- 1Department of Orthopaedics, First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
- 2Department of Orthopaedics and Traumatology, Cixi Hospital of Traditional Chinese Medicine, Ningbo, Zhejiang, China
- 3Department of Trauma Center, First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
Purpose: This meta-analysis aimed to compare the direct anterior approach (DAA) and posterior approach (PA) for total hip arthroplasty (THA) within the context of enhanced recovery after surgery (ERAS).
Methods: Studies comparing DAA and PA for THA were systematically retrieved from PubMed, Embase, Web of Science, Cochrane Library, and Google Scholar databases, covering the period from 2012 to 2024. A meta-analysis was conducted to compare the ERAS-related outcomes between DAA and PA for THA using RevMan 5.3 software, including surgical trauma, muscle damage, functional recovery, and complications. Heterogeneity was considered significant if I2 > 50%, in which case a random-effects model and subgroup analysis were applied. Continuous and dichotomous data were analyzed using 95% confidence intervals (CIs). Methodological quality and heterogeneity assessments were also conducted.
Results: A total of 48 studies, including 46,367 hips (13,285 in the DAA group and 33,082 in the PA group), were included. Compared with PA, DAA was associated with significantly lower blood transfusion rates [6.62% vs. 14.52%; odds ratio (OR) = 0.73; 95% CI: 0.59–0.91; P < 0.005], shorter hospital stay [mean difference (MD) = −0.88 days; 95% CI: −1.10 to −0.87; P < 0.001], and less gluteus minimus muscle damage on magnetic resonance imaging (MRI) (36.84% vs. 65.79%; OR = 0.28; 95% CI: 0.14–0.56; P < 0.005). Lower levels of creatine kinase (MD = −49.58; 95% CI: −56.43 to −43.26; P < 0.001) and C-reactive protein (MD = −4.48; 95% CI: −5.28 to −4.47; P < 0.001) were also observed in the DAA group. Functional outcomes, including Harris hip score (MD = 3.07; 95% CI: 0.08–6.07; P < 0.05) and short form (SF) score (MD = 1.53; 95% CI: 0.80–2.26; P < 0.001), were better with DAA. Dislocation rates were significantly lower with DAA (0.84% vs. 1.82%; OR = 0.32; 95% CI: 0.21–0.48; P < 0.001). However, there were no significant differences between DAA and PA in surgery time (MD = 2.43; 95% CI: −2.20 to 7.06; P = 0.30), gluteus medius muscle damage on MRI (17.34% vs. 15.15%; OR = 1.20; 95% CI: 0.53–2.71; P = 0.66), tensor fasciae latae muscle damage on MRI (25.51% vs. 38.38%; OR = 0.40; 95% CI: 0.03–4.97; P = 0.48), time to discontinuation of assistive devices (MD = −1.85; 95% CI: −4.05 to 0.35; P = 0.10), infection (1.09% vs. 0.60%; OR = 0.92; 95% CI: 0.48–1.77; P = 0.81), nerve injury (0.60% vs. 0.68%; OR = 1.06; 95% CI: 0.69–1.64; P = 0.79), intraoperative fracture (0.55% vs. 0.79%; OR = 0.68; 95% CI: 0.36–1.26; P = 0.22), or leg length discrepancy (MD = −1.85; 95% CI: −4.05 to 0.35; P = 0.10).
Conclusion: Within the framework of ERAS, the DAA was found to be associated with reduced muscle damage, fewer postoperative complications, and improved functional recovery compared with the PA in patients undergoing THA.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/recorddashboard.
Introduction
Total hip arthroplasty (THA) is one of the most effective treatments for end-stage hip disorders (1). Various surgical approaches have been developed for THA, including the posterior approach (PA), direct anterior approach (DAA), lateral approach, and minimally invasive techniques such as the orthopaedic chirurgie München (OCM) and the supercapsular percutaneously assisted total hip (SUPER-PATH) approach (2–4). Among these, the DAA has gained widespread clinical adoption as a representative minimally invasive technique. The approach was performed through the muscle gap between the broad fascia tensor and the sartorius muscle, allowing for muscle-sparing access to the hip joint (5). Compared with the PA, the DAA has been associated with several benefits from enhanced recovery after surgery (ERAS), including reduced muscle damage, faster postoperative recovery, and less pain (6). Although numerous studies have compared the DAA and PA in terms of complications, surgery time, length of hospital stay, muscle damage, and functional outcomes (7–13), few have systematically evaluated these parameters within the framework of ERAS. A comprehensive comparison of ERAS-related outcomes between these two approaches is essential to inform surgical decision-making and to identify which technique aligns more closely with ERAS principles. Therefore, we conducted a meta-analysis to compare the DAA and PA approaches in the context of ERAS. Key outcomes included surgical trauma (blood transfusion rate, hospital stay, and surgery time), muscle damage [magnetic resonance imaging (MRI) and serum creatine kinase (CK) and C-reactive protein (CRP) levels], functional recovery [Harris hip score (HHS), short form (SF) score], and postoperative complications (dislocation rate, nerve injury rate, intraoperative fracture, infection rate, and leg length discrepancy).
Materials and methods
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (14). Details of the protocol for this systematic review were registered on PROSPERO (CRD42051054229).
Literature search
A comprehensive literature search was performed across English-language databases, including PubMed, Embase, Web of Science, Cochrane Library, and Google Scholar, as well as Chinese-language databases, including the China National Knowledge Infrastructure (CNKI), WanFang, and VIP, covering the period from 2012 to 2024. The search terms used were as follows: (“direct anterior approach” OR “DAA” OR “Hueter approach” OR “SmithPetersen approach”) AND (“posterior approach” OR “posterior lateral approach” OR “Kocher approach” OR “Gibson approach” OR “PA” OR “posterolateral approach”) AND (“total hip arthroplasty” OR “total hip replacement” OR “THA”). In addition, relevant articles cited in the reference lists of systematic reviews or meta-analyses were screened and included if they met the eligibility criteria.
Inclusion criteria
Studies were included if they met the following criteria:
1. comparative studies evaluating DAA vs. PA or posterolateral approach in THA;
2. study design of randomized controlled trials (RCTs), prospective cohort studies, or retrospective studies; and
3. studies reported at least one of the following outcomes: hospital stay, surgery time, blood transfusion rate, MRI, CK level, CRP level, HHS, time to discontinuation of assistive devices, SF score, or postoperative complications;
Exclusion criteria
Studies were excluded if they met any of the following conditions:
1. studies involving revision of THA;
2. study design of case reports, systematic reviews, meta-analyses, letters to the editor, and fundamental research;
3. studies including the assistance of computer navigation- and robot-assisted THA, or hemiarthroplasty; and
4. studies containing incomplete or unavailable data;
Data extraction
Standardized data extraction forms were developed to collect the following information: (1) first author's surname; (2) year of publication; (3) methodological characteristics; (4) clinical data, including sample size, age range, and gender ratio; (5) follow-up duration; and (6) ERAS-related indicators, including blood transfusion rate, hospital stay, surgery time, MRI findings, CK and CRP levels, HHS, SF score, dislocation rate, infection rate, nerve injury rate, intraoperative fracture, and postoperative leg length discrepancy. Two reviewers (WX and JLa) independently extracted the data based on these forms. Any disagreements were resolved through consultation with a senior investigator (YX).
Assessment of risk of bias
The risk of bias (ROB) for the included studies was assessed using the Newcastle–Ottawa scale (NOS) (15), methodological index for non-randomized studies (MINORS) (16) for non-randomized studies, and the Cochrane Collaboration's Risk of Bias tool for RCTs (17). Two reviewers (WX and JLa) independently conducted the assessments. Any disagreements were resolved through consultation with a senior investigator (YX).
Statistical analysis
Statistical analyses were performed using Review Manager (RevMan) version 5.3 (Cochrane Collaboration, Oxford, UK). Odds ratios (ORs) were used for dichotomous outcomes, while weighted MDs were used for continuous variables. P < 0.05 was considered statistically significant. Heterogeneity among studies was assessed using the I² statistic derived from the chi-square test. An I² value of >50% indicated high heterogeneity, while an I² value of <50% suggested low heterogeneity. A fixed-effects model was applied when P > 0.1 and I² < 50%, whereas a random-effects model was used when I² exceeded 50%. When five or more studies were included, publication bias was assessed using Egger’s test with Stata software (version 17.0, StataCorp LP, College Station, TX, USA).
