- International Medical Department, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
Background: Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas (UC-OGCP) is an exceedingly rare malignant tumour that accounts for less than 1% of all pancreatic nonendocrine neoplasms.
Case presentation: We present a case of UC-OGCP in a 46-year-old male patient who was referred to our hospital after the incidental identification of a cystic tumour in the pancreas. Computed tomography (CT) revealed a 1.4 × 1.4 × 1.6-cm mass in the pancreatic head. Surgical resection was performed because it was difficult to determine the degree of malignancy of the tumour through clinical assessment. Intraoperative frozen-section pathology confirmed that the tumour was a malignant neoplasm. Pancreaticoduodenectomy was subsequently conducted. Histopathological studies confirmed the diagnosis of UC-OGCP.
Conclusion: The clinical symptoms of UC-OGCP are nonspecific, and there are no distinct serological markers. Diagnosis relies primarily on endoscopic biopsy or postoperative pathology. We report this case of UC-OGCP and provide a literature review on the clinicopathological features, differential diagnosis, treatment, and prognosis of UC-OGCP, aiming to improve the understanding of this disease.
1 Introduction
Undifferentiated carcinoma is a rare and highly aggressive subtype of pancreatic cancer, and undifferentiated carcinoma with osteoclast-like giant cells of the pancreas (UC-OGCP) accounts for less than 1% of all pancreatic cancer cases (1). UC-OGCP is composed of three main cell types, namely, nonneoplastic osteoclast-like multinucleated giant cells (OGCs), mononuclear histiocytes (MCHs), and neoplastic mononuclear cells, that can appear in different proportions, and the distribution of these cells affects prognosis; among these cell types, mononuclear tumour cells can include spindle-shaped cells that resemble epithelioid cells and pleomorphic giant cells, and the presence of these cell types is associated with more malignant tumours (2). The authors reasoned that UC-OGCP likely originates from epithelial and mesenchymal cells, and it is considered a rare morphological variant of pancreatic ductal adenocarcinoma (PDAC) (3). Although this condition was first described by Rosai (4) in 1968, only a limited number of cases have been reported, and the histological features and other characteristics of these tumours remain unclear or controversial. Imaging studies typically reveal the presence of a large mixed cystic or solid mass in the pancreas (5). The preoperative diagnosis of UC-OGCP presents significant challenges for clinicians, and diagnosis primarily depends on endoscopic biopsy or postoperative pathology (6). Here, we report a case of UC-OGCP and provide a literature review on the clinicopathological features, differential diagnosis, treatment, and prognosis of this disease, aiming to improve the understanding and awareness of this rare tumour.
2 Case presentation
A 46-year-old man was referred to our hospital after the incidental identification of a cystic tumour in his pancreas. The patient denied symptoms such as nausea, vomiting, diarrhoea, constipation, abdominal pain, appetite changes, or bloating. Moreover, his medical history was unremarkable, with no records of pancreatitis or solid organ malignancy. The patient also reported no allergies or significant social or family medical history, and physical examination revealed no obvious abnormalities. Serum biochemistry results revealed an elevated CA-19-9 level of 76.4 U/ml (normal range: <0–34 U/ml), while the patient's alpha-fetoprotein, carcinoembryonic antigen, carbohydrate antigen 125, carbohydrate antigen 15–3, and CEA levels were within normal ranges.
MRI revealed a space-occupying lesion in the head of the pancreas; the shape was regular, the boundary was clear, and the internal intensity was uneven. T1-weighted imaging (T1WI) showed hypointensity, T2-weighted imaging (T2WI) showed high intensity, diffusion-weighted imaging (DWI) showed isointensity, and the ADC showed high intensity (Figures 1A–D). Coronal T2WI revealed a ring-shaped low signal intensity at the periphery of the lesion, which suggested the possibility of haemosiderin deposition (Figure 1E). The mass showed heterogeneous peripheral progressive enhancement on T1 fat-sat postcontrast imaging (Figures 1F–H). Further investigation via enhanced computed tomography (CT) confirmed that the lesion was approximately 1.4 × 1.4 × 1.9 cm3 in size, and an enhanced CT scan revealed that the solid part of the lesion in the arterial stage showed uneven enhancement and that the extent of enhancement of the solid part decreased in the delayed period (Figures 2A–D). The preoperative evaluation suggested that the lesion was a cystic neoplasm, but it was unclear whether the lesion was benign or malignant. A discussion was had with the patient about the choice of surveillance or surgical resection, and the patient preferred the latter option. Thus, the patient underwent an elective exploratory laparotomy. The lesion was determined to be a malignant tumour intraoperatively via the use of frozen sections. The patient underwent standard pancreaticoduodenectomy. Histological examination revealed that the tumour comprised nonneoplastic osteoclast-like giant cells and neoplastic pleomorphic cells (Figure 3). Additionally, immunohistochemical staining revealed that the tumour was positive for CD163, P53, Ki-67 (Figure 4), CD68, INI-1, SATB2, PMS2, and MSH6 expression and negative for CK, CK7, CEA [M], and CAM5.2 expression.
