- 1Department of Colorectal Surgery, Zhejiang Hospital of Integrated Traditional Chinese and Western Medicine, Hangzhou, Zhejiang, China
- 2Department of General Surgery, Zhoushan Hospital, Wenzhou Medical University, Zhoushan, Zhejiang, China
- 3The Laboratory of Cytobiology and Molecular Biology, Zhoushan Hospital, Wenzhou Medical University, Zhoushan, Zhejiang, China
Background & aims: Extended cholecystectomy (EC) is recommended for T1b gallbladder cancer (GBC), but the optimal surgical procedure for T1b GBC remains controversial. This study aims to compare the prognosis of T1b GBC patients who underwent simple cholecystectomy (SC) vs. EC from a long-term survival perspective.
Methods: We performed a systematic search up to August 06, 2024, using MEDLINE (PubMed), EMBASE, Web of Science and Cochrane Library. The main outcomes were overall survival (OS) and disease-specific survival (DSS). We evaluated the quality of the studies included and the risk of bias, calculated the pooled hazard ratios (HRs) for OS and DSS and conducted the sensitivity analysis.
Results: A total of 8 retrospective studies involving 2,097 T1b GBC patients (SC = 1,263, EC = 408) were included. The pooled result of OS showed that the EC group had a significantly better OS than the SC group (pooled HR = 0.73; 95% CI = 0.59–0.89; P = 0.002). The pooled result of DSS indicated that EC significantly improved DSS of T1b GBC compared to SC (pooled HR = 0.47; 95% CI = 0.29–0.77; P = 0.003).
Conclusions: EC should be chosen as the optimal surgical procedure for patients with T1b GBC from the standpoint of long-term postoperative survival. However, further analysis of more comprehensive studies will be necessary in the future to improve the quality of evidence.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42023449431, PROSPERO CRD42023449431.
Introduction
Gallbladder cancer (GBC) is the most common malignant tumor of the biliary system with high lethality (1). Although the incidence of GBC is relatively low, with 115,949 new cases in 2020, ranking 25th among all 36 common tumors, the prognosis of GBC is poor with a five-year survival rate of less than 5% for advanced GBC, since the patients are always diagnosed at an advanced stage (2–4). Treatment of GBC includes surgery and adjuvant therapy, with surgery being the primary method of curing GBC, while the value of adjuvant therapy remains unclear (5–9). In terms of surgery, R0 resection (negative surgical margins) is considered as an important factor affecting the long-term prognosis of GBC, which has been a consensus stated by experts (10–12). The choice of surgical procedure mainly depends on the stage of GBC, however, the extent of R0 resection for T1 GBC has been controversial, especially for T1b GB (1, 13).
According to the eighth edition of the American Joint Committee on Cancer (AJCC) staging criteria, T1 GBC includes T1a and T1b GBC, which represents tumor invasion of the lamina propria and the muscular layer, respectively (14). The main controversy over the surgical strategy for T1 GBC has always been whether extended cholecystectomy (EC) is necessary or whether simple cholecystectomy (SC) is adequate (15, 16). SC indicates removal of the gallbladder only, and EC represents removal of the adjacent liver tissue (wedge resection or IVb and V hepatic segmental resection) with regional lymph node dissection in addition to removal of gallbladder (1, 14). Based on the high five-year cumulative survival rate of T1a GBC after SC, which is above 95%, SC alone as a treatment for T1a GBC is generally considered reasonable (12, 17–19). As for T1b GBC, the National Comprehensive Cancer Network (NCCN) guidelines and the Clinical practice guidelines of Japan recommend that T1b or greater lesions should receive radical surgery (20, 21). However, SC still accounts for a significant portion of the surgical volume for T1b GBC in clinical practice, which means that, as a recommended procedure for T1b GBC, EC is still under- recognized (22–24). In addition, several studies have concluded EC does not significantly improve the long-term prognosis of patients with T1b GBC compared to SC (23, 25). One study even noted that there is a trend toward worse survival in T1b GBC receiving EC, though no statistically significant difference is shown (26).
There was a systematic review and meta-analysis about this controversy, which concluded that SC and EC show no difference in patients with T1b GBC in terms of long-term survival. However, the authors of the study argued that the conclusions are limited by a number of factors, including insufficient survival data and lack of standardization of surgical methodology and pathology reports (16). After first meta-analysis was published, a number of higher-quality articles with more comprehensive data have been published in response to the controversy. Therefore, we collected related studies published up to the year 2024 and conducted an updated systematic review and meta-analysis to reevaluate which surgical procedure is better for T1b GBC from a long-term survival perspective.
