- 1Nursing Department, The Affiliated Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
- 2Department of Head and Neck Surgery, The Affiliated Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
Background: The impact of parathyroid gland autotransplantation on permanent hypoparathyroidism remains incompletely understood. This study aimed to ascertain how selective autotransplantation of parathyroid glands affects the occurrence of permanent hypoparathyroidism after total thyroidectomy with central neck dissection (CND).
Method: A retrospective cohort study encompassed consecutive patients with papillary thyroid carcinoma who underwent primary total thyroidectomy plus CND from January 2008 to December 2010 and January 2012 to December 2019. Patients were categorized into two groups (0 and ≥1 parathyroid glands autotransplanted, respectively).
Result: The autotransplantation group comprised 501 patients, while the non-autotransplantation group comprised 652 patients. The autotransplantation group showed significantly lower permanent hypoparathyroidism than the non-autotransplantation group [1.2% (6 of 501) vs. 4.4% (29 of 652), P = 0.001]. Out of the total 1,153 patients, 652 (56.5%) had no autotransplanted glands, and 358 (31.0%), 136 (11.8%), and 7 (0.6%) had 1, 2, and 3 glands autotransplanted, respectively. As the number of autotransplanted glands increased (from 0 to 3), the prevalence of permanent hypoparathyroidism was 4.4% (29 of 652), 1.4% (5 of 358), 0.7% (1 of 136), and 0.0% (0 of 7), respectively (P = 0.016). Multivariate logistic analysis revealed that parathyroid autotransplantation independently prevented postoperative permanent hypoparathyroidism.
Conclusion: Selective parathyroid autotransplantation is associated with a lower risk of permanent postoperative hypoparathyroidism. Autotransplantation is recommended for parathyroid glands that are devascularized or challenging to preserve in their original location.
1 Introduction
Permanent hypoparathyroidism is a troubling long-term complication following total thyroid removal. The reported occurrence ranges from 4% to 11% (1–8). Individuals enduring permanent hypoparathyroidism not only face the inconvenience of daily calcium/vitamin D supplementation but also encounter an elevated risk of renal insufficiency, any malignancy (7), and mortality (8).
Hypoparathyroidism arises from intraoperative harm to the parathyroid glands, such as mechanical or thermal damage, gland devascularization, or unintended removal of parathyroid tissue (9, 10). The general recommendation for averting postoperative parathyroid failure involves a meticulous surgical approach to safeguard the parathyroid glands along with their blood supply in situ. However, this proves challenging in practice, even for experienced surgeons with substantial caseloads (11). Due to their anatomical location, parathyroid glands may still be devascularized or occasionally identified in surgical specimens. Autotransplantation of devascularized or inadvertently excised parathyroid glands is commonly employed to prevent enduring postoperative hypoparathyroidism (12, 13). Some authors advocate routine autotransplantation of at least one parathyroid gland or all discernible parathyroid glands to minimize the occurrence of permanent hypoparathyroidism (14–17). Nevertheless, two studies have indicated that parathyroid autotransplantation escalates the risk of enduring hypoparathyroidism (11, 18). A recent meta-analysis has demonstrated that parathyroid autotransplantation does not diminish the occurrence of permanent hypoparathyroidism and that an increased number of autotransplanted parathyroid glands does not correlate with a lower incidence of postoperative enduring hypoparathyroidism (19).
Thus, the impact of parathyroid autotransplantation on postoperative permanent hypoparathyroidism remains unclear. The aim of the current study was to evaluate whether selective parathyroid autotransplantation influences the onset of permanent hypoparathyroidism in a sizable cohort of cases.
2 Materials and methods
2.1 Patients
A retrospective analysis was conducted on patients diagnosed with papillary thyroid carcinoma (PTC) who underwent total thyroidectomy with ipsilateral or bilateral central neck dissection (CND), including lateral neck dissection, at the Department of Head and Neck Surgery, Affiliated Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, from January 2008 to December 2010, and January 2012 to December 2019. Exclusion criteria comprised prior thyroidectomy, non-PTC cases, procedures less than total thyroidectomy, concurrent parathyroid gland disease, and absence of postoperative parathyroid hormone data.
The study protocol received approval from the Ethics Committee of Affiliated Sir Run Run Shaw Hospital, Zhejiang University School of Medicine.
2.2 Surgical decision-making
Following the American Thyroid Association (ATA) guidelines (2009) (20), total thyroidectomy was conducted for patients meeting one of the criteria from January 2008 to December 2015: bilateral nodularity, extra-thyroidal extension, tumor diameter >1.0 cm, multifocal lesions in the affected lobe, and regional or distant metastases. Post January 2016, total thyroidectomy indications were adjusted based on ATA guidelines 2015 (21): thyroid tumors >4 cm, gross extra-thyroidal extension, or clinically apparent metastasis to nodes (clinical N1) or distant sites (clinical M1).
In adherence to the China Thyroid Association expert consensus (22), ipsilateral CND was routinely performed on the affected side in PTC patients, irrespective of clinical metastasis in central neck lymph nodes. Bilateral CND was carried out in cases with tumor(s) in the isthmus and/or both thyroid lobes or in individuals with clinically apparent neck lymph node metastasis.
2.3 Surgical procedures
All procedures were conventional open surgeries conducted by three senior surgeons. Thyroidectomy strictly followed the capsular dissection technique recommended by Thompson et al. (23). Parathyroid glands were consistently identified and preserved under direct vision, except for any not found. Vascular supply assessment of parathyroid glands by surgeons relied on gland color and plumpness from January 2008 to December 2010. Post January 2012, blood supply confirmation employed the fine-needle pricking (FNP) test (24). Resected thyroid lobe specimens and fibrofatty tissue from the central compartment were scrutinized for inadvertently removed parathyroid tissue. Any devascularized or unintentionally removed parathyroid gland was cut into small granules and mixed with isotonic sodium chloride solution (0.3–0.5 ml). The suspension was aspirated into a 1-ml syringe and injected into the sternocleidomastoid muscle through the pinhead of a 20 ml syringe (25). Per ATA guidelines, bilateral CND involved removal of prelaryngeal, pretracheal, and both right and left paratracheal nodal basins; unilateral CND entailed removal of prelaryngeal, pretracheal, and the single paratracheal nodal basin (26).