Results
Search results
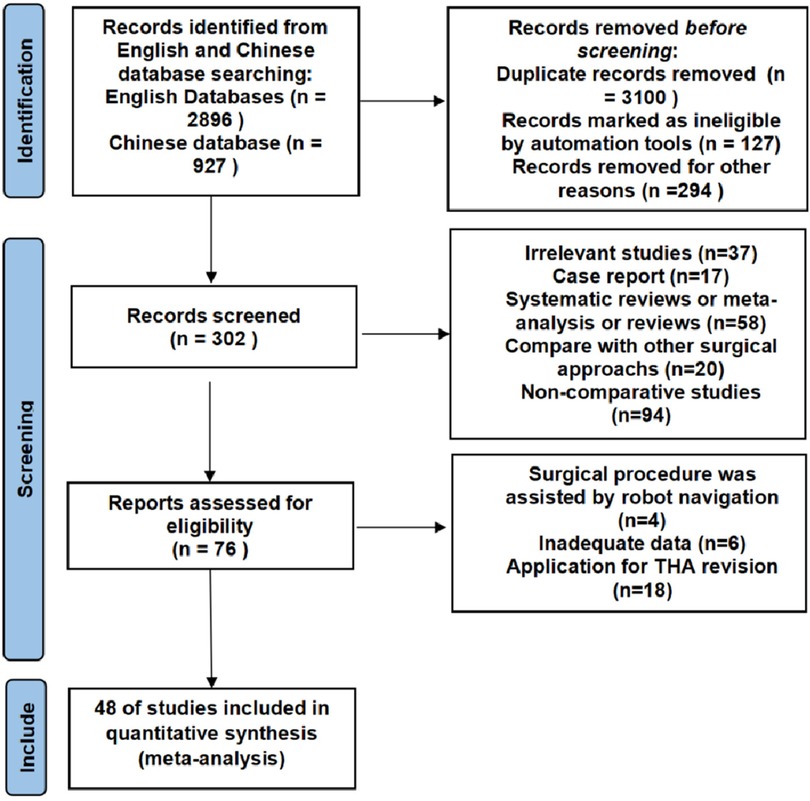
A total of 3,823 studies were initially identified, of which 3,579 were excluded due to duplication or irrelevance. An additional 226 articles were excluded after screening the titles and abstracts. Following full-text review, 48 English-language articles that met the inclusion criteria were selected for meta-analysis. The detailed search and screening process is listed in Figure 1.
Baseline characteristics of the included studies
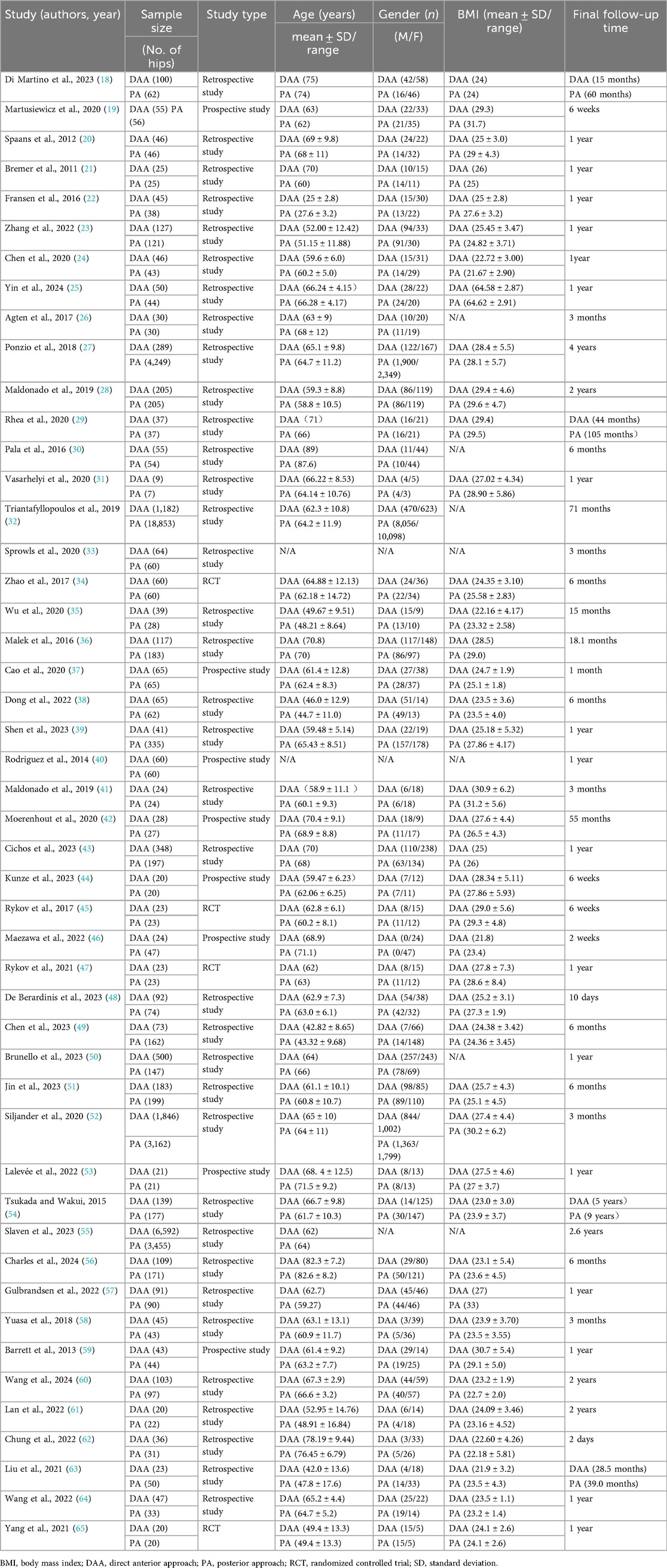
A total of 48 articles were included in the meta-analysis: 4 RCTs and 44 case–control studies, of which 8 were prospective and 36 were retrospective. These studies included data on 46,367 hips, with 13,285 in the DAA group and 33,082 in the PA group. The included studies were published between 2012 and 2024, with a maximum follow-up duration of 4 years. Detailed baseline characteristics are provided in Table 1.
Assessment of ROB in the included studies
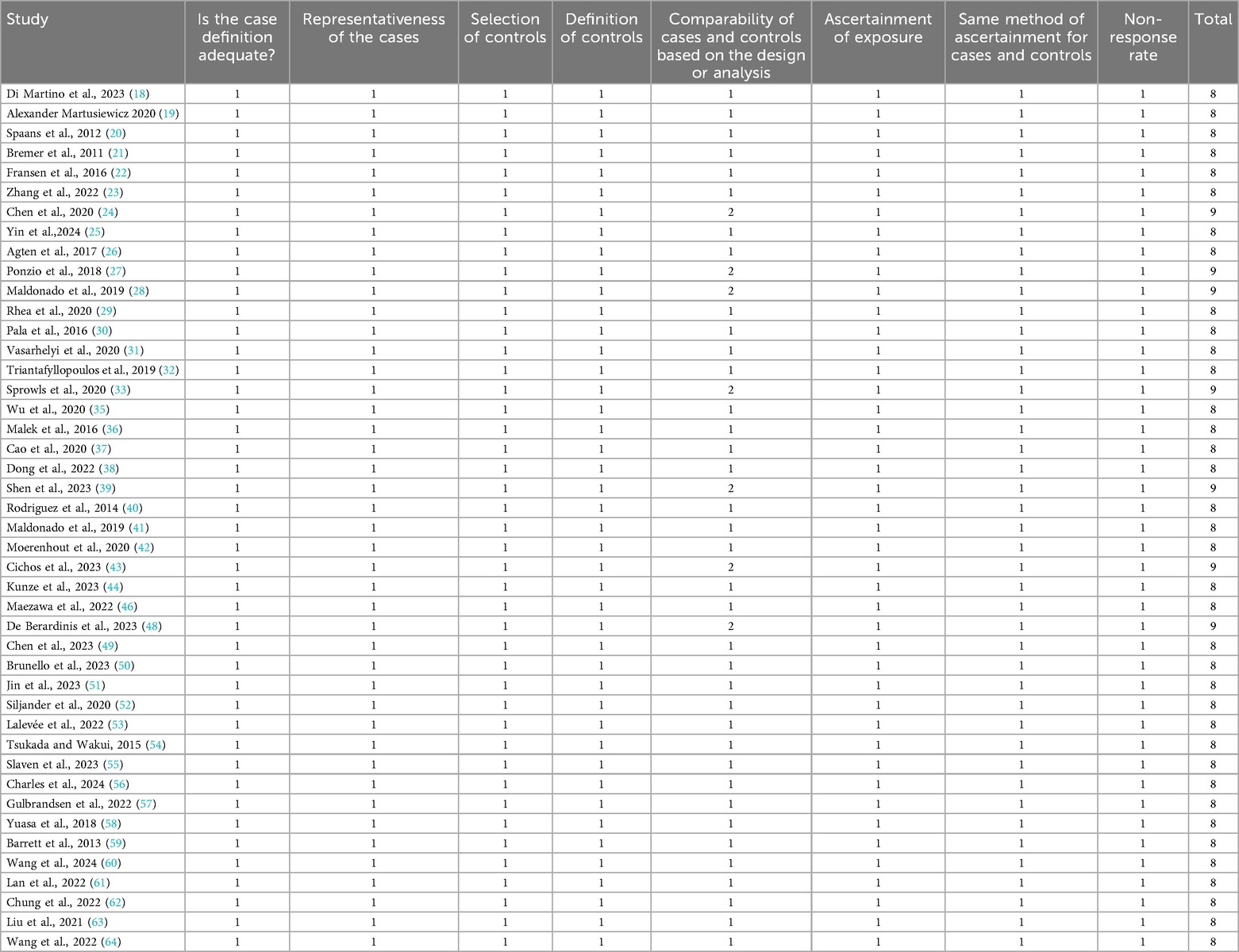
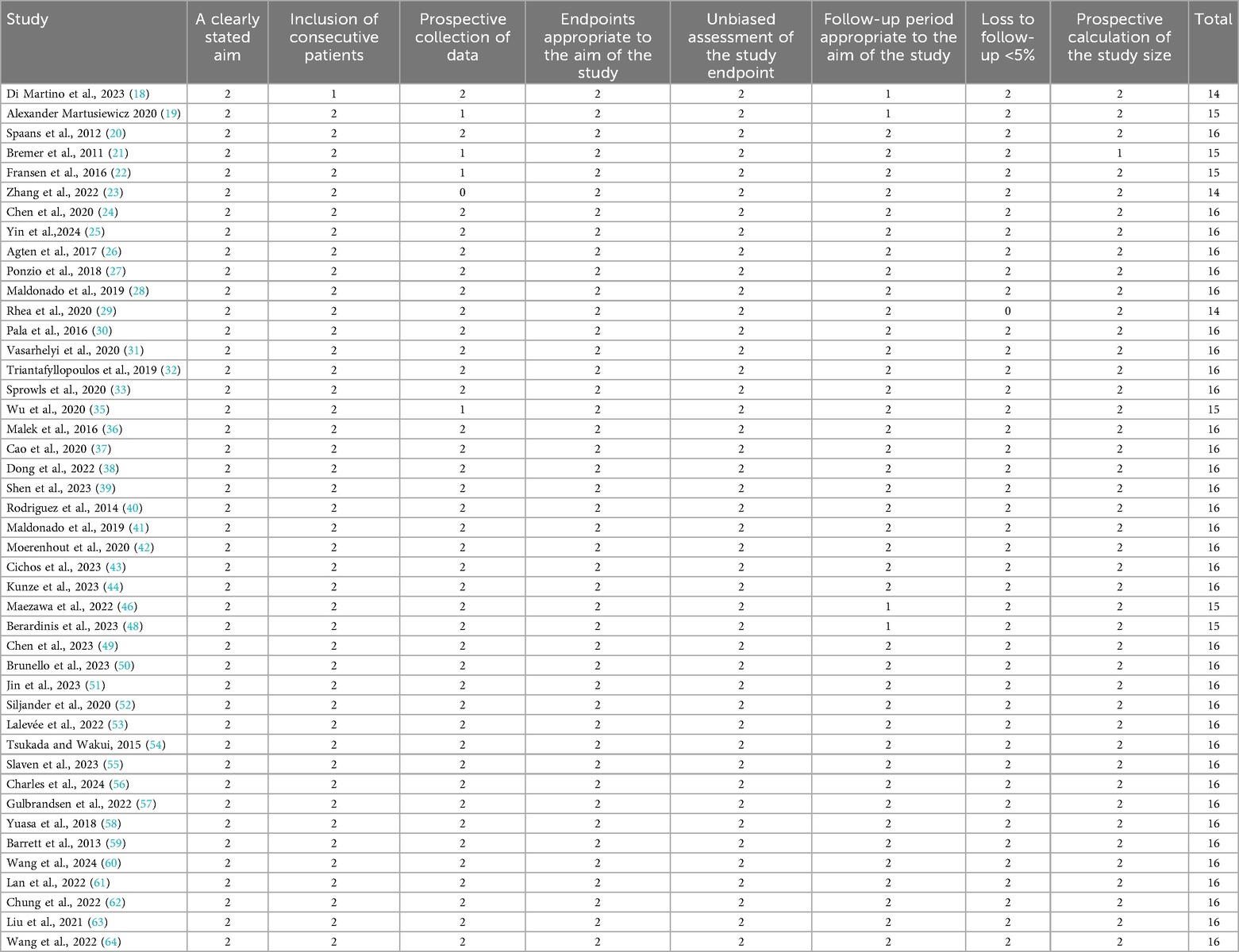