Methods
The protocol of this research was registered in advance in the International Prospective Register of Systematic Reviews. (CRD42023449431). This meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (27).
Data sources and search strategy
The literature search was performed using MEDLINE (PubMed), EMBASE, Web of Science and Cochrane Library, up to August 06, 2024, with language restricted to English. The literatures were retrieved by combining medical subject headings (MeSH) terms with entry terms. The details of search terms and search strategy are shown in Table 1.
Study selection
Two authors (G.H.P., Y.Z.) independently reviewed the titles, abstracts, and the full-text was evaluated when necessary. Disagreements were resolved by consulting a third team member (M.H.J.).
The inclusion criteria were as follows: (1) population: Patients with a final pathologic diagnosis of T1b GBC (gallbladder adenocarcinoma), whether it is GBC that has been clarified preoperatively by imaging or incidental GBC found by postoperative pathology; (2) intervention: the studies including the comparison of SC and EC; (3) study design: randomized controlled trials (RCTs) or cohort studies; (4) outcomes: sufficient data describing overall survival (OS) or disease-specific survival (DSS) with follow-up longer than 5 years.
The following studies were excluded: (1) reviews, case reports, letters, conference abstracts or guidelines; (2) studies with experimental or control group sample size of less than five; (3) when data were overlapped from the same database with same study periods, studies with insufficient data were excluded; (4) studies without hazard ratios (HRs) or Kaplan–Meier (KM) survival curves comparing the two surgical strategies. We defined the SC as cholecystectomy alone without lymphadenectomy, and the EC was identified as cholecystectomy including a wedge resection of the gallbladder bed in the liver or segmentectomy of liver segments IVb and V with regional lymphadenectomy regardless of whether open or laparoscopic, and whether extended surgery in the first procedure or secondary enlargement for incidental GBC. Therefore, studies that did not meet the definition of these two surgical strategies were also excluded.
Data extraction and quality assessment
The main outcomes were OS and DSS. Data were extracted by two authors (G.H.P., Y.Z.) independently according to the predefined data collection form. The following data were extracted from included studies: first author, publication year, study period, country of included patients, study design, median follow-up, sample size, gender, age, 5-year OS rate, 5-year DSS rate, HR of OS and HR of DSS. For studies with only KM survival curves but no HRs, data were extracted from KM survival curves using Engauge Digitizer11.1, and HRs were estimated following the methods provided by Tierney et al. (28).
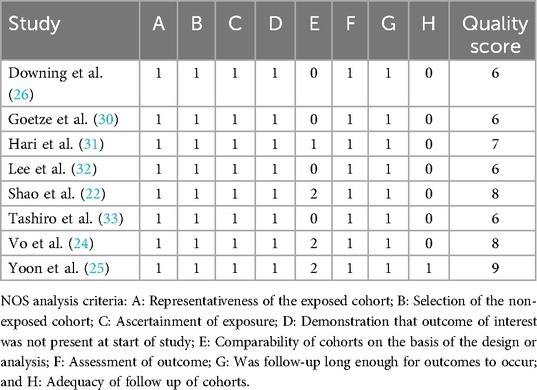
The quality of included studies was assessed using the Newcastle-Ottawa Scale (NOS) by two authors (G.H.P., Y.Z.) independently (29). Studies with a quality score ≤4 were considered low-quality studies and would be excluded. Disagreements were resolved by consulting a third team member (M.H.J.) during data extraction and quality assessment.
Data synthesis and statistical analysis
The pooled HR with 95% confidence interval (CI) was used to explore risk factors for OS and DSS of T1b GBC. Statistical heterogeneity was assessed using Q statistical test and I2 statistical test. Fixed-effects model was chosen when there was no significant heterogeneity (Q tests, P > 0.1; I2 < 50%), otherwise, random-effects model was used.
Sensitivity analysis was conducted to assess the stability and reliability of the pooled results of the meta-analysis by eliminating eligible studies one by one. Publication bias was assessed using funnel plots and the Egger's test.
Statistical analysis was performed using the Review Manager (RevMan) (version 5.3; The Cochrane Collaboration, The Nordic Cochrane Center, Copenhagen, Denmark) and Stata software Version 12.0 (Stata Corp. LP, College Station, Texas, USA). Statistical significance was defined as a two-tailed P value less than 0.05.