2.4 Clinical parameters
Collected parameters comprised sex, age, maximum tumor size, extra-thyroidal extension, multifocality, concurrent thyroiditis, type of central neck dissection (CND), number of harvested and metastatic lymph nodes in the central compartment, T and N classification, the American Joint Committee on Cancer (AJCC) stage (8th edition) (27), number of parathyroid gland autotransplantations, and number of inadvertently excised parathyroid glands. Verification of inadvertently excised parathyroid glands was conducted through the examination of paraffin-embedded specimens from thyroid lobes and fibrofatty tissue in the central compartment.
2.5 Laboratory assays
Serum calcium and intact parathyroid hormone (iPTH) levels were assessed in patients before surgery and every morning (06:00 a.m.) postoperative until discharge. Most patients were discharged on postoperative day 4, contingent on drainage fluid volume. Serum iPTH levels were measured using a Roche Cobas E601 instrument (Hitachi High-Technologies, Tokyo, Japan) (normal range 15–65 ng/L). Serum calcium levels were measured on an Abbott Aeroset Automated Instrument Analyzer (Toshiba Medical Systems, Tochigi-ken, Japan) (normal range 2.11–2.52 mmol/L). Ionized calcium amounts were not separately determined in this study.
2.6 Definition of hypoparathyroidism
Transient hypoparathyroidism was defined as subnormal serum iPTH level, serum calcium level below 2 mmol/L (8 mg/dl), or the need for calcium supplementation to alleviate clinical symptoms of hypocalcemia (e.g., numbness, paraesthesia, or carpopedal spasm) during the hospital stay (11, 28). Permanent hypoparathyroidism was defined as persistently subnormal serum iPTH level or hypocalcemia six months post-surgery, necessitating calcium and vitamin D supplements (4). Symptomatic hypocalcemia was treated with twice-daily oral calcium supplements containing 600 mg calcium carbonate plus 125 units of vitamin D3. Calcium gluconate injection was prescribed for persistent symptomatic hypocalcemia after oral calcium treatment. All patients were followed up for more than 6 months postoperatively to confirm the presence of permanent hypoparathyroidism.
2.7 Statistical analysis
Categorical data were summarized using frequencies and percentages, while continuous data were presented as mean (s.d.) for normally distributed variables or median (range) for non-normally distributed variables. Normal distribution was assessed with the Kolmogorov–Smirnov test. The χ2 test was employed for categorical variable comparisons. Continuous variable comparisons utilized Student's t-test (for normally distributed two samples), Mann–Whitney U-test (for non-normally distributed two samples), and Kruskal–Wallis test (for four samples). Variables demonstrating statistical significance in univariate analysis were subjected to multivariate analysis using binary logistic regression to identify risk factors for transient or permanent hypoparathyroidism. Multivariate analysis results were expressed as odds ratio (OR) and 95% confidence interval (CI). All P values were two-sided, with a significance level set at <0.05. The analyses were executed using SPSS® version 16.0 (IBM, Armonk, New York, USA).
3 Results
3.1 Patient characteristics
A total of 1,153 patients met the study criteria, with 501 patients in the autotransplantation group (at least one parathyroid gland autotransplanted) and the remaining 652 patients in the non-autotransplantation group (no parathyroid gland autotransplanted). All patients underwent total thyroidectomy with CND for PTC, with 712 (61.8%) undergoing unilateral CND and 441 (38.2%) undergoing bilateral CND. Simultaneous lateral neck dissections were performed in 219 patients.
Patient characteristics are presented in Table 1. Of the total, 904 were female, and 249 were male, with a mean age of 44.9 years. No differences were observed in the incidence of central neck lymph node metastases, with 262 of 501 autotransplantation patients (52.3%) and 334 of 652 non-autotransplantation patients (51.2%) having such metastases. Age, sex, tumor size, presence of Hashimoto's thyroiditis, N classification, and AJCC stage showed no significant differences between the two groups.
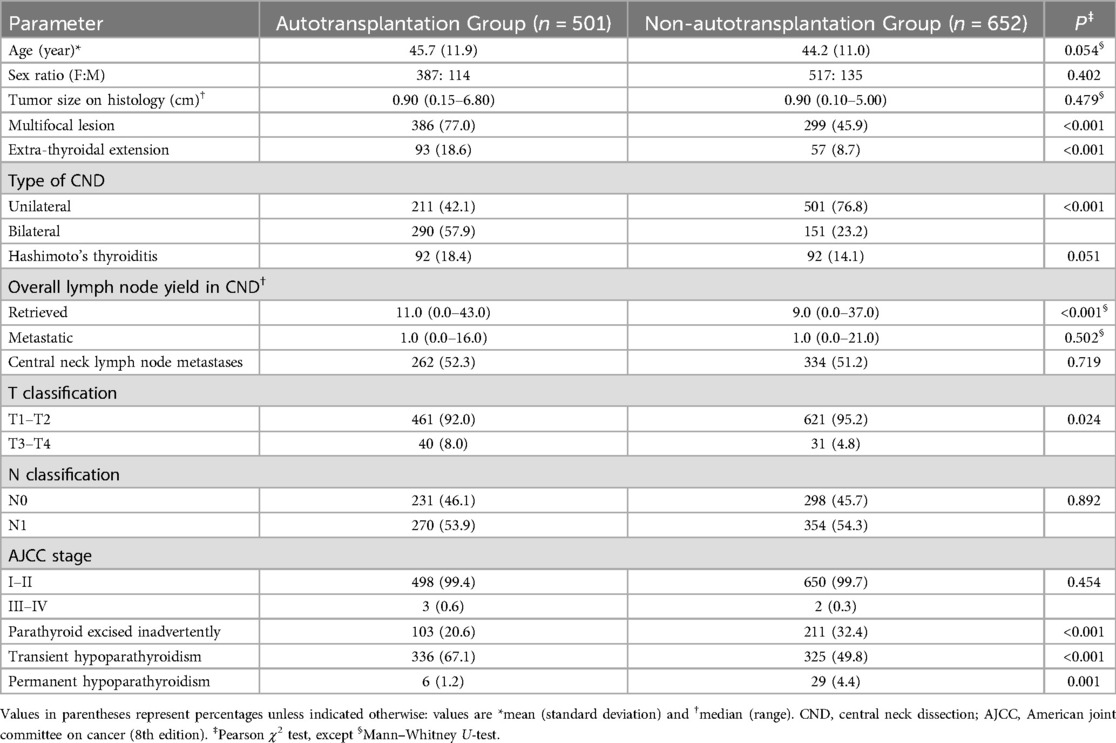
Table 1. Baseline characteristics and surgical results for patients undergoing total thyroidectomy and central neck dissection.
