization can reduce patient comfort (16, 18). This study aimed to explore the analgesic effects of SFNB using 0.2% ropivacaine (50 ml) to offer a reference for improved clinical pain management strategies.
In this study, we found that compared with traditional CFNB, there were no statistically significant differences in VAS scores at rest and during activity at 2 h, 6 h, 12 h, 24 h, 48 h, and 72 h after surgery, nor in the dosage of postoperative celecoxib used for remedial analgesia. These results suggest that SFNB can provide adequate analgesia lasting up to 72 h postoperatively (19). In addition to quadriceps muscle strength, we also examined the ROM and the AKS score. Muscle strength was observed to recover gradually starting 2 h after TKA, and knee joint mobility resumed within 6 h post-surgery. However, the MMT grade and ROM in the SFNB group were significantly higher than in the CFNB group at 6 h, 12 h, and 24 h after surgery. We also used GEE model to further prove the differences in VAS (rest pain and motion pain) and MTT score between the two groups at different time points. The results obtained by GEE model were all similar with those by Rank sum test and independent sample t test. All the findings indicate that SFNB not only provides stable and effective analgesia but also significantly promotes the recovery of postoperative muscle strength and joint mobility, supporting overall postoperative rehabilitation (20). By 3 days and 1-week post-surgery, there was no significant difference in AKS scores between the two groups, suggesting that patients in both groups experienced a steady recovery as the drugs were metabolized and maintained consistent efficacy.
In clinical, there are many factors, including the type, concentration and volume of local anesthetics. Moreover, the relationship between the perineuronal spatial anatomy and the target nerve/plexus may have a decisive influence on the effect of local anesthetics. The femoral nerve space is a large space, and the volume of local anesthetics has become an important factor affecting the anesthetic effect. Thus, compared with 0.2% ropivacaine (20 ml), 0.2% ropivacaine (50 ml) had a wider block plane, and could fully infiltrate the femoral nerve, with a longer total sensory block time.
Regarding complications, we discussed the puncture complications, circulatory complications, inflammation, central system complications, and so on. Pre-existing comorbidities significantly impact postoperative outcomes. Cardiovascular/cerebrovascular diseases may induce severe hemodynamic fluctuations. Pulmonary comorbidities and smoking history could predispose patients to pulmonary infections and pyrexia. Dementia and cerebrovascular disorders may lead to postoperative communication difficulties. Diabetes mellitus could increase risks of infections and postoperative pain. Thus, we firstly compared the dementia, cardiovascular disease, neurological disease, pulmonary disease, diabetes, and smoking between the two groups. There were no statistically significant difference, which implied that the two groups had comparable baseline characteristics, including physical status and comorbidities.
According to the postoperative complications, We found that: (1) there were no puncture complications, such as local hematoma, postoperative sensory disturbance and dyskinesia, which implied the SFNB and CFNB were safe. (2) According to the circulatory complications. there were two cases of bradycardia in SFNB group and one case of bradycardia in CFNB group, which had nothing to do with the FNB. We thought the bradycardia was related to the age and patient's basal heart rate. (3) Postoperative nausea and vomiting were relatively common in the two groups, which may be linked with the circulatory fluctuations or stimulation of vestibular function induced by moving the patient and postural changes. (4) Fever was also relatively common, which maybe caused by the weak resistance of the elderly patients and the operation stimulation. (5) Besides, femoral nerve block could facilitate early postoperative active movement and relieve pain for patients. However, continuous femoral nerve block, due to the presence of the catheter, could cause some discomfort during active training.
The results indicated that SFNB with a low concentration and high volume of anesthetic is safe and suitable for widespread clinical use. Although the difference in hospital stay between the two groups was not statistically significant, the average length of stay in the SFNB group was slightly shorter than in the CFNB group, suggesting that early postoperative training might help accelerate rehabilitation, which need to be further discussed.
Additionally, the General Comfort Questionnaire (GCQ) (21, 22) was used to evaluate patient comfort 72 h after surgery. We found that both the physiological and psychological assessment scores in the SFNB group were significantly higher than those in the CFNB group. Two-thirds of the patients in the CFNB group reported feeling psychological and physical discomfort during daily activities and rehabilitation exercises due to the presence of the catheter, seepage from the PCA, and pruritus caused by the adhesive tape (23, 24). These findings suggest that continuous femoral nerve block may increase patient discomfort after surgery. In contrast, single femoral nerve block not only provides effective pain relief and supports postoperative functional training but also improves overall patient comfort.
