- Department of Neurosurgery, University Hospital Leipzig, Leipzig, Germany
Objective: Hemorrhagic events in cavernous malformations (CCMs) are linked to significant morbidity and mortality. Identifying factors contributing to bleeding is crucial for effective clinical and surgical management.
Methods: This retrospective, observational, single-center study assessed potential and known risk factors for bleeding in patients with cerebral cavernous malformations. We evaluated age, gender, smoking habits, arterial hypertension, antithrombotic medication, diabetes mellitus, cavernoma shape, size changes observed in follow-up MRIs, the occurrence of epileptic seizures, and the localization of the lesion in regard of cerebral vascular territories.
Results: We identified 143 patients (43.4% male, 45.5% with hemorrhagic events, and 19.6% with epileptic seizures). Antithrombotic medication was associated with a lower frequency of hemorrhage (p = 0.012). Arterial hypertension, diabetes mellitus, smoking habits and localization in the border zone of a vascular territory or seizures were not significantly associated with bleeding events. An increased rate of hemorrhagic events was found in cavernomas with irregular MRI shape (p = 0.001), or with changes in size during follow-up (p = 0.012). Multivariate analysis confirmed that antithrombotic therapy was associated with a reduced risk of hemorrhage (OR: 0.151, 95% CI: 0.041–0.552, p = 0.004), while male gender (OR: 2.114, 95% CI: 1.047–4.269, p = 0.037), irregular cavernoma shape (p = 0.001), and cavernoma growth (p = 0.002) were independently associated with a higher bleeding risk. Cavernoma localisation in the border zone between median and posterior arterial territories was associated with a significant lower rate of bleeding events compared to localisation in other watershed areas.
Conclusion: We observed a reduced percentage of bleeding in cavernoma patients utilizing antithrombotic agents, compared to patients without antithrombotic medication. Factors such as smoking habits, irregular cavernoma shape on MRI, and changes in size during follow-up were associated with a higher frequency of bleeding events.
1 Introduction
Cerebral cavernous malformations (CCMs) are rare vascular anomalies occurring in approximately 0.4%–0.5% of the population, with an estimated annual incidence of hemorrhage about 0.7%–1% (1). Given their tendency to remain asymptomatic, many CCMs are discovered incidentally, approximately in 40.6% of cases (2).
Neurological symptoms depend on the occurrence of a bleeding event and from the localization of the lesion in the central nervous system (3). Cavernoma-related epilepsy has been found in about 40%–70% percent of patients (4, 5). Lesions in the temporal lobe are particularly epileptogenic and often require surgical intervention (6). Another frequent symptom leading to MR imaging and detection of CCMs is cephalgia. For incidentally detected and asymptomatic cavernomas, a conservative treatment approach, such as a watch-and-wait strategy, may be an appropriate option, as supported by prior studies (7, 8). However, this approach requires careful consideration, as there remains a risk of hemorrhage, which can lead to serious or even life-threatening consequences (9, 10). Therefore, accurate stratification of bleeding risk is essential to identify patients who are more likely to benefit from surgical intervention. This approach helps ensure that the risks associated with surgery, such as potential neurological deficits, are justified by a significant reduction in the probability of a major hemorrhagic event. Developing and refining predictive models for individual bleeding risk is crucial in supporting therapeutic decision-making and improving patient outcomes.
The pathophysiological mechanisms underlying cavernoma hemorrhage are still not completely understood. Endothelial shear stress, loss of cell-junctions and alteration of the coagulation cascade by surface proteins have been discussed (11). Experimental data points to a correlation of hypoxia and formation of thrombosis in cavernoma tissue, the subsequent venous congestion being involved in rupture of the vessel (12, 13). However, while these mechanisms are increasingly understood, their practical implications for patient management remain uncertain.
We hypothesized, that in arterial vascular border zones, the experimentally described mechanisms such as slow blood flow and relative hypoxia are more prevalent. This might then lead to a higher rate of cavernoma incidence and detection of hemorrhage in these areas. To test this hypothesis, in addition to the previously described risk factors for cavernoma hemorrhage, MR-imaging was assessed concerning the localization of cavernoma in arterial vascular border zones and the respective rates of hemorrhage.
2 Methods
2.1 Study design, setting, and participants
We conducted a single-center, retrospective cohort analysis using data from a database of pediatric and adult patients diagnosed with cavernous malformations at the Department of Neurosurgery, University Hospital of Leipzig, Germany, between 2000 and 2023. The study included patients with cranial and spinal lesions, encompassing both previously treated, or incidentally discovered cavernous malformations, as well as symptomatic and asymptomatic individuals.