Results
Systematic search and characteristics
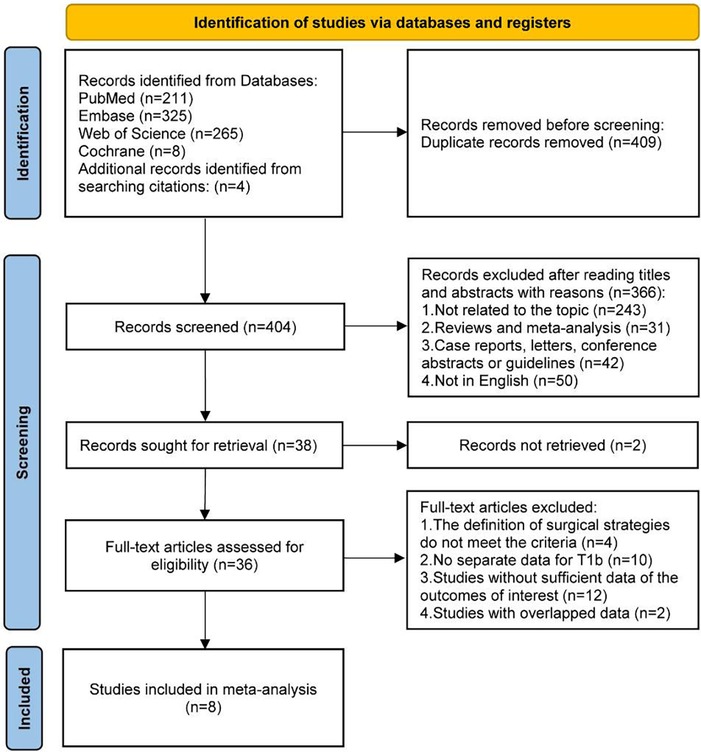
After an initial systematic search, a total of 813 studies were identified, of which 409 duplicate records were excluded. 366 studies were further excluded by reading the titles and abstracts, and 2 studies were also excluded for records not retrieved. The 36 remaining studies were assessed for eligibility through full-text reading, and 8 studies were included in the final analysis (Figure 1) (22, 24–26, 30–33).
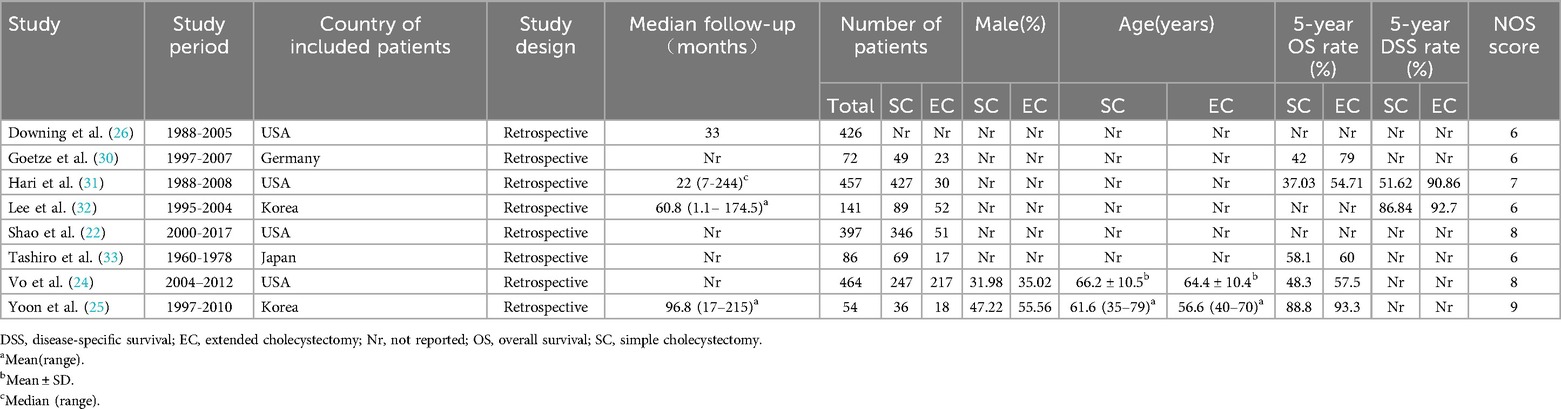
The characteristics of included studies are described in Table 2. The 8 included studies were all retrospective cohort studies and included a total of 2,097 patients with T1b GBC, of which 1,263 patients received SC and 408 patients received EC, with one study not describing the exact number of patients who received SC or EC (26). The lowest quality score was 6 among all studies and the details of quality assessment of all included studies are presented in Table 3.