More patients in the autotransplantation group exhibited multifocal lesions, extra-thyroidal extension, bilateral CND, and T3–T4 classification than in the non-autotransplantation group. Although there was no difference in the number of metastatic lymph nodes in CND between the two groups, significantly more lymph nodes were removed in the autotransplantation group than in the non-autotransplantation group. The rate of inadvertently excised parathyroid glands was significantly lower in the autotransplantation group than in the non-autotransplantation group [20.6% (103 of 501) vs. 32.4% (211 of 652), P < 0.001].
3.2 The prevalence of transient and permanent hypoparathyroidism
In the autotransplantation group, 336 of 501 patients (67.1%) experienced transient hypoparathyroidism, compared to 325 of 652 patients (49.8%) in the non-autotransplantation group (P < 0.001). Permanent hypoparathyroidism occurred in 6 patients (1.2%) in the autotransplantation group and 29 patients (4.4%) in the non-autotransplantation group (P = 0.001). While parathyroid autotransplantation increased the incidence of transient hypoparathyroidism, the autotransplantation group showed significantly lower permanent hypoparathyroidism.
Among 651 parathyroid glands that underwent autotransplantation, 274 (42.1%) glands were transplanted from inadvertently removed glands, and 377 (57.9%) glands were removed due to devascularization. The number of autotransplanted parathyroid glands was as follows: 0 gland in 56.5% patients (652 of 1,153; group 0), 1 gland in 31.0% patients (358 of 1,153; group 1), 2 glands in 11.8% patients (136 of 1,153; group 2), and 3 glands in 0.6% patients (7 of 1,153; group 3). None of the patients underwent autotransplantation of 4 parathyroid glands (Table 2). The number of parathyroids identified during each surgery among the four groups has no significantly difference (P = 0.178) (Table 2). The incidence of transient hypoparathyroidism was 49.8% (325 of 652), 59.5% (213 of 358), 86.0% (117 of 136), and 85.7% (6 of 7) for groups with 0, 1, 2, and 3 parathyroid glands autotransplanted, respectively (P < 0.001). The incidence of permanent hypoparathyroidism in these groups was 4.4% (29 of 652), 1.4% (5 of 358), 0.7% (1 of 136), and 0.0% (0 of 7), respectively (P = 0.016). The group 0 patients had lowest incidence of transient hypoparathyroidism, but highest prevalence of permanent hypoparathyroidism.
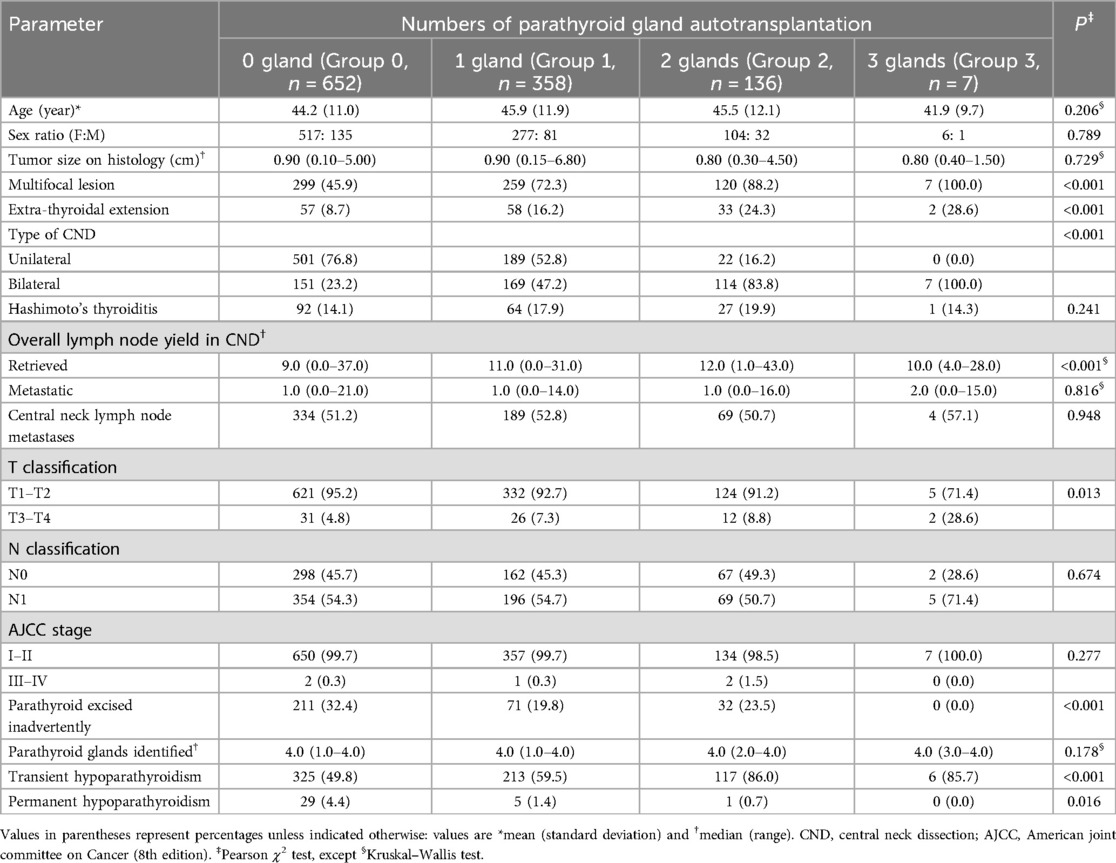
Table 2. Comparison of different numbers of parathyroid gland autotransplantation among patients undergoing total thyroidectomy plus CND.