However, this study has certain limitations: (1) The study was limited to two groups comparing the analgesic effects of SFNB and CFNB combined with PCIA. The minimum effective concentration of ropivacaine for this procedure in elderly patients needs further investigation. (2) This study was conducted at a single center with a relatively small sample size. Future multicenter randomized controlled trials with larger sample sizes are necessary to provide more robust evidence for pain management strategies.
5 Conclusion
SFNB with 0.2% ropivacaine (50 ml) offers effective and stable postoperative analgesia that is both safe and straightforward. Using low-concentration, high-volume ropivacaine can accelerate the recovery of muscle strength following TKA, facilitating early postoperative training and promoting faster recovery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Beijing Jishuitan Hospital, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
YT: Project administration, Writing – original draft. NC: Methodology, Project administration, Writing – original draft. JZ: Project administration, Writing – original draft. PL: Conceptualization, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bergstein V, Weinblatt A, Taylor W, Long W. Total knee arthroplasty survivorship and outcomes in young patients: a review of the literature and 40-year update to a longitudinal study. Arch Orthop Trauma Surg. (2024) 144(9):4077–83. doi: 10.1007/s00402-024-05198-5
2. Jurewicz A, Gasiorowska A, Leźnicka K, Pawlak M, Sochacka M, Machoy-Mokrzyńska A, et al. Individual factors modifying postoperative pain management in elective total hip and total knee replacement surgery. Life. (2024) 14(2):211. doi: 10.3390/life14020211
3. Yu D, Wu Y, Han S, Wang X, Jiang L. Analgesic efficacy of local infiltration anaesthesia versus femoral nerve block in alleviating postoperative wound pain following total knee arthroplasty: a systematic review and meta-analysis. Int Wound J. (2024) 21(2):e14766. doi: 10.1111/iwj.14766
4. Dong J, Jin Z, Chen H, Bao N, Xia F. Sufentanil improves the analgesia effect of continuous femoral nerve block after total knee arthroplasty. J Pain Res. (2023) 16:4209–16. doi: 10.2147/jpr.S409668
5. Pippa P, Cuomo P, Panchetti A, Scarchini M, Poggi G, D'Arienzo M. High volume and low concentration of anaesthetic solution in the perivascular interscalene sheath determines quality of block and incidence of complications. Eur J Anaesthesiol. (2006) 23(10):855–60. doi: 10.1017/S0265021506001074
6. Albrecht E, Morfey D, Chan V, Gandhi R, Koshkin A, Chin K, et al. Single-injection or continuous femoral nerve block for total knee arthroplasty? Clin Orthop Relat Res. (2014) 472(5):1384–93. doi: 10.1007/s11999-013-3192-3
7. de Padua A, Renfro C, Grabnar M, Kilgore K, Bryden A, Roach M, et al. Can the electrically stimulated manual muscle test differentiate upper from lower motor neuron injury in persons with acute SCI? Neurol Res. (2023) 45(10):906–11. doi: 10.1080/01616412.2020.1824417
8. Ma J, Zhang L, Kuang M, Zhao J, Wang Y, Lu B, et al. The effect of preoperative training on functional recovery in patients undergoing total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. (2018) 51:205–12. doi: 10.1016/j.ijsu.2018.01.015
9. Gonzalez-Baz M, Pacheco Del Cerro E, Ferrer-Ferrándiz E, Araque-Criado I, Merchán-Arjona R, de la Rubia Gonzalez T, et al. Psychometric validation of the kolcaba general comfort questionnaire in critically ill patients. Aust Crit Care. (2023) 36(6):1025–34. doi: 10.1016/j.aucc.2022.12.013
10. Alrawashdeh W, Eschweiler J, Migliorini F, El Mansy Y, Tingart M, Rath B. Effectiveness of total knee arthroplasty rehabilitation programmes: a systematic review and meta-analysis. J Rehabil Med. (2021) 53(6):jrm00200. doi: 10.2340/16501977-2827
11. Lavand'homme P, Kehlet H, Rawal N, Joshi G. Pain management after total knee arthroplasty: PROcedure SPEcific postoperative pain management recommendations. Eur J Anaesthesiol. (2022) 39(9):743–57. doi: 10.1097/eja.0000000000001691
