- 1Department of Oncology, Xi Chang People’s Hospital, Xi Chang, China
- 2Department of Radiotherapy, XuZhou Central Hospital, Xuzhou, China
- 3Department of Urology, Kidney and Urology Center, Pelvic Floor Disorders Center, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China
- 4School of Medicine, Shenzhen Campus of Sun Yat-sen University, Sun Yat-sen University, Shenzhen, China
- 5Department of Medical Administration, Xi Chang People’s Hospital, Xi Chang, China
- 6Department of Emergency, The Affiliated Hospital, Southwest Medical University, Luzhou, China
Background: Kidney cancer is a highly heterogeneous oncologic disease with historically poor prognosis. Precise assessment of the risk of distal metastasis can facilitate risk stratification and improve prognosis for kidney cancer patients.
Methods: Data from the Surveillance, Epidemiology, and End Results (SEER) database, we identified 40,527 kidney cancer patients diagnosed between 2010 and 2017 were obtained. LASSO, univariate and multivariate logistic regression analyses were employed to screen independent risk factors for distal metastasis. Six machine learning (ML) algorithms including logistic regression (LR), Naïve Bayes Classifier (NBC), Decision Tree (DT), Random Forest (RF), Gradient boosting machine (GBM) and Extreme gradient boosting (XGB), were further applied to build the predictive models. After testing with ten-fold cross-validation and receiver operating characteristic (ROC) analysis, the model with the highest area under curve (AUC) was selected as the best performing model to establish the risk predictive nomogram and web calculator.
Results: In distal metastasis risk prediction, the XGB model had the best performance in both training (AUC = 0.91) and testing (AUC = 0.851) datasets among the six ML algorithms. Variables including marital status, sequence number, primary site, grade, pathological type, T-stage, N-stage, the calculated risk of XGB, surgical and radiation treatment were incorporated to establish a nomogram to predict the 1-, 3-, and 5-years survival probability. The calibration plots, decision curve analysis (DCA), ROC curves and Kaplan–Meier (KM) curves all verified the predictive utility of the nomogram.
Conclusions: We established a favorable prediction for the occurrence of distal metastasis with the ML model. The nomogram based on XGB algorithm can contribute to identify high-risk patients and provide optimal clinical strategies.
1 Introduction
Kidney cancer is among the 10 most malignancy in USA with estimated 76,080 new cases and 13,780 deaths in 2021 (1, 2). It is a highly heterogeneous oncologic disease originating from the urinary system with historically poor prognosis (3). The 5-year survival rate was about 74% for all patients with kidney cancer, 53% for locoregional disease and 8% for metastatic disease (4, 5). Among all patients, nearly 91% cases were diagnosed at an age of 45 or older, making kidney cancer a disease of the middle- and old-aged (4). Renal-cell carcinoma (RCC) occurs in 90% kidney cancer and has diverse molecular and histologic subtypes (5).
Though more low-stage and indolent tumors were identified with the improvement of early-detection techniques, there are still one third of kidney cancer patients present with metastasis (4, 6). And nearly 25% localized RCC present with relapses in distal sites after treating with nephrectomy (7–9). The common sites of RCC metastasis are the lungs (45%), bones and brain (10). Due to the immunogenicity of metastatic RCC (mRCC), immune checkpoint inhibitors (ICIs), such as nivolumab plus ipilimumab and nivolumab monotherapy, were validated to improve the prognosis of mRCC (11, 12). Systemic therapies targeting angiogenesis and modulating immunity, such as sunitinib, bevacizumab and axitinib, have also been optimized (13–18). Nevertheless, mRCC still has a limited median survival of approximately 12 months (19). Thus, novel targets or predictive tools to predict distal metastasis and identify high-risk mRCCs are urgently required.
In the 1950s, artificial intelligence (AI) became a branch of computer science dedicated to developing algorithms to enable machines to perform complex tasks that would normally require human intelligence to accomplish. Machine learning (ML) is the main area of AI research, The integration of artificial intelligence in the medical field is developing rapidly, and there have been breakthroughs especially in the diagnosis, treatment and efficacy assessment of medicine (20).
Nomogram is a simple and practical tool widely applied in prognosis prediction. A few literatures have established nomograms to instruct clinical treatment and predict prognosis targeting metastasis from kidney cancer (21–23). While our study employed six ML algorithms including logistic regression (LR), Naïve Bayes Classifier (NBC), Decision Tree (DT), Random Forest (RF), Gradient boosting machine (GBM) and Extreme gradient boosting (XGB) to analyze the kidney cancer data from the SEER database, aimed to obtain the best ML algorithm and construct an insightful risk prediction nomogram for distal metastasis.