3.3 Comparison between autotransplantation and non-autotransplantation groups among patients without inadvertently parathyroidectomy
To eliminate the impact of unintentional parathyroid gland resection on hypoparathyroidism incidence, a subgroup analysis was conducted on patients with no inadvertently excised parathyroid glands (Table 3). In this subgroup, there were 398 and 441 patients in the autotransplantation and non-autotransplantation groups, respectively. The incidence of transient hypoparathyroidism in the autotransplantation group was significantly higher than in the non-autotransplantation group [66.8% (266 of 398) vs. 47.2% (208 of 441), P < 0.001], while permanent hypoparathyroidism was significantly lower [0.3% (1 of 398) vs. 3.2% (14 of 441), P = 0.001)]. No differences were observed in age, sex, tumor size, presence of Hashimoto's thyroiditis, number of metastatic lymph nodes, T and N classification, and AJCC stage among patients without inadvertent parathyroidectomy in the autotransplantation vs. non-autotransplantation groups (Table 3). However, more patients in the autotransplantation group had multifocal lesions, extra-thyroidal extension, bilateral CND, and a higher number of retrieved lymph nodes compared to the non-autotransplantation group (Table 3).
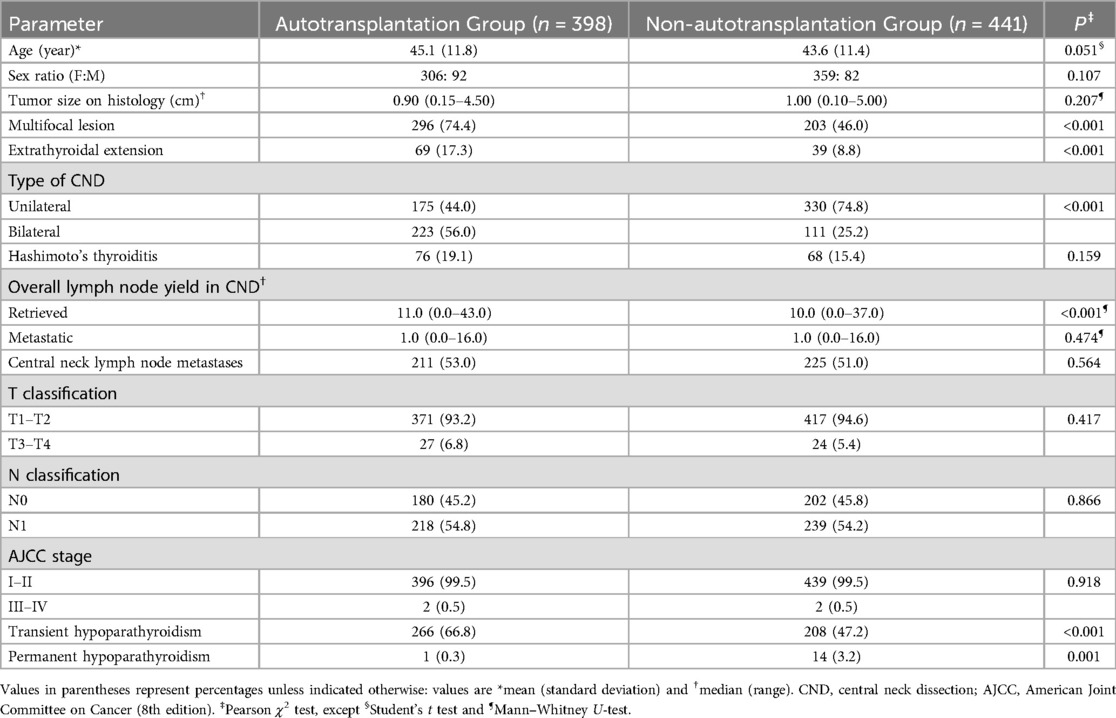
Table 3. Comparison between autotransplantation and Non-autotransplantation groups among patients without inadvertent parathyroidectomy.
3.4 Risk factors of transient and permanent hypoparathyroidism in patients underwent total thyroidectomy with CND
The patients were categorized into three groups: Normal, Transient and Permanent. Separate univariate and multivariate analyses were conducted to compare “Transient vs. Normal” and “Permanent vs. Transient” groups. Univariate analysis revealed five significant variables associated with transient hypoparathyroidism compared to normal (sex, multifocal lesion, type of CND, metastatic lymph nodes in the central compartment, and parathyroid autotransplantation), while two variables showed significance for permanent vs. transient hypoparathyroidism (inadvertent parathyroid excision and parathyroid autotransplantation) (Table 4).
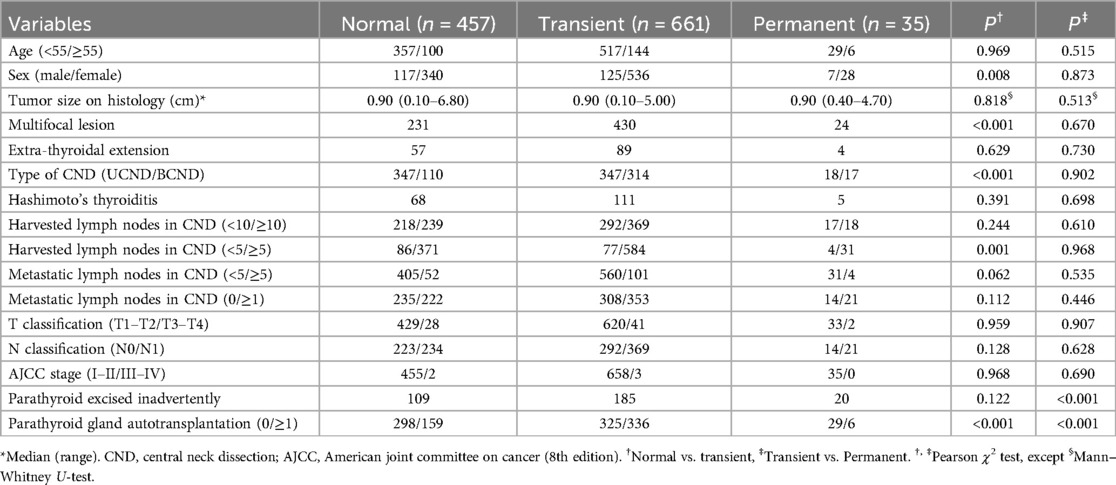
Table 4. Univariate analysis of risk factors for transient and permanent hypoparathyroidism in patients undergoing total thyroidectomy plus CND.
Multivariate logistic regression analysis identified female sex, bilateral CND, and parathyroid autotransplantation as independent risk factors for transient hypoparathyroidism (Table 5). Notably, inadvertent parathyroid excision was predictive of progression from transient to permanent hyporathyroidism, whereas parathyroid autotransplantation acted as a protective factor against this progression (Table 6).
