- 1Department of Clinical Oncology, The University of Hong Kong-Shenzhen Hospital, Shenzhen, Guangdong, China
- 2Department of Ultrasound Medicine, The Eighth Affiliated Hospital of Sun Yat-sen University, Shenzhen, Guangdong, China
- 3Department of Ultrasound, Peking University Shenzhen Hospital, Shenzhen Key Laboratory for Drug Addiction and Medication Safety, Institute of Ultrasound Medicine, Shenzhen-PKU-HKUST Medical Center, Shenzhen, Guangdong, China
Objective: This meta-analysis aims to assess the efficacy and safety of ultrasound-guided percutaneous microwave ablation (MWA) in the treatment of hepatocellular carcinoma (HCC) located at specific anatomic sites of the liver with those of non-specific sites.
Methods: A systematic search was conducted across five databases, covering the period from the establishment of each database to September 30, 2024. Prospective and retrospective studies involving ultrasound-guided percutaneous MWA for the treatment of HCC were included. Data extraction and statistical analysis were performed using Stata 15.1 software. The main outcomes were 1-year, 3-year, and 5-year overall survival rates, complete ablation rates, and major complication rates. The results were presented as odds ratios (OR) with 95% confidence intervals (CI).
Results: A total of 9 studies were included, involving 2,381 patients, of which 1,047 had HCC at specific anatomic sites, and 1,334 had HCC at non-specific sites. The OR values (95% CI) for overall survival at 1 year, 3 years, and 5 years for patients with HCC at specific anatomic sites compared to non-specific sites were 0.89 (0.59, 1.35), 0.83 (0.66, 1.05), and 1.12 (0.91, 1.38), respectively. The OR for complete ablation rate was 0.97 (0.61, 1.53), and the OR for major complications was 1.44 (0.59, 3.51).
Conclusion: Ultrasound-guided percutaneous MWA for HCC at specific anatomic sites shows similar efficacy and safety to that at non-specific sites, with no significant differences in survival rates, complete ablation rates, or complication rates.
Highlights
• Ultrasound-guided percutaneous MWA is effective for HCC in special anatomical liver locations, comparable to non-special locations.
• No significant differences in 1-year, 3-year, and 5-year survival rates between special and non-special location groups.
• MWA's advantages include reduced heat sink effect, higher temperatures, and applicability in complex anatomical areas.
• Auxiliary techniques and individualized ablation plans enhance efficacy and safety for treating HCC in special locations.
• Findings support expanding MWA use in patients unsuitable for surgery or those refusing surgery.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide, accounting for approximately 85%–90% of primary liver cancers. Its incidence and mortality rates continue to rise, making it a major global health issue (1, 2). Although liver transplantation is the optimal treatment, its application is limited by donor shortages. Surgical resection is the preferred treatment (3), but many patients cannot undergo surgery due to impaired liver function or unsuitable tumor location (3). Therefore, there is an urgent need to explore alternative treatment strategies.
Percutaneous thermal ablation techniques, including radiofrequency ablation (RFA), microwave ablation (MWA), and laser interstitial heat therapy, have significantly advanced HCC treatment (4). Radiofrequency ablation is the preferred treatment for small HCCs due to its minimal trauma and fewer complications. The American Association for the Study of Liver Diseases recommends that, for tumors smaller than 2.5 cm, radiofrequency ablation offers similar outcomes to surgical resection (4, 5). However, the efficacy of radiofrequency ablation is limited by the tumor's location, especially when the tumor is near blood vessels, the liver hilum, the gastrointestinal tract, the gallbladder, or the diaphragm. In these areas, the heat sink effect can result in incomplete ablation, with local progression rates reaching as high as 30%. The heat sink effect describes the cooling phenomenon that occurs during thermal ablation procedures, such as MWA or RFA, when the target tissue is near a large blood vessel. The flowing blood within the vessel acts as a heat dissipater, drawing thermal energy away from the ablation zone and potentially compromising treatment efficacy (6, 7).
Microwave ablation offers several advantages, including higher temperatures, faster heating rates, and reduced heat sink effects (8). Unlike radiofrequency ablation, microwave conduction is unaffected by tissue drying and carbonization, making it more suitable for complex anatomical sites (8). Recent meta-analyses have shown no significant differences between radiofrequency ablation and microwave ablation in terms of complete ablation rate, 5-year survival rate, and local recurrence rate. However, microwave ablation has shown superior outcomes in reducing distant recurrence and improving 5-year disease-free survival (9). Ultrasound-guided microwave ablation, through real-time monitoring, has become an important approach for treating HCC at specific anatomic sites (9).
Although studies have shown similar rates of local tumor progression in ultrasound-guided microwave ablation for HCC at specific and non-specific sites, there remains a risk of incomplete ablation due to the tumor's proximity to vital organs (10, 11). For example, subphrenic tumors, which are difficult to locate due to respiratory movement, are traditionally considered unsuitable for ablation due to risks such as diaphragmatic perforation and hernia (12, 13).
In light of the above, this study aims to systematically evaluate the efficacy and safety of ultrasound-guided percutaneous microwave ablation for HCC at specific liver sites. Through a comprehensive analysis of existing literature, the goal is to provide strong evidence to support the optimization of non-surgical treatment strategies and the improvement of patient prognosis.
