- 1Graduate School, Hebei North University, Zhangjiakou, Hebei, China
- 2Department of Hepatobiliary Surgery, Hebei General Hospital, Shijiazhuang, Hebei, China
- 3School of Clinical Medicine, Hebei Medical University, Shijiazhuang, Hebei, China
Background: The purpose of this study was to explore the risk factors for prolonging the operative time of fluorescence laparoscopic cholecystectomy (LC). In addition, we aimed to construct predictive models to identify patients with potentially prolonged operative times (OT) using machine learning (Ml) methods.
Methods: Clinical data of patients who underwent fluorescent LC for gallbladder stones in the Department of Hepatobiliary Surgery at our hospital from April 2023 to July 2024 were retrospectively analyzed, with the 75th percentile of operative time as the cut-off point. Parameters screened by univariate and multifactor analysis and LASSO regression were incorporated into the model, and the optimal model was analyzed and determined by integrating 11 Ml classification models.
Results: The 85 min or more was defined as prolonged OT, and 29% (223/726) of patients had prolonged OT. The variables screened by univariate, multivariate analysis and lasso regression included type of cholecystitis, number of puncture ports, gallbladder adhesion, conservative antibiotic treatment before surgery, gallbladder thickness (mm). The above five parameters were incorporated into the Ml model. Comprehensive analysis revealed that the Light Gradient Boosting Machine (LightGBM) classification model was the optimal model, with the area under the curve (AUC) of the validation cohort was 0.876, the 95% confidence interval was 0.8139–0.938, the accuracy was 0.843, the sensitivity was 0.805, and the specificity was 0.857, with AUC of validation cohort was 0.876. The calibration curves showed good agreement between the actual and predicted probabilities of the LightGBM classification model; The decision curve analysis showed that the model had good net clinical benefit in most of the threshold probability range.
Conclusions: We created a nomogram for assessing the risk of prolonged fluorescent LC time using the LightGBM classification model, which may help surgeon identify patients whose OT may be prolonged.
1 Introduction
About 6% of the global population suffers from gallbladder stones and the trend is increasing (1), with about 20% of them presenting with clinical symptoms such as epigastric pain, and requiring gallbladder removal for symptomatic gallbladder stones and asymptomatic gallbladder stones with risk factors for gallbladder cancer (2, 3). LC as the main operation for benign gallbladder diseases (4), it may be the most common surgical operations in the world (5). OT can influence LC outcomes, with up to 87 min of OT associated with postoperative superficial surgical site infections, organ-space infections, dehiscence, and septic shock, and prolonged hospitalization compared with 46 min (6). Traditional LC is mostly completed within 2 h (7). Ml can assist surgeons in making clinical decisions that are beneficial to patients (8), and has previously been used to assess the difficulty of LC but cannot infer the OT (9). In recent years, video imaging technology has made significant progress, and indocyanine green (ICG) fluorescence has been introduced into laparoscopic surgery (10). ICG can be rapidly discharged into the bile duct after intravenous injection, while near-infrared light penetrates 0.5–1 cm of human tissue and is absorbed by IGG and re-emitted at a specific wavelength, and the intraoperative fluorescence imaging system enables visualization of the extra-hepatic bile ducts (11, 12). Dissecting the Calot's triangle is a critical and time-consuming step in LC, and IGG fluorescence laparoscopy improves the visibility of the extrahepatic bile ducts, especially the Calot's triangle, compared to xenon white light imaging (12), allowing surgeons to easily identify critical anatomical landmarks in LC (13). Preoperative IGG injection reduces LC time to 21–46 min (14). In addition, inexperienced residents participation in LC will prolong the OT (15), while others believe that the ineffective guidance of the attending physician to the residents leads to the extension of the OT (16).
The aim of this study was to find out the factors that prolong the OT of fluorescent LC under the guidance of experienced chief physicians and deputy chief physicians. Developing an effective and practical predictive tool that would visualize the probability of the event, avoid or even eliminate the risk factors for prolonging the OT, reduce the occurrence of postoperative adverse events, and shorten the length of hospital stay. This study is not only applicable to traditional three or four port laparoscopy and single port laparoscopy, but also has important reference significance for 3D laparoscopy and robotic surgery.
2 Materials and methods
2.1 Participants
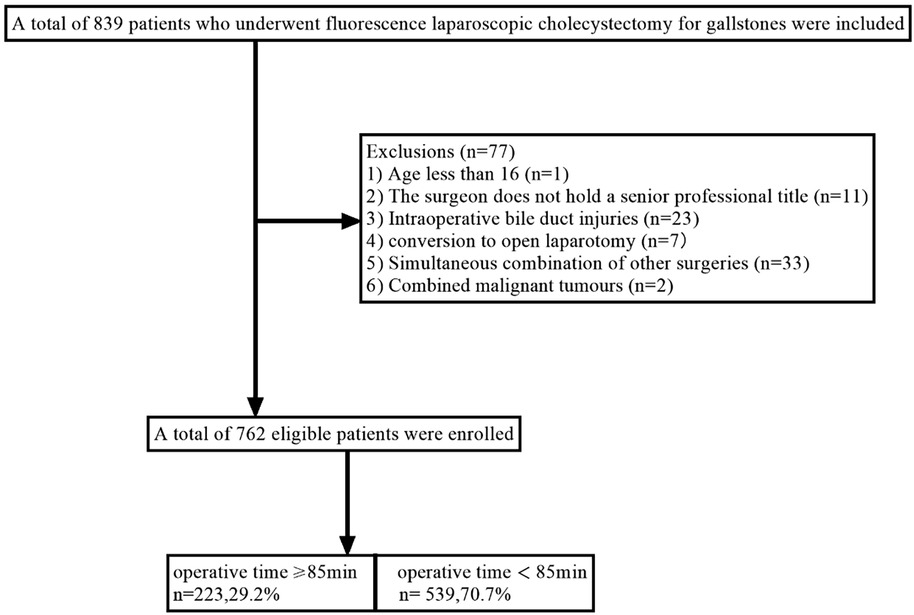
This study included patients who underwent fluorescent LC for gallstones in the Department of hepatobiliary surgery of Hebei General Hospital from April 1, 2023, to July 31, 2024. The chief physician or deputy chief physician served as the surgeon. The exclusion criteria are patients who meet one of the following characteristics. (1) Age less than 16 (n = 1); (2) The surgeon does not have a senior professional title (n = 11); (3) Intraoperative bile duct injuries (n = 23); (4) Conversion to open laparotomy (n = 7); (5) Simultaneous combination of other surgeries (n = 33); (6) Combined malignant tumours (n = 2). Finally, 762 patients were included in this study, and the patient selection process is shown in Figure 1.
2.2 Data collection
Baseline parameters of patients were collected, including age, gender, body mass index (BMI), and diabetes history. Preoperative information includes cholecystitis type, history of percutaneous transhepatic gallbladder drainage (PTGD), epigastric pain, conservative antibiotic treatment before surgery, white blood cell count, alanine aminotransferase, aspartate transaminase, bilirubin, gallbladder thickness and size. Intraoperative information included surgical methods (single-incision, three-port, and four-port laparoscopy) and gallbladder adhesion. The OT was calculated from skin incision to the end of skin suture. The 75th percentile of operative time (85 min) was used as the cut-off point, and an OT of 85 min or more was defined as prolonged OT.