The quality of the included RCTs and case–control studies was evaluated by the Cochrane Collaboration tool, NOS, and MINORS. As shown in Figure 2, all four RCTs were assessed as high quality. Additionally, 44 case–control studies scored at least eight points on the NOS, suggesting relatively stable methodological quality (Tables 2, 3).
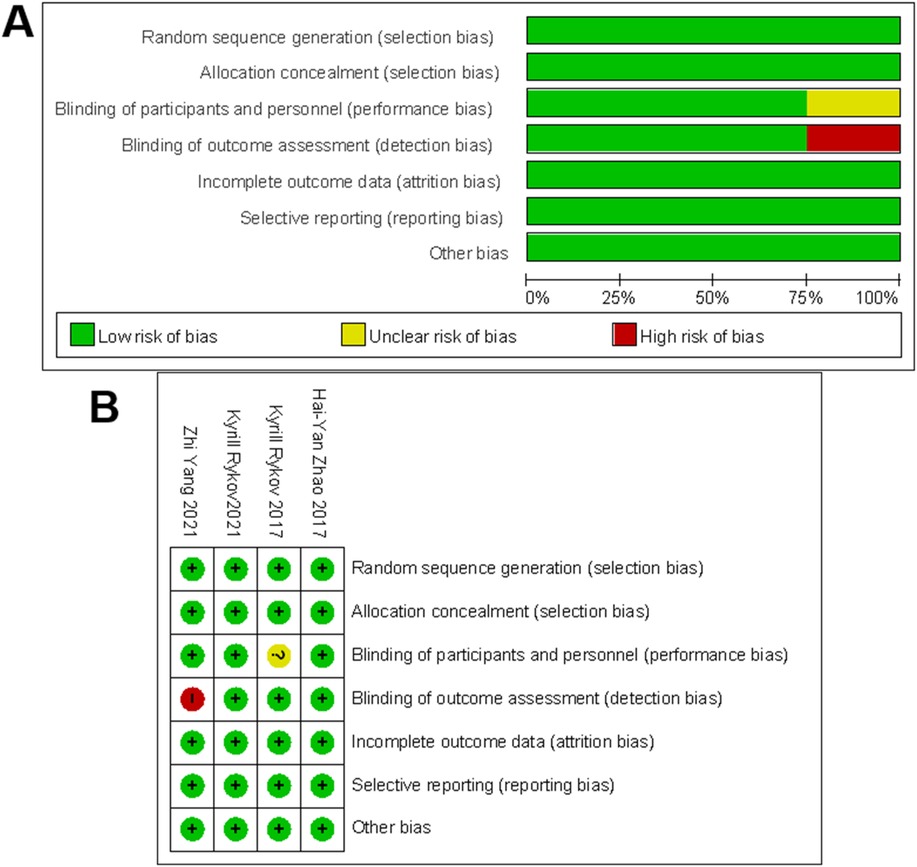
Figure 2. The methodological quality assessment for RCTs. (A) Risk-of-bias graph for included studies. (B) Risk-of-bias summary for included studies. +, no bias; −, bias; ?, bias unknown.
Surgical trauma
Blood transfusion rate
As shown in Figure 3A1, 12 articles (18, 22, 27, 29, 34, 37, 38, 43, 49, 50, 52, 57) evaluated the blood transfusion rate. Egger's test (Figure 3A2) indicated no publication bias (P > 0.05). The initial fixed-effects model revealed heterogeneity (I2 = 62%, P = 0.21). The study by Ponzio et al. (27) was identified as the primary source of heterogeneity due to significant sample size imbalance between groups and was excluded from further analysis. After exclusion, the fixed-effects model showed a significantly lower blood transfusion rate in the DAA group compared with that in the PA group [6.62% vs. 14.52%; I 2 = 42%, OR = 0.73, 95% confidence interval (CI): 0.59–0.91, P < 0.005].