Methods
Retrieval strategy
To comprehensively collect literature on ultrasound-guided percutaneous microwave ablation (MWA) for hepatocellular carcinoma (HCC) at specific anatomical sites. Special anatomical locations has been explicitly defined as follows: “tumors within 5 mm of the diaphragm, liver capsule, gallbladder, gastrointestinal tract, hepatic hilum, or within 5 mm of major blood vessels (e.g., branches of the portal vein, hepatic veins, inferior vena cava)”. We conducted systematic searches in multiple databases, including PubMed, Web of Science, Cochrane Library, CNKI, and Wanfang, covering publications up to September 30, 2024. The search end date was intentional to include the most recent preprints and unpublished data available at the time of our search. We used a combination of controlled vocabulary and free-text terms such as “Carcinoma, Hepatocellular”, “Hepatocellular Carcinomas”, “microwave ablation”, “MWA”, and “ultrasou*”. Wildcards were applied to capture variations of the terms. Additionally, references of included studies were manually reviewed to ensure comprehensive coverage.
Inclusion and exclusion criteria inclusion criteria
Inclusion criteria
Eligible studies included patients diagnosed with HCC located in both specific and non-specific liver regions. A specific site was defined as a tumor located within 5 mm of the diaphragm, liver capsule, gallbladder, gastrointestinal tract, hepatic portal, right kidney, or heart, or within 5 mm of a major blood vessel (e.g., portal vein branch, hepatic vein, inferior vena cava). The 5-mm threshold was chosen because tumors in such close proximity to these structures pose higher risks for thermal injury, incomplete ablation, or damage to adjacent organs, and this distance has been referenced in prior studies evaluating technical challenges and complication rates in ablation procedures (14, 15). Studies comparing ultrasound-guided percutaneous MWA for specific (experimental group) and non-specific (control group) sites of HCC were included, ensuring identical interventions in both groups. Primary outcomes included 1-year, 3-year, and 5-year survival rates, complete ablation rate, and safety outcomes such as complications. Eligible study designs were prospective and retrospective cohort studies.
Exclusion criteria
Studies were excluded if they involved benign liver tumors, pediatric populations, or MWA combined with other treatments (e.g., systemic chemotherapy, radiofrequency ablation). We also excluded studies with incomplete data, fewer than 20 cases, or lacking a control group. Additionally, reviews, case reports, meta-analyses, conference abstracts, animal studies, and AI-assisted ablation studies were not considered.
Study selection, data extraction, and quality assessment
The study selection process followed a systematic approach. A literature search was conducted across five databases (PubMed, Web of Science, Cochrane Library, China National Knowledge Infrastructure, and WanFang), followed by the removal of duplicate records. Two independent researchers screened titles and abstracts, excluding irrelevant or ineligible studies. Full-text assessments were then performed to further exclude studies that did not meet inclusion criteria. Any disagreements during the selection process were resolved through discussion or adjudicated by a third reviewer, adhering strictly to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA guidelines) (16).
Data extraction was also performed independently by Researcher A and Researcher B. Key information collected from each study comprised the first author's name, publication year, study design, patient characteristics (including sample size, age, gender distribution, and specific inclusion/exclusion criteria), and intervention details (such as the MWA device used, power settings, ablation duration, and any auxiliary techniques like artificial ascites). We also recorded relevant outcomes, including 1-year, 3-year, and 5-year overall survival, complete ablation rate, and incidence of major complications (e.g., hemorrhage, biliary leakage, infection). In cases of missing or ambiguous data, attempts were made to contact the corresponding authors for clarification. Discrepancies in data extraction were resolved by re-evaluating the full text or consulting a third reviewer when necessary.
The risk of bias in each study was assessed using the Newcastle-Ottawa Scale (NOS) (17). This tool evaluates the selection of study groups, the comparability of groups, and the ascertainment of outcomes, assigning a maximum of nine stars. A total score of six or above generally indicated moderate-to-high quality, whereas scores below six suggested a higher risk of bias. Both researchers independently assigned NOS ratings and documented the rationale for their judgments. Any disagreements were addressed through discussion, or if needed, adjudicated by a third reviewer. Studies with potential sources of bias, such as limited follow-up or unaddressed baseline imbalances, were closely scrutinized during sensitivity analyses to ensure the robustness of the meta-analysis findings.
Statistical analysis
Statistical analysis was performed using Stata 15.1 software (18, 19). For binary outcomes, such as survival rates, complete ablation rate, and complication rates, odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated. Heterogeneity was assessed using the Cochrane Q test and I2 statistics, where I2 ≤ 25% indicated low heterogeneity, 25% < I2 ≤ 50% indicated moderate heterogeneity, and I2 > 50% indicated high heterogeneity. When I2 ≤ 50% and P ≥ 0.10, the fixed-effect model was used; otherwise, the random-effect model was adopted. The robustness of the results was further assessed through Duval and Tweedie's trim-and-fill analysis (20). Publication bias was evaluated using the Egger linear regression test, with P ≥ 0.05 indicating a low risk of bias (21). All statistical tests were two-sided, and the significance level was set at P < 0.05. The results were presented in the form of forest plots.
Results
Literature search results and characteristics of included studies
A total of 1,634 studies were retrieved according to the predefined search strategy. After removing duplicates and excluding studies that did not meet the inclusion criteria, such as studies lacking control groups, reviews, meta-analyses, conference abstracts, and animal studies, 98 studies were included for full-text evaluation. After further assessment, nine studies met all inclusion criteria and were included in the meta-analysis (3, 10, 22–28). The research selection process is shown in Figure 1.