All patients had undergone high-resolution MRI or CT scans. MRI protocols included T1-, T2-weighted imaging and susceptibility-weighted imaging (SWI). Patient data was extracted from clinical medical records. In three cases, diagnosis was based on CT imaging alone due to emergency conditions or contraindications for MRI. All CT-based diagnoses were confirmed within an interdisciplinary team and followed recognized imaging criteria for cavernoma.
2.2 Terms and definitions
Hemorrhage at the time of diagnosis was assessed based on radiologic evidence of recent blood products within or surrounding the cavernous malformation. All patients with suspicion of cavernoma hemorrhage received an emergency scan (MRI/CT). We classified hemorrhage according to the Angioma Alliance reporting standards, ensuring consistency with international guidelines. A symptomatic hemorrhage was defined as radiologically confirmed acute hemorrhage (via CT or MRI) accompanied by acute or subacute clinical symptoms such as focal neurological deficits, seizures, or severe headache (14).
Lesion size was measured using axial, sagittal, and coronal MRI images (T2 sequences, T1 sequences, and SWI) or CT scans, based on the greatest diameter observed in the first imaging at diagnosis, or as documented in outpatient reports where the diameter was already specified. Only the visible cavernoma was measured; associated hematoma, if present, was not included in the measurement. Changes in size during follow-up were defined as an increase of ≥1 mm in any dimension, measured using the same imaging modality. Cavernomas were categorized based on their morphology as cyclic (rounded or symmetrical), polycyclic (irregular or lobulated), or unknown (indeterminate due to imaging limitations).
The vascular border zone was defined as the area located at the junction of major cerebral arterial territories, as the so-called watershed areas. In the context of cavernous malformations, these zones represent regions where blood flow is slowing down in the capillary bed, potentially increasing the risk for relative hypoxia or thrombotic events (12). Due to reduced oxygen transfer in these areas, the risk of relative hypoxia is further exacerbated, which may contribute to the pathophysiology of cavernoma formation or hemorrhage. The arterial territories and border zones were defined according to a clinical neuroanatomic atlas (15), MRI localization was assessed in axial, sagittal and coronal views by two examiners in consensus (AG, UN, Figure 1).
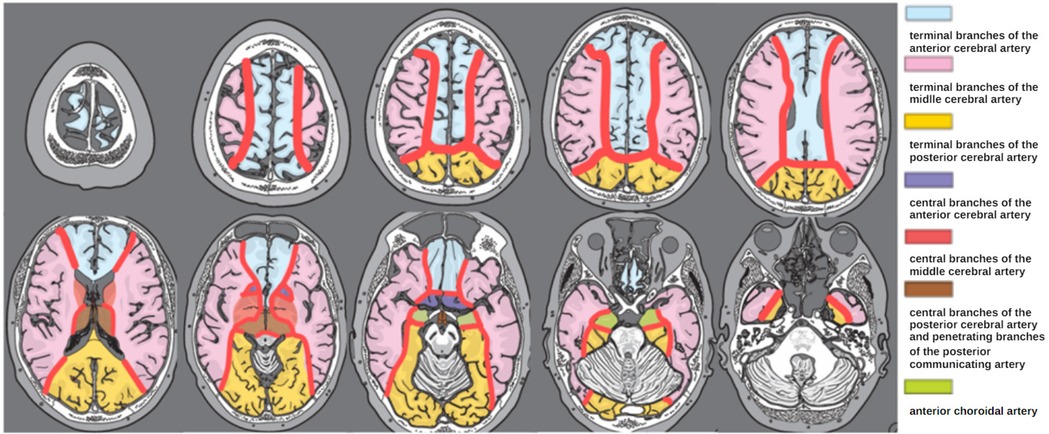
Figure 1. Schematic representation of the areas of arterial vascular border zones (red) employed for MRI-based cavernoma localization in axial views. Modified from Lanfermann et al. (15).
For detailed localization of cavernomas within vascular border zones, lesions were classified according to arterial supply territories into the following subtypes: ACA/ACM (border zone between the anterior cerebral artery and middle cerebral artery), ACM/ACP (middle cerebral artery and posterior cerebral artery), ACP/AB (posterior cerebral artery and basilar artery), and ACA/ACP (anterior and posterior cerebral artery) (Figure 1).
2.3 Baseline characteristics and management data
We investigated the known risk factors for bleeding in cerebral cavernous malformations from the archived records and radiographic images, including age, gender, smoking status, hypertension, antithrombotic medication usage, diabetes mellitus, cavernoma morphology, changes in size observed in follow-up MRIs and occurrence of epileptic seizures.