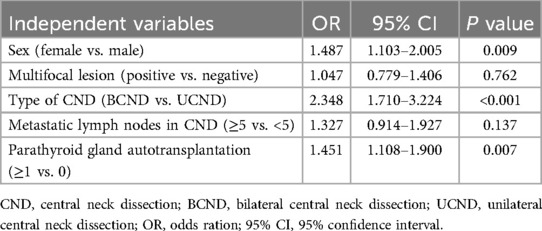
Table 5. Multivariate logistic regression analysis of risk factors for the development of normal to transient hypoparathyroidism in patients undergoing total thyroidectomy plus CND.
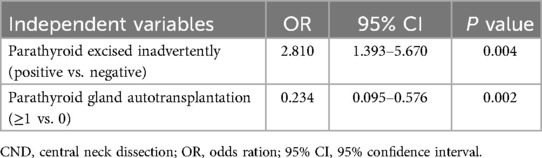
Table 6. Multivariate logistic regression analysis of risk factors for the development of transient to permanent hypoparathyroidism in patients undergoing total thyroidectomy plus CND.
4 Discussion
Permanent hypoparathyroidism constitutes a significant complication following total thyroidectomy with CND, presenting a challenge for most surgeons. Our study reveals that parathyroid autotransplantation heightens the occurrence of transient hypoparathyroidism but substantially diminishes the risk of permanent hypoparathyroidism. Multivariate analysis underscores parathyroid autotransplantation as a preventive factor against the progression from transient to permanent hyporathyroidism.
Undoubtedly, parathyroid autotransplantation stands as an efficacious method to restore parathyroid gland function after devascularization or identification in surgical specimens (13, 29). Numerous studies demonstrate the long-term survival of the majority of parathyroid grafts (17, 30). Presently, a widespread consensus associates parathyroid autotransplantation during total thyroidectomy with elevated rates of postoperative transient hypoparathyroidism (11, 14, 15, 31–37). Consequently, the primary controversy centers on its potential to prevent permanent hypoparathyroidism.
Some investigations assert that autotransplanting at least one parathyroid gland can reduce or eliminate permanent hypoparathyroidism (14, 15, 30, 33, 38). For instance, Yuxuan Qiu and colleagues demonstrated that the autotransplantation of one or two parathyroid glands independently increased the risk of transient hypoparathyroidism while decreasing the risk of permanent hypoparathyroidism (38). Our study similarly reveals that, although parathyroid autotransplantation increases the incidence of transient hypoparathyroidism, it significantly diminishes the risk of permanent hypoparathyroidism. Multivariate analysis highlights parathyroid autotransplantation as the sole protective factor for permanent hypoparathyroidism.
Contrastingly, other studies argue that parathyroid autotransplantation does not reduce the frequency of permanent hypoparathyroidism (31, 39–43). A meta-analysis concurs, indicating that parathyroid autotransplantation exerts no influence on the risk of permanent hypoparathyroidism (19). Furthermore, two studies suggest that parathyroid autotransplantation heightens the risk of permanent hypoparathyroidism (11, 18). Lorente-Poch L and colleagues report that autotransplantation not only correlates with higher rates of postoperative hypocalcemia but also entails a three-fold increase in permanent hypoparathyroidism rates (11). It's noteworthy that only 117 out of a total of 657 patients underwent CND in the study by Lorente-Poch L, and the authors did not clarify the incidence of inadvertent parathyroidectomy between the autotransplantation and non-autotransplantation groups.
The controversy surrounding the impact of parathyroid autotransplantation on permanent hypoparathyroidism primarily stems from three key issues.
Firstly, the subjective and inconclusive nature of surgeons' assessments of parathyroid gland blood supply contributes to the debate. Relying on observations of gland color and plumpness proves unreliable. Autotransplanted parathyroid glands exhibit more predictable functionality compared to those left in situ without blood supply. It is crucial to assess whether a parathyroid gland preserved in situ after thyroidectomy is “live” (vascularized) or not. If a devascularized parathyroid gland (appearing intact) is autotransplanted, the likelihood of the patient experiencing permanent hypoparathyroidism is reduced compared to preserving the devascularized gland in situ.
Secondly, the lack of uniformity in the technique of parathyroid autotransplantation across different studies adds complexity to the evaluation. Variations in procedural steps, such as whether the gland is immediately autografted (14, 40, 44) or at the end of thyroidectomy (11, 15, 30, 34, 35), the chosen autotransplantation technique (slicing vs. injecting a solution of suspended parathyroid tissue) (45), and the surgeon's criteria for carrying out autotransplantation (routine vs. selective), make it challenging to determine the substantial differences in outcomes.
Thirdly, the differing incidence of inadvertent parathyroidectomy between autotransplantation and non-autotransplantation groups can influence the effect of parathyroid autotransplantation on permanent hypoparathyroidism. Inadvertent parathyroidectomy is a relatively common finding in pathology specimens after thyroid operation. Sitges-Serra et al. reported a 28% prevalence in their specimens after total thyroidectomy with CND (46). Mayer AW et al. reported parathyroid tissue was removed inadvertently in 50 of 378 hemithyroidectomies (13.2%), 73 of 404 near-total or total thyroidectomies (18.1%), 89 of 743 thyroidectomies without CND (12.0%), and 76 of 154 thyroidectomies with CND (49.4%) (47). Other studies of varying extent of thyroid surgery have reported rates between 5.8% and 28% (46–53). The incidence of inadvertent parathyroidectomy, of 27.2% (314 of total 1,153 patients) reported in this study after total thyroidectomy with CND was in keeping with these previously reported series. Certain studies showed inadvertent parathyroidectomy increases the risk of permanent hypoparathyroidism (36, 46, 53). In this study, multivariate analysis identified inadvertent parathyroidectomy as the sole independent risk factor for permanent hypoparathyroidism.
The rate of inadvertently excised parathyroid glands in the non-autotransplantation group was higher than in the autotransplantation group [32.4% (211 of 652) vs. 20.6% (103 of 501), P < 0.001] in our study. To eliminate the impact of unintentional parathyroid gland resection on hypoparathyroidism incidence, we compared the prevalence of permanent hypoparathyroidism on patients with no inadvertently excised parathyroid glands. The incidence of permanent hypoparathyroidism in the autotransplantation group was also significantly lower than in the non-autotransplantation group [0.3% (1 of 398) vs. 3.2% (14 of 441), P = 0.001)] in these subgroup patients.