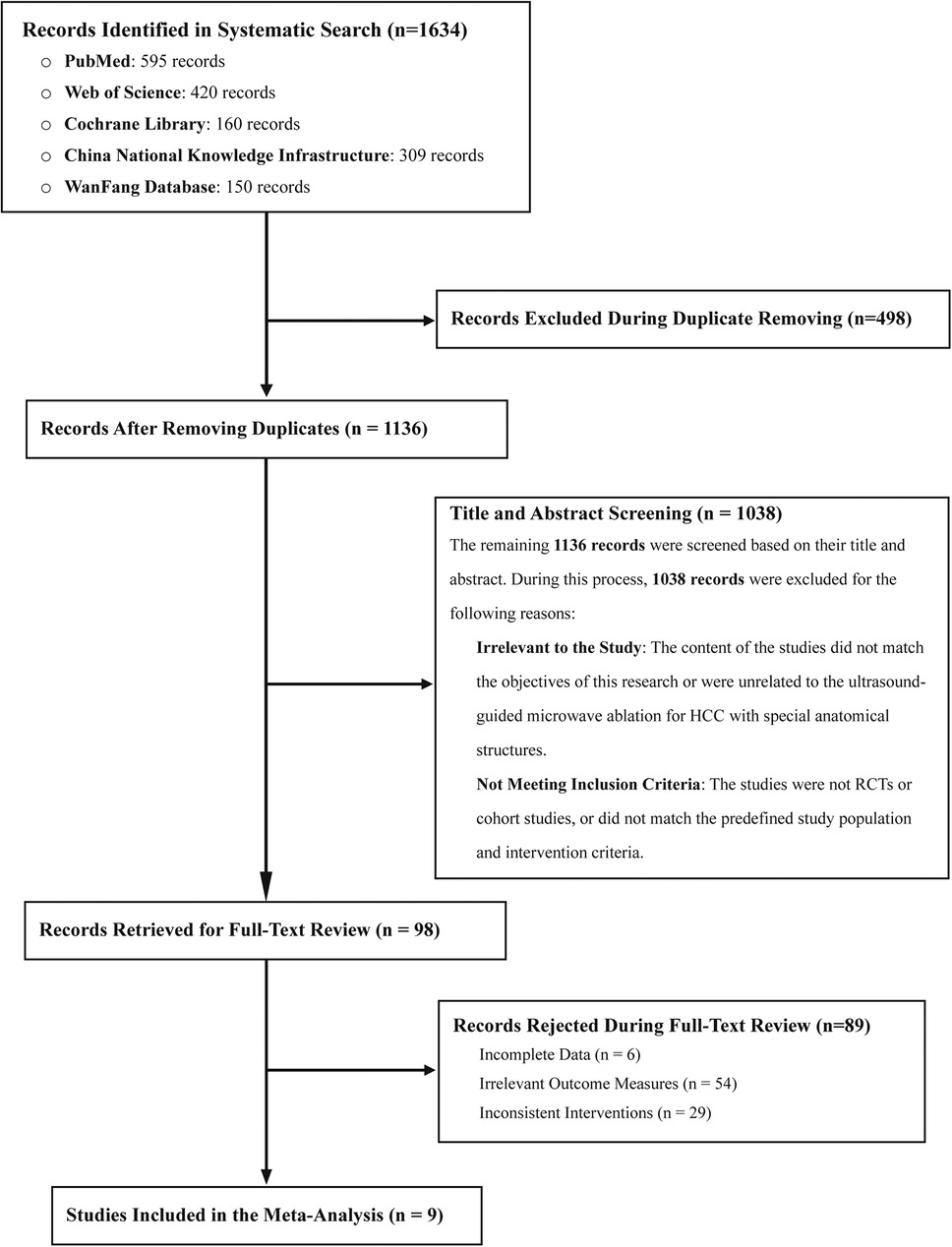
Figure 1. Flow diagram illustrating the literature screening process for identifying eligible studies for inclusion in the meta-analysis.
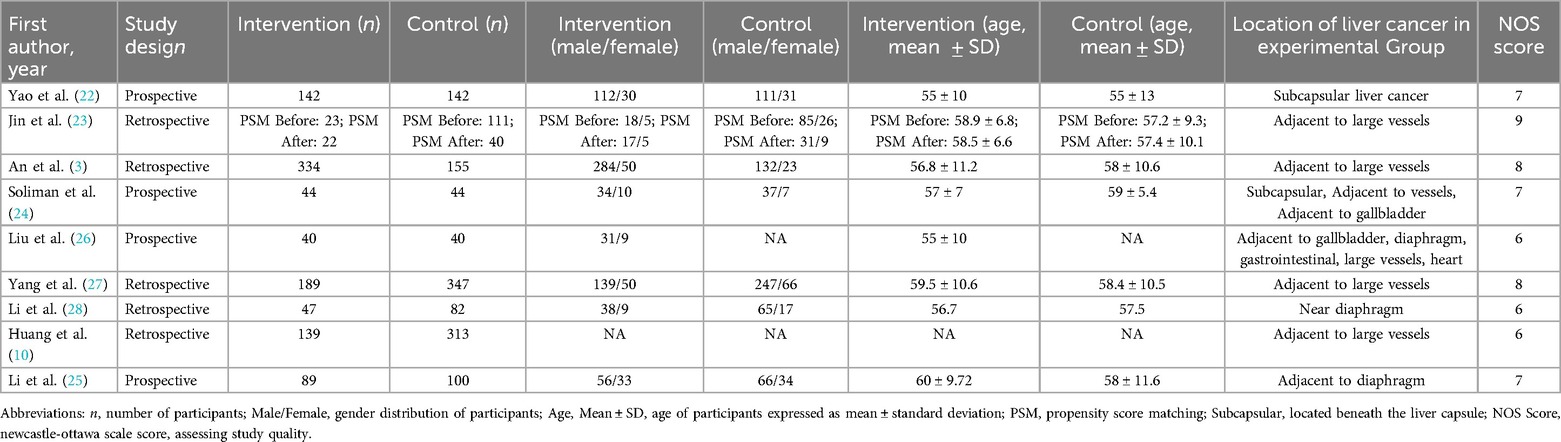
The nine included studies involved a total of 2,381 patients, of whom 1,047 had specific anatomical site HCC and 1,334 had non-specific anatomical site HCC. All studies were cohort studies, some of which were prospective. All patients received ultrasound-guided percutaneous MWA therapy. The primary outcomes were 1-, 3-, and 5-year overall survival rates and complete ablation rates. Secondary outcomes included the incidence of major complications. The characteristics of the included studies are summarized in Table 1 (3, 10, 22–28).