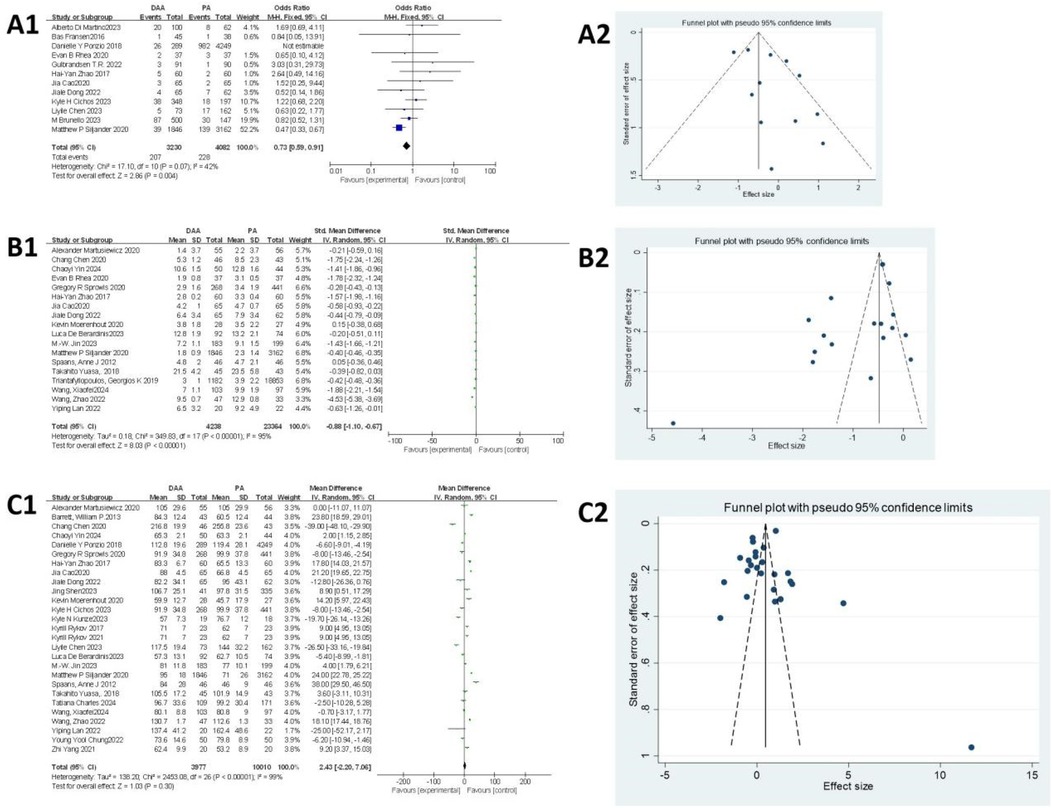
Figure 3. Comparison between DAA and PA in the surgical trauma-related subject for (A1, A2) blood transfusion rate, (B1, B2) hospital stay, and (C1, C2) surgery time. DAA, direct anterior approach; PA, posterior approach; Fixed, fixed-effects model; Random, random-effects model; M–H, Mantel–Haenszel; CI, confidence intervals; MD, mean difference.
Hospital stay
As shown in Figure 3B1, 18 articles (19, 20, 24, 25, 29, 32–34, 37, 38, 42, 48, 51, 52, 58, 60, 61, 64) assessed hospital stay. Egger's test (Figure 3B2) revealed significant publication bias (P < 0.05), primarily attributed to the studies by Chen et al. (24), Rhea et al. (29), Zhao et al. (34), Jin et al. (51), Wang et al. (60), and Wang et al. (64). After excluding these seven studies, heterogeneity was significantly reduced, allowing the use of a fixed-effects model. The results indicated that hospital stay was significantly shorter in the DAA group than that in the PA group (I2 = 36%, 95% CI: −0.43 to −0.36, P < 0.001).
Surgery time
As shown in Figure 3C1, surgery time was reported in 27 studies (19, 20, 24, 25, 27, 33, 34, 37–39, 42–45, 47–49, 51, 52, 56, 58–62, 64, 65), involving 13,987 hips (3,977 in the DAA group and 10,010 in the PA group). Egger's test (Figure 3C2) indicated no publication bias (P > 0.05). Due to significant heterogeneity in the fixed-effects model, subgroup analysis based on body mass index (BMI) was performed; however, this did not substantially reduce heterogeneity (Attachment 1). A random-effects model was therefore applied, revealing no significant difference in surgery time between the DAA and PA groups (I2 = 99%, 95% CI: −2.20 to 7.06, P = 0.30).
Muscle damage
MRI findings
Muscle damage around the hip joint was assessed using the Goutallier score (66), where a score of >2 was considered to indicate muscle damage according to previous reports (47). As shown in Figures 4A1–C1, four studies (21, 26, 45, 53) assessed muscle damage using MRI, including damage to the gluteus minimus, gluteus medius, and tensor fasciae latae muscles. For the gluteus minimus muscle damage, the fixed-effects model initially showed significant heterogeneity (I 2 = 76%, P = 0.02). After excluding the study by Rykov K, which was identified as the primary source of heterogeneity, the heterogeneity was reduced (I2 = 21%, P < 0.05). The final analysis showed that the DAA resulted in less muscle damage compared with the PA (36.84% vs. 65.79%, OR = 0.28; 95% CI: 0.14−0.56; P < 0.005). For the gluteus medius muscle damage, the fixed-effects model indicated no significant difference between the DAA and PA groups (OR = 1.20; 95% CI: 0.53–2.71; I2 = 0%, P = 0.66). For the tensor fasciae latae muscle damage, substantial heterogeneity was observed in the fixed-effects model (I 2 = 88%, P = 0.07). Therefore, a random-effects model was applied. The analysis showed no significant difference between the two approaches (OR = 0.40; 95% CI: 0.03−4.97; I2 = 88%, P = 0.48). Due to the small sample sizes, Egger's test could not be used to assess publication bias.
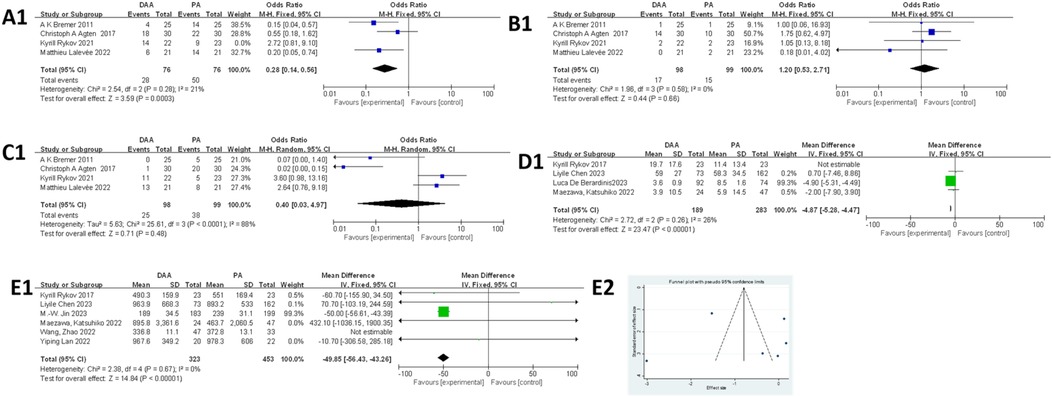
Figure 4. Comparison between DAA and PA in muscle damage-related factor for (A1) the glutes minimus damage on MRI, (B1) the gluteus medius damage on MRI, (C1) the tensor fasciae latae damage on MRI, and (D1) CRP and (E1, E2) CK levels. DAA, direct anterior approach; PA, posterior approach; Fixed, fixed-effects model; Random, random-effects model; M–H, Mantel–Haenszel; CI, confidence intervals; MD, mean difference.
CRP levels
As shown in Figure 4D1, four studies (45, 46, 48, 49) were included in the CRP level analysis. The fixed-effects model revealed significant heterogeneity (I2 = 72%, P < 0.00001). After excluding the study by Rykov et al., which was identified as the primary source of heterogeneity, the heterogeneity was substantially reduced (I2 = 26%, P < 0.00001). The final analysis, involving 472 hips (189 in the DAA group and 283 in the PA group), showed that CRP levels were significantly lower in the DAA group compared with those in the PA group [mean difference (MD) = −4.87; 95% CI: −5.28 to −4.47; P < 0.00001]. Due to the small sample size, Egger's test could not be used to assess publication bias.
CK levels
As shown in Figure 4E1, six studies (45, 46, 49, 51, 61, 64) were included in the meta-analysis of CK levels. Egger’s test (Figure 4E2) showed no evidence of publication bias (P > 0.05). The initial fixed-effects model indicated significant heterogeneity (I2 = 60%, P < 0.00001). After excluding the study by Wang et al. (64), identified as the main source of heterogeneity, the heterogeneity was eliminated (I2 = 0%, P < 0.00001). The final results showed that CK levels were lower in the DAA group compared with those in the PA group.
Complications
Infection
As shown in Figure 5A1, 11 studies (27, 36, 49, 51, 52, 54, 56–58, 63, 64) involving 6,628 hips (1,276 in the DAA group and 5,352 in the PA group) were included in the infection rate analysis. Egger's test (Figure 5A2) indicated publication bias (P < 0.05). In addition, the fixed-effects model showed low heterogeneity (I2 = 0%, P = 0.81), and there was no significant difference in infection rates between the two groups (OR = 0.92; 95% CI: 0.48–1.77; P = 0.81).