OS
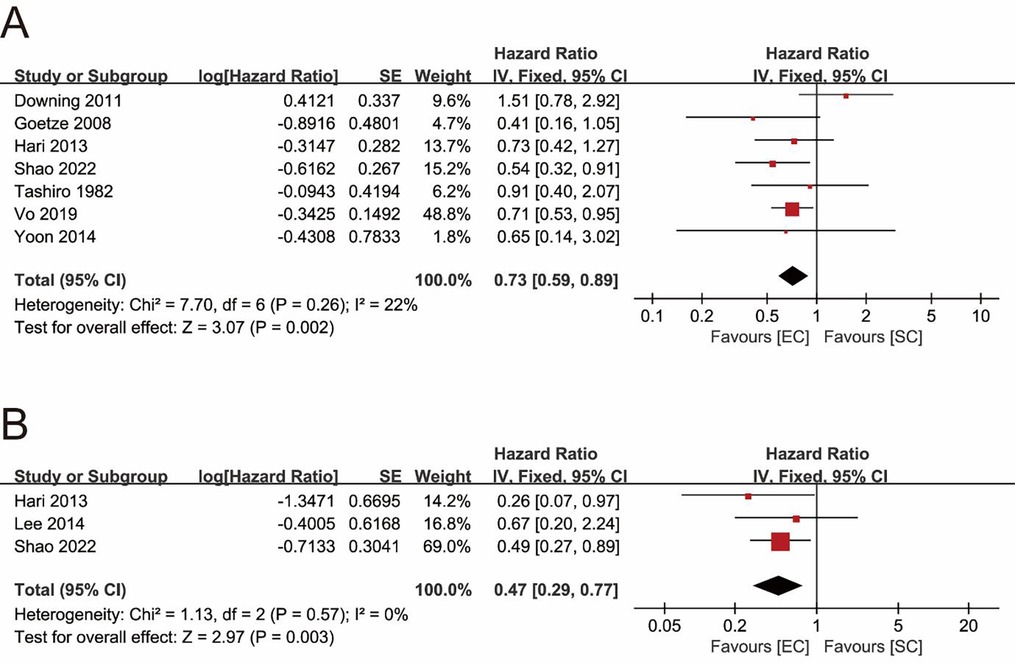
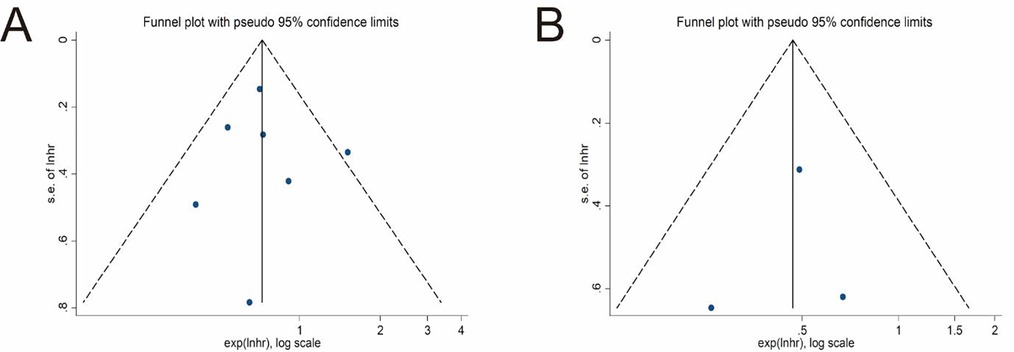
Seven studies explored the impact of surgical procedures (SC vs. EC) on the long-term survival of T1b GBC by providing HR values or KM survival curves for OS (22, 24–26, 30, 31, 33). The pooled result showed that the EC group had a significantly better OS (pooled HR = 0.73; 95% CI = 0.59- 0.89; P = 0.002) (Figure 2A). No significant heterogeneity (I2 = 22%, P = 0.26) and statistical (Egger's test, P = 0.915) or visual (Figure 3A) evidence of publication bias were observed.
DSS
Only 3 studies compared the differences in DSS between the SC and EC groups (22, 31, 32). The pooled result indicated that EC significantly improved DSS of T1b GBC compared to SC (pooled HR = 0.47; 95% CI = 0.29–0.77; P = 0.003) (Figure 2B) without significant heterogeneity (I2 = 0%, P = 0.57) and statistical (Egger's test, P = 0.816) or visual (Figure 3B) evidence of publication bias.
Sensitivity analysis
Sensitivity analyses were conducted on the pooled results of OS and DSS. The pooled result of OS did not change after eliminating any individual study, which confirmed the stability and reliability of the pool result (Figure 4A). However, when the study reported by Shao et al. was excluded, the significance of the pooled result of DSS disappeared (Figure 4B), suggesting that the pooled result of DSS was not robust and greatly influenced by the data reported by Shao et al. (22).