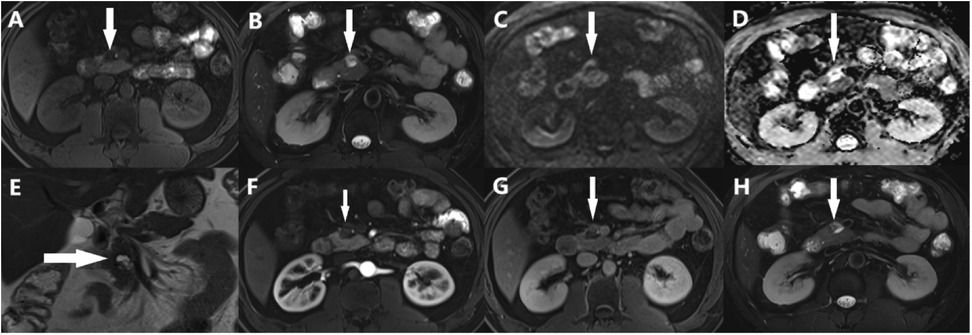
Figure 1. Abdominal MRI showed a solid lesion with cystic lesion, (A) T1WI showed hypointensity (arrows), (B) T2WI showed high-intensity (arrows), (C) DWI showed iso-intensity (arrows), (D) ADC showed high-intensity (arrows), (E) Coronal T2WI showed a ring-shaped low signal on T2WI at the periphery of the lesion (arrows), (F-H) the lesion had obvious progressive enhancement.
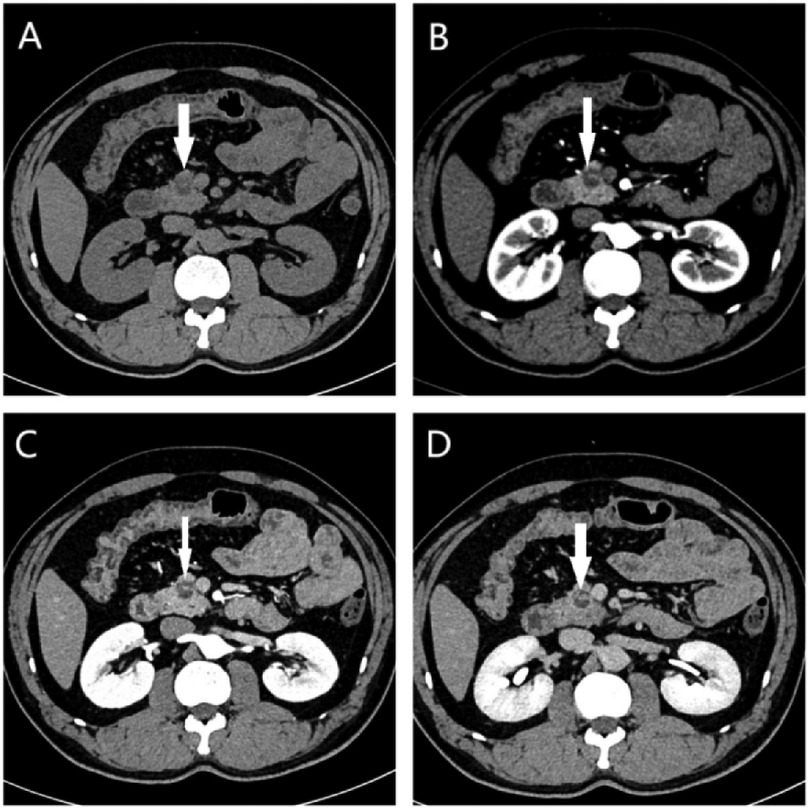
Figure 2. (A) On the plain CT scan, a small lesion with heterogeneous density and indistinct borders located in the pancreatic head (white arrow) were observed. Contrast-enhanced axial CT images showed the mass (white arrow) with heterogeneous enhancement in the (B) arterial, (C) venous and (D) delay phase.