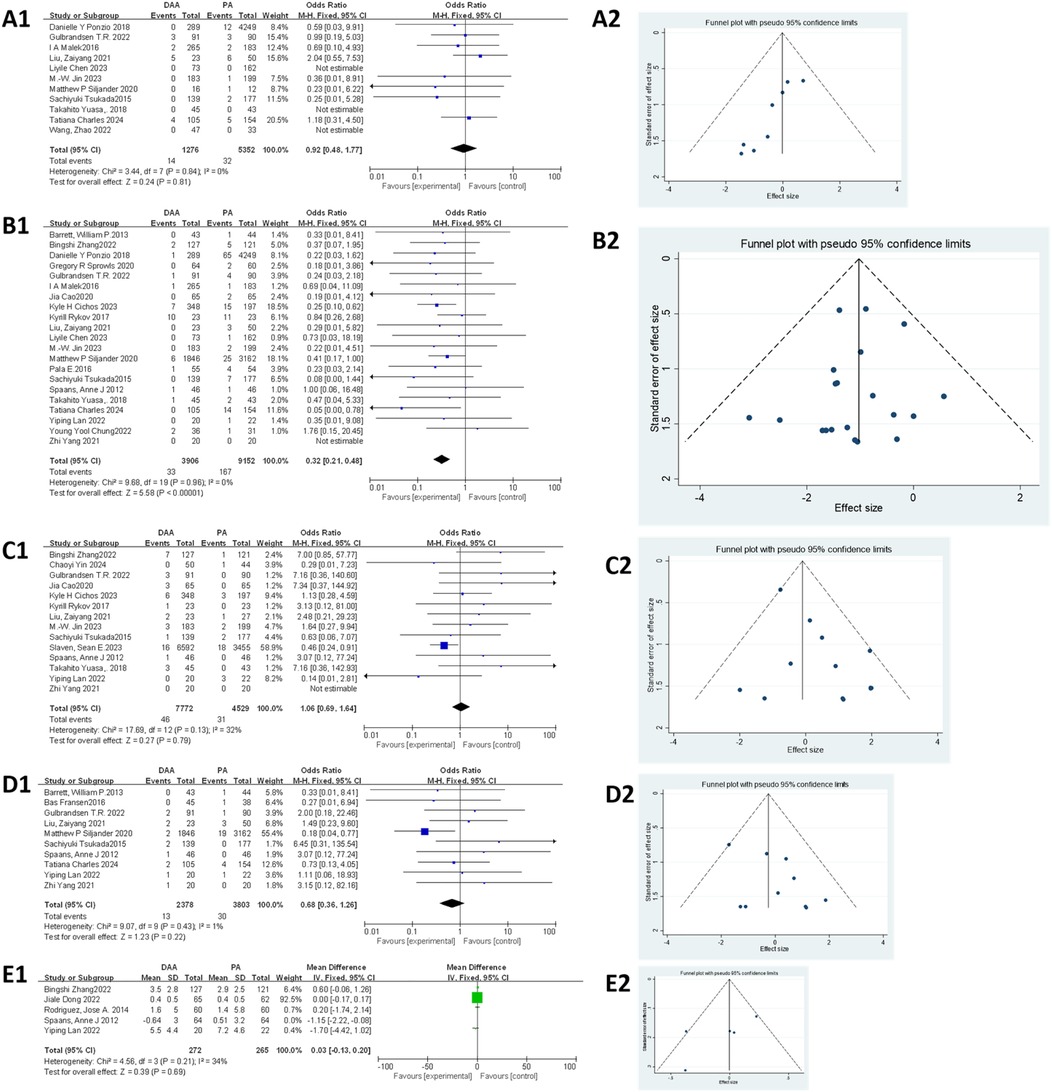
Figure 5. Comparison between DAA and PA in complications-related factor for (A1, A2) infection rate, (B1, B2) dislocation rate, (C1, C2) nerve injury rate, (D1, D2) intraoperative fracture rate, and (E1, E2) posterior leg length discrepancy. DAA, direct anterior approach; PA, posterior approach; Fixed, fixed-effects model; Random, random-effects model; M–H, Mantel–Haenszel; CI, confidence intervals; MD, mean difference.
Dislocation
As shown in Figure 5B1, 21 studies (20, 23, 27, 30, 33, 36, 37, 43, 45, 49, 51, 52, 54, 56–59, 61–63, 65) involving 13,058 hips (3,902 in the DAA group and 9,856 in the PA group) were analyzed for dislocation rates. Egger's test (Figure 5B2) showed no publication bias (P > 0.05). The fixed-effects model (I2 = 0%, P < 0.001) revealed that the dislocation rate was significantly lower in the DAA group compared with that in the PA group (0.84% vs. 1.82%; 95% CI: 0.20–0.48; P < 0.00001; I2 = 0%).
Nerve injury
As shown in Figure 5C1, 14 studies (20, 23, 25, 37, 43, 45, 51, 54, 55, 57, 58, 61, 63, 65) involving 12,301 hips (7,772 in the DAA group and 4,529 in the PA group) were included in the nerve injury analysis. Egger's test (Figure 5C2) indicated publication bias (P < 0.05). In addition, the fixed-effects model showed low heterogeneity (I2 = 32%, P = 0.79), and the analysis revealed no significant difference in nerve injury between the two groups (95% CI: 0.69–1.64; P = 0.79).
Intraoperative fracture
As shown in Figure 5D1, 10 studies (20, 22, 52, 54, 56, 57, 59–61, 65) involving 6,181 hips (2,378 in the DAA group and 3,803 in the PA group) were included in the meta-analysis. Egger's test (Figure 5D2) indicated no publication bias (P > 0.05). In addition, the fixed-effects model showed low heterogeneity (I2 = 1%, P = 0.22), and the results demonstrated no significant difference in intraoperative fracture rates between the DAA and PA groups (95% CI: 0.36–1.26; P = 0.22).
Leg length discrepancy
As shown in Figure 5E1, five studies (20, 23, 38, 40, 61) involving 665 hips (336 in the DAA group and 329 in the PA group) were included in the meta-analysis. Egger's test (Figure 5E2) indicated no publication bias (P > 0.05). In addition, the fixed-effects model initially showed moderate heterogeneity (I2 = 56%, P = 0.95), which was significantly reduced after excluding the study by Spaans et al. (20) (I2 = 34%, P = 0.69). The final results showed no significant difference in postoperative leg length discrepancy between the DAA and PA groups (95% CI: −0.13 to 0.20; P = 0.69).
Function scores
HHS
As shown in Figure 6A1, 10 studies (23, 34, 35, 37, 40, 42, 51, 59, 64, 65) were eligible for this meta-analysis. Egger's test (Figure 6A2) indicated no publication bias (P > 0.05). Due to significant heterogeneity in the fixed-effects model (I2 = 99%, P < 0.00001), subgroup analysis based on BMI was performed but did not reduce heterogeneity (Attachment 2). A random-effects model was applied, and the results indicated that the DAA group had significantly higher HHS compared with that in the PA group (MD = 3.07, 95% CI: 0.08–6.07; I2 = 99%, P < 0.05).
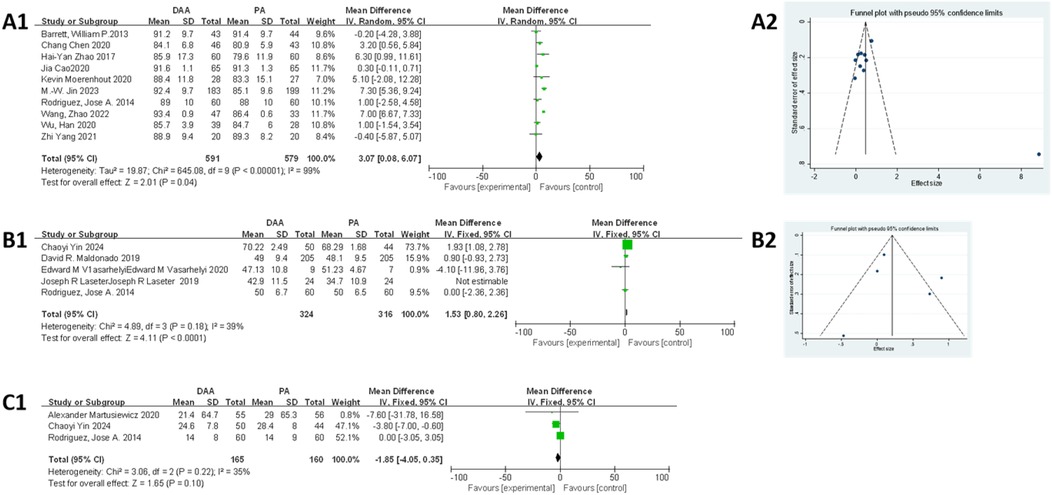
Figure 6. Comparison between DAA and PA in function score for (A1, A2) postoperative HHS score, (B1, B2) postoperative SF score, (C1) time to discontinuation of a walker after surgery. DAA, direct anterior approach; PA, posterior approach; Fixed, fixed-effects model; Random, random-effects model; M–H, Mantel–Haenszel; CI, confidence intervals; MD, mean difference.
SF score
As shown in Figure 6B1, five studies (25, 28, 31, 40, 41) were included in the meta-analysis. Egger's test (Figure 6B2) indicated no publication bias (P > 0.05). However, the fixed-effects model showed moderate heterogeneity (I2 = 56%, P < 0.00001), which was clearly reduced after excluding the study by Maldonado et al. (41) (I2 = 39%, P < 0.00001). The final results showed that patients in the DAA group had significantly higher SF scores compared with those in the PA group (MD = 1.53, 95% CI: 0.80–2.26; P < 0.01).