2.3 Surgical procedures
In this study, three kinds of laparoscopic surgery methods (single-incision, three-port, and four-port laparoscopic) were used.
Single-incision laparoscopy: after successful anesthesia, the patient was placed in the supine position and disinfected three times according to the standard process. Make an arc incision at the lower edge of the umbilicus, insert a trocar with a diameter of 20 mm, place a camera to observe whether the abdominal cavity was damaged, and establish a 14 mmHg pneumoperitoneum with a CO2 pneumoperitoneum machine. The gallbladder and the Calot's triangle were isolated and exposed under the guidance of ICG fluorescence, followed by separation of the cystic duct using ultrasonic scalpel, closure of the cystic duct using bioabsorbable clips about 0.5 cm from the common bile duct, and the neck of the gallbladder using Hem-o-lock clips, with the cystic duct being cut between them. The cystic artery was clamped with bioabsorbable clip, and the distal end was cut off with ultrasonic scalpel. After removing the gallbladder through the abdominal incision, checked whether there was bleeding point and bile duct injury, wash the abdominal cavity with normal saline and anti-adhesion liquid, empty the CO2 in the abdominal cavity, and sutured the abdominal incision. 4-port laparoscope was performed using a 10 mm trocar in the umbilicus accompanied by three 5 mm trocars placed in the epigastric, middle right upper, or lower right lateral regions. Compared with four port-laparoscopy, three port laparoscopy omits the right lower abdominal lateral trocar.
2.4 Clinical features selected
The 17 clinical predictive variables were preprocessed, and the data with missing rate below 20% were processed by random forest interpolation, and the abnormal values in the data were cleared. Then, the predictive variables were standardized, and the clinical variables related to the research results were determined by univariate analysis. The odds ratio (OR) and 95% confidence interval (95% CI) of the variables were calculated, and then the normal group and the delay group were compared. Variables with significance in univariate analyses were included in multivariate analyses, and variables with significance in multivariate analyses were likely to be independent risk factors affecting the results. Lasso regression was used to filter variables to avoid poor fitting of the model, and variables with non-zero regression coefficients were included in the construction of the final prediction model.
2.5 Construction and performances assessment of the machine learning models
The patients were randomly divided into training cohort (534 cases) and validation cohort (228 cases) according to the ratio of 8:2. According to the selected prediction parameters, 11 Ml models such as Logistic Regression, Naive Bayes, Support Vector Machine, K-Nearest Neighbor, Random Forest, Extra Trees, eXtreme Gradient Boosting, LightGBM, Gradient Boosting, Adaptive Boosting, and Multilayer perceptron were constructed. 10-fold cross validation was performed on the training cohort to determine the hyperparameters in the final model. The models were evaluated and compared by sensitivity, specificity, positive predictive value, negative predictive value, accuracy, and area under the curve (AUC). The closer the AUC to 1, the better the performance of the model and best model was selected by plotting decision curve analysis (DCA). To improve the clinical practicability of the prediction model, we developed a nomogram. According to the contribution of each hyperparameter in the model to the results, each hyperparameter was scored, and then the total score was obtained by adding the scores. Through the total score, the probability of event occurrence can be calculated on the probability axis. The nomogram visualizes the complex regression equation, making the prediction results more readable.
2.6 Statistical analysis
The categorical variables in the data were expressed in percentages and figures, the continuous variables in normal distribution were expressed in mean ± standard deviation (SD), and non-normally distributed data were expressed as median and Inter-Quartile Range (IQR). Categorical variables were compared by chi-square test or Fisher exact test, and continuous variables were compared by independent t test or Mann–Whitney U-test. Variables with P < 0.05 were further included in the analysis. SPSS (version 22.0).
3 Results
3.1 Clinical characteristics
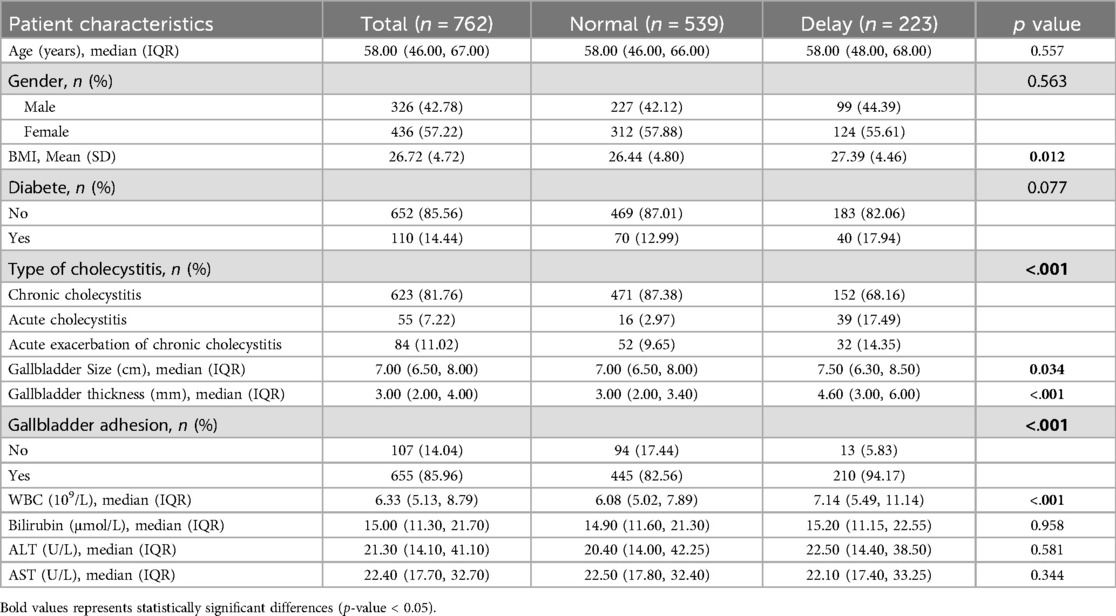
Table 1 summarized the clinical characteristics of 762 patients. The average age of the patients was 58 years old. 55 cases (7.22%) had acute cholecystitis and 84 cases (11.02%) had acute exacerbation of chronic cholecystitis. There were 655 cases of gallbladder adhesion (85.96%). 40 cases (5.24%) underwent preoperative PTGD, and 239 cases (31.36%) received conservative antibiotic therapy before surgery. Of all the patients, single-incision laparoscopy was used in 27 cases (3.64%), four-port laparoscopy in 313 cases (41.07%), and three-port laparoscopy in 422 cases (55.38%). We randomly assigned these patients, 543cases (70%) of whom were assigned to the training cohort, and the remaining 228cases (30%) to the validation cohort.