2 Methods
2.1 Source of data
In our study, data were extracted from the SEER database. The inclusion criteria were adopted as follows: (1) with primary kidney cancer; (2) diagnosed based on positive histology from 2010 to 2017, and the included histological subtypes including RCC, transitional cell carcinoma, clear cell adenocarcinoma, and other kidney cancer; (3) with complete survival and follow-up data until 2017; (4) age ≥18 years. Exclusion criteria were practiced as follows: (1) multiple primary malignant tumors; (2) unknown tumor characteristics and demographic information; (3) diagnosed via a death certificate; (4) with unknown distal metastasis and survival status; (5) died of causes other than kidney cancer. This research was conducted after obtaining informed consent from all patients and was approved by the ethics committee.
2.2 Data collection and follow-up
The included demographic features include marital status, age at diagnosis, race ethnicity and sex. We also extracted the following clinicopathological characteristics: tumor size, sequence number, primary site, grade, laterality, pathological type, TNM-staging, surgical approaches, the status of radiotherapy, chemotherapy and systemic therapy. Based on AJCC staging system, histological grades were divided into grade 1–4, corresponding to well-differentiated, moderately differentiated, poorly differentiated and undifferentiated in turn. CT examination, radionuclide bone scan and PET-CT are recommended to identify and evaluate the suspected metastatic lesions. While pathological biopsy is the gold criterion of diagnosis for the metastatic sites. The presence of distal metastasis was defined as the primary endpoint event, while survival time was the sub-endpoint event. All enrolled patients were followed up through outpatient review or telephone calls.