FNP test emerges as a simple and reliable tool for evaluating the blood supply of the parathyroid gland (24). Every parathyroid gland preserved in situ during thyroidectomy was suggested to undergo FNP testing. Parathyroid glands with no blood supply, as determined by the FNP test, should be promptly autotransplanted into muscle, presenting an effective method to prevent permanent hypoparathyroidism. Current evidence suggests that both indocyanine green (ICG) imaging with SPY camera and parathyroid autofluorescence serve as reliable techniques for evaluating the vascularity and viability of in situ preserved parathyroid glands (54–56). Future comparative studies are warranted to evaluate the efficacy of FNP test against ICG imaging and parathyroid autofluorescence in assessing the perfusion status and functional integrity of in situ parathyroid glands.
The “thymus-blood vessel-inferior parathyroid gland” layer concept proves to be an effective method for preserving the inferior parathyroid gland (IPTG) in situ during CND (57). However, the IPTG and its blood supply are often implicated in dorsal extra-thyroidal invasion of the primary tumor or extra-nodal metastasis of paratracheal lymph nodes. Consequently, autotransplantation of IPTGs and en bloc resection of the thyroid and central neck lymph nodes are recommended for these patients. Autotransplanting IPTGs during the initial operation in PTC patients with high-volume central neck lymph node metastasis may prevent permanent hypoparathyroidism after subsequent reoperation of the central neck compartment (58).
The present study has several limitations. Firstly, the data are derived from a retrospective chart review. More patients in the autotransplantation group exhibited multifocal lesions, extra-thyroidal extension, bilateral CND, and T3-T4 classification than in the non-autotransplantation group. These different characteristics between the two groups may cause bias. Secondly, from January 2008 to December 2010, the state of parathyroid gland blood supply was subjectively judged by surgeons based on the gland's color and plumpness, potentially introducing selection bias. Thirdly, the duration of follow up is 6 month postoperatively to identify the presence of permanent hypoparahtyoidism, which is short. Finally, the function of autotransplanted glands cannot be assessed in our study. The recovery of postoperative serum iPTH levels may predominantly depend on the in situ preserved parathyroid glands, as no patients underwent autotransplantation of all four parathyroid glands.
5 Conclusion
Selective parathyroid autotransplantation appears to increase the incidence of transient hypoparathyroidism after total thyroidectomy with CND, but it serves as a preventive factor for permanent hypoparathyroidism. Autotransplantation is advisable for parathyroid glands that are devascularized or challenging to preserve in situ.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: The data that support the findings of this study are available in the following link. https://doi.org/10.6084/m9.figshare.24679035.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Affiliated Sir Run Run Shaw Hospital, Zhejiang University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HS: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft. LG: Data curation, Methodology, Resources, Software, Writing – original draft, Conceptualization, Project administration. GX: Conceptualization, Data curation, Project administration, Formal analysis, Supervision, Validation, Writing – original draft. LX: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. YZ: Conceptualization, Investigation, Project administration, Supervision, Validation, Writing – review & editing. JW: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Project from the Health Commission of Zhejiang Province (grant no. 2020KY588).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PTC, papillary thyroid carcinoma; CND, central neck dissection; ATA, American thyroid association; FNP, fine-needle pricking; AJCC, American joint committee on cancer; iPTH, Intact parathyroid hormone; OR, odds ratio; CI, confidence interval; IPTG, inferior parathyroid gland.
References
1. Henry JF, Gramatica L, Denizot A, Kvachenyuk A, Puccini M, Defechereux T. Morbidity of prophylactic lymph node dissection in the central neck area in patients with papillary thyroid carcinomas. Langenbecks Arch Surg. (1998) 383:167–9. doi: 10.1007/s004230050111
2. Pereira JA, Jimeno J, Miquel J, Iglesias M, Munné A, Sancho JJ, et al. Nodal yield, morbidity, and recurrence after central neck dissection for papillary thyroid carcinoma. Surgery. (2005) 138:1095–101. doi: 10.1016/j.surg.2005.09.013
3. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg. (2006) 30:91–9. doi: 10.1007/s00268-005-0113-y
4. Giordano D, Valcavi R, Thompson GB, Pedroni C, Renna L, Gradoni P, et al. Complications of central neck dissection in patients with papillary thyroid carcinoma: results of a study on 1087 patients and review of the literature. Thyroid. (2012) 22:911–7. doi: 10.1089/thy.2012.0011
5. Ito Y, Miyauchi A, Masuoka H, Fukushima M, Kihara M, Miya A. Excellent prognosis of central lymph node recurrence-free survival for cN0M0 papillary thyroid carcinoma patients who underwent routine prophylactic central node dissection. World J Surg. (2018) 42:2462–8. doi: 10.1007/s00268-018-4497-x
6. Chadwick DR. Hypocalcaemia and permanent hypoparathyroidism after total/bilateral thyroidectomy in the BAETS registry. Gland Surg. (2017) 6(1):S69–74. doi: 10.21037/gs.2017.09.14
7. Bergenfelz A, Nordenström E, Almquist M. Morbidity in patients with permanent hypoparathyroidism after total thyroidectomy. Surgery. (2020) 167:124–8. doi: 10.1016/j.surg.2019.06.056