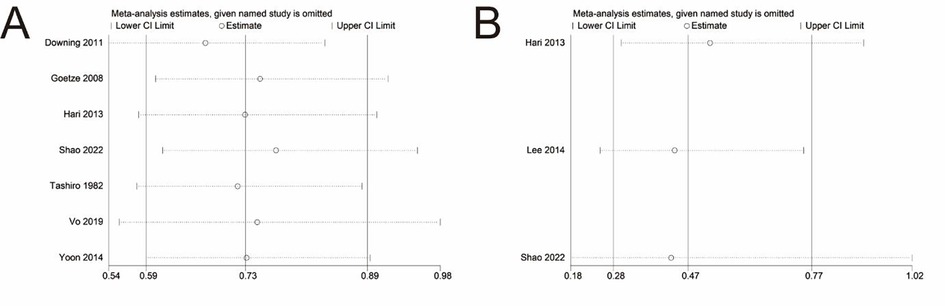
Figure 4. Sensitivity analysis of main outcomes. (A) Overall survival. (B) Disease-specific survival.
Discussion
This systematic review and meta-analysis of 8 retrospective cohort studies provided an overview of the evidence comparing different surgical procedures on the long-term survival of T1b GBC. Our study found that EC led to better long-term survival outcomes for T1b GBC compared to SC. From an OS perspective, EC reduced the risk of outcome events in T1b GBC by 27% and reduced the risk of outcome events by 53% in terms of DSS.
The recurrence and lymph node metastasis rates of GBC are as high as approximately 50%, meanwhile, the deeper the invasion of GBC, the higher the degree of malignancy and the higher the risk of metastasis (34–36). From a more specific perspective, the incidence of residual or metastatic lesions after SC for GBC is similarly high (11, 37). Moreover, GBC spreads early through lymph, blood, or direct infiltration into the liver. Although T1b GBC does not penetrate the entire gallbladder wall and cannot directly infiltrate the liver, it may produce undetectable micrometastases through lymph and venous blood. Anatomically, cholecystic venous blood most frequently enters the peripheral portal vein branches of hepatic segment IV and V (38), and there are small veins in the connective tissue between the gallbladder and liver that directly enter the liver parenchyma (39, 40), through which GBC cells can metastasize to the liver parenchyma of the IVb and V segments surrounding the gallbladder and form local intrahepatic metastases. Consequently, as opposed to SC, EC removes the liver tissue at high risk for GBC metastasis, which are the liver tissue adjacent to the gallbladder bed and the segment of the liver with the most frequent inflow of the gallbladder vein, and the regional lymph nodes, which means the potential micro-metastatic lesions are removed as well. It may be in this way that EC reduces the postoperative metastasis or recurrence of T1b GBC, thereby significantly improving their long-term survival outcomes. Meanwhile, the conclusion of our study suggested that EC was more effective in reducing the risk of outcome events of DSS than reducing the risk of outcome events of OS in T1b GBC (53% vs. 27%), which laterally verified that EC might play a role by improving the postoperative metastasis or recurrence in T1b GBC. However, due to the lack of data related to recurrence or metastasis in most of the studies included in this meta-analysis, it is not possible to validate the above speculation by data analysis for the time being.
The diagnosis of T1b GBC can be broadly categorized into two types, T1b GBC clinically diagnosed with the aid of imaging, namely cT1b GBC, and pT1b incidental GBC, which is definitively diagnosed by intraoperative or postoperative pathology. In fact, close to half of late GBC and two-thirds of early GBC are diagnosed incidentally (not suspected before or during surgery) and are usually concealed by mucosal changes caused by acute cholecystitis during routine examination of the gallbladder (1). It is in view of this situation that both cT1b GBC and pT1b incidental GBC were included in our study. Therefore, we did not specifically differentiate between the above two cases during the search, but rather targeted GBC with a final pathologic diagnosis of T1b. Consequently, when we searched for extended cholecystectomy, we included the literature based on the scope of the procedure only, including both the first surgery with extended cholecystectomy and the second extended surgery due to a diagnosis of pT1b incidental GBC after simple cholecystectomy. For this reason, the results of our study can be interpreted as follows: for cT1b GBC, extended cholecystectomy is the appropriate surgical procedure, whereas for pT1b incidental GBC, given the risk of residual lesions in the gallbladder fossa or regional lymph nodes, a second extended procedure is usually recommended, including removal of the adjacent liver tissue (wedge resection or IVb and V hepatic segmental resection) with regional lymph node dissection.