2.3 Statistical analysis
Qualitative data including demographics and clinicopathological characteristics were compared via Pearson Chi-square test. T-test were utilized to compare quantitative data on normal distribution, while Wilcoxon rank test for abnormal distribution. Six different machine learning algorithms were utilized to analyze our data: LR, NBC, DT, RF, GBM and XGB. The model having the highest AUC was regarded as the best performing model. All analyses were conducted utilizing R version 4.3.1 and SPSS version 25.0. P < 0.05 indicated statistical significance in all analyses.
3 Results
3.1 Baseline characteristics
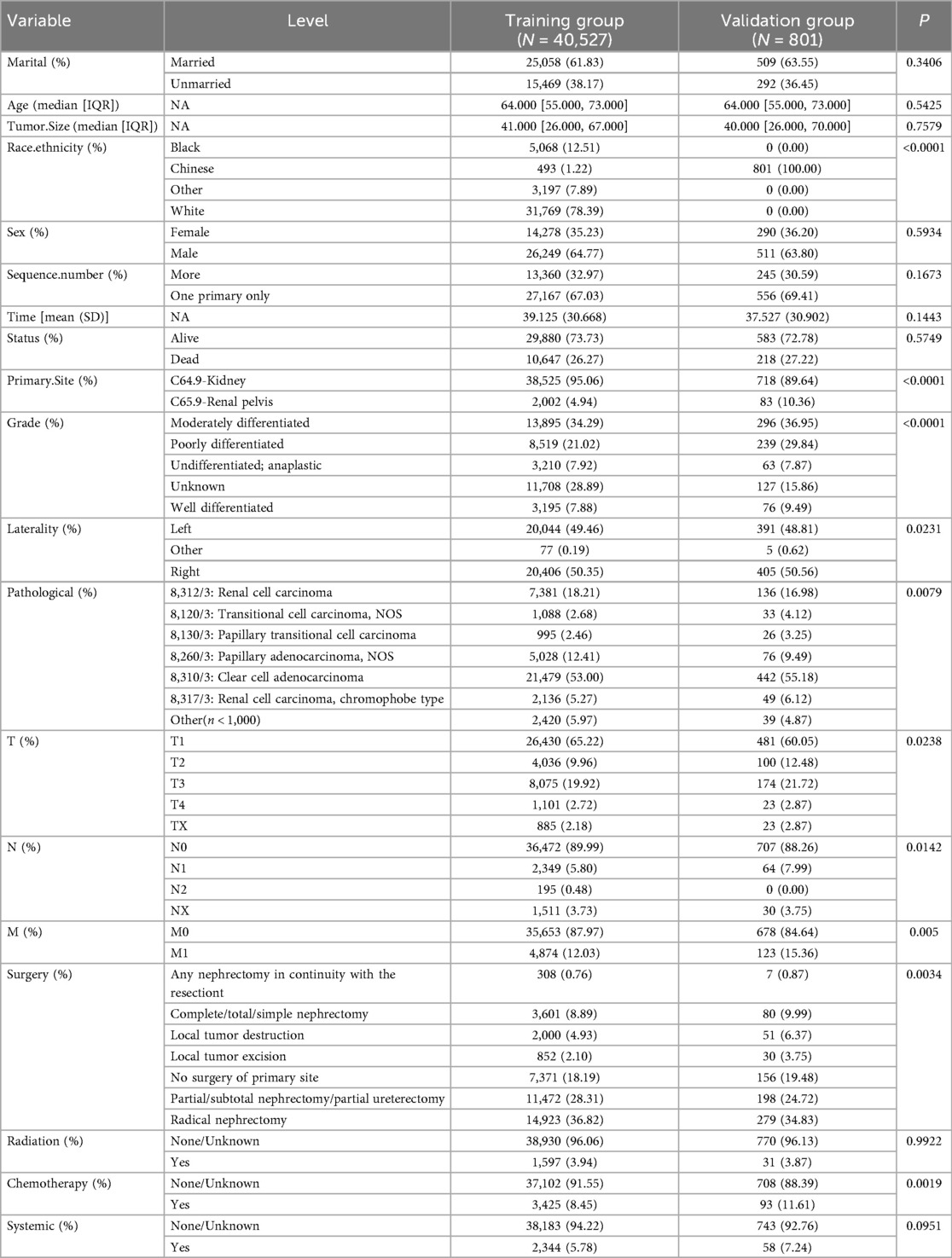
A total of 40,527 patients from SEER database diagnosed between 2010 and 2017 were enrolled in this study. Among these patients, there were 38,525 kidney cancer patients and 2002 renal pelvis cancer patients at initial diagnosis. The detailed clinicopathological features of the whole cohort were presented in Table 1. The training group included 40,527 patients and validation group included 801 patients. And the correlation analysis of these features was displayed in Figures 1A,B.
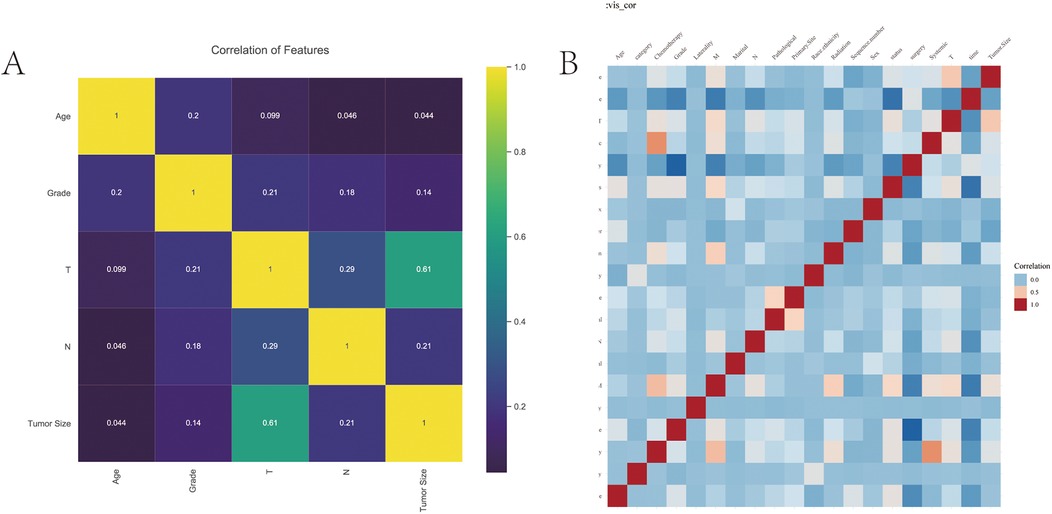
Figure 1. The correlation analysis of features. (A) The correlation of variables. Yellow indicates positive correlation and purple indicates negative correlation. (B) Correlation heat map.