8. Almquist M, Ivarsson K, Nordenström E, Bergenfelz A. Mortality in patients with permanent hypoparathyroidism after total thyroidectomy. Br J Surg. (2018) 105:1313–8. doi: 10.1002/bjs.10843
9. Fahad Al-Dhahri S, Al-Ghonaim YA, Sulieman Terkawi A. Accuracy of postthyroidectomy parathyroid hormone and corrected calcium levels as early predictors of clinical hypocalcemia. J Otolaryngol Head Neck Surg. (2010) 39:342–8. doi: 10.2310/7070.2010.090239
10. Bliss RD, Gauger PG, Delbridge LW. Surgeon’s approach to the thyroid gland: surgical anatomy and the importance of technique. World J Surg. (2000) 24:891–7. doi: 10.1007/s002680010173
11. Lorente-Poch L, Sancho JJ, Ruiz S, Sitges-Serra A. Importance of in situ preservation of parathyroid glands during total thyroidectomy. Bri J Surg. (2015) 102:359–67. doi: 10.1002/bjs.9676
12. Shaha AR, Burnett C, Jaffe BM. Parathyroid autotransplantation during thyroid surgery. J Surg Oncol. (1991) 46:21–4. doi: 10.1002/jso.2930460106
13. Lo CY, Lam KY. Routine parathyroid autotransplantation during thyroidectomy. Surgery. (2001) 129:318–23. doi: 10.1067/msy.2001.111125
14. Zedenius J, Wadstrom C, Delbridge L. Routine autotransplantation of at least one parathyroid gland during total thyroidectomy may reduce permanent hypoparathyroidism to zero. ANZ J Surg. (1999) 69:794–7. doi: 10.1046/j.1440-1622.1999.01697.x
15. Ahmed N, Aurangzeb M, Muslim M, Zarin M. Routine parathyroid autotransplantation during total thyroidectomy: a procedure with predictable outcome. J Pak Med Assoc. (2013) 63:190–3.23894893
16. Funahashi H, Satoh Y, Imai T, Ohno M, Narita T, Katoh M, et al. Our technique of parathyroid autotransplantation in operation for papillary thyroid carcinoma. Surgery. (1993) 114:92–6.8356534
17. Kikumori T, Imai T, Tanaka Y, Oiwa M, Mase T, Funahashi H. Parathyroid autotransplantation with total thyroidectomy for thyroid carcinoma: long-term follow-up of grafted parathyroid function. Surgery. (1999) 125:504–8. doi: 10.1016/S0039-6060(99)70201-1
18. Kihara M, Miyauchi A, Kontani K, Yamauchi A, Yokomise H. Recovery of parathyroid function after total thyroidectomy: long-term follow-up study. ANZ J Surg. (2005) 75:532–6. doi: 10.1111/j.1445-2197.2005.03435.x
19. Wang B, Zhu C-R, Liu H, Wu J. The effectiveness of parathyroid gland autotransplantation in preserving parathyroid function during thyroid surgery for thyroid neoplasms: a meta-analysis. PLoS One. (2019) 14:e0221173. doi: 10.1371/journal.pone.0221173
20. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. (2009) 19:1167–214. doi: 10.1089/thy.2009.0110
21. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2015) 26:1–133. doi: 10.1089/thy.2015.0020
22. Chinese Society of Nuclear Medicine. Clinical management guidelines for thyroid nodules and differentiated thyroid carcinoma. Chin J Clin Oncol. (2012) 39:1249–72. (in Chinese). doi: 10.3969/j.issn.1000-8179.2012.17.001
23. Thompson NW, Olsen WR, Hoffman GL. The continuing development of the technique of thyroidectomy. Surgery. (1973) 73:913–27.4703492
24. Wu YJ, Wang JB, Li FB, Jin L, Zhou L, Xie L. Fine-needle pricking test of the parathyroid gland during thyroid surgery in predicting parathyroid function. Int J Endocrinol. (2022) 2022:8747680. doi: 10.1155/2022/8747680
25. Zhang D, Gao L, He G, Chen J, Fang L. Predictors of graft function after parathyroid autotransplantation during thyroid surgery. Head Neck. (2018) 40:2476–81. doi: 10.1002/hed.25371
26. American Thyroid Association Surgery Working GroupAmerican Association of Endocrine SurgeonsAmerican Academy of Otolaryngology-Head and Neck Surgery, American Head and Neck Society, Carty SE, Cooper DS, et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. (2009) 19:1153–8. doi: 10.1089/thy.2009.0159
27. Zanoni DK, Patel SG, Shah JP. Changes in the 8th edition of the American joint committee on cancer (AJCC) staging of head and neck cancer: rationale and implications. Curr Oncol Rep. (2019) 21:52. doi: 10.1007/s11912-019-0799-x
28. Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, Dralle H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery. (2003) 133:180–5. doi: 10.1067/msy.2003.61
29. Cavallaro G, Iorio O, Centanni M, Gargano L, Del Duca S, Gurrado A, et al. Parathyroid reimplantation with PR-FaST technique in unselected patients during thyroidectomy. A case series with long term follow up confirming graft vitality and parathormone production. Int J Surg. (2017) 39:202–5. doi: 10.1016/j.ijsu.2017.01.117
30. Olson JA Jr., DeBenedetti MK, Baumann DS, Wells SA Jr. Parathyroid autotransplantation during thyroidectomy. Results of long-term follow-up. Ann Surg. (1996) 223:472–8; discussion 478–480. doi: 10.1097/00000658-199605000-00003
31. Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian SP. Systematic review and meta-analysis of predictors of post thyroidectomy hypocalcaemia. Br J Surg. (2014) 101:307–20. doi: 10.1002/bjs.9384
32. Almquist M, Hallgrimsson P, Nordenström E, Bergenfelz A. Prediction of permanent hypoparathyroidism after total thyroidectomy. World J Surg. (2014) 38:2613–20. doi: 10.1007/s00268-014-2622-z
33. Abboud B, Sleilaty G, Zeineddine S, Braidy C, Aouad R, Tohme C, et al. Is therapy with calcium and vitamin D and parathyroid autotransplantation useful in total thyroidectomy for preventing hypocalcemia? Head Neck. (2008) 30:1148–54. doi: 10.1002/hed.20836
34. Sitges-Serra A, Ruiz S, Girvent M, Manjón H, Dueñas JP, Sancho JJ. Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg. (2010) 97:1687–95. doi: 10.1002/bjs.7219
35. Asari R, Passler C, Kaczirek K, Scheuba C, Niederle B. Hypoparathyroidism after total thyroidectomy: a prospective study. Arch Surg. (2008) 143:132–7. doi: 10.1001/archsurg.2007.55
36. Paek SH, Lee YM, Min SY, Kim SW, Chung KW, Youn YK. Risk factors of hypoparathyroidism following total thyroidectomy for thyroid cancer. World J Surg. (2013) 37:94–101. doi: 10.1007/s00268-012-1809-4