3.2 Clinical characteristic differences between the study groups
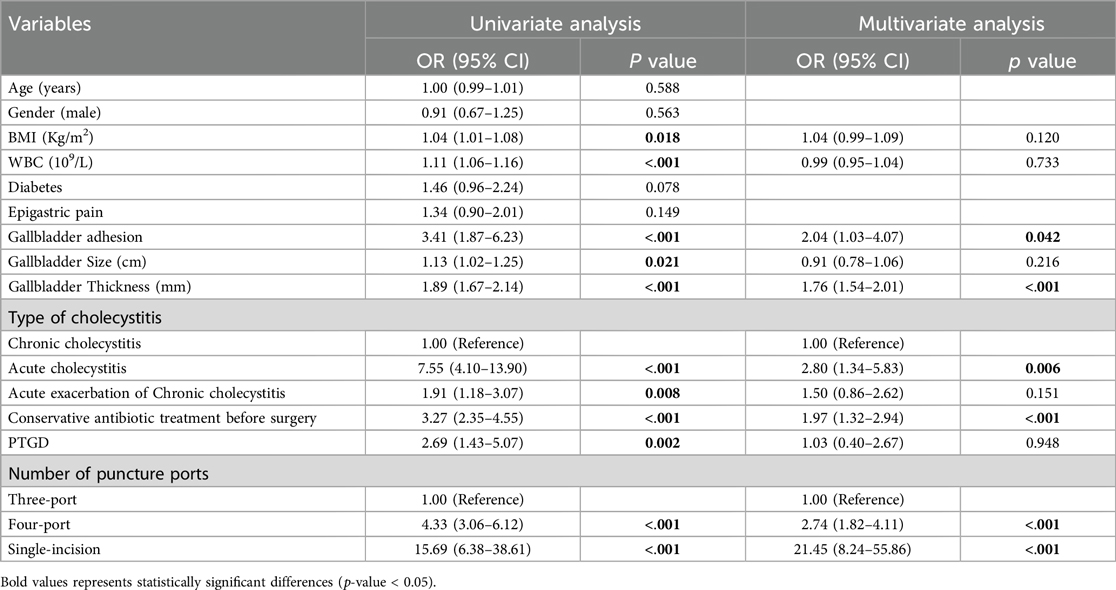
In univariate analysis (Table 2), the related factors included BMI, white blood cell count, gallbladder adhesion, gallbladder size, gallbladder thickness, acute cholecystitis, acute exacerbation of chronic cholecystitis, conservative antibiotic treatment before surgery, PTGD, single-incision and four-port surgical methods. In multivariate analysis, gallbladder adhesion, acute cholecystitis, conservative antimicrobial treatment before surgery, single-incision and four-port were independent predictors of prolonged OT (Table 2). Lasso regression was used to analyze the features with significant differences in the training cohort, which can also compress the variable coefficients, prevent over fitting, and solve the problem of collinearity. Finally, five features with non-zero regression coefficients were selected for model construction, including type of cholecystitis, number of puncture ports, gallbladder adhesion, conservative antibiotic treatment before surgery, gallbladder thickness (mm) (Figure 2).
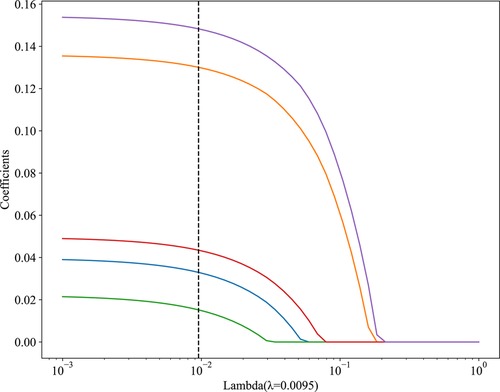
Figure 2. The results of the LASSO regression analysis. The abscissa λ represents regularization strengths, and the vertical axis represents the coefficients of features, as λ increases, features with non-zero coefficients at the dashed line have a greater impact on the results.
3.3 Construction of the predictive model
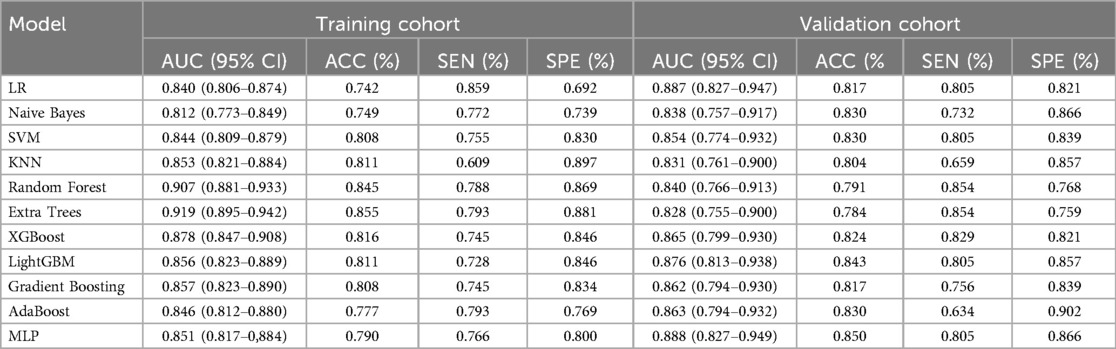
11 Ml models were constructed using predictors, and the predictive performance of each Ml model was compared in the training and validation cohort (Table 3), with satisfactory results for each model in the validation cohort (Figure 3A). Combined with the analysis of model performance (Figure 3B) and DCA (Figure 4A), LightGBM model is an ideal model for identifying patients with potential prolonged OT, with an AUC value of 0.876 (95% CI:0.813–0.938), and its SEN (80%), SPE (85%), ACC (84%), positive predictive value (67%), negative predictive value (92%). The calibration curves for the LightGBM prediction model are as follows (Figure 4B), and the curves show that the actual probabilities in the validation cohort are in good agreement with the predicted probabilities.
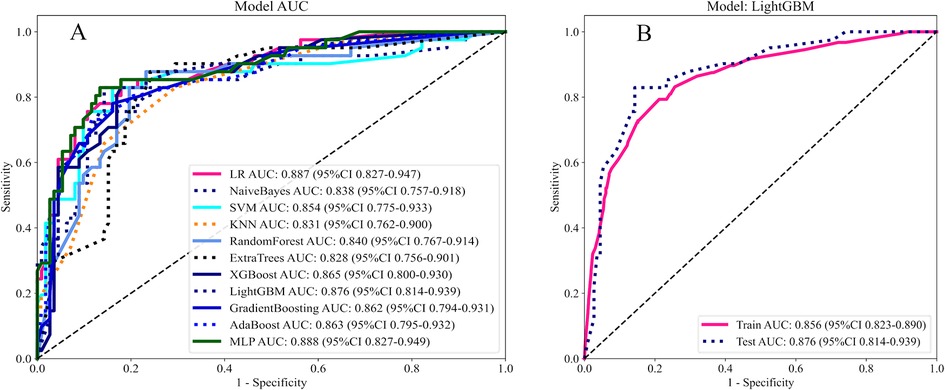
Figure 3. (A) AUC of 11 Ml models. (B) AUC of LightGBM model. Model performance is evaluated by training cohort, validation cohort.