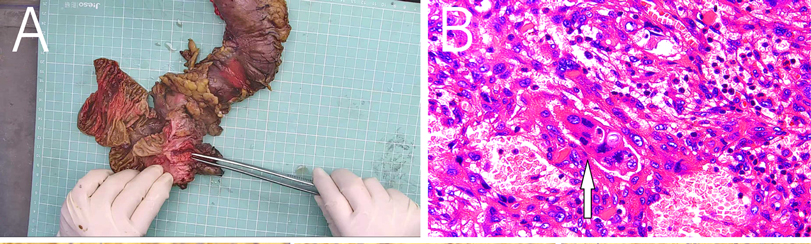
Figure 3. (A) Postoperative mass specimen of undifferentiated carcinoma with osteoclast-like giant cells of the pancreas, (B) The white arrow indicates osteoclast-like giant cells. The image is magnified 400 times.
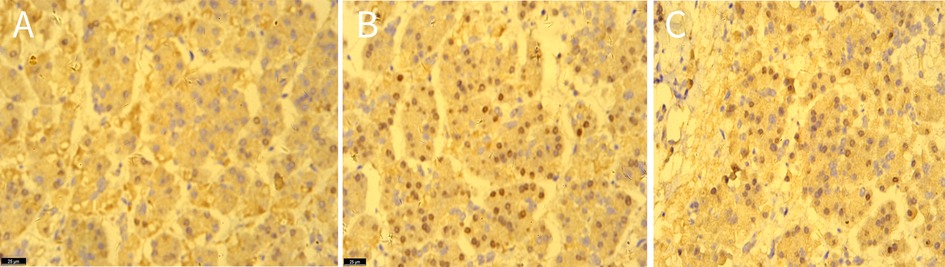
Figure 4. The immunohistochemical staining showed the expression of CD163 (A), P53 (B), ki-67 (C) in tissues, original amplification ×400.
Appropriate imaging studies did not reveal metastases. The final pathological stage of the tumour was the Union for International Cancer Control pT1cN0M0 stage I AJCC 8th edition. Following surgery, the patient's progression was uneventful, and imaging data revealed no evidence of recurrence at the 6-month follow-up visit.
3 Discussion
UC-OGCP is an extremely rare and highly aggressive type of pancreatic malignancy (7). The 2010 WHO classification of pancreatic tumours considers UC-OGCP to be a subtype of PDAC, although its pathogenesis remains largely unclear (8). UC-OGCP can affect patients across a wide range of ages, with no apparent sex predilection (9). Owing to the lack of distinctive clinical symptoms, physical signs, or serological markers, patients are often diagnosed at an advanced stage of disease, which contributes to the generally poor prognosis (10). According to previous reports, patients commonly present with nonspecific gastrointestinal symptoms, including abdominal pain, distension, fatigue, jaundice, and weight loss (11). Tumour marker levels are typically within normal ranges, although elevated CA199 levels are observed in some patients (12). In the present case, the patient's pancreatic lesion was incidentally discovered through imaging, which enabled timely intervention. As a result, the tumour was identified at an early stage, and the patient experienced a favourable prognosis.