A previous systematic review and meta-analyses have reported that SC is comparable to EC with regard to overall survival in T1b GBC (16), but this study was limited by the number of cases included in the study and the lack of standardization of key factors. Our study excluded the studies with less than 5 samples in the experimental or control group included in the previous study and included newly published research on this topic. Meanwhile, we also standardized the definition of extent of surgical procedures. In addition, we included DSS as a new outcome indicator and used HR as a new effect value, which better and more comprehensively reflected the risk profile throughout the postoperative period of T1b GBC by excluding time factors. Therefore, despite the inconsistency with previous findings, our conclusions are more current and can be used as additional evidence to the mainstream guidelines as well as consensus.
However, there are some limitations that should be acknowledged in our study. First, the unstable results of sensitivity analysis on DSS may be attributed to insufficient studies included in the analysis. Second, the HRs estimated from KM survival curves were the result of log-rank tests that did not exclude the effect of confounding factors, and the process of extracting data from the KM survival curves caused slight errors as well. Third, our study only considered the long-term survival outcomes of patients and did not evaluate the impact of SC and EC on T1b GBC in other ways, such as postoperative complications, quality of life, and so on. Fourth, the influence of the type of surgical approach, whether open or laparoscopic, was not considered in our study. Finally, the eight studies included in our analysis are all retrospective cohort studies, with five studies having moderate quality, therefore the evidence level of these studies is relatively low.
Robotic surgery in gallbladder cancer has demonstrated an advantage in the precision of lymph node dissection in key areas such as hilar liver and peripancreatic head. For example, a case of single-hole robotic surgery showed that using the flexibility of the robotic arm and the high-definition three-dimensional field of view, the 7th, 8th, 12th, and 13th lymph nodes required for gallbladder cancer could be completely removed, and the postoperative pathologically confirmed negative margin rate was as high as 91% (41). Compared with traditional laparoscopy, the robotic system reduces the problem of limited field of view due to instrument conflict (42). Robotic surgery has demonstrated technical feasibility and short-term efficacy advantages in the treatment of gallbladder cancer, but its full promotion needs to address key issues such as device suitability, cost-effectiveness and insufficient long-term oncologic evidence. In the next 5–10 years, as technology iterations and high-quality evidence accumulate, robotic surgery is expected to become an important option for minimally invasive treatment of early gallbladder cancer.
Conclusions
The findings of our research supported that EC should be chosen as the optimal surgical procedure for patients with T1b GBC from the standpoint of long-term postoperative survival. Specifically, for cT1b GBC, extended cholecystectomy is the appropriate surgical procedure, whereas for pT1b incidental GBC, a second extended procedure is usually recommended. However, more future studies with large-scale and more comprehensive data are essential to strengthen the current findings and guide the clinical treatment through an evidence-based approach.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
HG: Data curation, Methodology, Project administration, Validation, Writing – original draft. GZ: Investigation, Supervision, Visualization, Writing – original draft. HM: Investigation, Project administration, Resources, Supervision, Writing – review & editing. ZY: Conceptualization, Formal analysis, Funding acquisition, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Zhejiang Medical and Health Science and Technology Project (2024KY517).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Roa JC, García P, Kapoor VK, Maithel SK, Javle M, Koshiol J. Gallbladder cancer. Nat Rev Dis Primers. (2022) 8:69. doi: 10.1038/s41572-022-00398-y