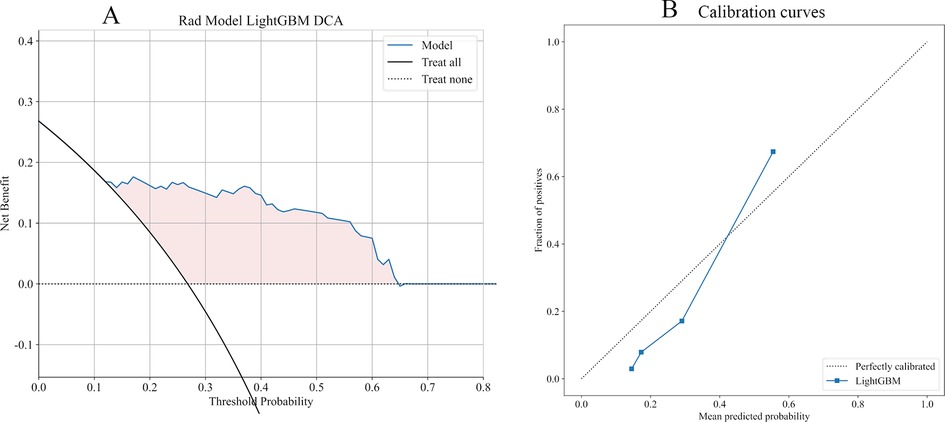
Figure 4. (A) DCA for LightGBM model. The curve shows that the model has a good clinical net benefit in most of the threshold probability range. (B) The dotted line represents the actual probability, and the blue line represents the predicted probability of the model, with closer proximity between the two representing better predictive performance.
LR, Logistic Regression; SVM, Support Vector Machine; KNM, K-Nearest Neighbor; Extra Trees, Extremely randomized trees; XGBoost, eXtreme Gradient Boosting; AdaBoost, Adaptive Boosting; MLP, Multilayer Perceptron.
3.4 Clinical value of the nomogram
We established a nomogram (Figure 5) to predict prolonged OT, assigning points to the predictors in the nomogram, with gallbladder thickness scoring the highest (0–100 points), followed by number of holes (0–22 points), conservative antibiotic treatment before surgery (6 points), type of cholecystitis (0–5 points), and gallbladder adhesions (4 points). The probability of prolongation of the operative time for each patient was visually estimated by summing the points for the corresponding variables and locating the corresponding score on the total score axis.
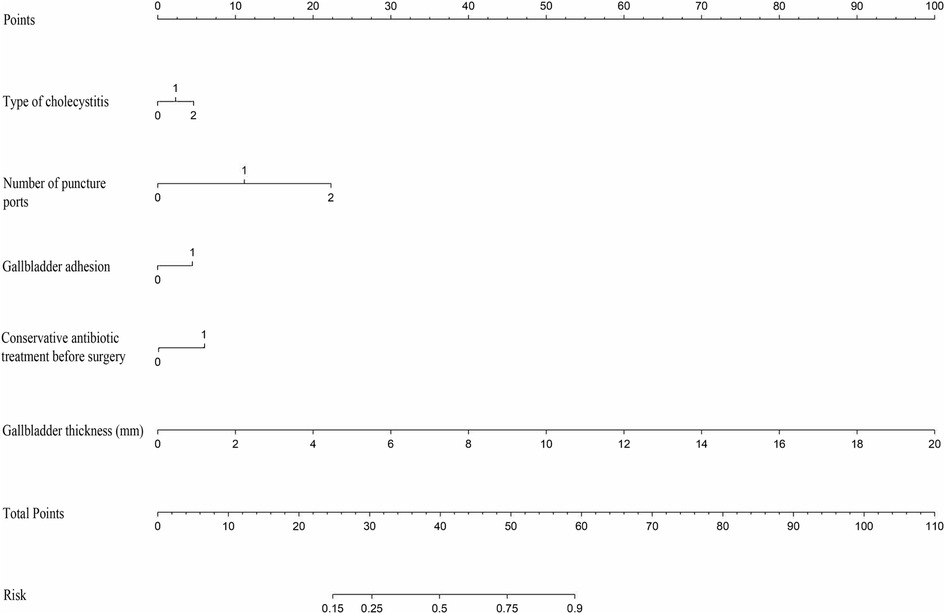
Figure 5. Use nomogram to determine the possibility of prolonged operative time. In the type of cholecystitis, 1 represents acute cholecystitis and 2 represents acute exacerbation of chronic cholecystitis; in number of puncture ports, 1 represents four holes and 2 represents single-incision.
4 Discussion
ICG fluorescence imaging in cholecystectomy has the characteristics of fast, easy and safe exposure of the bile ducts, and the learning curve is similar to the traditional LC, which is expected to become the mainstream surgical procedure for cholecystectomy (17–19). There have been previous prediction models for cholecystectomy OT. Cornelius et al. used a classification tree model to predict LC OT, incorporating four factors: gender, BMI, American Society of Anesthesiologists Classification, and abnormal liver function, but the model fit was poor (20). Bharamgoudar et al. developed a scoring tool to predict operative time for elective LC based on ten risk factors such as gallbladder thickness, common bile duct diameter, etc., assigning a score to each factor, with higher total scores being associated with longer OT (21). Bourgouin et al. developed a simple and reliable scoring tool based on preoperative variables: gender, previous cholecystitis attack, neutrophil count, fibrinogen, and alkaline phosphatase, which had the best predictive effect with an AUC of 0.8 (22). Previous studies have considered the effect of the absence of ICG fluorescence imaging and operator experience on OT.
Similar to previous studies, we found that increased BMI was a risk factor for prolonging the OT (23, 24). Obesity and abdominal fat cause the umbilicus downward displacement and difficult in identifying the umbilical fascia, leading to obvious difficulty in the placement of the umbilical port and restricted port movement (25, 26). In addition, fat accumulation may result in the inability of near-infrared light to penetrate the bile ducts and the inability of IGG to expose the cystic ducts and extrahepatic bile ducts (11, 27). Fernando found that each 1-unit increase in BMI reduced visualization of the accessory bile duct by 10% and the cystic ducts by 3% prior to dissection, and that even after dissection some biliary structures remained less well visualized, with an overall average reduction in biliary visualization of 6% per unit of BMI increase (12). Lean patients had no effect on LC time (28), and obese patients receiving a very low-calorie diet (947 kcal per day) for a fortnight prior to surgery made the Calot's triangle more recognisable and reduced OT by 20% (29), which confirmed the impact of BMI on surgery from the side.
About 12% of gallbladder stones progress to acute cholecystitis (30), and according to the Tokyo Guidelines 2018, elevated white blood cells indicate the presence of acute cholecystitis (31). The recognized optimal time for surgery in acute cholecystitis is within 3 days (32–36), however, patients often miss the optimal time for surgery when they attend the clinic (the average waiting time for operation in this study is 4.03 days) (37). In the early stages of acute inflammation, adhesions in the plane of oedema around the gallbladder are loose, and over time, inflammation and oedema are replaced by severe fibrotic adhesions between the gallbladder and the surrounding tissues, and separation of the gallbladder becomes extremely difficult (38). Solid adhesion between gallbladder and surrounding organs and greater omentum can be seen in the course of more than 7 days (39), and the adhesion of gallbladder delayed for 3 weeks is more dense (33). In addition, ICG fluorescence is difficult to observe in acute cholecystitis because of edema and severe inflammation of the tissues surrounding the gallbladder, the cystic duct, and the hepatic pedicle (13). Fernando et al. found that bile duct visualization was 50% to 100% higher in mild inflammation than in moderate to severe inflammation (12). Lack of intraoperative IGG fluorescence to guide the surgery resulted in prolonged time.