In terms of quality assessment, the Newcastle-Ottawa Scale (NOS) was used. The results showed that all studies were of high quality, with scores of 6 or above. However, some studies had limitations regarding intergroup comparability and follow-up completeness. Detailed quality assessment results are shown in Table 1 (3, 10, 22–28).
Meta-analysis results
1-year overall survival rate
Six studies reported the 1-year overall survival rate. The heterogeneity test showed I2 = 0.0%, P = 0.678, and a fixed-effect model was used. The combined results showed no statistically significant difference in 1-year overall survival between patients with specific and non-specific HCC sites (OR = 0.89, 95% CI: 0.59–1.35) (Figure 2A).
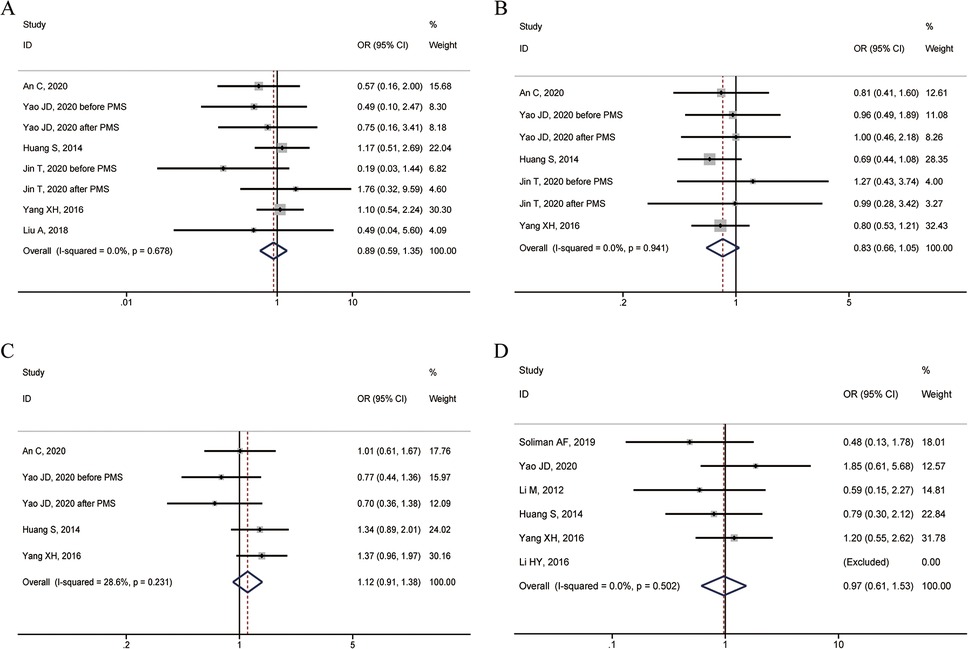
Figure 2. Forest plots of overall survival rates between HCC in special and non-special liver locations. (A) 1-year overall survival rate; (B) 3-year overall survival rate; (C) 5-year overall survival rate; (D) Complete ablation rate.
3-year overall survival rate
Five studies reported the 3-year overall survival rate. The heterogeneity test showed I2 = 0.0%, P = 0.941, and a fixed-effect model was used. The combined results showed no statistically significant difference in 3-year overall survival between patients with specific and non-specific HCC sites (OR = 0.83, 95% CI: 0.66–1.05) (Figure 2B).
5-year overall survival rate
Four studies reported the 5-year overall survival rate. The heterogeneity test showed I2 = 28.6%, P = 0.231, and a fixed-effect model was used. The combined results showed no statistically significant difference in 5-year overall survival between patients with specific and non-specific HCC sites (OR = 1.12, 95% CI: 0.91–1.38) (Figure 2C).
Complete ablation rate
Six studies reported the complete ablation rate. The heterogeneity test showed I2 = 0.0%, P = 0.502, and a fixed-effect model was used. The combined results showed no statistically significant difference in the rate of complete ablation between HCC patients with specific and non-specific sites (OR = 0.97, 95% CI: 0.61–1.53) (Figure 2D).
Major complication rate
Four studies reported the incidence of major complications. The heterogeneity test showed I2 = 0.0%, P = 0.896, and a fixed-effect model was used. The combined results showed no statistically significant difference in the incidence of major complications between patients with specific and non-specific HCC sites (OR = 1.44, 95% CI: 0.59–3.51) (Figure 3).