37. Hallgrimsson P, Nordenstrom E, Almquist M, Bergenfelz AO. Risk factors for medically treated hypocalcaemia after surgery for Graves’ disease: a Swedish multicenter study of 1,157 patients. World J Surg. (2012) 36:1933–42. doi: 10.1007/s00268-012-1574-4
38. Qiu Y, Xing Z, Qian Y, Fei Y, Luo Y, Su A. Selective parathyroid autotransplantation during total thyroidectomy for papillary thyroid carcinoma: a cohort study. Front Surg. (2021) 8:683041. doi: 10.3389/fsurg.2021.683041
39. Bhattacharyya N, Fried MP. Assessment of the morbidity and complications of total thyroidectomy. Arch Otolaryngol Head Neck Surg. (2002) 128:389–92. doi: 10.1001/archotol.128.4.389
40. Palazzo FF, Sywak MS, Sidhu SB, Barraclough BH, Delbridge LW. Parathyroid autotransplantation during total thyroidectomy—does the number of glands transplanted affect outcome? World J Surg. (2005) 29:629–31. doi: 10.1007/s00268-005-7729-9
41. Testini M, Rosato L, Avenia N, Basile F, Portincasa P, Piccinni G, et al. The impact of single parathyroid gland autotransplantation during thyroid surgery on postoperative hypoparathyroidism: a multicenter study. Transplant Proc. (2007) 39:225–30. doi: 10.1016/j.transproceed.2006.10.192
42. Kirdak T, Dundar HZ, Uysal E, Ocakoglu G, Korun N. Outcomes of parathyroid autotransplantation during total thyroidectomy: a comparison with age- and sex-matched controls. J Invest Surg. (2017) 30:201–9. doi: 10.1080/08941939.2016.1232768
43. Tartaglia F, Blasi S, Giuliani A, Merola R, Livadoti G, Krizzuk D, et al. Parathyroid autotransplantation during total thyroidectomy. Results of a retrospective study. Int J Surg. (2016) 28:S79–83. doi: 10.1016/j.ijsu.2015.05.059
44. Lo CY, Lam KY. Parathyroid autotransplantation during thyroidectomy: is frozen section necessary? Arch Surg. (1999) 134:258–60. doi: 10.1001/archsurg.134.3.258
45. Barczyński M, Gołkowski F, Nawrot I. Parathyroid transplantation in thyroid surgery. Gland Surg. (2017) 6:530–6. doi: 10.21037/gs.2017.06.07
46. Sitges-Serra A, Gallego-Otaegui L, Suarez S, Munné A, Sancho JJ. Inadvertent parathyroidectomy during total thyroidectomy and central neck dissection for papillary thyroid carcinoma. Surgery. (2017) 161:712–9. doi: 10.1016/j.surg.2016.08.021
47. Mayer AW, Sharp A, Aziz S, Balasubramanian SP. Distribution of inadvertently excised parathyroid glands during thyroid surgery and the link with postsurgical hypoparathyroidism. J Laryngol Otol. (2023) 137:1226–32. doi: 10.1017/S002221512300035X
48. Özden S, Erdoğan A, Simsek B, Saylam B, Yıldız B, Tez M. Clinical course of incidental parathyroidectomy: single center experience. Auris Nasus Larynx. (2018) 45:574–7. doi: 10.1016/j.anl.2017.07.019
49. Ataş H, Akkurt G, Saylam B, Tez M. Central neck dissection is an independent risk factor for incidental parathyroidectomy. Acta Chir Belg. (2021) 121:36–41. doi: 10.1080/00015458.2020.1828677
50. Barrios L, Shafqat I, Alam U, Ali N, Patio C, Filarski CF, et al. Incidental parathyroidectomy in thyroidectomy and central neck dissection. Surgery. (2021) 169:1145–51. doi: 10.1016/j.surg.2020.11.023
51. Chew C, Li R, Ng MK, Chan STF, Fleming B. Incidental parathyroidectomy during total thyroidectomy is not a direct cause of post-operative hypocalcaemia. ANZ J Surg. (2018) 88:158–61. doi: 10.1111/ans.13939
52. Mencio M, Calcatera N, Ogola G, Mahady S, Shiller M, Roe E, et al. Factors contributing to unintentional parathyroidectomy during thyroid surgery. Proc (Bayl Univ Med Cent). (2020) 33:19–23. doi: 10.1080/08998280.2019.1680911
53. Bai B, Chen Z, Chen W. Risk factors and outcomes of incidental parathyroidectomy in thyroidectomy: a systematic review and meta-analysis. PLoS One. (2018) 13:e0207088. doi: 10.1371/journal.pone.0207088
54. Noltes ME, Metman MJH, Jansen L, Peeperkorn EWM, Engelsman AF, Kruijff S. Parathyroid function saving total thyroidectomy using autofluorescence and quantified indocyanine green angiography. VideoEndocrinology. (2021) 8(2):ve.2021.0008. doi: 10.1089/ve.2021.0008
55. Priyanka S, Sam ST, Rebekah G, Sen S, Thomas V, Wankhar S, et al. The utility of indocyanine green (ICG) for the identification and assessment of viability of the parathyroid glands during thyroidectomy. Updates Surg. (2022) 74(1):97–105. doi: 10.1007/s13304-021-01202-4
56. Wapnir I, Dua M, Kieryn A, Paro J, Morrison D, Kahn D, et al. Intraoperative imaging of nipple perfusion patterns and ischemic complications in nipple-sparing mastectomies. Ann Surg Oncol. (2014) 21(1):100–6. doi: 10.1245/s10434-013-3214-0
57. Wang JB, Wu K, Shi LH, Sun YY, Li FB, Xie L. In situ preservation of the inferior parathyroid gland during central neck dissection for papillary thyroid carcinoma. Br J Surg. (2017) 104:1514–22. doi: 10.1002/bjs.10581
Keywords: parathyroid gland, autotransplantation, in situ preservation, transient hypoparathyroidism, permanent hypoparathyroidism
Citation: Sun H, Gao L, Xiao G, Xie L, Zhuang Y and Wang J (2025) Selective parathyroid autotransplantation prevent permanent hypoparathyroidism after total thyroidectomy with central neck dissection. Front. Surg. 12:1565581. doi: 10.3389/fsurg.2025.1565581
Received: 23 January 2025; Accepted: 10 April 2025;
Published: 24 April 2025.
Edited by:
Fabio Medas, University of Cagliari, ItalyReviewed by:
Matteo Mascherini, Ospedale Policlinico San Martino, ItalyMohamad Sidani, Texas Tech University Health Sciences Center, United States
Copyright: © 2025 Sun, Gao, Xiao, Xie, Zhuang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiyu Zhuang, emh1YW5neXlAemp1LmVkdS5jbg==; Jianbiao Wang, ZHJ3YW5namlhbmJpYW9Aemp1LmVkdS5jbg==
†These authors have contributed equally to this work
 Haili Sun1
Haili Sun1 Lei Xie
Lei Xie Jianbiao Wang
Jianbiao Wang