The imaging features of UC-OGCP provide valuable insights into tumour characteristics. On CT, UC-OGCP lesions generally appear as well-defined cystic or cystic‒solid masses, often exceeding 8 cm in diameter, and they are typically located in the body or tail of the pancreas (13). UC-OGCP is frequently associated with internal necrosis and haemorrhage, with occasional calcifications (14). When located in the pancreatic head or neck, UC-OGCP often causes dilation of the pancreatic and bile ducts (15). After contrast enhancement, visible enhancement is observed in the solid areas, septations, and lesion margins (16). On MRI, UC-OGCP tumours commonly show mixed signal intensities. T1WI reveals areas of low to slightly low signal intensity that are occasionally interspersed with patchy regions with high signal intensity. T2WI displays a heterogeneous mix of high and low signal intensity, with visible septations and cystic cavities of irregular thickness; fluid‒fluid levels are frequently observed within these cavities, and a rim of low signal intensity is often observed around the lesion margins (17). In most cases, the tumour exhibits low signal intensity on DWI, likely due to haemosiderin deposits from the phagocytosis of erythrocytes by osteoclast-like giant cells (18). On PET-CT, UC-OGCP lesions typically show high uptake of fluorodeoxyglucose (FDG), reflecting high metabolic activity that is consistent with the rapid growth of tumour cells (19).
The definitive diagnosis of UC-OGCP relies primarily on endoscopic biopsy or postoperative pathological examination of three main types of cells: nonneoplastic OGCs, neoplastic mononuclear cells, and MCHs (20). Mononuclear tumour cells can include spindle-shaped cells that resemble epithelioid and pleomorphic giant cells (21). OGCs contain eosinophilic cytoplasm and multiple nuclei and are generally located in haemorrhagic and necrotic regions (22). Immunohistochemical staining has revealed that MCHs express CD163, which is a marker of tumour-associated macrophages, whereas OGCs and some MCHs express CD68 (23). In UC-OGCP, elevated Ki-67 expression is predominantly observed in the mononuclear tumour cell population, particularly in histiocyte-like sarcomatoid cells (HSCs) and pleomorphic giant carcinoma cells (PCs). These cell types exhibit marked proliferative activity, with Ki-67 labelling indices typically ranging from 15% to 45%, which indicates the high degree of malignancy and aggressive biological behaviour of these cells (11). In contrast, OGCs, although present within the tumour microenvironment, display low Ki-67 expression. These cells are generally considered to be nonneoplastic, macrophage-derived reactive cells that lack proliferative potential (24). Thus, the aggressive nature of UC-OGCP is driven primarily by mononuclear tumour components with high Ki-67 expression, especially HSCs and PCs. The elevated proliferative index of these cells is strongly correlated with rapid tumour progression, a propensity for metastasis, and poor clinical outcomes.
Currently, there is no standard protocol for treating UC-OGCP. For early-stage cases, surgical resection is the primary treatment option (25). However, owing to the lack of specific clinical features, most patients are diagnosed at an advanced stage of disease or after metastasis has occurred. UC-OGCP, which is a variant of PDAC, has a striking genetic similarity to PDAC (26). Recent genomic studies suggest that UC-OGCP shares key molecular alterations with PDAC, including recurrent mutations in KRAS, TP53, and SMAD4 (27). Given the genomic similarities of UCOGCP and PDAC, the use of therapeutic strategies that are effective in treating PDAC for the treatment of aggressive UC-OGCP, particularly in the treatment of chemotherapy-resistant tumours, should be explored. In certain locally advanced, unresectable cases, gemcitabine may be recommended as palliative therapy, although the available data are limited, and no clear consensus has been established (28). Studies have demonstrated that PD-L1 is expressed in tumour cells in approximately 63% of UC-OGCP patients, and PD-L1 expression is strongly associated with a poor prognosis, potentially offering valuable insights for the development and optimization of treatment strategies (29). PD-L1 expression has been associated with poor prognosis in various cancers, including PDAC, where its presence is associated with immune evasion and resistance to therapy (30). Several studies have highlighted the prognostic value of PD-L1 expression and demonstrated that high PD-L1 levels are correlated with advanced disease stages and worse overall survival in multiple cancer patient population. Notably, Hrudka et al. (31) reported that patients with PD-L1-negative UC-OGCP exhibit a more favourable prognosis than patients with PD-L1-positive UC-OGCP. Obayashi et al. (32) reported a durable response to pembrolizumab in a patient with metastatic UC-OGCP, emphasizing the potential of immune checkpoint blockade in patients with high PD-L1 expression. Similarly, Besaw et al. (33) reported a favourable response to anti-PD-L1 therapy in a patient with recurrent UC-OGCP, further supporting the clinical importance of immune modulation in managing this aggressive variant of pancreatic cancer.