PTGD is a transitional treatment for acute cholecystitis with delayed cholecystectomy. Compared with emergency cholecystectomy, patients with acute cholecystitis of more than 3 days' course who undergo PTGD followed by cholecystectomy have a shorter operative time (40). However, prolonged drainage produces more fibrous exudates and adhesions, and preoperative PTGD of more than 8 weeks can cause a significant increase in the difficulty and duration of surgery (41). Presently, there is no consensus on the optimal timing for LC after PTGD. Some studies suggest that the OT of cholecystectomy within 3–5 days (42), 7 days (43), 7–26 days (44), and 35 days (45) after PTGD is shorter, and duration of PTGD more than 60 days will increase the risk of cholecystitis recurrence and tube detachment (45). It is suggested that PTGD should not exceed 60 days. Without extubation before operation, the OT will be also prolonged (46). Wei et al. found that Elevated preoperative inflammatory markers increased the risk of LC for more than 90 min after PTGD (47). In a randomized controlled trial, compared with PTGD, ENBD (Endoscopic Naso-gallbladder Drainage) has lighter gallbladder inflammation, less intraoperative adhesion and shorter OT, ENBD can be used as a safe and effective treatment to replace PTGD (48).
Normal gallbladder thickness is less than 3 mm on ultrasonography, and varying degrees of gallbladder thickening can be seen in chronic cholecystitis and acute cholecystitis (49, 50). Nikolaos et al. analysed 1,089 cholecystectomy patients and found that thickening of the gallbladder caused abnormal distortion of the Calot's triangle and difficulty in grasping the gallbladder, making separation of the gallbladder from the gallbladder fossa difficult, and that for every 1-mm increase in the thickness of the gallbladder, the operative time increased by 4.2 min (51). Abdulrahman believe that the thickening of gallbladder wall may be related to the increase of intraoperative blood loss (52), which requires more time to stop bleeding.
Acute cholecystitis is often converted from chronic cholecystitis (53) and always relapse frequently after remission (54). Approximately 13% of patients relapsed around 100 days after conservative antibiotic treatment (55), and another study found that patients with acute cholecystitis who underwent delayed LC had a relapse rate of up to 44.6%, with a median time to relapse of 2.8 months (56). Recurrent episodes of cholecystitis can cause thickening of the gallbladder and dense adhesions at the Calot's triangle and gallbladder fossa, resulting in increased difficulty in LC (30, 57). Acute exacerbations of chronic cholecystitis and a history of conservative treatment with antibiotics are indicative of cholecystitis attacks. Patients undergoing conservative treatment often choose to refuse surgery for other reasons such as medical background and fear of surgery (54), which may result in patients deciding to undergo surgery when they have already experienced a higher number of recurrences. The nomogram we created similarly suggest that patients receiving conservative antibiotic therapy have longer operative times than those with acute exacerbation of chronic cholecystitis. The more recurrent cholecystitis, the longer the OT.
Cholecystitis with gallbladder adhesion to omentum or bowel can make laparoscopic surgery difficult (58). The presence of adhesions restricts the surgical field of view and obscures critical anatomy, and attempts to separate these adhesions take more time so as not to injure the surrounding vital organs and tissues (59). Kapoor et al. used acoustic radiation force impulse to acquire images of the gallbladder fundus, body, and neck region, and the virtual touch imaging function to indicate the adhesion site in red, which can accurately detect gallbladder adhesions (60).
Gallbladder distension is also one of the causes of difficulty in LC (61). A distended gallbladder is not easily grasped because it tends to slip away, and the presence of pericholecystic inflammation makes the gallbladder wall friable and oedematous, thus making grasp difficult. A distended gallbladder can make it difficult to remove the specimen through a small incision, so the gallbladder needs to be aspirated and the epigastric incision lengthened (26).
Like the study by Lin et al, three-port laparoscopic cholecystectomy took the shortest time, followed by four-port LC, and single-port LC was the longest (62). The shorter time for three-port LC compared to four-port is associated with less time spent on port establishment and subsequent closure (63). Several studies have confirmed that higher surgical difficulty is the main reason for significantly longer single-port LC times (64–66). Improved surgical and imaging modalities may reduce OT. Yun et al. concluded that 3D laparoscopic imaging may reduce OT by providing a better view and easier identification of the calot's triangle and gallbladder structures than 2D (67). Fundus first laparoscopic cholecystectomy is similar to open surgery and reduces the OT in patients with difficult cholecystectomies such as acute cholecystitis and tight adhesions around the Calot's triangle (68, 69).
Nomograms can guide the clinician's surgical decisions. Patients with acute cholecystitis should be operated on as early as possible, and those who miss the optimal time for surgery should be operated on within 60 days of PTGD. For obese patients undergoing elective surgery, low calorie diet should be given for two weeks before cholecystectomy. Preoperative ultrasonography to clarify gallbladder adhesions is necessary to assess the difficulty of surgery. As cholecystitis recurs frequently, patients should be informed in detail that cholecystitis still has a high risk of recurrence after conservative treatment and that the gallbladder should be removed early.
Unlike previously, this study did not find gender, and age to be associated with prolonged OT. This may be due to update of surgical equipment and improved surgeon expertise and surgical techniques. In addition, our study has some limitations; as a retrospective study, we may have missed some important clinical factors associated with prolongation surgery and secondly, there is a possibility of bias. Therefore, predictive models need to be externally validated in larger retrospective studies to confirm model prediction accuracy.
5 Conclusion
In summary, this study identified factors associated with prolonged fluorescent laparoscopic cholecystectomy time, and for the first time established a nomogram, which can objectively and individually predict the duration of surgery. This study may help clinicians to better improve preoperative preparation, rationally arrange surgery, and reduce patients' postoperative complications and hospitalization time.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of HEBEI GENERAL HOSPITAL. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
CW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft. JW: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. ZS: Data curation, Project administration, Writing – original draft. HY: Data curation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from Hebei Provincial Department of Finance, China (ZF2023203).