3.2 Risk factors for distal metastasis
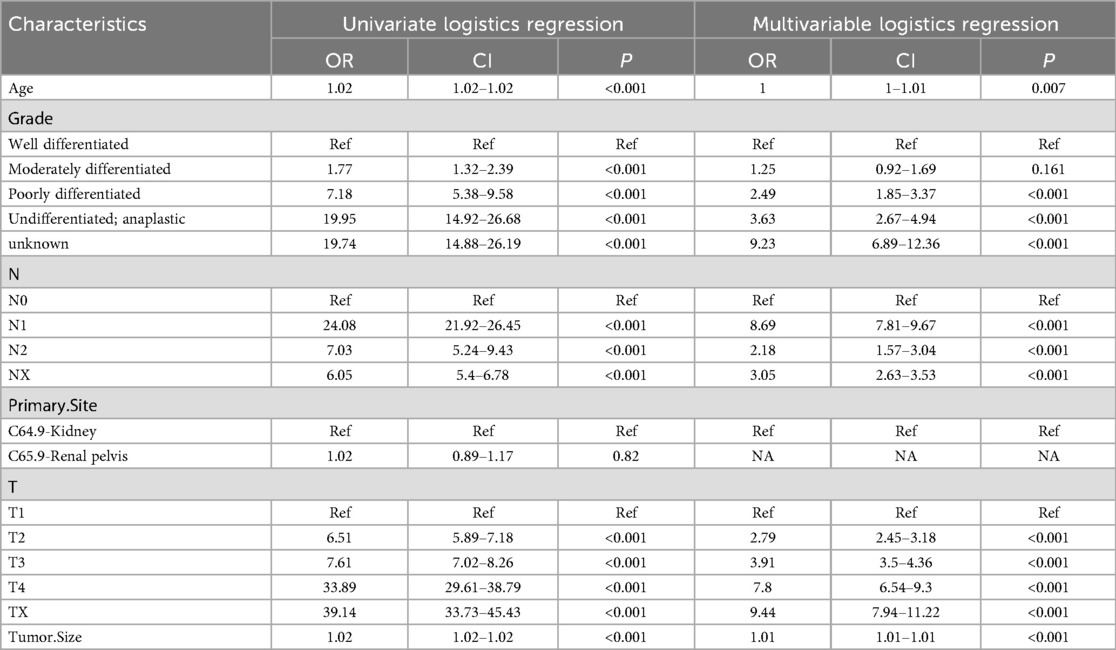
There were 4,874 metastatic patients during the follow-up. To identify independent risk factors for distal metastasis, LASSO (Figures 2A,B), univariate and multivariate regression analyses (Table 2) were utilized in order. The results of univariate analysis demonstrated that age, grade, T-stage, N-stage and tumor size (p = 0.007, p < 0.001, p < 0.001, p < 0.001, respectively) were related to distal metastasis. While multivariate analysis further confirmed that these variables can independently influence the distal metastasis of kidney cancer patients.
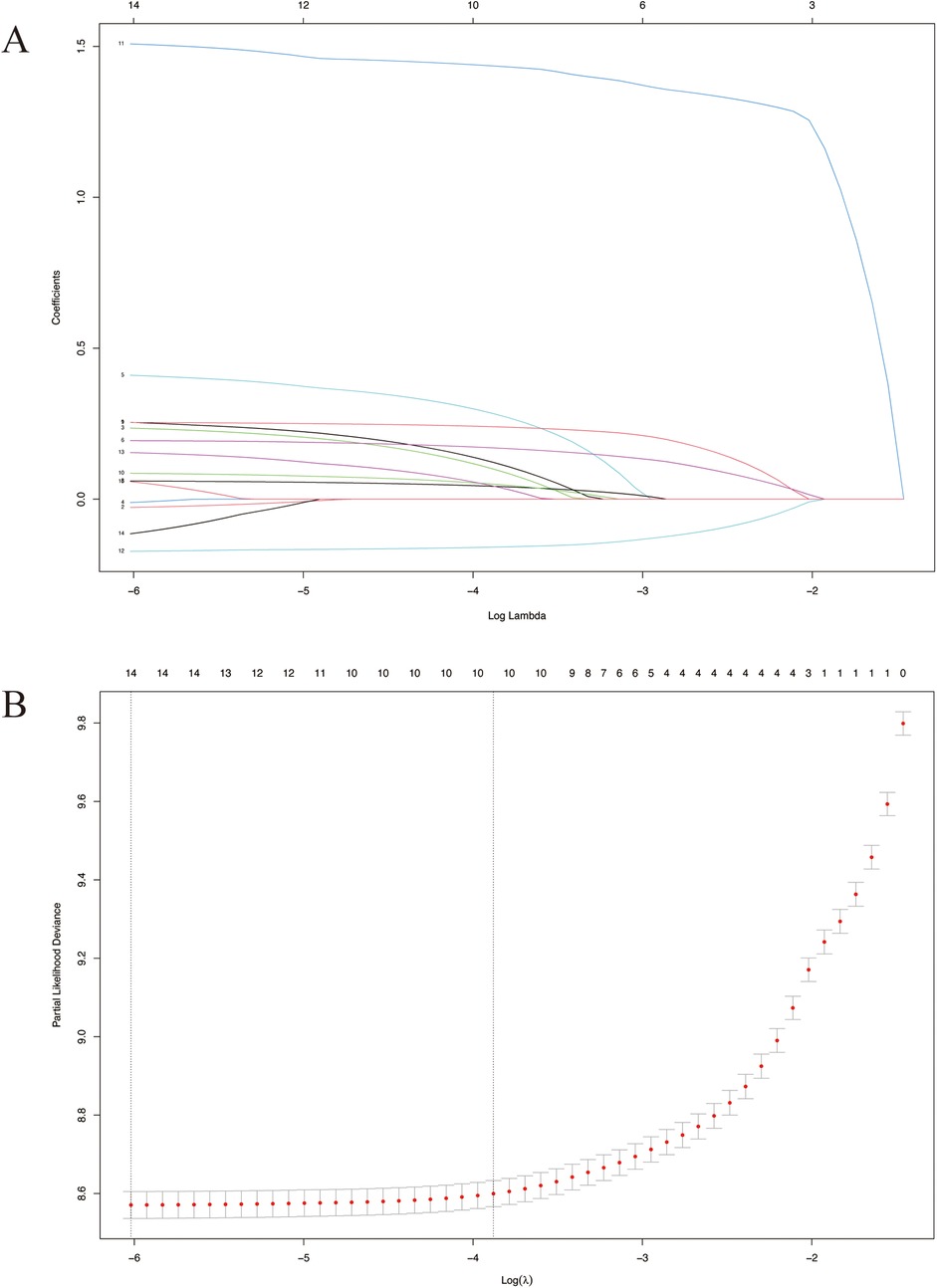
Figure 2. LASSO survival analysis. (A) Coefficient profile plots showing how the size of the coefficients of clinical factors shrinks with increasing value of the penalty, with the factors and their regression coefficients selected for the model based on the optimal for the LASSO model. (B) Penalty plot for the LASSO model; color error bars indicate standard error. LASSO, least absolute shrinkage and selection operator.
3.3 Performance of six machine learning algorithms
The predictive performance of six machine learning algorithms was compared via 10-fold cross validation in inner training dataset and ROC analysis in testing dataset. We found XGB had the best performance in predicting distal metastasis in both training (AUC = 0.91) and testing (AUC = 0.851) datasets (Figure 3A). Then T-stage, N-stage, grade, tumor size and age were arranged as per their relative importance in each algorithm (Figure 3B). This order was derived using the built-in gain-based importance metric of the XGBoost algorithm, which measures the average improvement in model accuracy brought by each feature across all trees. The fact that tumor size, N stage, and Grade emerged as the top three most important features suggests that these factors are strongly associated with the occurrence of distant metastasis in our model. This finding aligns well with established clinical knowledge, as larger tumor size, presence of nodal involvement, and higher histological grade are widely recognized as key indicators of aggressive disease and metastatic potential (24). A heatmap showing the predictive accuracy rate of six algorithms and the actual survival status of the testing dataset was displayed in Figure 3C. The cut-off value of XGB algorithm calculated by ROC curve was 0.492 (Figure 3D). The probability density function (PDF) for patients with non-distal metastases was concentrating on a metastasis risk between 0.0 and 0.5, while the PDF for patients with distal metastases was concentrated in a portion representing the metastasis risk (Figure 3E). The clinical utility curves (CUCs) of the XGB algorithm was also conducted, which exhibited the significant clinical utility (Figure 3F).