Our study highlights the importance of further investigation when cystic‒solid pancreatic lesions are identified, especially in patients with elevated tumour marker levels. Optimal diagnostic procedures include EUS-guided fine-needle aspiration or fine-needle biopsy, which allows the use of haematoxylin and eosin staining and immunohistochemical analysis to inform subsequent treatment decisions. This approach can facilitate the early diagnosis and effective management of malignancies. In our patient, the tumour was discovered at an early stage during routine examination, allowing prompt intervention, which contributed to a favourable prognosis.
4 Conclusion
UC-OGCP is a rare malignant tumour that typically presents with nonspecific clinical symptoms. While imaging techniques such as CT and MRI can assist in the diagnostic process, definitive diagnosis relies on pathological examination and immunohistochemical analysis. Surgical intervention remains the mainstay of treatment. For patients who are ineligible for radical resection or those with metastatic disease or postoperative recurrence, adjuvant therapies, including radiotherapy and chemotherapy, may be employed. This study presents a confirmed case of UC-OGCP, reviews its clinical data and integrates insights from the literature to discuss the clinicopathological features, key points in diagnosis and differential diagnosis, treatment strategies, and prognosis of UC-OGCP. The aim of this study was to advance the understanding and awareness of this rare condition.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The present study involving humans was approved by the Ethics Committee of the Guangxi Zhuang Autonomous Region People's Hospital (ethical approval no. V1.0 2024.12.16; Guangxi, China). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. YL: Software, Supervision, Visualization, Writing – original draft. ZLi: Investigation, Supervision, Writing – original draft. QQ: Conceptualization, Writing – original draft. YZ: Supervision, Writing – original draft. DD: Writing – review & editing, Supervision. ZLu: Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The mechanism by which erythromycin alleviates NET-stimulated autophagy-related inflammation in monocytes via PI3K/Akt/mTOR pathway inhibition was studied.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mylonakis A, Driva TS, Lykoudis P, Frountzas M, Machairas N, Tsapralis D, et al. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas: an individual participant data meta-analysis. Ann Hepatobiliary Pancreat Surg. (2024) 28(2):125–33. doi: 10.14701/ahbps.23-161
2. Tambasco ML, Echelard P, Perrault F, Temmar R, Trinh VQ, Collin Y. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells, a two cases report. Int J Surg Case Rep. (2024) 116:109419. doi: 10.1016/j.ijscr.2024.109419
3. Wu H. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas: a narrative review. Front Oncol. (2024) 14:1409197. doi: 10.3389/fonc.2024.1409197
4. Rosai J. Carcinoma of pancreas simulating giant cell tumor of bone. Electron-microscopic evidence of its acinar cell origin. Cancer. (1968) 22(2):333–44. doi: 10.1002/1097-0142(196808)22:2%3C333::AID-CNCR2820220210%3E3.0.CO;2-A
5. Cavalcanti E, Schena N, Serino G, Lantone G, Armentano R. Assessment and management of undifferentiated carcinoma with osteoclastic like giant cells of the pancreas: a case report and revision of literature. BMC Gastroenterol. (2021) 21(1):247. doi: 10.1186/s12876-021-01779-5
6. Jiang J, Luo J. Osteoclast-like giant cell undifferentiated carcinoma of the pancreas: a case report. Int J Clin Exp Pathol. (2021) 14(2):179–85.33564350