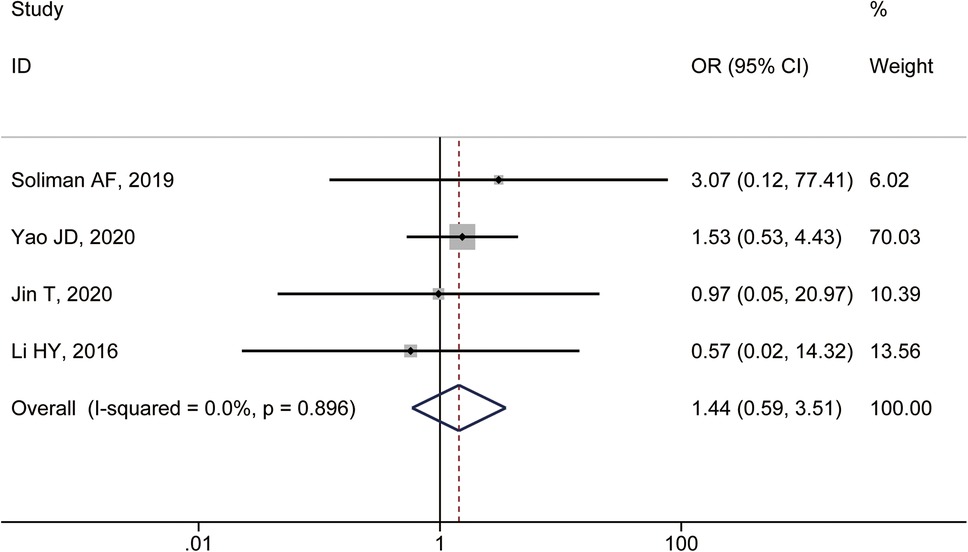
Figure 3. Forest plot comparing the incidence of major complications between HCC patients treated with MWA in special and non-special liver locations.
Publication bias and sensitivity analysis
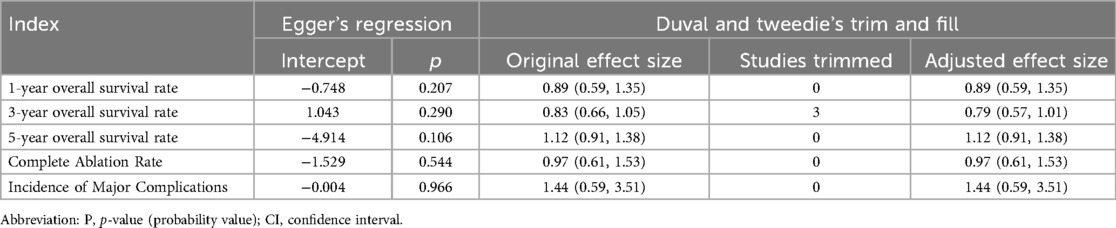
Publication bias was assessed using the Egger test. The results showed that all P-values were greater than 0.05, indicating no significant publication bias (Table 2). Additionally, sensitivity analysis was performed, and the results showed no significant change in the combined effect size, further confirming the robustness of our analysis. This indicates that our conclusions are reliable and stable (Table 2).
Discussion
This meta-analysis included nine studies on ultrasound-guided percutaneous microwave ablation (MWA) for the treatment of hepatocellular carcinoma (HCC), involving a total of 2,381 patients, of whom 1,047 had HCC at specific anatomical sites and 1,334 had non-specific anatomical site HCC. The results showed no significant differences in 1-year, 3-year, and 5-year overall survival rates, complete ablation rates, and major complication rates between patients with specific and non-specific HCC sites (P > 0.05), with low heterogeneity among studies. While the statistical heterogeneity in our analysis was minimal, the possibility of clinical heterogeneity resulting from variations in MWA equipment, power settings, and operator experience, which could influence outcomes. This suggests that the efficacy and safety of ultrasound-guided percutaneous MWA for treating liver HCC at specific anatomic sites is comparable to that at non-specific sites.
These findings contrast with conventional wisdom. Previously, tumors located in specific anatomic areas of the liver, such as those near the diaphragm, gallbladder, gastrointestinal tract, and large blood vessels, were thought to increase the risk of ablation, leading to higher complication rates and reduced outcomes (24). However, the results of this study indicate that survival and complete ablation rates were not significantly affected in patients with specific site HCC who received ultrasound-guided MWA, and the incidence of major complications was not increased. This is consistent with recent studies. For example, Li et al. reported that HCC patients near the diaphragm had local tumor control and survival rates similar to those of patients with non-specific sites after MWA treatment (28). Wang et al. also supported the efficacy of MWA in treating site-specific HCC (29).
When comparing radiofrequency ablation (RFA) and microwave ablation (MWA) for hepatocellular carcinoma, especially in complex or high-risk anatomical locations, MWA presents several theoretical and practical advantages. In particular, MWA is less susceptible to the heat-sink effect caused by blood flow, which can otherwise lead to incomplete ablation in tumors situated near major vessels (30, 31). By generating higher temperatures more quickly, MWA can create larger and more uniform ablation zones, thereby minimizing residual tumor tissue and potentially reducing marginal recurrence (32, 33). Unlike RFA—which relies on electrical conductivity and can be influenced by tissue carbonization and dehydration—MWA's energy delivery is relatively unaffected by these factors, enabling more consistent performance in challenging lesions. These benefits may be especially relevant for tumors in close proximity to critical structures or in regions with substantial vascularity, where rapid and predictable heating is crucial for achieving complete and safe ablation (32, 33). Nonetheless, some studies within this meta-analysis found similar clinical outcomes between RFA and MWA for smaller tumors, indicating that the choice of modality may also depend on tumor size, operator expertise, and equipment availability. Moreover, data remain sparse for large or anatomically complex lesions, underscoring the need for head-to-head comparisons of RFA and MWA in prospective trials with standardized endpoints such as local tumor progression, overall survival, and procedure-related complications. A clearer understanding of each modality's efficacy and safety profile under similar clinical conditions would guide more tailored treatment strategies and improve outcomes for patients with high-risk or anatomically challenging hepatocellular carcinoma.