Acknowledgments
Thank you for all participants for accomplishing this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang X, Yu W, Jiang G, Li H, Li S, Xie L, et al. Global epidemiology of gallstones in the 21st century: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2024) 22(8):1586–95. doi: 10.1016/j.cgh.2024.01.051
2. Lammert F, Gurusamy K, Ko CW, Miquel JF, Méndez-Sánchez N, Portincasa P, et al. Gallstones. Nat Rev Dis Primers. (2016) 2:16024. doi: 10.1038/nrdp.2016.24
3. Lynen Jansen P, Gutt C, Jenssen C, Barreiros AP, Stokes CS, Neubrand M, et al. Leitlinienreport zur aktualisierten S3-leitlinie der deutschen gesellschaft für gastroenterologie, verdauungs- und stoffwechselkrankheiten (DGVS) und der deutschen gesellschaft für allgemein- und viszeralchirurgie (DGAV) zur prävention, diagnostik und behandlung von gallensteinen. Z Gastroenterol. (2018) 56(8):e116–e80. doi: 10.1055/a-0643-4420
4. Alli VV, Yang J, Xu J, Bates AT, Pryor AD, Talamini MA, et al. Nineteen-year trends in incidence and indications for laparoscopic cholecystectomy: the NY State experience. Surg Endosc. (2017) 31(4):1651–8. doi: 10.1007/s00464-016-5154-9
5. Harrison E, Kathir Kamarajah S. Global evaluation and outcomes of cholecystectomy: protocol for a multicentre, international, prospective cohort study (GlobalSurg 4). BMJ Open. (2024) 14(7):e079599. doi: 10.1136/bmjopen-2023-079599
6. Wang DE, Bakshi C, Sugiyama G, Coppa G, Alfonso A, Chung P. Does operative time affect complication rate in laparoscopic cholecystectomy. Am Surg. (2022) 89(11):4479–84. doi: 10.1177/00031348221117032
7. Martínez-Mier G, Mendez-Rico D, Reyes-Ruiz JM, Moreno-Ley PI, Bernal-Dolores V, Avila-Mercado O. External validation of two scoring tools to predict the operative duration and open conversion of elective laparoscopic cholecystectomy in a Mexican population. Dig Surg. (2023) 40(3-4):108–13. doi: 10.1159/000531087
8. Isaac T-E, Bill W, Tim E, Saxon C. Bayesian Process mapping for intraoperative decision making in laparoscopic cholecystectomy. Artif Intell Surg. (2024) 4(2):77–91. doi: 10.20517/ais.2024.04
9. Isaac T-E, Saxon C, Tim E, Thomas HJ. Operative difficulty in laparoscopic cholecystectomy: considering the role of machine learning platforms in clinical practice. Artif Intell Surg. (2022) 2(1):46–56. doi: 10.20517/ais.2022.01
10. De Simone B, Abu-Zidan FM, Saeidi S, Deeken G, Biffl WL, Moore EE, et al. Knowledge, attitudes and practices of using indocyanine green (ICG) fluorescence in emergency surgery: an international web-based survey in the ARtificial intelligence in emergency and trauma surgery (ARIES)—WSES project. Updates Surg. (2024) 76(5):1969–81. doi: 10.1007/s13304-024-01853-z
11. Ishizawa T, Fukushima N, Shibahara J, Masuda K, Tamura S, Aoki T, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. (2009) 115(11):2491–504. doi: 10.1002/cncr.24291
12. Dip F, LoMenzo E, Sarotto L, Phillips E, Todeschini H, Nahmod M, et al. Randomized trial of near-infrared incisionless fluorescent cholangiography. Ann Surg. (2019) 270(6):992–9. doi: 10.1097/sla.0000000000003178
13. Vu VQ, Le VT, Nguyen HNA, Dang KK, Luong MVA. Preliminary results of laparoscopic cholecystectomy using real-time indocyanine green fluorescence: a cross-sectional study. Ann Med Surg. (2023) 85(3):402–6. doi: 10.1097/ms9.0000000000000261
14. Jin H, Yang J, Lu L, Cui M. Propensity score matching between conventional laparoscopic cholecystectomy and indocyanine green cholangiography-guided laparoscopic cholecystectomy: observational study. Lasers Med Sci. (2022) 37(2):1351–9. doi: 10.1007/s10103-021-03401-2
15. Lelovic N, Reif R, Jensen H, Villafranca AA, Kimbrough MK, Sexton K. Resident level is associated with operative time in laparoscopic cholecystectomy. Surgery in Practice and Science. (2024) 17:100251. doi: 10.1016/j.sipas.2024.100251
16. Wiseman JE, Morris-Wiseman LF, Hsu C-H, Riall TS. Attending surgeon influences operative time more than resident level in laparoscopic cholecystectomy. J Surg Res. (2022) 270:564–70. doi: 10.1016/j.jss.2021.09.038
17. Tseng C, Huang PW, Huang SW, Chen YC, Hung MC, Wong HP, et al. Study of learning curve in a surgeon for near-infrared fluorescence cholangiography during laparoscopic cholecystectomy-A retrospective evaluation. Surg Innov. (2022) 29(4):519–25. doi: 10.1177/15533506221093239
18. Lombardi PM, Mazzola M, Veronesi V, Granieri S, Cioffi SPB, Baia M, et al. Learning curve of laparoscopic cholecystectomy: a risk-adjusted cumulative summation (RA-CUSUM) analysis of six general surgery residents. Surg Endosc. (2023) 37(10):8133–43. doi: 10.1007/s00464-023-10345-x
19. Strigalev M, Tzedakis S, Nassar A, Dhote A, Gavignet C, Gaillard M, et al. Intra-operative indocyanine green fluorescence imaging in hepatobiliary surgery: a narrative review of the literature as a useful guide for the surgeon. Updates Surg. (2023) 75(1):23–9. doi: 10.1007/s13304-022-01388-1
20. Thiels CA, Yu D, Abdelrahman AM, Habermann EB, Hallbeck S, Pasupathy KS, et al. The use of patient factors to improve the prediction of operative duration using laparoscopic cholecystectomy. Surg Endosc. (2017) 31(1):333–40. doi: 10.1007/s00464-016-4976-9
21. Bharamgoudar R, Sonsale A, Hodson J, Griffiths E. The development and validation of a scoring tool to predict the operative duration of elective laparoscopic cholecystectomy. Surg Endosc. (2018) 32(7):3149–57. doi: 10.1007/s00464-018-6030-6
22. Bourgouin S, Mancini J, Monchal T, Calvary R, Bordes J, Balandraud P. How to predict difficult laparoscopic cholecystectomy? Proposal for a simple preoperative scoring system. Am J Surg. (2016) 212(5):873–81. doi: 10.1016/j.amjsurg.2016.04.003
23. Jang EJ, Roh YH, Choi CJ, Kim MC, Kim KW, Choi HJ. Comparison of outcomes after single-port laparoscopic cholecystectomy in relation to patient body mass index. JSLS. (2014) 18(3):e2014.00048. doi: 10.4293/jsls.2014.00048
24. Janik MR, Jędras K, Golik D, Sroczyński P. The influence of obesity on the safety of laparoscopic cholecystectomy: a retrospective analysis. Wideochir Inne Tech Maloinwazyjne. (2024) 19(1):68–75. doi: 10.5114/wiitm.2023.134121