Time to discontinuation of a walker after surgery
As shown in Figure 6C1, three studies (19, 25, 40) were included in this meta-analysis. The fixed-effects model demonstrated low heterogeneity (I2 = 35%), and the results showed no significant difference between the DAA and PA groups in terms of time to discontinuation of a walker postoperatively (MD = −1.85, 95% CI: −4.05 to 0.35; P = 0.10). Due to the small sample size, Egger's test could not be used to assess publication bias.
Discussion
This meta-analysis compared ERAS-related outcomes between the DAA and PA in THA. The results showed that the DAA group had a lower blood transfusion rate, shorter hospital stay, reduced gluteus minimus muscle damage on MRI, lower postoperative levels of CK and CRP, and significantly higher HHS and SF scores. Additionally, the DAA group had a lower dislocation rate compared with that in the PA group. However, no significant differences were found in surgery time, damage to the gluteus medius and tensor fasciae latae muscles on MRI, time to discontinuation of a walker after surgery, or complication rates (infection, nerve injury, intraoperative fracture, and postoperative leg length discrepancy).
ERAS can reduce surgical trauma, minimize postoperative complications, and promote faster recovery (67). Muscle damage indicators such as MRI-based Goutallier score and serum CK (68) and CRP (60) levels have been widely used to assess perioperative soft tissue injury (59, 63, 68). In the present analysis, the DAA demonstrated less gluteus minimus muscle damage and lower postoperative CK and CRP levels, highlighting its minimally invasive feature. No significant differences were observed in gluteus medius or tensor fasciae latae muscle damage, which may be attributed to the fact that both approaches have minimal involvement with these muscles. Although previous studies suggested that the DAA caused greater damage to the tensor fasciae latae and required longer surgery time (5), more recent evidence suggests that surgeons' experience can significantly reduce this impact, especially after overcoming the learning curve (69). In addition, variations in healthcare systems, rehabilitation protocols, and patient populations may contribute to discrepancies in hospitalization duration. Collectively, these findings indicate that the DAA, with less muscle injury, is associated with a lower blood transfusion rate and reduced length of hospital stay.
This meta-analysis evaluated the rate of complications, including infection, dislocation, nerve injury, intraoperative fracture, and postoperative leg length discrepancy, in the DAA and PA groups. Among these, the DAA showed a significant advantage only in the dislocation rate. While some previous studies reported a higher rate of femoral fractures with the DAA, our meta-analysis found no significant difference between the two groups. It could be related to surgeons' proficiency and the occurrence of minor proximal femoral cleavages that do not compromise the stability of the prosthesis, which also commonly occur within the PA. Regarding nerve injury, the impact of both approaches on nerve damage was inconsistent. It is generally believed that the DAA approach is more likely to cause lateral femoral cutaneous nerve injury, while the PA approach is more associated with sciatic nerve injury, resulting in no significant difference in nerve injury rates between the two approaches. Additionally, it was previously thought that the DAA provided better control over leg length due to its performance in the supine position (67, 70). However, with the widespread adoption of the DAA in the lateral decubitus position, this advantage has diminished (71). Therefore, the transition from the supine to the lateral decubitus position may account for the loss of DAA's advantage in leg length control.
Functional outcomes, such as the HHS, SF score, and time to discontinuation of a walker after surgery, were closely related to surgical trauma, muscle damage, and postoperative complications. The DAA, with its lower dislocation rate and reduced muscle damage, resulted in better functional scores, especially in pain relief and mental improvement. The DAA group also had significantly higher SF scores compared with those in the PA group, which is likely due to less muscle damage, lower dislocation rates, and shorter hospital stay. However, these significant differences may still not necessarily translate into meaningful real-world benefits for patients.
Several limitations should be considered in this meta-analysis. (1) Only four RCTs were included, resulting in a relatively low level of evidence. (2) Methodological inconsistencies across the included studies led to data deviations that may have affected the findings. (3) The analysis did not account for surgeons' proficiency due to the absence of a learning curve evaluation. Learning curves are an important factor, as previous studies (72) have shown that even experienced surgeons can experience a significant increase in surgical time and postoperative complications during the early stages of learning. Unfortunately, studies comparing learning curves between the DAA and PA were lacking and therefore not included in this analysis. (4) High heterogeneity in the measurement of some outcomes may have affected the results, potentially due to publication bias and selection bias. Future research should adopt stricter inclusion criteria and standardized outcome reporting to reduce heterogeneity and improve the reliability of findings. (5) The two approaches were not the same in terms of muscle injury. The DAA was performed between the tensor fasciae latae and sartorius, making significant damage to the gluteus medius and minimus very unlikely. Similarly, the PA passes through the gluteus maximus, meaning damage to the gluteus medius and minimus was also not expected. So the future studies should focus on assessing potential damage to the gluteus maximus and tensor fasciae latae.
Conclusion
Based on the results of our meta-analysis, the DAA demonstrated the advantages of minimally invasive surgery, including less muscle damage, fewer postoperative complications, and better functional outcomes compared with the PA in the context of ERAS protocols. Therefore, we recommend that surgeons consider adopting the DAA in ERAS protocols, provided that the patient meets the surgical criteria for this approach.
Author contributions
WX: Writing – original draft, Writing – review & editing. JLa: Writing – review & editing. JLi: Writing – review & editing. ZZ: Writing – review & editing. XW: Writing – review & editing. ZC: Writing – review & editing. XH: Writing – review & editing. NC: Writing – review & editing. YX: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (Grant number: 82260427); Science and Technology Plan Project of Yunnan Province Technology Hall (Grant number: 202301AT070134); Yunnan Revitalization Talent Support Program (grant number: XDYC-QNRC-2023-0198); Yunnan Province medical discipline reserve talent project (grant number: H-2024030); and PhD Research Fund Project of the First Affiliated Hospital of Kunming Medical University (grant number: 2021BS016).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ackerman IN, Bohensky MA, Zomer E, Tacey M, Gorelik A, Brand CA, et al. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskelet Disord. (2019) 20(1):90. doi: 10.1186/s12891-019-2411-9
2. Supra R, Supra R, Agrawal DK. Surgical approaches in total hip arthroplasty. J Orthop Sports Med. (2023) 5(2):232–40. doi: 10.26502/josm.511500106
3. Hagio K, Aikawa K. Minimally invasive surgery supercapsular percutaneously-assisted total hip (SuperPath) arthroplasty. Acta Biomed. (2023 ) 94(3):e2023069. doi: 10.23750/abm.v94i3.139225
4. Röttinger H. Minimalinvasiver zugang zum hüftgelenk (OCM) zur implantation von hüftendoprothesen [minimally invasive anterolateral approach for total hip replacement (OCM technique)]. Oper Orthop Traumatol. (2010) 22(4):421–30. doi: 10.1007/s00064-010-8035-8
5. Nogler M, Randelli F, Macheras GA, Thaler M. Hemiarthroplasty of the hip using the direct anterior approach. Oper Orthop Traumatol. (2021) 33(4):304–17. doi: 10.1007/s00064-021-00727-6
6. Faldini C, Perna F, Mazzotti A. Direct anterior approach versus posterolateral approach in total hip arthroplasty: effects on early post-operative rehabilitation period. J Biol Regul Homeost Agents. (2017) 31(4 suppl 1):75–81.29185307