In this meta-analysis, several studies noted the use of auxiliary or assistive techniques—most commonly, the induction of artificial ascites or pleural effusion—to facilitate safer and more complete ablation of HCC in anatomically challenging regions (34, 35). Among the nine included studies, only four explicitly reported how often such measures were employed, with usage rates ranging from 15% to 40% for tumors near the diaphragm or gastrointestinal tract. This approach can isolate the lesion from surrounding organs, providing thermal insulation and reducing collateral injury, while simultaneously enhancing the clarity of ultrasound imaging for more precise ablation (34, 35). Although there was a general trend suggesting that these techniques lower the risk of complications and improve treatment outcomes, none of the included studies performed a dedicated subgroup analysis to quantify their direct impact on local tumor control or overall survival. Advances in imaging, such as contrast-enhanced ultrasound, CT/MRI fusion, and real-time three-dimensional visualization, have likewise heightened the accuracy of needle placement and real-time monitoring, minimizing adverse events and improving efficacy (34, 36). However, the limited and inconsistent reporting of auxiliary methods in our included studies precluded a robust, quantitative assessment of their precise benefit. Future research that systematically documents the use of artificial ascites, pleural effusion, and other imaging-guided enhancements would help clarify the full potential of these modalities in improving ablative treatment for hepatocellular carcinoma at special anatomical sites.
Improved operating techniques and increased physician experience are also crucial factors. Accurate needle positioning and real-time temperature monitoring ensure adequate tumor ablation while avoiding thermal damage to adjacent normal tissue (37). Individualized ablation protocols, based on tumor size, location, and surrounding anatomy, help select appropriate ablation parameters and needle placement to maximize efficacy (38). These factors collectively contribute to the success of treating specific-site HCC.
The use of combination treatment strategies should not be overlooked. Prior to ablation, transarterial chemoembolization or percutaneous ethanol injection reduces tumor volume and blood flow, enhancing the ablation effect and reducing thermal damage to surrounding tissue (39). This combined approach further improves the therapeutic outcomes for patients with site-specific HCC.
The results of this study have significant implications for clinical practice. First, the study expands the scope of MWA application, showing that ultrasound-guided percutaneous MWA can be safely and effectively used for liver HCC at specific anatomical sites, providing a new treatment option for patients who are not candidates for surgery or who refuse surgery. Second, the study alleviates concerns about the risks of ablation for site-specific HCC and encourages clinicians to adopt MWA with greater confidence, thereby improving patient survival and quality of life. Furthermore, the study demonstrates that the advantages of MWA technology, combined with assistive techniques and individualized protocols, can achieve optimal outcomes in the treatment of site-specific HCC.
Despite the robustness of our findings, this study has several limitations. First, the included studies were primarily prospective and retrospective cohort studies, lacking high-quality randomized controlled trials (RCTs). Such non-randomized designs may introduce selection bias, confounding factors, and variability in outcome assessment, potentially affecting the reliability of the results. Future research would benefit from high-quality RCTs that standardize patient selection, ablation protocols, and follow-up procedures, allowing for more definitive conclusions regarding the efficacy and safety of ultrasound-guided percutaneous MWA for HCC at special anatomical sites. Second, variability across studies in patient characteristics, tumor size, MWA devices, and ablation parameters limited the ability to perform detailed subgroup analyses, which may have impacted the accuracy of the results. Incorporating larger, multicenter cohorts and stratified analyses based on tumor characteristics and underlying liver function could help refine patient selection criteria and optimize therapeutic strategies. Finally, this study focused mainly on survival rates, complete ablation rates, and major complication rates, while neglecting important clinical outcomes such as quality of life, tumor recurrence, and disease-free survival, which are crucial for a comprehensive understanding of patient prognosis. Future studies should incorporate these key clinical endpoints to provide a more holistic evaluation of treatment effectiveness.
Conclusion
This meta-analysis indicates that the efficacy and safety of ultrasound-guided percutaneous MWA for treating liver HCC at specific anatomical sites is comparable to that for non-specific sites, with no significant differences in survival rates, complete ablation rates, and major complication rates. The advantages of MWA technology, the application of assistive technologies, improved operational skills, and combination treatment strategies all contributed to this outcome. Although this study has some limitations, it provides strong evidence for the effectiveness of MWA in treating site-specific HCC. Future high-quality studies are needed to further validate and refine the use of MWA for treating specific-site HCC, providing a stronger scientific basis for optimizing clinical decision-making and improving patient prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
JZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FZ: Data curation, Formal analysis, Methodology, Validation, Writing – review & editing. TC: Data curation, Formal analysis, Methodology, Writing – review & editing. SL: Data curation, Formal analysis, Methodology, Writing – review & editing. XS: Data curation, Formal analysis, Funding acquisition, Methodology, Writing – review & editing. LX: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. WC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by Shenzhen Science and Technology Program (ZDSYS20210623091811035, JCYJ20220530142203008), Shenzhen Science and Technology Program (KQTD20180411185028798), Guangdong Basic and Applied Basic Research Foundation (2022A1515110156), Sanming Project of Medicine in Shenzhen (SZSM202211017), Futian Healthcare Research Program (FTWS2022051), Young Scientists Fund of the National Natural Science Foundation of China (82403259), and the Shenzhen Key Laboratory for cancer metastasis and personalized therapy (ZDSYS20210623091811035).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HCC, hepatocellular carcinoma; MWA, microwave ablation; RFA, radiofrequency ablation; OR, odds ratio; CI, confidence interval; NOS, newcastle-ottawa scale; CNKI, China national knowledge infrastructure; PRISMA, preferred reporting items for systematic reviews and meta-analyses; CT, computed tomography; MRI, magnetic resonance imaging.