Arterial hypertension was documented when recorded as a clinical diagnosis, or if patients were on antihypertensive medication. Data on antithrombotic medication, smoking, seizure occurrence and diabetes mellitus were extracted from available inpatient and outpatient records.
2.4 Study endpoint
The statistical analyses were performed using IBM SPSS Statistics (Version 29; IBM Corp., Armonk, NY, USA). Descriptive statistics were generated for all baseline characteristics. Continuous variables were expressed as means ± standard deviation (SD) or medians, depending on data distribution, while categorical variables were reported as frequencies and percentages.
For univariate analysis, Fisher's exact tests were used for categorical variables. Binary logistic regression models were built to assess the independent effects of risk factors on hemorrhage, with odds ratios (ORs) and 95% confidence intervals (CIs) reported for all variables. Multicollinearity was assessed using the variance inflation factor (VIF).
All p-values were two-tailed, and a p-value <0.05 was considered to be statistically significant.
3 Results
3.1 Baseline characteristics
In total, 143 patients were included, of whom 65 experienced a hemorrhage (Table 1). The mean age of the cohort was 46.57 years (SD ± 18.39). When categorized according to bleeding status, the mean age in the bleeding group was 44.83 years (SD ± 15.50, range 8–78), while the non-bleeding group had a mean age of 48.01 years (SD ± 20.46, range 7–85, p = 0.30).
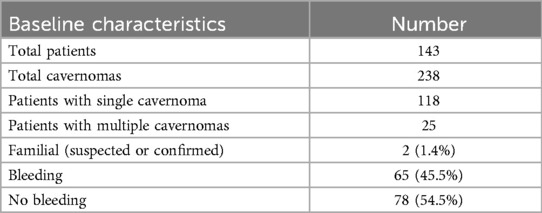
Table 1. Baseline characteristics of the reported patient cohort with cerebral cavernous malformations percentages refer to the total number of patients (n = 143).
62 patients (43.4%) were male, indicating a slight female predominance. In the patient group with cavernoma bleeding 34 from 65 were male (52.3%), against 28 from 78 without bleeding (35.9%, p = 0.6, two-sided Fisher's exact test).
Regarding cavernoma prevalence, 118 patients (82.5%) had a single cavernoma, while 25 patients (17.5%) had multiple cavernomas. Notably, only two patients were identified with suspected or confirmed familial cavernomatosis.
The mean follow-up time after diagnosis was 52.00 months (SD ± 60.54) in the bleeding group and 49.56 months (SD ± 57.44) in the non-bleeding group.
3.2 Symptoms
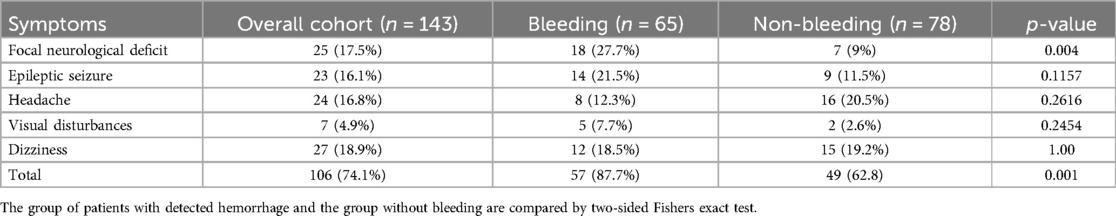
Among the 143 patients 35 (24.5%) were diagnosed with incidental cerebral cavernous malformations, while 106 patients presented with clinical symptoms (Table 2). Two additional patients were diagnosed during family screening due to suspected familial cavernomatosis. Of the 65 patients with hemorrhage, 57 were symptomatic. The remaining 8 were asymptomatic at the time of diagnosis—six were detected incidentally, and two were identified during familial screening.
The most frequently reported symptoms were dizziness (18.9%) and focal neurological deficits (17.5%), followed by headache (16.8%) and epileptic seizures (16.1%). Visual disturbances were the least common, observed in only 4.9% of patients. Notably, clinical symptoms, and especially focal neurological deficits were significantly more frequent in the bleeding group compared to the non-bleeding group (Table 2). No significant differences between the two groups were observed for symptoms, such as headache, epileptic seizures, dizziness, and visual disturbances.
3.3 Lesion characteristics
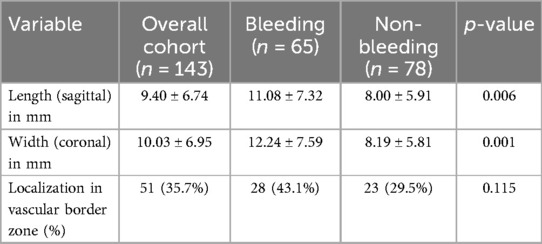
The mean sagittal length of cavernomas in the overall cohort was 9.40 mm, with significant differences observed between the groups (11.08 mm vs. 8.00 mm, Table 3). Among the 143 patients, 51 lesions (35.7%) were located in arterial vascular border zones. The proportion of cavernomas in vascular border zones was 43.1% (28/65) in the bleeding group and 29.5% (23/78) in the non-bleeding group, without reaching significant difference between the two subsets (p = 0.115).