25. Hughes DL, Elmasry M, Wilson I, El Kafsi J. Evaluating the evidence for a liver shrinkage diet for obese patients prior to laparoscopic cholecystectomy: a systematic review and meta-analysis. J Minim Access Surg. (2024) 20(1):1–6. doi: 10.4103/jmas.jmas_142_23
26. Vivek MA, Augustine AJ, Rao R. A comprehensive predictive scoring method for difficult laparoscopic cholecystectomy. J Minim Access Surg. (2014) 10(2):62–7. doi: 10.4103/0972-9941.129947
27. Nassar AHM, Khan KS, Ng HJ, Sallam M. Operative difficulty, morbidity and mortality are unrelated to obesity in elective or emergency laparoscopic cholecystectomy and bile duct exploration. J Gastrointest Surg. (2022) 26(9):1863–72. doi: 10.1007/s11605-022-05344-7
28. Bhandari TR, Khan SA, Jha JL. Prediction of difficult laparoscopic cholecystectomy: an observational study. Ann Med Surg. (2021) 72:103060. doi: 10.1016/j.amsu.2021.103060
29. Burnand KM, Lahiri RP, Burr N, Jansen van Rensburg L, Lewis MP. A randomised, single blinded trial, assessing the effect of a two week preoperative very low calorie diet on laparoscopic cholecystectomy in obese patients. HPB. (2016) 18(5):456–61. doi: 10.1016/j.hpb.2016.01.545
30. Nassar A, Elshahat I, Forsyth K, Shaikh S, Ghazanfar M. Outcome of early cholecystectomy compared to percutaneous drainage of gallbladder and delayed cholecystectomy for patients with acute cholecystitis: systematic review and meta-analysis. HPB. (2022) 24(10):1622–33. doi: 10.1016/j.hpb.2022.04.010
31. Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. (2018) 25(1):41–54. doi: 10.1002/jhbp.515
32. Oymaci E, Ucar AD, Yakan S, Carti EB, Coskun A, Erkan N, et al. Determination of optimal operation time for the management of acute cholecystitis: a clinical trial. Prz Gastroenterol. (2014) 9(3):147–52. doi: 10.5114/pg.2014.43576
33. Gupta G, Shahbaj A, Pipal DK, Saini P, Verma V, Gupta S, et al. Evaluation of early versus delayed laparoscopic cholecystectomy in acute calculous cholecystitis: a prospective, randomized study. J Minim Invasive Surg. (2022) 25(4):139–44. doi: 10.7602/jmis.2022.25.4.139
34. Ohya H, Maeda A, Takayama Y, Takahashi T, Seita K, Kaneoka Y. Preoperative risk factors for technical difficulty in emergent laparoscopic cholecystectomy for acute cholecystitis. Asian J Endosc Surg. (2022) 15(1):82–9. doi: 10.1111/ases.12969
35. Barka M, Jarrar MS, Sahli J, Abdessalem ZB, Hamila F, Youssef S. Early laparoscopic cholecystectomy for acute cholecystitis: should we operate beyond the first week? Langenbecks Arch Surg. (2023) 408(1):68. doi: 10.1007/s00423-023-02816-5
36. Woong Choi JD, John Fong M, Shanmugalingam A, Aslam A, Aqeel Abbas Kazmi S, Kulkarni R, et al. Safe postoperative outcomes following early cholecystectomy for acute calculus cholecystitis regardless of symptom onset. Turk J Surg. (2023) 39(4):321–7. doi: 10.47717/turkjsurg.2023.6165
37. Cheng X, Cheng P, Xu P, Hu P, Zhao G, Tao K, et al. Safety and feasibility of prolonged versus early laparoscopic cholecystectomy for acute cholecystitis: a single-center retrospective study. Surg Endosc. (2021) 35(5):2297–305. doi: 10.1007/s00464-020-07643-z
38. Casillas RA, Yegiyants S, Collins JC. Early laparoscopic cholecystectomy is the preferred management of acute cholecystitis. Arch Surg. (2008) 143(6):533–7. doi: 10.1001/archsurg.143.6.533
39. Lyu Y, Cheng Y, Wang B, Zhao S, Chen L. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis: an up-to-date meta-analysis of randomized controlled trials. Surg Endosc. (2018) 32(12):4728–41. doi: 10.1007/s00464-018-6400-0
40. El-Gendi A, El-Shafei M, Emara D. Emergency versus delayed cholecystectomy after percutaneous transhepatic gallbladder drainage in grade II acute cholecystitis patients. J Gastrointest Surg. (2017) 21(2):284–93. doi: 10.1007/s11605-016-3304-y
41. Liu YQ, Cai X, Zheng ZX, Xu FJ, Bi JT. Increased difficulty and complications of delayed laparoscopic cholecystectomy following percutaneous transhepatic gallbladder drainage in acute cholecystitis: a retrospective study. BMC Surg. (2023) 23(1):277. doi: 10.1186/s12893-023-02185-2
42. Choi JW, Park SH, Choi SY, Kim HS, Kim TH. Comparison of clinical result between early laparoscopic cholecystectomy and delayed laparoscopic cholecystectomy after percutaneous transhepatic gallbladder drainage for patients with complicated acute cholecystitis. Korean J Hepatobiliary Pancreat Surg. (2012) 16(4):147–53. doi: 10.14701/kjhbps.2012.16.4.147
43. Bao J, Wang J, Shang H, Hao C, Liu J, Zhang D, et al. The choice of operation timing of laparoscopic cholecystectomy (LC) after percutaneous transhepatic gallbladder drainage (PTGBD) for acute cholecystitis: a retrospective clinical analysis. Ann Palliat Med. (2021) 10(8):9096–104. doi: 10.21037/apm-21-1906
44. Sakamoto T, Fujiogi M, Matsui H, Fushimi K, Yasunaga H. Timing of cholecystectomy after percutaneous transhepatic gallbladder drainage for acute cholecystitis: a nationwide inpatient database study. HPB. (2020) 22(6):920–6. doi: 10.1016/j.hpb.2019.10.2438
45. Lyu Y, Wang B. Predictors of the difficulty of laparoscopic cholecystectomy after percutaneous transhepatic gallbladder drainage for grade II acute cholecystitis. Surg Laparosc Endosc Percutan Tech. (2024) 34:479–84. doi: 10.1097/sle.0000000000001304
46. Tomimaru Y, Fukuchi N, Yokoyama S, Mori T, Tanemura M, Sakai K, et al. Effect of preserving the percutaneous gallbladder drainage tube before laparoscopic cholecystectomy on surgical outcome: post hoc analysis of the CSGO-HBP-017. J Gastrointest Surg. (2022) 26(6):1224–32. doi: 10.1007/s11605-022-05291-3
47. Wei H-H, Wang Y-X, Xu B, Zhang Y-G. Preoperative systemic and local inflammation are independent risk factors for difficult laparoscopic cholecystectomy after percutaneous transhepatic gallbladder drainage. Heliyon. (2024) 10(16):e36081. doi: 10.1016/j.heliyon.2024.e36081
48. Mu P, Lin Y, Zhang X, Lu Y, Yang M, Da Z, et al. The evaluation of ENGBD versus PTGBD in high-risk acute cholecystitis: a single-center prospective randomized controlled trial. EClinicalMedicine. (2021) 31:100668. doi: 10.1016/j.eclinm.2020.100668