References
1. Vogl TJ, Basten LM, Nour-Eldin NA, Kaltenbach B, Bodelle B, Wichmann JL, et al. Evaluation of microwave ablation of liver malignancy with enabled constant spatial energy control to achieve a predictable spherical ablation zone. Int J Hyperthermia. (2018) 34:492–500. doi: 10.1080/02656736.2017.1358408
2. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. (2020) 9:452–63. doi: 10.21037/hbsn-20-480
3. An C, Cheng Z, Yu X, Han Z, Liu F, Li X, et al. Ultrasound-guided percutaneous microwave ablation of hepatocellular carcinoma in challenging locations: oncologic outcomes and advanced assistive technology. Int J Hyperthermia. (2020) 37:89–100. doi: 10.1080/02656736.2019.1711203
4. Sun X, Li RU, Zhang B, Yang Y, Cui Z. Treatment of liver cancer of middle and advanced stages using ultrasound-guided percutaneous ethanol injection combined with radiofrequency ablation: a clinical analysis. Oncol Lett. (2016) 11:2096–100. doi: 10.3892/ol.2016.4180
5. Laimer G, Schullian P, Jaschke N, Putzer D, Eberle G, Alzaga A, et al. Minimal ablative margin (MAM) assessment with image fusion: an independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur Radiol. (2020) 30:2463–72. doi: 10.1007/s00330-019-06609-7
6. Pillai K, Akhter J, Chua TC, Shehata M, Alzahrani N, Al-Alem I, et al. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine. (2015) 94:e580. doi: 10.1097/MD.0000000000000580
7. Huang H, Liang P, Yu XL, Cheng ZG, Han ZY, Yu J, et al. Safety assessment and therapeutic efficacy of percutaneous microwave ablation therapy combined with percutaneous ethanol injection for hepatocellular carcinoma adjacent to the gallbladder. Int J Hyperthermia. (2015) 31:40–7. doi: 10.3109/02656736.2014.999017
8. Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, et al. Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. (2019) 24:e990–e1005. doi: 10.1634/theoncologist.2018-0337
9. Spiliotis AE, Gabelein G, Hollander S, Scherber PR, Glanemann M, Patel B. Microwave ablation compared with radiofrequency ablation for the treatment of liver cancer: a systematic review and meta-analysis. Radiol Oncol. (2021) 55:247–58. doi: 10.2478/raon-2021-0030
10. Huang S, Yu J, Liang P, Yu X, Cheng Z, Han Z, et al. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J Radiol. (2014) 83:552–8. doi: 10.1016/j.ejrad.2013.12.015
11. Qin S, Liu GJ, Huang M, Huang J, Luo Y, Wen Y, et al. The local efficacy and influencing factors of ultrasound-guided percutaneous microwave ablation in colorectal liver metastases: a review of a 4-year experience at a single center. Int J Hyperthermia. (2019) 36:36–43. doi: 10.1080/02656736.2018.1528511
12. Smolock AR, Lubner MG, Ziemlewicz TJ, Hinshaw JL, Kitchin DR, Brace CL, et al. Microwave ablation of hepatic tumors abutting the diaphragm is safe and effective. AJR Am J Roentgenol. (2015) 204:197–203. doi: 10.2214/AJR.14.12879
13. Saito T, Chiba T, Ogasawara S, Inoue M, Wakamatsu T, Motoyama T, et al. Fatal diaphragmatic hernia following radiofrequency ablation for hepatocellular carcinoma: a case report and literature review. Case Rep Oncol. (2015) 8:238–45. doi: 10.1159/000431310
14. Schaible J, Pregler B, Baumler W, Einspieler I, Jung EM, Stroszczynski C, et al. Safety margin assessment after microwave ablation of liver tumors: inter- and intrareader variability. Radiol Oncol. (2020) 54:57–61. doi: 10.2478/raon-2020-0004
15. Verdonschot KHM, Arts S, Van den Boezem PB, de Wilt JHW, Futterer JJ, Stommel MWJ, et al. Ablative margins in percutaneous thermal ablation of hepatic tumors: a systematic review. Expert Rev Anticancer Ther. (2023) 23:977–93. doi: 10.1080/14737140.2023.2247564
16. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
17. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2000). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
18. Harris RJ, Deeks JJ, Altman DG, Bradburn MJ, Harbord RM, Sterne JAC. Metan: fixed- and random-effects meta-analysis. Stata J. (2008) 8:3–28. doi: 10.1177/1536867X0800800102
19. Nyaga VN, Arbyn M, Aerts M. Metaprop: a stata command to perform meta-analysis of binomial data. Arch Public Health. (2014) 72:39. doi: 10.1186/2049-3258-72-39
20. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
21. Egger M, Davey Smith G, Schneider M. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
22. Yao J, Liu B, Wang X, Yu J, Cheng Z, Han Z, et al. Long-term efficacy of microwave ablation in the treatment of subcapsular hepatocellular carcinomas of ≤3 cm in diameter: a multicenter, propensity score-matched study. Int J Hyperthermia. (2022) 39:209–16. doi: 10.1080/02656736.2021.2023228
23. Jin T, Liu X, Zhang H, Cao Y, Dai C, Tang S, et al. Ultrasound-guided percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a propensity score matching analysis. Int J Hyperthermia. (2020) 37:955–64. doi: 10.1080/02656736.2020.1804076
24. Soliman AF, Abouelkhair MM, Hasab Allah MS, El-Kady NM, Ezzat WM, Gabr HA, et al. Efficacy and safety of microwave ablation (MWA) for hepatocellular carcinoma (HCC) in difficult anatomical sites in Egyptian patients with liver cirrhosis. Asian Pac J Cancer Prev. (2019) 20:295–301. doi: 10.31557/APJCP.2019.20.1.295
25. Li M, Yu XL, Liang P, Liu F, Dong B, Zhou P. Percutaneous microwave ablation for liver cancer adjacent to the diaphragm. Int J Hyperthermia. (2012) 28:218–26. doi: 10.3109/02656736.2012.665565
26. Liu A, Xu W, Xu H, Lu J, Qi PJ, Gu YM. Efficacy analysis of low-power microwave ablation under ultrasound guidance in treating liver cancer in special locations. J Pract Radiol. (2018) 34:1925–8. doi: 10.3969/j.issn.1002-1671.2018.12.028
27. Yang XH, Huang SJ, Yu J, Liang P, Yu XL, Cheng ZG, et al. Long-term efficacy of ultrasound-guided percutaneous microwave ablation for primary liver cancer adjacent to blood vessels. J Oncol. (2016) 22:24–8. doi: 10.11735/j.issn.1671-170X.2016.01.B005
28. Li HY, Wu JH, Qiao Q, Zhang L, Cheng W. Clinical study of microwave ablation combined with artificial ascites for liver cancer near the diaphragm. J Med Imaging. (2016) 26:1829–32.