7. Imaoka H, Ikeda M, Umemoto K, Sunakawa Y, Ueno M, Ueno H, et al. Comprehensive review of undifferentiated carcinoma of the pancreas: from epidemiology to treatment. Jpn J Clin Oncol. (2023) 53(9):764–73. doi: 10.1093/jjco/hyad062
8. Takahashi Y, Hioki M, Ohno K, Sadamori H, Takakura N. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells localized in the main pancreatic duct without extraductal invasion: a case report and literature review. Cureus. (2025) 17(3):e80592. doi: 10.7759/cureus.80592
9. Sah SK, Li Y, Li Y. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells: a rare case report and review of the literature. Int J Clin Exp Pathol. (2015) 8(9):11785–91.26617927
10. Gao HQ, Yang YM, Zhuang Y, Liu P. Locally advanced undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. World J Gastroenterol. (2015) 21(2):694–8. doi: 10.3748/wjg.v21.i2.694
11. Muraki T, Reid MD, Basturk O, Jang KT, Bedolla G, Bagci P, et al. Undifferentiated carcinoma with osteoclastic giant cells of the pancreas: clinicopathologic analysis of 38 cases highlights a more protracted clinical course than currently appreciated. Am J Surg Pathol. (2016) 40(9):1203–16. doi: 10.1097/PAS.0000000000000689
12. Tomihara H, Hashimoto K, Ishikawa H, Terashita D, Gakuhara A, Fukuda S, et al. Spontaneous rupture of an undifferentiated carcinoma with osteoclast-like giant cells of the pancreas presenting as intra-abdominal bleeding: a case report. Surg Case Rep. (2022) 8(1):79. doi: 10.1186/s40792-022-01437-2
13. Guo YL, Ruan LT, Wang QP, Lian J. Undifferentiated carcinoma with osteoclast-like giant cells of pancreas: a case report with review of the computed tomography findings. Medicine (Baltimore). (2018) 97(48):e13516. doi: 10.1097/MD.0000000000013516
14. Zhan K, Zhang S, Hu P, Chen J, Liu W, Niu Z. Undifferentiated carcinoma of the pancreas with osteoclast like giant cells: literature review with CT/MR imaging findings in 3 cases. Radiol Case Rep. (2022) 17(7):2529–33. doi: 10.1016/j.radcr.2022.03.032
15. Shiozawa M, Imada T, Ishiwa N, Rino Y, Hasuo K, Takanashi Y, et al. Osteoclast-like giant cell tumor of the pancreas. Int J Clin Oncol. (2002) 7(6):376–80. doi: 10.1007/s101470200059
16. Shindoh N, Ozaki Y, Kyogoku S, Nakanishi A, Sumi Y, Katayama H. Osteoclast-type giant cell tumor of the pancreas: helical CT scans. AJR Am J Roentgenol. (1998) 170(3):653–4. doi: 10.2214/ajr.170.3.9490947
17. Fukukura Y, Kumagae Y, Hirahara M, Hakamada H, Nagano H, Nakajo M, et al. CT and MRI features of undifferentiated carcinomas with osteoclast-like giant cells of the pancreas: a case series. Abdom Radiol (NY). (2019) 44(4):1246–55. doi: 10.1007/s00261-019-01958-9
18. Yang KY, Choi JI, Choi MH, Park MY, Rha SE, Byun JY, et al. Magnetic resonance imaging findings of undifferentiated carcinoma with osteoclast-like giant cells of pancreas. Clin Imaging. (2016) 40(1):148–51. doi: 10.1016/j.clinimag.2015.09.013
19. Fu LP, Cheng AP, Wang XG, Fu JL, Jin L. 18F-FDG PET/CT in the detection of undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. Clin Nucl Med. (2017) 42(8):615–6. doi: 10.1097/RLU.0000000000001719
20. Gupta K, Goyal S, Chaudhary D, Sakhuja P, Narang P, Nag HH. Undifferentiated carcinoma with osteoclast-like giant cells of pancreas on cytology: a case report with review of literature. Diagn Cytopathol. (2022) 50(10):E289–E94. doi: 10.1002/dc.25001
21. Saito H, Kashiyama H, Murohashi T, Sasaki K, Misawa R, Ohwada S. Case of six-year disease-free survival with undifferentiated carcinoma of the pancreas. Case Rep Gastroenterol. (2016) 10(2):472–8. doi: 10.1159/000448878
22. Demetter P, Marechal R, Puleo F, Delhaye M, Debroux S, Charara F, et al. Undifferentiated pancreatic carcinoma with osteoclast-like giant cells: what do we know so far? Front Oncol. (2021) 11:630086. doi: 10.3389/fonc.2021.630086