To provide a more detailed view, vascular border zones were further subclassified based on anatomical territories into ACA ACM, ACM ACP, ACP AB, and ACA ACP. Lesions in the ACM ACP region were significantly less frequently associated with bleeding (27.3%) compared to the other subtypes (p = 0.0007). No statistically significant differences were observed for the remaining sublocations (Table 4).
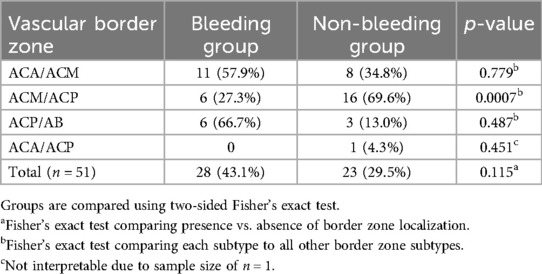
Table 4. Frequency of vascular border zone localizations among patients with and without hemorrhage.
3.4 Risk factors
The univariate analysis revealed significant associations with bleeding for antithrombotic therapy (note the negative correlation, p = 0.012). Among the 143 patients, 24 (16.8%) received antithrombotic therapy. Within this subgroup, 13 patients (54.2%) were treated with cyclooxygenase inhibitors (e.g., acetylsalicylic acid), 6 (25.%) with DOACs NOACs (e.g., rivaroxaban, apixaban, dabigatran), 4 (16.7%) with vitamin K antagonists (e.g., phenprocoumon), and 1 patient (4.2%) with a P2Y12 inhibitor (e.g., clopidogrel). No patient received dual antiplatelet therapy.
Associations with bleeding events were also observed for increase in size during follow-up of the cavernous malformation (p = 0.002), and for the cavernomas shape (p = 0.001). In contrast, arterial hypertension, diabetes mellitus, gender and smoking habits were not found to have a significant association with cavernoma hemorrhage in the univariate model (Table 5).
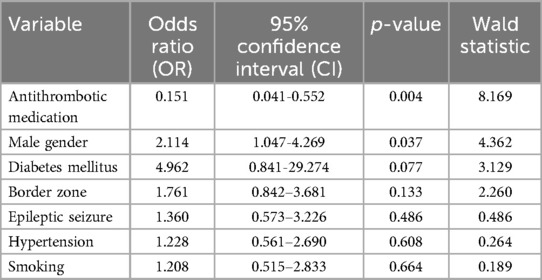
To further evaluate the independent effects of the variables on bleeding risk, a binary logistic regression model was constructed. Multicollinearity was assessed using variance inflation factors (VIF), and all variables included in the model exhibited VIF values below 2, indicating no significant multicollinearity among predictors (Table 6).
The use of antithrombotic medication remained significantly associated with a reduced risk of bleeding (OR: 0.151, 95% CI: 0.041–0.552, p = 0.004, Table 6). Male gender, which was not significant in the univariate analysis, was identified as an independent predictor of bleeding in the multivariate model (OR: 2.114, 95% CI: 1.047–4.269, p = 0.037). In contrast, the further factors, including diabetes mellitus, vascular border zone localization of the cavernoma, epileptic seizures, hypertension, and smoking habits, did not reveal significant associations to bleeding risk in the present cohort.
4 Discussion
Currently, the risk factors influencing hemorrhage in patients with cerebral cavernous malformations remain ambiguous, and clear therapeutic recommendations cannot be given. In this single-center retrospective analysis over a period of 23 years, 68 of the 143 patients experienced a hemorrhagic event. Additionally, we found that over 70% of patients presented with symptoms at the time of the initial diagnosis.
The central hypothesis of our study was that the anatomical localization of cavernomas within vascular border zones—also known as watershed areas—may be associated with an increased risk of hemorrhage. Border zones are regions located at the junctions of major cerebral arterial territories, where blood supply may be relatively compromised (16, 17). These territories are particularly vulnerable to slow capillary flow and relative hypoxia, which may lead to microthrombosis, venous congestion, and secondary rupture. This hemodynamic vulnerability is especially relevant in the context of CCMs, which are already prone to vascular instability. Thus, we hypothesized that lesions located in these territories might be more susceptible to bleeding.