7. Sun X, Zhao X, Zhou L, Su Z. Direct anterior approach versus posterolateral approach in total hip arthroplasty: a meta-analysis of results on early post-operative period. J Orthop Surg Res. (2021) 16(1):69. doi: 10.1186/s13018-021-02218-7
8. Ang JJM, Onggo JR, Stokes CM, Ambikaipalan A. Comparing direct anterior approach versus posterior approach or lateral approach in total hip arthroplasty: a systematic review and meta-analysis. Eur J Orthop Surg Traumatol. (2023) 33(7):2773–92. doi: 10.1007/s00590-023-03528-8
9. Jin Z, Wang L, Qin J, Hu H, Wei Q. Direct anterior approach versus posterolateral approach for total hip arthroplasty in the treatment of femoral neck fractures in elderly patients: a meta-analysis and systematic review. Ann Med. (2023) 55(1):1378–92. doi: 10.1080/07853890.2023.2193424
10. Zhou Z, Li Y, Peng Y, Jiang J, Zuo J. Clinical efficacy of direct anterior approach vs. other surgical approaches for total hip arthroplasty: a systematic review and meta-analysis based on RCTs. Front Surg. (2022) 9:1022937. doi: 10.3389/fsurg.2022.1022937
11. Ramadanov N, Bueschges S, Liu K, Lazaru P, Marintschev I. Direct and indirect comparisons in network meta-analysis of SuperPATH, direct anterior and posterior approaches in total hip arthroplasty. Sci Rep. (2022) 12(1):16778. doi: 10.1038/s41598-022-20242-3
12. Yang XT, Huang HF, Sun L, Yang Z, Deng CY, Tian XB, et al. Direct anterior approach versus posterolateral approach in total hip arthroplasty: a systematic review and meta-analysis of randomized controlled studies. Orthop Surg. (2020) 12(4):1065–73. doi: 10.1111/os.12669
13. Huang XT, Liu DG, Jia B, Xu YX. Comparisons between direct anterior approach and lateral approach for primary total hip arthroplasty in postoperative orthopaedic complications: a systematic review and meta-analysis. Orthop Surg. (2021) 13(6):1707–20. doi: 10.1111/os.13101
14. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
15. Cook DA, Reed DA. Appraising the quality of medical education research methods: the medical education research study quality instrument and the Newcastle-Ottawa scale-education. Acad Med. (2015) 90(8):1067–76. doi: 10.1097/ACM.0000000000000786
16. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
17. Higgins JP, Altman DG, Gøtzsche PC. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
18. Di Martino A, Pederiva D, Brunello M. Outcomes of direct anterior approach for uncemented total hip replacement in medial femoral neck fractures: a retrospective comparative study on the first 100 consecutive patients. BMC Musculoskelet Disord. (2023) 24(1):776. doi: 10.1186/s12891-023-06919-4
19. Martusiewicz A, Delagrammaticas D, Harold RE, Bhatt S, Beal MD, Manning DW, et al. Anterior versus posterior approach total hip arthroplasty: patient-reported and functional outcomes in the early postoperative period. Hip Int. (2020) 30(6):695–702. doi: 10.1177/1120700019881413
20. Spaans AJ, van den Hout JA, Bolder SB. High complication rate in the early experience of minimally invasive total hip arthroplasty by the direct anterior approach. Acta Orthop. (2012) 83(4):342–6. doi: 10.3109/17453674.2012.711701
21. Bremer AK, Kalberer F, Pfirrmann CW, Dora C. Soft-tissue changes in hip abductor muscles and tendons after total hip replacement: comparison between the direct anterior and the transgluteal approaches. J Bone Joint Surg Br. (2011) 93(7):886–9. doi: 10.1302/0301-620X.93B7.25058
22. Fransen B, Hoozemans M, Vos S. Direct anterior approach versus posterolateral approach in total hip arthroplasty : one surgeon, two approaches. Acta Orthop Belg. (2016) 82(2):240–8.27831458
23. Zhang B, Liu S, Liu Z. Clinical and radiologic outcomes in patients undergoing primary total hip arthroplasty with collum femoris preserving stems: a comparison between the direct anterior approach and the posterior approach. BMC Musculoskelet Disord. (2022) 23(1):77. doi: 10.1186/s12891-022-05040-2
24. Chen C, Yin Y, Juncai L, Chen G. The direct anterior approach for simultaneous bilateral total hip arthroplasty: a short-term efficacy analysis. Arthroplasty. (2020) 2(1):21. doi: 10.1186/s42836-020-00040-w
25. Yin C, Wen H, Chen Z, Zhang B. Exploring the clinical value of direct anterior approach THA for short-term hip function improvement: a single-center retrospective analysis of short-term outcomes. Medicine (Baltimore. (2024) 103(24):e38479. doi: 10.1097/MD.0000000000038479
26. Agten CA, Sutter R, Dora C, Pfirrmann CW. MR Imaging of soft tissue alterations after total hip arthroplasty: comparison of classic surgical approaches. Eur Radiol. (2017) 27(3):1312–21. doi: 10.1007/s00330-016-4455-7
27. Ponzio DY, Poultsides LA, Salvatore A, Lee YY, Memtsoudis SG, Alexiades MM, et al. In-hospital morbidity and postoperative revisions after direct anterior vs posterior total hip arthroplasty. J Arthroplasty. (2018) 33(5):1421–1425.e1. doi: 10.1016/j.arth.2017.11.053
28. Maldonado DR, Kyin C, Walker-Santiago R. Direct anterior approach versus posterior approach in primary total hip replacement: comparison of minimum 2-year outcomes. Hip Int. (2021) 31(2):166–73. doi: 10.1177/1120700019881937
29. Rhea EB, Iman DJ, Wilke BK, Sherman CE, Ledford CK, Blasser KE, et al. A crossover cohort of direct anterior vs posterolateral approach in primary total hip arthroplasty: what does the patient prefer? Arthroplast Today. (2020) 6(4):792–5. doi: 10.1016/j.artd.2020.07.015
30. Pala E, Trono M, Bitonti A, Lucidi G. Hip hemiarthroplasty for femur neck fractures: minimally invasive direct anterior approach versus postero-lateral approach. Eur J Orthop Surg Traumatol. (2016) 26(4):423–7. doi: 10.1007/s00590-016-1767-x
31. Vasarhelyi EM, Williams HA, Howard JL, Petis S, Barfett J, Lanting BA, et al. The effect of total hip arthroplasty surgical technique on postoperative muscle atrophy. Orthopedics. (2020) 43(6):361–6. doi: 10.3928/01477447-20200910-01
32. Triantafyllopoulos GK, Memtsoudis SG, Wang H, Ma Y, Alexiades MM, Poultsides LA, et al. Surgical approach does not affect deep infection rate after primary total hip arthroplasty. Hip Int. (2019) 29(6):597–602. doi: 10.1177/1120700018825237
33. Sprowls GR, Allen BC, Lundquist KF, Sager LN, Barnett CD. Incision site fat thickness and 90-day complications for direct anterior and posterior approach total hip arthroplasty. Hip Int. (2022) 32(4):431–7. doi: 10.1177/1120700020977166
34. Zhao HY, Kang PD, Xia YY, Shi XJ, Nie Y, Pei FX, et al. Comparison of early functional recovery after total hip arthroplasty using a direct anterior or posterolateral approach: a randomized controlled trial. J Arthroplasty. (2017) 32(11):3421–8. doi: 10.1016/j.arth.2017.05.056
35. Wu H, Cheng WD, Jing J. Total hip arthroplasty by direct anterior approach in the lateral position for the treatment of ankylosed hips. Eur J Orthop Surg Traumatol. (2020) 30(6):993–1001. doi: 10.1007/s00590-020-02655-w
36. Malek IA, Royce G, Bhatti SU. A comparison between the direct anterior and posterior approaches for total hip arthroplasty: the role of an ‘enhanced recovery’ pathway. Bone Joint J. (2016) 98-B(6):754–60. doi: 10.1302/0301-620X.98B6.36608
37. Cao J, Zhou Y, Xin W. Natural outcome of hemoglobin and functional recovery after the direct anterior versus the posterolateral approach for total hip arthroplasty: a randomized study. J Orthop Surg Res. (2020) 15(1):200. doi: 10.1186/s13018-020-01716-4
38. Dong J, Kong L, Zhang S. Conversion of a fused or ankylosed hip to total hip arthroplasty: is the direct anterior approach in the lateral decubitus position an ideal solution? Front Surg. (2022) 9:819530. doi: 10.3389/fsurg.2022.819530
39. Shen J, Ji R, Yao S. Direct anterior approach provides superior prosthesis adaptability in the early postoperative period of total hip arthroplasty. Orthop Surg. (2023) 15(3):679–86. doi: 10.1111/os.13640
40. Rodriguez JA, Deshmukh AJ, Rathod PA. Does the direct anterior approach in THA offer faster rehabilitation and comparable safety to the posterior approach? Clin Orthop Relat Res. (2014) 472(2):455–63. doi: 10.1007/s11999-013-3231-0
41. Maldonado DR, Laseter JR, Kyin C, Lall AC, Domb BG. Direct anterior approach in total hip arthroplasty leads to superior outcomes at 3-month follow-up when compared with the posterior approach: a matched study using propensity score analysis. J Am Acad Orthop Surg Glob Res Rev. (2019) 3(12):e19. doi: 10.5435/JAAOSGlobal-D-19-00118
42. Moerenhout K, Derome P, Laflamme GY, Leduc S, Gaspard HS, Benoit B, et al. Direct anterior versus posterior approach for total hip arthroplasty: a multicentre, prospective, randomized clinical trial. Can J Surg. (2020) 63(5):E412–7. doi: 10.1503/cjs.012019