12. Kim S, Han H, Lee M, Ro D. Single shot adductor canal block combined with intravenous patient-controlled analgesia can be effective as continuous adductor canal block in reducing opioid consumption and breakthrough pain after total knee arthroplasty. J Exp Orthop. (2022) 9(1):84. doi: 10.1186/s40634-022-00523-6
13. Yang X, Dong J, Xiong W, Huang F. Early postoperative pain control and inflammation for total knee arthroplasty: a retrospective comparison of continuous adductor canal block versus single-shot adductor canal block combined with patient-controlled intravenous analgesia. Emerg Med Int. (2022) 2022:1351480. doi: 10.1155/2022/1351480
14. Grape S, Kirkham K, Baeriswyl M, Albrecht E. The analgesic efficacy of sciatic nerve block in addition to femoral nerve block in patients undergoing total knee arthroplasty: a systematic review and meta-analysis. Anaesthesia. (2016) 71(10):1198–209. doi: 10.1111/anae.13568
15. Ren Y, Tian M, Duan Y, Sun Y, Yang T, Hou W, et al. Was femoral nerve block effective for pain control of medial opening-wedge high tibial osteotomy?: a single blinded randomized controlled study. Medicine. (2021) 100(3):e23978. doi: 10.1097/md.0000000000023978
16. Li J, Ma Y, Xiao L. Postoperative pain management in total knee arthroplasty. Orthop Surg. (2019) 11(5):755–61. doi: 10.1111/os.12535
17. Elmallah R, Chughtai M, Khlopas A, Newman J, Stearns K, Roche M, et al. Pain control in total knee arthroplasty. J Knee Surg. (2018) 31(6):504–13. doi: 10.1055/s-0037-1604152
18. Gunaratne R, Pratt D, Banda J, Fick D, Khan R, Robertson B. Patient dissatisfaction following total knee arthroplasty: a systematic review of the literature. J Arthroplasty. (2017) 32(12):3854–60. doi: 10.1016/j.arth.2017.07.021
19. Gandhi H, Trivedi L, Tripathi D, Dash D, Khare A, Gupta M. A randomized, controlled trial of comparison of a continuous femoral nerve block (CFNB) and continuous epidural infusion (CEI) using 0.2% ropivacaine for postoperative analgesia and knee rehabilitation after total knee arthroplasty (TKA). J Anaesthesiol Clin Pharmacol. (2019) 35(3):386–9. doi: 10.4103/joacp.JOACP_134_16
20. Babu S, Menon G, Vasu B, George M, Thilak J, Iyer S. Postoperative ultrasound guided continuous femoral nerve blockade for unilateral total knee arthroplasty: a comparison of 0.125% bupivacaine and 0.2% ropivacaine. Anesth Essays Res. (2017) 11(4):1026–9. doi: 10.4103/aer.AER_155_17
21. Balci H, Saglam Y, Pehlivanoglu T, Sen C, Eralp L, Kocaoglu M. Knee arthrodesis in persistently infected total knee arthroplasty. J Knee Surg. (2016) 29(7):580–8. doi: 10.1055/s-0035-1569479
22. Gao C, Huang T, Wu K, Zhang W, Wang S, Chai X, et al. Multimodal analgesia for accelerated rehabilitation after total knee arthroplasty: a randomized, double-blind, controlled trial on the effect of the co-application of local infiltration analgesia and femoral nerve block combined with dexmedetomidine. Brain Sci. (2022) 12(12):1652. doi: 10.3390/brainsci12121652
23. Boussemart P, Quintard H. Continuous peripheral nerve block for analgesia in the severe polytraumatized patient: better analgesia for fewer opioids used? Anaesth Crit Care Pain Med. (2023) 42(2):101215. doi: 10.1016/j.accpm.2023.101215
Keywords: ropivacaine, femoral nerve block, perioperative analgesia, total knee arthroplasty, the elderly
Citation: Tao Y, Cai N, Zhang J, Zhou Y and Liu P (2025) Effects of single femoral nerve block and continuous femoral nerve block on perioperative analgesia and muscle strength in elderly patients undergoing total knee arthroplasty, a randomized clinical trial. Front. Surg. 12:1403280. doi: 10.3389/fsurg.2025.1403280
Received: 26 March 2024; Accepted: 9 April 2025;
Published: 16 June 2025.
Edited by:
Ziyi Wang, Johns Hopkins University, United StatesReviewed by:
Jianda Xu, Changzhou Traditional Chinese Medicine Hospital, ChinaAlfredo Lerín Calvo, La Salle Centro Universitario, Spain
Yatan Li, Xinjiang Medical University, China
Copyright: © 2025 Tao, Cai, Zhang, Zhou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zhou, emhvdXlhbnN0dWR5QHNpbmEuY29t; Pengfei Liu, c2ZmbHBmQDEyNi5jb20=
 Yan Tao1
Yan Tao1 Nan Cai
Nan Cai Juxia Zhang
Juxia Zhang Pengfei Liu
Pengfei Liu