In our cohort, 51 lesions (35.7%) were located in arterial vascular border zones. The proportion of cavernomas in vascular border zones was 43.1% (28/65) in the bleeding group and 29.5% (23/78) in the non-bleeding group, without reaching significant difference between the two subsets (p = 0.115). Notably, further sub-analysis revealed a significantly lower hemorrhage rate in the ACM/ACP subgroup (27.3%) compared to other border zone sublocations (p = 0.0007), potentially indicating heterogeneity within these territories. Although the global hypothesis of an increased bleeding risk in border zones was not statistically confirmed, these findings suggest that individual watershed areas may differ in their susceptibility to hemorrhage. This anatomical approach may still serve as a relevant framework for future investigations and risk stratification models. The negative result in our primary hypothesis does not diminish its clinical relevance, but rather calls for refined prospective studies incorporating microvascular imaging and hemodynamic mapping.
To illustrate the clinical relevance of vascular border zone localization, we included two representative cases with cavernomas located in different arterial watershed areas (Figure 2).
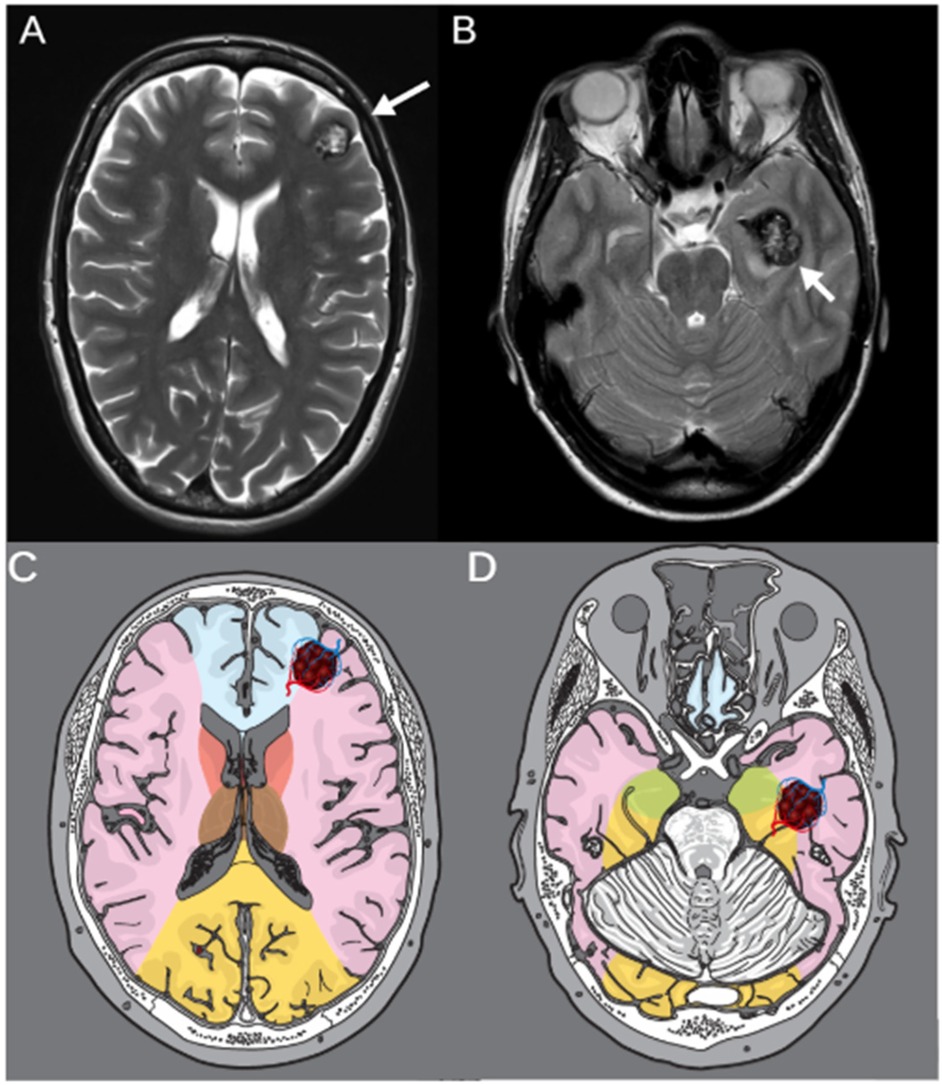
Figure 2. T2-weighted MRI of two representative cases with cerebral cavernomas located in vascular border zones. (A) Axial image of a 46-year-old female patient with a left frontal cavernoma in the ACA/ACM border zone. (B) Axial image of a 53-year-old patient with a cavernoma in the lefttemporal lobe, located in the ACM/ACP border zone. White arrows indicate the respective lesions. (C,D) Corresponding schematic illustrations of the same axial planes showing vascular territories and border zones. The vascular border zones are marked in red, and the respective cavernoma lesions are highlighted illustratively within the anatomical maps.