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Roa I, Ibacache G, Muñoz S, de Aretxabala X. Gallbladder cancer in Chile: pathologic characteristics of survival and prognostic factors: analysis of 1,366 cases. Am J Clin Pathol. (2014) 141:675–82. doi: 10.1309/AJCPQT3ELN2BBCKA
4. Bertran E, Heise K, Andia ME, Ferreccio C. Gallbladder cancer: incidence and survival in a high-risk area of Chile. Int J Cancer. (2010) 127:2446–54. doi: 10.1002/ijc.25421
6. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. (2012) 30:1934–40. doi: 10.1200/JCO.2011.40.5381
7. Ma N, Cheng H, Qin B, Zhong R, Wang B. Adjuvant therapy in the treatment of gallbladder cancer: a meta-analysis. BMC Cancer. (2015) 15:615. doi: 10.1186/s12885-015-1617-y
8. Saluja SS, Nekarakanti PK, Mishra PK, Srivastava A, Singh K. Prospective randomized controlled trial comparing adjuvant chemotherapy vs. No chemotherapy for patients with carcinoma of gallbladder undergoing curative resection. J Gastrointest Surg. (2022) 26:398–407. doi: 10.1007/s11605-021-05143-6
9. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. (2019) 20:663–73. doi: 10.1016/S1470-2045(18)30915-X
10. D'Hondt M, Lapointe R, Benamira Z, Pottel H, Plasse M, Letourneau R, et al. Carcinoma of the gallbladder: patterns of presentation, prognostic factors and survival rate. An 11-year single centre experience. Eur J Surg Oncol. (2013) 39:548–53. doi: 10.1016/j.ejso.2013.02.010
11. Fuks D, Regimbeau JM, Treut L, Bachellier YP, Raventos P, Pruvot A, et al. Incidental gallbladder cancer by the AFC- GBC-2009 study group. World J Surg. (2011) 35:1887–97. doi: 10.1007/s00268-011-1134-3
12. Aloia TA, Járufe N, Javle M, Maithel SK, Roa JC, Adsay V, et al. Gallbladder cancer: expert consensus statement. HPB (Oxford). (2015) 17:681–90. doi: 10.1111/hpb.12444
13. Krell RW, Wei AC. Gallbladder cancer: surgical management. Chin Clin Oncol. (2019) 8:36. doi: 10.21037/cco.2019.06.06
14. Hickman L, Contreras C. Gallbladder cancer: diagnosis, surgical management, and adjuvant therapies. Surg Clin North Am. (2019) 99:337–55. doi: 10.1016/j.suc.2018.12.008
15. Lee SE, Jang JY, Lim CS, Kang MJ, Kim SW. Systematic review on the surgical treatment for T1 gallbladder cancer. World J Gastroenterol. (2011) 17:174–80. doi: 10.3748/wjg.v17.i2.174
16. Lee H, Kwon W, Han Y, Kim JR, Kim SW, Jang JY. Optimal extent of surgery for early gallbladder cancer with regard to long-term survival: a meta-analysis. J Hepatobiliary Pancreat Sci. (2018) 25:131–41. doi: 10.1002/jhbp.521
17. Cangemi V, Fiori E, Picchi C, De Cesare A, Cangemi R, Galati G, et al. Early gallbladder carcinoma: a single-center experience. Tumori. (2006) 92:487–90. doi: 10.1177/030089160609200604
18. Kwon AH, Imamura A, Kitade H, Kamiyama Y. Unsuspected gallbladder cancer diagnosed during or after laparoscopic cholecystectomy. J Surg Oncol. (2008) 97:241–5. doi: 10.1002/jso.20944
19. You DD, Lee HG, Paik KY, Heo JS, Choi SH, Choi DW. What is an adequate extent of resection for T1 gallbladder cancers? Ann Surg. (2008) 247:835–8. doi: 10.1097/SLA.0b013e3181675842
20. Benson AB 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN Clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. (2009) 7:350–91. doi: 10.6004/jnccn.2009.0027
21. Nagino M, Hirano S, Yoshitomi H, Aoki T, Uesaka K, Unno M, et al. Clinical practice guidelines for the management of biliary tract cancers 2019: the 3rd English edition. J Hepatobiliary Pancreat Sci. (2021) 28:26–54. doi: 10.1002/jhbp.870
22. Shao J, Lu HC, Wu LQ, Lei J, Yuan RF, Shao JH. Simple cholecystectomy is an adequate treatment for grade I T1bN0M0 gallbladder carcinoma: evidence from 528 patients. World J Gastroenterol. (2022) 28:4431–41. doi: 10.3748/wjg.v28.i31.4431
23. Xu L, Tan H, Liu X, Huang J, Liu L, Si S, et al. Survival benefits of simple versus extended cholecystectomy and lymphadenectomy for patients with T1b gallbladder cancer: an analysis of the surveillance, epidemiology, and end results database (2004 to 2013). Cancer Med. (2020) 9:3668–79. doi: 10.1002/cam4.2989