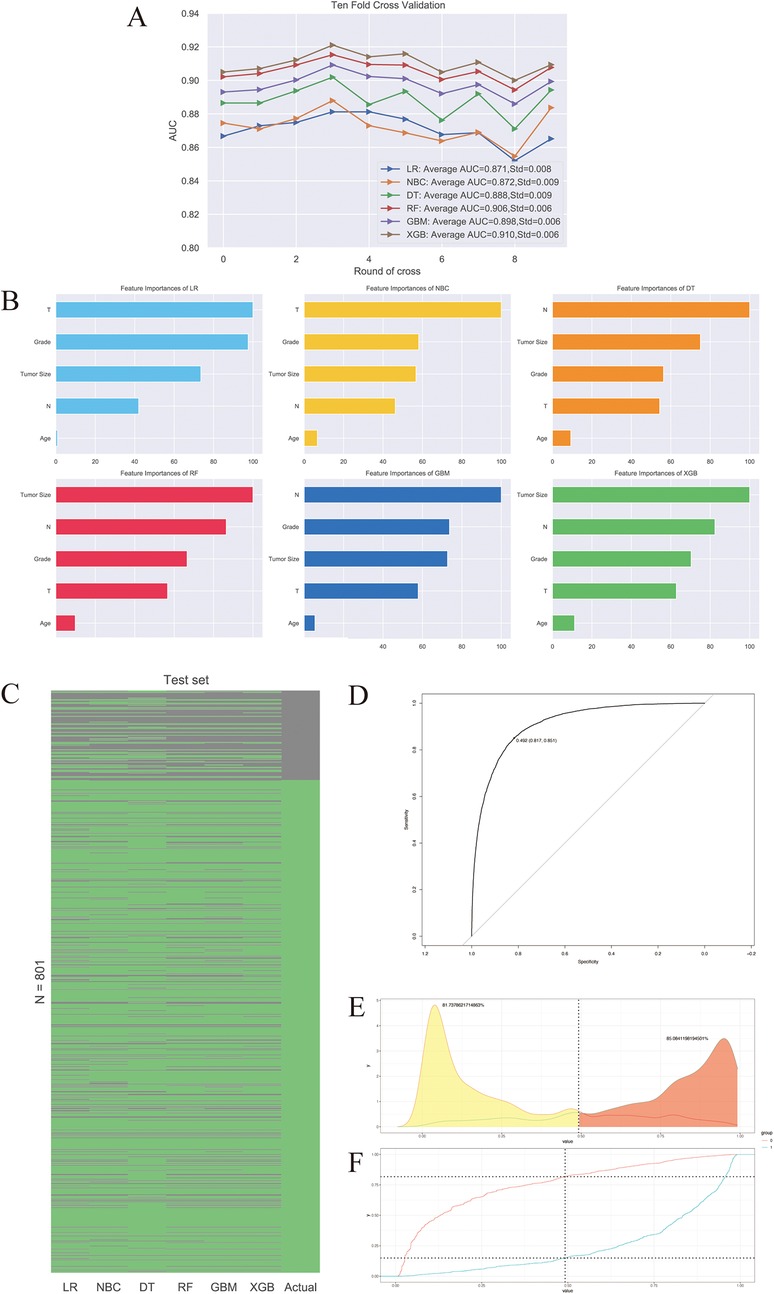
Figure 3. The predictive performance of six machine learning algorithms. (A) 10-fold cross-validation of machine learning algorithms. (B) Relative importance ranking of features. (C) Heat map of accuracy rate of prediction results. (D) ROC curve of XGB algorithm. (E) Transfer risk density. (F) The clinical utility curves (CUCs) of the XGB algorithm.
3.4 Establish the nomogram prediction model
Based on the clinicopathological characteristics listed in Table 1, together with the predicted risk of the XGB algorithm, we next employed LASSO Cox analysis to screen independent risk factors to predict survival possibility. Variables including marital status, sequence number, primary site, grade, pathological type, T-stage, N-stage, the calculated risk of XGB, surgical and radiation treatment were incorporated to establish a nomogram to predict the 1-, 3-, and 5-years survival probability (Figure 4A). The ROC and calibration curves of both training and test sets at 1, 3, and 5 years all displayed good consistency between actual and predictive values (Figures 4B,C). And then, the DCA was applied to check the clinical practicability (Figure 4D). The net benefits of the nomogram, in 1-, 3-, 5-year OS prediction, were all superior to the states when all patients survived or none. Furthermore, the Kaplan–Meier curves for samples stratified by above incorporated variables demonstrated (Figure 4E).
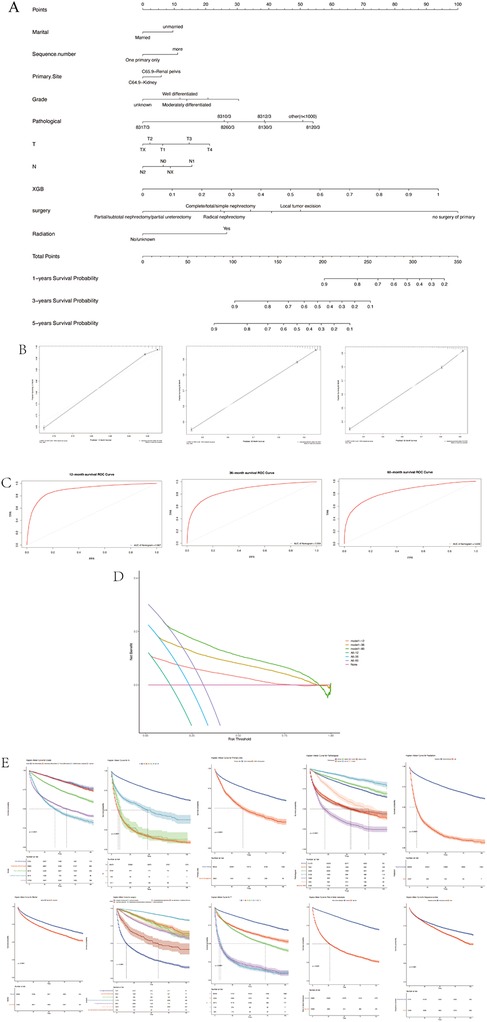
Figure 4. The survival prediction. (A) Nomogram. (B) The calibration curve. (C) ROC curves. (D) Decision curve analysis. (E) The Kaplan–Meier curves for samples stratified.