29. Wang CC, Kao JH. Artificial ascites is feasible and effective for difficult-to-ablate hepatocellular carcinoma. Hepatol Int. (2015) 9:514–9. doi: 10.1007/s12072-015-9639-8
30. Abd El Baset OA, Werida RH, Askar S, El-Mohamdy M, Omran GA, Hagag RS. Comprehensive Management of Hepatocellular Carcinoma: A Review of Current Surgical, Locoregional, and Systemic Therapies.
31. Di Muzio B, Wutami I, Cheung W. Enhancing Safety in Liver Microwave Ablation: Thermoprotection Techniques Under US Guidance.
32. Shaqran TM, Alharbi J, Al-Hunbusi SK, Alharbi RA, Alawaji M, Diqarshawi AM, et al. Comparison of radiofrequency ablation and microwave ablation for the management of hepatocellular carcinoma: a systematic review and meta-analysis of randomized controlled trials. Cureus. (2024) 16:e67938. doi: 10.7759/cureus.67938 39328664
33. Han S, Sung PS, Park SY, Kim JW, Hong HP, Yoon JH, et al. Local ablation for hepatocellular carcinoma: 2024 expert consensus-based practical recommendation of the Korean liver cancer association. J Liver Cancer. (2024) 24:131–44. doi: 10.17998/jlc.2024.08.04
34. Ai Q, Liu D, Liang F, Kong Z, Pan Y, Zhang X. Artificial ascites-assisted microwave ablation for liver cancer adjacent to the diaphragm and perioperative nursing care. Oncol Lett. (2024) 28:382. doi: 10.3892/ol.2024.14515
35. Liu LN, Xu HX, Lu MD, Xie XY. Percutaneous ultrasound-guided thermal ablation for liver tumor with artificial pleural effusion or ascites. Chin J Cancer. (2010) 29:830–5. doi: 10.5732/cjc.010.10095
36. Hsieh YC, Limquiaco JL, Lin CC, Chen WT, Lin SM. Radiofrequency ablation following artificial ascites and pleural effusion creation may improve outcomes for hepatocellular carcinoma in high-risk locations. Abdom Radiol. (2019) 44:1141–51. doi: 10.1007/s00261-018-1831-6
37. Li M, Yu X, Liang P, Dong B, Liu F. Ultrasound-guided percutaneous microwave ablation for hepatic malignancy adjacent to the gallbladder. Int J Hyperthermia. (2015) 31:579–87. doi: 10.3109/02656736.2015.1014869
38. Hao MZ, Hu YB, Chen QZ, Chen ZX, Lin HL. Efficacy and safety of computed tomography-guided microwave ablation with fine needle-assisted puncture positioning technique for hepatocellular carcinoma. World J Gastrointest Oncol. (2022) 14:1727–38. doi: 10.4251/wjgo.v14.i9.1727
Keywords: ultrasound guidance, microwave ablation, liver cancer, specific anatomical sites, meta-analysis
Citation: Zha J, Zhang F, Cao T, Li S, Shen X, Xu L and Chen W (2025) Efficacy and safety of ultrasound-guided percutaneous microwave ablation for hepatocellular carcinoma at specific anatomic sites of the liver: a systematic review and meta-analysis. Front. Surg. 12:1587435. doi: 10.3389/fsurg.2025.1587435
Received: 4 March 2025; Accepted: 27 March 2025;
Published: 22 May 2025.
Edited by:
Mingyuan Wang, Central South University, ChinaReviewed by:
Huan Chen, Zhuzhou Central Hospital, ChinaGuangquan Zhang, Sun Yat-sen University, China
Copyright: © 2025 Zha, Zhang, Cao, Li, Shen, Xu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liangliang Xu, bGlhbmdsaWFuZ3h1ODlAMTI2LmNvbQ==; Wenqi Chen, Y2hlbndlbnFpQGhrdS1zemgub3Jn
 Jiandong Zha1
Jiandong Zha1 Liangliang Xu
Liangliang Xu