24. Vo E, Curley SA, Chai CY, Massarweh NN, Tran Cao HS. National failure of surgical staging for T1b gallbladder cancer. Ann Surg Oncol. (2019) 26:604–10. doi: 10.1245/s10434-018-7064-7
25. Yoon JH, Lee YJ, Kim SC, Lee JH, Song KB, Hwang JW, et al. What is the better choice for T1b gallbladder cancer: simple versus extended cholecystectomy. World J Surg. (2014) 38:3222–7. doi: 10.1007/s00268-014-2713-x
26. Downing SR, Cadogan KA, Ortega G, Oyetunji TA, Siram SM, Chang DC, et al. Early- stage gallbladder cancer in the surveillance, epidemiology, and End results database: effect of extended surgical resection. Arch Surg. (2011) 146:734–8. doi: 10.1001/archsurg.2011.128
27. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J. (2009) 339:b2700. doi: 10.1136/bmj.b2700
28. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
29. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
30. Goetze TO, Paolucci V. Immediate re-resection of T1 incidental gallbladder carcinomas: a survival analysis of the German registry. Surg Endosc. (2008) 22:2462–5. doi: 10.1007/s00464-008-9747-9
31. Hari DM, Howard JH, Leung AM, Chui CG, Sim MS, Bilchik AJ. A 21-year analysis of stage I gallbladder carcinoma: is cholecystectomy alone adequate? HPB (Oxford). (2013) 15:40–8. doi: 10.1111/j.1477-2574.2012.00559.x
32. Lee SE, Jang JY, Kim SW, Han HS, Kim HJ, Yun SS, et al. Surgical strategy for T1 gallbladder cancer: a nationwide multicenter survey in South Korea. Ann Surg Oncol. (2014) 21:3654–60. doi: 10.1245/s10434-014-3527-7
33. Tashiro S, Konno T, Mochinaga M, Nakakuma K, Murata E, Yokoyama I. Treatment of carcinoma of the gallbladder in Japan. Jpn J Surg. (1982) 12:98–104. doi: 10.1007/BF02469375
34. Yuan Z, Shui Y, Liu L, Guo Y, Wei Q. Postoperative recurrent patterns of gallbladder cancer: possible implications for adjuvant therapy. Radiat Oncol. (2022) 17:118. doi: 10.1186/s13014-022-02091-6
35. Goel M, Pandrowala S, Patel P, Patkar S. Node positivity in T1b gallbladder cancer: a high volume centre experience. Eur J Surg Oncol. (2022) 48:1585–9. doi: 10.1016/j.ejso.2022.03.013
36. Kim WJ, Lim TW, Park PJ, Choi SB, Kim WB. Clinicopathological differences in T2 gallbladder cancer according to tumor location. Cancer Control. (2020) 27:1073274820915514. doi: 10.1177/1073274820915514
37. Zou Q, Yang ZL, Yuan Y, Li JH, Liang LF, Zeng GX, et al. Clinicopathological features and CCT2 and PDIA2 expression in gallbladder squamous/adenosquamous carcinoma and gallbladder adenocarcinoma. World J Surg Oncol. (2013) 11:143. doi: 10.1186/1477-7819-11-143
38. Hanazawa T, Fukami Y, Osawa T, Kurahashi S, Matsumura T, Saito T, et al. A case of resected hepatocellular carcinoma with gallbladder metastasis. Surg Case Rep. (2021) 7:145. doi: 10.1186/s40792-021-01222-7
39. Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D, et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2016) 27:v28–37. doi: 10.1093/annonc/mdw324
40. Huang L, Zhang C, Tian Y, Liao C, Yan M, Qiu F, et al. Laparoscopic segment 4b+5 liver resection for stage T3 gallbladder cancer. Surg Endosc. (2022) 36:8893–907. doi: 10.1007/s00464-022-09325-4
41. Jang EJ, Kim KW. Exploring the potential of robotic single-port surgery for gallbladder cancer: initial insights and future prospects. Ann Surg Oncol. (2025) 32:440–2. doi: 10.1245/s10434-024-16252-2
Keywords: gallbladder cancer, simple cholecystectomy, extended cholecystectomy, prognosis, meta-analysis
Citation: Gu H, Zhang G, Ma H and Yu Z (2025) Effect of simple vs. extended cholecystectomy on prognosis of T1b gallbladder cancer: a systematic review and meta-analysis. Front. Surg. 12:1477301. doi: 10.3389/fsurg.2025.1477301
Received: 7 August 2024; Accepted: 13 May 2025;
Published: 26 May 2025.
Edited by:
Ren Lang, Capital Medical University, ChinaReviewed by:
Natale Calomino, University of Siena, ItalyRahul Gupta, Synergy Institute of Medical Sciences, India
Shi Chen, Fujian Provincial Hospital, China
Copyright: © 2025 Gu, Zhang, Ma and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ze Yu, emV5dW5mdUAxNjMuY29t; Haijie Ma, SGFpamllOTI3QDE2My5jb20=
 Hongpeng Gu
Hongpeng Gu Guoqiang Zhang2
Guoqiang Zhang2 Ze Yu
Ze Yu