23. Wang X, Miao J, Wang S, Shen R, Zhang S, Tian Y, et al. Single-cell RNA-Seq reveals the genesis and heterogeneity of tumor microenvironment in pancreatic undifferentiated carcinoma with osteoclast-like giant-cells. Mol Cancer. (2022) 21(1):133. doi: 10.1186/s12943-022-01596-8
24. Olayinka O, Kaur G, Gupta G. Undifferentiated pancreatic carcinoma with osteoclast-like giant cells and associated ductal adenocarcinoma with focal signet-ring features. Cureus. (2021) 13(5):e14988. doi: 10.7759/cureus.14988
25. Swaid MB, Vitale E, Alatassi N, Siddiqui H, Yazdani H. Metastatic undifferentiated osteoclast-like giant cell pancreatic carcinoma. Cureus. (2022) 14(8):e27586. doi: 10.7759/cureus.27586
26. Luchini C, Pea A, Lionheart G, Mafficini A, Nottegar A, Veronese N, et al. Pancreatic undifferentiated carcinoma with osteoclast-like giant cells is genetically similar to, but clinically distinct from, conventional ductal adenocarcinoma. J Pathol. (2017) 243(2):148–54. doi: 10.1002/path.4941
27. Hrudka J, Kalinová M, Ciprová V, Moravcová J, Dvořák R, Matěj R. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas: molecular genetic analysis of 13 cases. Int J Mol Sci. (2024) 25(6):3285. doi: 10.3390/ijms25063285
28. Yazawa T, Watanabe A, Araki K, Segawa A, Hirai K, Kubo N, et al. Complete resection of a huge pancreatic undifferentiated carcinoma with osteoclast-like giant cells. Int Cancer Conf J. (2017) 6(4):193–6. doi: 10.1007/s13691-017-0305-y
29. Luchini C, Cros J, Pea A, Pilati C, Veronese N, Rusev B, et al. PD-1, PD-L1, and CD163 in pancreatic undifferentiated carcinoma with osteoclast-like giant cells: expression patterns and clinical implications. Hum Pathol. (2018) 81:157–65. doi: 10.1016/j.humpath.2018.07.006
30. Lehrke HD, Graham RP, McWilliams RR, Lam-Himlin DM, Smyrk TC, Jenkins S, et al. Undifferentiated pancreatic carcinomas display enrichment for frequency and extent of PD-L1 expression by tumor cells. Am J Clin Pathol. (2017) 148(5):441–9. doi: 10.1093/ajcp/aqx092
31. Hrudka J, Lawrie K, Waldauf P, Ciprová V, Moravcová J, Matěj R. Negative prognostic impact of PD-L1 expression in tumor cells of undifferentiated (anaplastic) carcinoma with osteoclast-like giant cells of the pancreas: study of 13 cases comparing ductal pancreatic carcinoma and review of the literature. Virchows Arch. (2020) 477(5):687–96. doi: 10.1007/s00428-020-02830-8
32. Obayashi M, Shibasaki Y, Koakutsu T, Hayashi Y, Shoji T, Hirayama K, et al. Pancreatic undifferentiated carcinoma with osteoclast-like giant cells curatively resected after pembrolizumab therapy for lung metastases: a case report. BMC Gastroenterol. (2020) 20(1):220. doi: 10.1186/s12876-020-01362-4
Keywords: undifferentiated carcinoma, osteoclast-like giant cells, pancreas, computed tomography, immunotherapy
Citation: Si L, Liu Y, Lin Z, Qin Q, Zhang Y, Deng D and Lu Z (2025) Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas: a rare case report and review of the literature. Front. Surg. 12:1584200. doi: 10.3389/fsurg.2025.1584200
Received: 27 February 2025; Accepted: 16 June 2025;
Published: 18 July 2025.
Edited by:
Shaocheng Lyu, Capital Medical University, ChinaReviewed by:
Jan Hrudka, Charles University, CzechiaHaifeng Ran, The Affiliated Hospital of Zunyi Medical University, China
Gargi Kapatia, All India Institute of Medical Sciences, India
Copyright: © 2025 Si, Liu, Lin, Qin, Zhang, Deng and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Demao Deng, ZGVtYW9kZW5nQDE2My5jb20=; Zhao Lu, NTY0MjUyMTczQHFxLmNvbQ==
†ORCID:
Zhao Lu
orcid.org/0009-0007-0470-9796
 Lu Si
Lu Si Yihan Liu
Yihan Liu Zhiyu Lin
Zhiyu Lin Demao Deng
Demao Deng Zhao Lu
Zhao Lu