Our study supports the results of previous studies that had shown an association between a larger size of the cavernoma and hemorrhage (18, 19). The retrospective analysis also confirms that antithrombotic therapy in patients with cavernomas is inversely associated with bleeding, suggesting a protective effect against hemorrhagic events. This observation aligns with prior studies that support antithrombotic therapy's potential role in reducing bleeding risk in cerebral cavernous malformations (20, 21).
Antithrombotic agents are a broad category that includes both anticoagulants (e.g., vitamin K antagonist, DOACs) and antiplatelet medications (e.g., acetylsalicylic acid, clopidogrel), which act through distinct pathways (22). By preventing thrombus formation, these agents may help to avoid venous congestion and thus mitigate hemorrhagic events. Furthermore, certain antithrombotic agents improve cerebral blood flow by reducing microvascular occlusions (23), which may enhance the perfusion of surrounding brain tissue, potentially lowering ischemic injury and rupture risk. Some antithrombotic medications also promote endothelial stability (24), potentially reinforcing vascular integrity within CCMs. In addition, the anti-inflammatory properties of certain antithrombotic agents can stabilize these lesions (25), as inflammation has been linked to vascular fragility and increased rupture likelihood. Together, these mechanisms underscore an eventual therapeutic potential of antithrombotic therapy in managing bleeding risk in cavernoma patients.
Gender emerged as a significant risk factor for bleeding in our study, with male patients demonstrating a higher risk. This finding is consistent with published results, which identified male gender as a significant predictor of hemorrhage risk in patients with cavernous malformations (16). However, the literature on the influence of gender remains inconsistent. Some studies have suggested female sex as a risk factor for cavernoma-associated hemorrhage (4, 8, 17, 26), while others reported no clear gender predilection (27). These discrepancies may be attributed to differences in study design, population characteristics, or the inclusion of genetic and sporadic cases.
Smoking emerged as a potential risk factor for bleeding in our study, although it did not reach statistical significance. This observation aligns with the hypothesis that smoking-induced atherosclerosis exacerbates hemorheological changes and promotes blood flow disturbances within cavernomas (28). The literature presents conflicting evidence on this topic. Chen et al. found no significant association between nicotine abuse and hemorrhage risk in sporadic cavernomas (29), while Flemming et al. reported a significant link between tobacco use and increased lesion burden in familial cases, which could indirectly elevate the risk of bleeding (30). These contrasting findings underline the complex interplay between genetic predisposition, environmental factors, and cavernoma characteristics, underscoring the need for further research to clarify the role of smoking in cerebral cavernoma pathophysiology.
In our cohort, the size and the shape of the lesions are significantly correlated to hemorrhagic events (Tables 3, 5). Since hemorrhage by itself increases the size of the cavernoma and changes its shape, and since in this retrospective study 45% of the patients presented at the time of bleeding, this correlation is a symptom of the hemorrhage, rather than a prediction factor. On the other hand, after detection of an incidental cavernoma, increasing size and varying contours during follow-up MRIs should prompt alertness and re-discussion of the neurosurgical indication.
5 Limitations
The retrospective, single center design restricts causal conclusions, and potential bias may arise from reliance on medical records. In three emergency cases, diagnosis was based on CT imaging without MRI confirmation. Although these cases were reviewed and agreed upon in an interdisciplinary setting, the absence of MRI remains a diagnostic limitation.
The sample size limits statistical power, particularly in subgroup analyses, such as concerning the vascular border zone hemorrhage risk. Although we observed an absolute difference of over 10% between bleeding and non-bleeding groups regarding border zone localization, this did not reach statistical significance — likely due to insufficient statistical power. This limitation should be considered when interpreting the somewhat negative finding.
Furthermore, we did not differentiate detailed dosages of antithrombotic agents, which may have an impact on bleeding outcomes. Lastly, genetic factors were not analyzed, which could impact bleeding risk. Concerning analysis of brain imaging studies on shape and size of the cavernoma, these two items are strongly altered by the occurrence of the hemorrhage, thus making them associated to hemorrhage, but impeding to define them as true prognostic factors.
6 Conclusion
Our study confirms a reduced risk of bleeding in cavernoma patients treated with antithrombotic agents compared to those without such medication. Male gender was not significantly associated with bleeding in univariate analysis but emerged as an independent risk factor in the multivariate model. Irregular cavernoma shape, and changes in size during follow-up were also significantly associated with bleeding. Smoking habits were associated with a higher frequency of bleeding events, here, without reaching statistical significance.