4 Discussion
The advent of the era of precision medicine has provided more advanced research tools for the development of clinical medicine. AI, as a branch of computer science, is gradually penetrating into the research field of precision medicine through algorithms that simulate human intelligence (25). AI uses intelligent algorithms to mine and extract medical data resources in order to improve the accuracy and effectiveness of clinical treatment. The combination of AI and medical research is one of the key research directions in biomedicine, especially in oncology, which provides a more accurate aid for clinicians' diagnosis and treatment (26). In recent years, AI has been rapidly developed and fruitful results have been achieved in the field of kidney cancer research. As a malignant tumor with high heterogeneity, kidney cancer has various pathological types, and there are obvious individualized differences in patients' treatment effects and clinical prognosis (27). The technologies of AI can provide important support for individualized diagnosis and treatment of kidney cancer.
Over the past two decades, the detection rate of small renal masses has risen significantly, largely attributable to advances in cross-sectional imaging. Partial nephrectomy (PN) is now widely established as the standard treatment for T1 renal parenchymal tumors (28). However, 20%-50% of kidney cancer patients have a distal metastasis or local invasion at initial diagnosis (24). The therapeutic landscape for metastatic renal cell carcinoma has significantly broadened. Interferon alfa, once a conventional option, has been largely superseded by newer agents that demonstrate superior efficacy, including improved response rates and/or prolonged progression-free survival. These advancements comprise antiangiogenic agents directed against VEGF and its receptors, mTOR inhibitors, and immune checkpoint inhibitors, collectively leading to enhanced clinical outcomes and a wider array of therapeutic strategies for this challenging malignancy (5). However, despite these advancements, the treatment of distantly metastatic renal cancer remains a formidable clinical challenge. The early detection of distal metastasis is a crucial measure for clinical decision-making and appropriate management of RCC patients. In this research, a nomogram was built for predicting the risk of distal metastasis in 40,527 kidney cancer patients extracted from the SEER database. We identified ten clinicopathological and demographic features as risk and prognostic predictors, including marital status, sequence number, primary site, grade, pathological type, T-stage, N-stage, the calculated risk of XGB, surgical and radiation treatment.
The impact of marital status on the survival possibility of mRCC was explored previously, which displayed the favorable prognostic effect of marriage on mRCC patients (29–32). Married patients tended to enjoy better survival outcomes than widowed patients in the aspects of both overall survival (OS) and cancer-specific survival (CSS). This may due to the unhealthy lifestyles and scanty financial resources of unmarried patients. Unmarried status was proved to be a barrier for obtaining treatment in mRCC patients (33). While married patients were more likely to receive financial and psychological support from their spouses, so that they can get timely medical care and medication reminders, and avoid psychological distress and depression (31, 34–37).
In our nomogram, we incorporated some vital tumor biological characteristics. The influence of histologic subtype on the metastatic potential of RCC was demonstrated in this study. Indeed, previous studies have found that ccRCC owned the highest metastasis risk, followed by pRCC and chRCC (38). Besides, poorly differentiated RCC generally had inferior prognosis (39–41). With the degree of RCC differentiation from well to poor, the rate of distal metastasis increased (42). This rate can increase by 50% with regional lymph node involvement (43).The tumor size was also an independent risk predictor, with a 2% metastatic proportion for RCC with mean size at 23 mm. When the size of renal neoplasms ≥3 cm, the risk of distal metastasis was higher (44, 45). A linear positive connection can be seen between tumor size and the metastatic rate.
The six applied algorithms are objective, reliable and repeatable in processing big data and can contribute to the inherent paradigm shift in healthcare, thus widely applied in identifying disease progression, improving early diagnosis and predicting survival outcomes. These advantages can facilitate the rational and effective employment of healthcare sources (46). By comparing the AUC values, XGB was found to have the best predictive performance. PDF and CUC further proved its powerful predictions.