49. Miyoshi H, Inui K, Katano Y, Tachi Y, Yamamoto S. B-mode ultrasonographic diagnosis in gallbladder wall thickening. J Med Ultrason. ((2001). 2021) 48(2):175–86. doi: 10.1007/s10396-020-01018-6
50. Khan I, Yadav P, Saran RK, Sharma S, Sharma AK. A study of the degree of gall bladder wall thickness and its impact on patients undergoing laparoscopic cholecystectomy. Cureus. (2023) 15(5):e38990. doi: 10.7759/cureus.38990
51. Kokoroskos N, Peponis T, Lee JM, El Hechi M, Naar L, Elahad JA, et al. Gallbladder wall thickness as a predictor of intraoperative events during laparoscopic cholecystectomy: a prospective study of 1089 patients. Am J Surg. (2020) 220(4):1031–7. doi: 10.1016/j.amjsurg.2020.03.007
52. Alotaibi AM. Gallbladder wall thickness adversely impacts the surgical outcome. Ann Hepatobiliary Pancreat Surg. (2023) 27(1):63–9. doi: 10.14701/ahbps.22-067
53. Yeo DM, Jung SE. Differentiation of acute cholecystitis from chronic cholecystitis: determination of useful multidetector computed tomography findings. Medicine. (2018) 97(33):e11851. doi: 10.1097/md.0000000000011851
54. Handler C, Kaplan U, Hershko D, Abu-Hatoum O, Kopelman D. High rates of recurrence of gallstone associated episodes following acute cholecystitis during long term follow-up: a retrospective comparative study of patients who did not receive surgery. Eur J Trauma Emerg Surg. (2023) 49(2):1157–61. doi: 10.1007/s00068-022-02106-7
55. Wang CH, Chou HC, Liu KL, Lien WC, Wang HP, Wu YM. Long-term outcome of patients with acute cholecystitis receiving antibiotic treatment: a retrospective cohort study. World J Surg. (2014) 38(2):347–54. doi: 10.1007/s00268-013-2311-3
56. Busto Bea V, Caro Paton A, Aller Dela Fuente R, Gonzalez Sagrado M, Garcia-Alonso FJ, Perez-Miranda Castillo M. Acute calculous cholecystitis: a real-life management study in a tertiary teaching hospital. Rev Esp Enferm Dig. (2019) 111(9):667–71. doi: 10.17235/reed.2019.6260/2019
57. Sanmoto Y, Hasegawa M, Kinuta S. Factors contributing to prolonged operative time for laparoscopic cholecystectomy performed by trainee surgeons: a retrospective single-center study. Surg Today. (2024) 16(2):177–82. doi: 10.1007/s00595-024-02857-3
58. Yuda Handaya A, Werdana VAP, Fauzi AR, Andrew J, Hanif AS, Tjendra KR, et al. Gallbladder adhesion degree as predictor of conversion surgery, common bile duct injury and resurgery in laparoscopic cholecystectomy: a cross-sectional study. Ann Med Surg (Lond). (2021) 68:102631. doi: 10.1016/j.amsu.2021.102631
59. Gadiyaram S, Nachiappan M. Laparoscopic ‘D2 first’ approach for obscure gallbladders. Ann Hepatobiliary Pancreat Surg. (2021) 25(4):523–7. doi: 10.14701/ahbps.2021.25.4.523
60. Kapoor A, Sidhu BS, Singh J, Brar N, Singh P, Kapur A. Adhesions detection and staging classification for preoperative assessment of difficult laparoscopic cholecystectomies: a prospective case-control study. J Med Ultrasound. (2023) 31(2):137–43. doi: 10.4103/jmu.jmu_36_22
61. Jameel SM, Bahaddin MM, Mohammed AA. Grading operative findings at laparoscopic cholecystectomy following the new scoring system in Duhok governorate: cross sectional study. Ann Med Surg. (2020) 60:266–70. doi: 10.1016/j.amsu.2020.10.035
62. Lin H, Zhang J, Li X, Li Y, Su S. Comparative outcomes of single-incision laparoscopic, mini-laparoscopic, four-port laparoscopic, three-port laparoscopic, and single-incision robotic cholecystectomy: a systematic review and network meta-analysis. Updates Surg. (2023) 75(1):41–51. doi: 10.1007/s13304-022-01387-2
63. Nip L, Tong KS, Borg CM. Three-port versus four-port technique for laparoscopic cholecystectomy: systematic review and meta-analysis. BJS Open. (2022) 6(2):zrac013. doi: 10.1093/bjsopen/zrac013
64. Garg P, Thakur JD, Garg M, Menon GR. Single-incision laparoscopic cholecystectomy vs. Conventional laparoscopic cholecystectomy: a meta-analysis of randomized controlled trials. J Gastrointest Surg. (2012) 16(8):1618–28. doi: 10.1007/s11605-012-1906-6
65. Khorgami Z, Shoar S, Anbara T, Soroush A, Nasiri S, Movafegh A, et al. A randomized clinical trial comparing 4-port, 3-port, and single-incision laparoscopic cholecystectomy. J Invest Surg. (2014) 27(3):147–54. doi: 10.3109/08941939.2013.856497
66. Subirana H, Rey FJ, Barri J, Robres J, Parra L, Martín M, et al. Single-incision versus four-port laparoscopic cholecystectomy in an ambulatory surgery setting: a prospective randomised double-blind controlled trial. J Minim Access Surg. (2021) 17(3):311–7. doi: 10.4103/jmas.JMAS_97_20
67. Yun JJ, Kim EY, Ahn EJ, Kim JK, Choi JH, Park JM, et al. A retrospective single-center study comparing clinical outcomes of 3-dimensional and 2-dimensional laparoscopic cholecystectomy in acute cholecystitis. Ann Hepatobiliary Pancreat Surg. (2019) 23(4):339–43. doi: 10.14701/ahbps.2019.23.4.339
68. Garzali IU, Aburumman A, Alsardia Y, Alabdallat B, Wraikat S, Aloun A. Is fundus first laparoscopic cholecystectomy a better option than conventional laparoscopic cholecystectomy for difficult cholecystectomy? A systematic review and meta-analysis. Updates Surg. (2022) 74(6):1797–803. doi: 10.1007/s13304-022-01403-5
Keywords: indocyanine green, cholecystectomy, laparoscopic, operative time, gallstones, predictive model
Citation: Wang C, Wen J, Su Z and Yu H (2025) Machine learning algorithms as early diagnostic tools for prolonged operative time in patients with fluorescent laparoscopic cholecystectomy: a retrospective cohort study. Front. Surg. 12:1582425. doi: 10.3389/fsurg.2025.1582425
Received: 24 February 2025; Accepted: 6 June 2025;
Published: 23 June 2025.
Edited by:
Giuseppe Salamone, University of Palermo, ItalyReviewed by:
Heba Taher, Cairo University, EgyptGiuseppe Massimiliano De Luca, University of Bari Aldo Moro, Italy
Copyright: © 2025 Wang, Wen, Su and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: JunYe Wen, d2VuanVueWFuNkAxNjMuY29t
 Chu Wang
Chu Wang JunYe Wen
JunYe Wen ZiYi Su3
ZiYi Su3