While an overall impact of the localization of the lesion in an arterial watershed territory on the rate of hemorrhage could not be detected, we observed a higher bleeding rate in border zone cavernomas, together with a significantly lower bleeding frequency in lesions of the ACM ACP subgroup. The results presented here, thus remain equivocal regarding the clinical impact of cavernoma localization in vascular border zones.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Writing – original draft, Writing – review & editing. TW: Investigation, Visualization, Writing – review & editing. MV: Investigation, Visualization, Writing – review & editing. EG: Supervision, Visualization, Writing – review & editing. UN: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ene C, Kaul A, Kim L. Natural history of cerebral cavernous malformations. Handb Clin Neurol. (2017) 143:227–32. doi: 10.1016/b978-0-444-63640-9.00021-7
2. Flemming KD, Kumar S, Brown RD Jr, Lanzino G. Predictors of initial presentation with hemorrhage in patients with cavernous malformations. World Neurosurg. (2020) 133:e767–73. doi: 10.1016/j.wneu.2019.09.161
3. Rauschenbach L, Dammann P, Sure U. Recent novelties in research and management of cerebrospinal cavernous malformations. Acta Neurochir (Wien). (2024) 166:489. doi: 10.1007/s00701-024-06378-3
4. Del Curling O Jr, Kelly DL Jr, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg. (1991) 75:702–8. doi: 10.3171/jns.1991.75.5.0702
5. Moriarity JL, Clatterbuck RE, Rigamonti D. The natural history of cavernous malformations. Neurosurg Clin N Am. (1999) 10:411–7. doi: 10.1016/S1042-3680(18)30175-X
6. Schuss P, Marx J, Borger V, Brandecker S, Güresir Á, Hadjiathanasiou A, et al. Cavernoma-related epilepsy in cavernous malformations located within the temporal lobe: surgical management and seizure outcome. Neurosurg Focus. (2020) 48:E6. doi: 10.3171/2020.1.Focus19920
7. Shoubash L, Baldauf J, Matthes M, Kirsch M, Rath M, Felbor U, et al. Long-term outcome and quality of life after CNS cavernoma resection: eloquent vs. non-eloquent areas. Neurosurg Rev. (2022) 45:649–60. doi: 10.1007/s10143-021-01572-8
8. Kupersmith MJ, Kalish H, Epstein F, Yu G, Berenstein A, Woo H, et al. Natural history of brainstem cavernous malformations. Neurosurgery. (2001) 48:47–53. discussion 53-44. doi: 10.1097/00006123-200101000-00007
9. Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Angioma alliance scientific advisory board. Stroke. (2008) 39:3222–30. doi: 10.1161/strokeaha.108.515544
10. Gross BA, Du R. Hemorrhage from cerebral cavernous malformations: a systematic pooled analysis. J Neurosurg. (2017) 126:1079–87. doi: 10.3171/2016.3.Jns152419
11. Snellings DA, Hong CC, Ren AA, Lopez-Ramirez MA, Girard R, Srinath A, et al. Cerebral cavernous malformation: from mechanism to therapy. Circ Res. (2021) 129:195–215. doi: 10.1161/circresaha.121.318174
12. Globisch MA, Onyeogaziri FC, Jauhiainen S, Yau ACY, Orsenigo F, Conze LL, et al. Immunothrombosis and vascular heterogeneity in cerebral cavernous malformation. Blood. (2022) 140:2154–69. doi: 10.1182/blood.2021015350
13. Musmar B, Salim H, Abdelgadir J, Spellicy S, Adeeb N, Zomorodi A, et al. Antithrombotic therapy in cerebral cavernous malformations: a systematic review, meta-analysis, and network meta-analysis. J Am Heart Assoc. (2024) 13:e032910. doi: 10.1161/JAHA.123.032910
14. Akers A, Al-Shahi Salman R, Issam AA, Dahlem K, Flemming K, Hart B, et al. Synopsis of guidelines for the clinical management of cerebral cavernous malformations: consensus recommendations based on systematic literature review by the angioma alliance scientific advisory board clinical experts panel. Neurosurgery. (2017) 80:665–80. doi: 10.1093/neuros/nyx091
15. Lanfermann H, Raab P, Kretschmann H-J, Weinrich W. Klinische Neuroanatomie—Kranielle MRT und CT. 4th ed Stuttgart, Germany: Thieme (2015). p. 536.