43. Cichos KH, McGwin G Jr, Boyd B, Arthroplasty for Hip Fracture Consortium, Ghanem ES. Direct anterior approach total hip arthroplasty is associated with reduced 1-year mortality and surgical complications after femoral neck fracture. J Arthroplasty. (2023) 38(11):2347–2354.e2. doi: 10.1016/j.arth.2023.05.045
44. Kunze KN, McLawhorn AS, Jules-Elysee KM. Effect of anterior approach compared with posterolateral approach on readiness for discharge and thrombogenic markers in patients undergoing unilateral total hip arthroplasty: a prospective cohort study. Arch Orthop Trauma Surg. (2023) 143(4):2217–26. doi: 10.1007/s00402-022-04484-4
45. Rykov K, Reininga IHF, Sietsma MS, Knobben BAS, Ten Have BLEF. Posterolateral vs direct anterior approach in total hip arthroplasty (POLADA trial): a randomized controlled trial to assess differences in serum markers. J Arthroplasty. (2017) 32(12):3652–3658.e1. doi: 10.1016/j.arth.2017.07.008
46. Maezawa K, Nozawa M, Gomi M, Sugimoto M, Maruyama Y. Changes in serum creatine kinase and C-reactive protein after posterior and direct anterior approaches in total hip arthroplasty. Hip Int. (2022) 32(5):591–5. doi: 10.1177/1120700020978643
47. Rykov K, Meys TWGM, Knobben BAS, Sietsma MS, Reininga IHF, Ten Have BLEF, et al. MRI assessment of muscle damage after the posterolateral versus direct anterior approach for THA (Polada Trial). A randomized controlled trial. J Arthroplasty. (2021) 36(9):3248–3258.e1. doi: 10.1016/j.arth.2021.05.009
48. De Berardinis L, Senarighi M, Farinelli L. In primary total hip arthroplasty, the direct anterior approach leads to higher levels of creatine kinase and lower levels of C-reactive protein compared with the posterolateral approach: a propensity score matching analysis of short-term follow-up data. J Orthop Surg Res. (2023) 18(1):594. doi: 10.1186/s13018-023-04084-x
49. Chen L, Sun S, Wang Q, Bahete A, Cai L, Kang P, et al. Comparison of perioperative outcomes and early complications between a direct anterior approach or posterolateral approach in simultaneous bilateral total hip arthroplasty: a retrospective study. HSS J. (2023) 19(2):172–9. doi: 10.1177/15563316221145688
50. Brunello M, Di Martino A, Ruta F. Which patient benefit most from minimally invasive direct anterior approach total hip arthroplasty in terms of perioperative blood loss? A retrospective comparative study from a cohort of patients with primary degenerative hips. Musculoskelet Surg. (2023) 107(4):431–7. doi: 10.1007/s12306-023-00792-z
51. Jin MW, Zhang L, Chu XB. Comparison of clinical efficacy between direct anterior approach and posterolateral approach in primary total hip arthroplasty. Eur Rev Med Pharmacol Sci. (2023) 27(12):5604–13. doi: 10.26355/eurrev_202306_32799
52. Siljander MP, Whaley JD, Koueiter DM, Alsaleh M, Karadsheh MS. Length of stay, discharge disposition, and 90-day complications and revisions following primary total hip arthroplasty: a comparison of the direct anterior, posterolateral, and direct superior approaches. J Arthroplasty. (2020) 35(6):1658–61. doi: 10.1016/j.arth.2020.01.082
53. Lalevée M, Curado J, Matsoukis J. Comparative MRI assessment of three minimally invasive approaches in total hip arthroplasty. Orthop Traumatol Surg Res. (2022) 108(6):103354. doi: 10.1016/j.otsr.2022.103354
54. Tsukada S, Wakui M. Lower dislocation rate following total hip arthroplasty via direct anterior approach than via posterior approach: five-year-average follow-up results. Open Orthop J. (2015) 9:157–62. doi: 10.2174/1874325001509010157
55. Slaven SE, Ho H, Sershon RA, Fricka KB, Hamilton WG. Motor nerve palsy after direct anterior versus posterior total hip arthroplasty: incidence, risk factors, and recovery. J Arthroplasty. (2023) 38(7S):S242–6. doi: 10.1016/j.arth.2023.03.086
56. Charles T, Bloemers N, Kapanci B, Jayankura M. Complication rates after direct anterior vs posterior approach for hip hemiarthroplasty in elderly individuals with femoral neck fractures. World J Orthop. (2024) 15(1):22–9. doi: 10.5312/wjo.v15.i1.22
57. Gulbrandsen TR, Muffly SA, Shamrock A. Total hip arthroplasty: direct anterior approach versus posterior approach in the first year of practice. Iowa Orthop J. (2022) 42(1):127–36.35821938
58. Yuasa T, Maezawa K, Sato H, Maruyama Y, Kaneko K. Safely transitioning to the direct anterior from posterior approach for total hip arthroplasty. J Orthop. (2018) 15(2):420–3. doi: 10.1016/j.jor.2018.03.013
59. Barrett WP, Turner SE, Leopold JP. Prospective randomized study of direct anterior vs postero-lateral approach for total hip arthroplasty. J Arthroplasty. (2013 Oct) 28(9):1634–8. doi: 10.1016/j.arth.2013.01.034
60. Wang X, Dai J, Wu Z. Direct anterior approach total hip arthroplasty for femoral neck fractures in the lateral position. Clin Interv Aging. (2024) 19:883–9. doi: 10.2147/CIA.S458179
61. Lan Y, Feng E, Lin B, Lu Z, Lin F, Weng Y, et al. Correction: direct anterior versus posteriorlateral approachs for clinical outcomes after total hip arthroplasty in the treatment of severe DDH. BMC Musculoskelet Disord. (2022) 23(1):1034. doi: 10.1186/s12891-022-06011-3
62. Chung YY, Lee SM, Baek SN, Park TG. Direct anterior approach for total hip arthroplasty in the elderly with femoral neck fractures: comparison with conventional posterolateral approach. Clin Orthop Surg. (2022) 14(1):35–40. doi: 10.4055/cios21008
63. Liu Z, Bell CD, Ong AC, Zhang J, Li J, Zhang Y, et al. Clinical evaluation of direct anterior approach total hip arthroplasty for severe developmental dysplasia of the hip. Sci Rep. (2021) 11(1):8105. doi: 10.1038/s41598-021-87543-x
64. Wang Z, Bao HW, Hou JZ. The direct anterior approach versus the posterolateral approach on the outcome of total hip arthroplasty: a retrospective clinical study. Orthop Surg. (2022) 14(10):2563–70. doi: 10.1111/os.13444
65. Yang Z, Feng S, Guo KJ, Zha GC. Patient-reported results of simultaneous direct anterior approach and posterolateral approach total hip arthroplasties performed in the same patients. J Orthop Traumatol. (2021) 22(1):46. doi: 10.1186/s10195-021-00611-w
66. Engelken F, Wassilew GI, Köhlitz T. Assessment of fatty degeneration of the gluteal muscles in patients with THA using MRI: reliability and accuracy of the Goutallier and quartile classification systems. J Arthroplasty. (2014) 29(1):149–53. doi: 10.1016/j.arth.2013.04.045
67. Zhu S, Qian W, Jiang C, Ye C, Chen X. Enhanced recovery after surgery for hip and knee arthroplasty: a systematic review and meta-analysis. Postgrad Med J. (2017) 93(1106):736–42. doi: 10.1136/postgradmedj-2017-134991
69. Nairn L, Gyemi L, Gouveia K, Ekhtiari S, Khanna V. The learning curve for the direct anterior total hip arthroplasty: a systematic review. Int Orthop. (2021) 45(8):1971–82. doi: 10.1007/s00264-021-04986-7
70. Tassinari L, Di Martino A, Brunello M, Rossomando V, Traina F, Faldini C, et al. Leg length discrepancy after total hip arthroplasty performed by direct anterior approach: a systematic review comparing surgical approaches and strategies for prevention. EFORT Open Rev. (2024) 9(8):733–44. doi: 10.1530/EOR-23-0116
71. Camenzind RS, Stoffel K, Lash NJ, Beck M. Direct anterior approach to the hip joint in the lateral decubitus position for joint replacement. Oper Orthop Traumatol. (2018) 30(4):276–85. doi: 10.1007/s00064-018-0550-z
72. Peters RM, Ten Have BLEF, Rykov K, Van Steenbergen L, Putter H, Rutgers M, et al. The learning curve of the direct anterior approach is 100 cases: an analysis based on 15,875 total hip arthroplasties in the Dutch Arthroplasty Register. Acta Orthop. (2022) 93:775–82. doi: 10.2340/17453674.2022.4802
Keywords: direct anterior approach, posterior approach, total hip arthroplasty, enhanced recovery after surgery, meta-analysis
Citation: Xu W, Lao J, Liu J, Zhang Z, Wan X, Chen Z, Huang X, Chen N and Xu Y (2025) Comparison of direct anterior vs. posterior approach in primary total hip arthroplasty: a systematic review and meta-analysis on enhanced recovery after surgery. Front. Surg. 12:1586187. doi: 10.3389/fsurg.2025.1586187
Received: 2 March 2025; Accepted: 9 October 2025;
Published: 6 November 2025.
Edited by:
Michela Saracco, University of Naples Federico II, ItalyReviewed by:
Alberto Fioruzzi, Gaetano Pini Specialist Orthopedic Trauma Center, ItalyNikolai Ramadanov, Brandenburg Medical School Theodor Fontane, Germany
Copyright: © 2025 Xu, Lao, Liu, Zhang, Wan, Chen, Huang, Chen and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingxing Xu, MTM3MDg3NzYyMjdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Wenqian Xu1,†
Wenqian Xu1,† Yingxing Xu
Yingxing Xu