16. Flemming KD, Link MJ, Christianson TJ, Brown RD Jr. Prospective hemorrhage risk of intracerebral cavernous malformations. Neurology. (2012) 78:632–6. doi: 10.1212/WNL.0b013e318248de9b
17. Gross BA, Du R. Cerebral cavernous malformations: natural history and clinical management. Expert Rev Neurother. (2015) 15:771–7. doi: 10.1586/14737175.2015.1055323
18. Gomez-Paz S, Maragkos GA, Salem MM, Ascanio LC, Lee M, Enriquez-Marulanda A, et al. Symptomatic hemorrhage from cerebral cavernous malformations: evidence from a cohort study. World Neurosurg. (2020) 135:e477–87. doi: 10.1016/j.wneu.2019.12.035
19. Gillespie CS, Alnaham KE, Richardson GE, Mustafa MA, Taweel BA, Islim AI, et al. Predictors of future haemorrhage from cerebral cavernous malformations: a retrospective cohort study. Neurosurg Rev. (2023) 46:52. doi: 10.1007/s10143-023-01949-x
20. Zuurbier SM, Hickman CR, Tolias CS, Rinkel LA, Leyrer R, Flemming KD, et al. Long-term antithrombotic therapy and risk of intracranial haemorrhage from cerebral cavernous malformations: a population-based cohort study, systematic review, and meta-analysis. Lancet Neurol. (2019) 18:935–41. doi: 10.1016/s1474-4422(19)30231-5
21. Bervini D, Jaeggi C, Mordasini P, Schucht P, Raabe A. Antithrombotic medication and bleeding risk in patients with cerebral cavernous malformations: a cohort study. J Neurosurg. (2019) 130:1922–30. doi: 10.3171/2018.1.Jns172547
22. Li T, Yuan D, Yuan J. Antithrombotic drugs-pharmacology and perspectives. Adv Exp Med Biol. (2020) 1177:101–31. doi: 10.1007/978-981-15-2517-9_4
23. Takeuchi H. 350Six-month Treatment with novel oral anticoagulants ameliorates cerebral blood flow, as evaluated with brain perfusion SPECT. Eur Heart J. (2018) 39:350. doi: 10.1093/eurheartj/ehy564.350
24. Wang M, Hao H, Leeper NJ, Zhu L. Thrombotic regulation from the endothelial cell perspectives. Arterioscler Thromb Vasc Biol. (2018) 38:e90–5. doi: 10.1161/atvbaha.118.310367
25. Muhlestein JB. Effect of antiplatelet therapy on inflammatory markers in atherothrombotic patients. Thromb Haemost. (2010) 103:71–82. doi: 10.1160/th09-03-0177
26. Vaquero J, Leunda G, Martínez R, Bravo G. Cavernomas of the brain. Neurosurgery. (1983) 12:208–10. doi: 10.1227/00006123-198302000-00013
27. Washington CW, McCoy KE, Zipfel GJ. Update on the natural history of cavernous malformations and factors predicting aggressive clinical presentation. Neurosurg Focus. (2010) 29:E7. doi: 10.3171/2010.5.Focus10149
28. Zhang S, Zhou C, Liu D, Piao Y, Zhang F, Hu J, et al. Is smoking a risk factor for bleeding in adult men with cerebral arteriovenous malformations? A single-center regression study from China. J Stroke Cerebrovasc Dis. (2020) 29:105084. doi: 10.1016/j.jstrokecerebrovasdis.2020.105084
29. Chen B, Saban D, Rauscher S, Herten A, Rauschenbach L, Santos A, et al. Modifiable cardiovascular risk factors in patients with sporadic cerebral cavernous malformations. Stroke. (2021) 52:1259–64. doi: 10.1161/STROKEAHA.120.031569
Keywords: cavernous malformation, cavernoma, bleeding risk, vascular border zone, antithrombotic treatment
Citation: Guranda A, Wende T, Vychopen M, Güresir E and Nestler U (2025) Antithrombotic therapy and cavernoma bleeding: does vascular border zone localization matter?. Front. Surg. 12:1577820. doi: 10.3389/fsurg.2025.1577820
Received: 16 February 2025; Accepted: 15 April 2025;
Published: 30 April 2025.
Edited by:
Jon Ramm-Pettersen, Oslo University Hospital, NorwayReviewed by:
Ulas Cikla, University of Florida Health, United StatesAlejandra Mosteiro, Hospital Clinic of Barcelona, Spain
Copyright: © 2025 Guranda, Wende, Vychopen, Güresir and Nestler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandru Guranda, YWxleGFuZHJ1Lmd1cmFuZGFAbWVkaXppbi51bmktbGVpcHppZy5kZQ==
 Alexandru Guranda
Alexandru Guranda Tim Wende
Tim Wende Martin Vychopen
Martin Vychopen Erdem Güresir
Erdem Güresir Ulf Nestler
Ulf Nestler