Surgical treatment is very crucial for the primary lesion of RCC patients, because the metastasis risk can remarkably increase without nephrectomy (47). Brain metastasis is a typical site of metastasis and its metastatic rate ranged from 2% to 16% in mRCC (48). RCC patients with brain metastasis displayed limited responses to current treatment options with a short median overall survival of only 5–8 months (5, 47, 49, 50). And nonsurgical treatment was a risk factor for brain metastasis from RCC. Bone is another common metastatic site and bone metastasis often occurs in the mid-shaft bone, including osteolytic, osteogenic and mixed lesions. Bone metastasis can lead to skeletal-related events (SRE), such as fractures, hypercalcemia and spinal cord compression, which can have severe influence on patients' quality of life and survival outcomes (51). Although kidney cancer was insensitive to radiotherapy, it can reduce the risk of above SREs (52, 53). According to the findings of Hua et al, radiotherapy can not reduce the all-cause mortality (ACM) and kidney cancer-special mortality (KCSM) of kidney cancer patients with bone metastasis. While for bone metastasis patients, the conclusions about the surgery were discordant. For intermediate-risk patients, the effect of using sunitinib alone was no less than nephrectomy followed by sunitinib (2). While another study proved that ACM and KCSM of patients were markedly improved after surgery. The indications for surgery yet to be explored. And when analyzing the metastatic status and frequencies of renal pelvis cell carcinoma (RPCC), lung and brain were found to be the most and least common metastatic lesions, respectively (54, 55). The influence of the sequence number was also explored. In a previous study, RCC patients with only one primary tumor were more likely to develop bone metastasis. The lack of necessary survival time to form bone metastasis may explain it. While our study demonstrated that more sequence number was related to a worse prognosis.
The ethical implications of applying our predictive model clinically are crucial, particularly concerning patient privacy and data protection in real-world implementation. To address these concerns in potential future applications, we propose the following safeguards: (1) All patient data used by the model will be rigorously anonymized and encrypted both at rest and during transmission. (2) Where feasible, we recommend implementing federated learning techniques that allow the model to be trained and updated across institutions without transferring sensitive patient data. (3) Compliance with Regulations: Any clinical implementation will strictly follow established regulations and other relevant data protection frameworks. (4) Robust access controls and detailed audit trails will be implemented to monitor data usage and prevent unauthorized access.
Although this study included a sufficient number of patients and summarized their information as detailed as possible, the limitations of this study should be notified. First, this was a retrospective study and had inevitable selection bias. Second, apart from the included variables, we may miss some vital biomarkers, genetic mutations, tumor markers, comorbidities, clinical symptoms and treatment responses. Third, we only knew whether these patients received radiation or chemotherapy, but the detailed radiotherapy dose or toxic effects of chemotherapy were unknown, which can also affect the risk prediction. The information about immunotherapy was also lack. Moreover, more external multi-center data are required to verify the accuracy of prediction model.
5 Conclusion
The current study identified marital status, sequence number, primary site, grade, pathological type, T-stage, N-stage, the calculated risk of XGB, surgical and radiation treatment as independent prognostic factors of survival possibility in RCC patients. These DM-related risk factors were included to establish a predictive nomogram to screen RCC patients with a high risk of DM.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
TZ: Writing – original draft, Data curation, Methodology, Formal analysis. TT: Writing – original draft, Data curation, Methodology, Formal analysis. YiZ: Data curation, Writing – original draft, Formal analysis. QT: Data curation, Formal analysis, Methodology, Writing – original draft, Conceptualization. YuZ: Methodology, Resources, Writing – original draft. JP: Supervision, Visualization, Writing – review & editing. YW: Writing – review & editing, Supervision, Project administration, Investigation. CY: Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Shenzhen Medical Research Fund (to Q.T., Grant No. A2302036).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71(1):7–33. doi: 10.3322/caac.21654. Erratum in: CA Cancer J Clin. (2021) 71(4):359. doi: 10.3322/caac.2166933433946
2. Méjean A, Ravaud A, Thezenas S, Colas S, Beauval J-B, Bensalah K, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. (2018) 379(5):417–27. doi: 10.1056/NEJMoa1803675
3. Zhang C, He H, Hu X, Liu A, Huang D, Xu Y, et al. Development and validation of a metastasis-associated prognostic signature based on single-cell RNA-Seq in clear cell renal cell carcinoma. Aging (Albany NY). (2019) 11(22):10183. doi: 10.18632/aging.102434
4. Surveillance E, Program ER. SEER Stat Fact Sheets: Kidney and Renal Pelvis Cancer. Bethesda, MD: National Cancer Institute (2016).
5. Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. (2017) 376(4):354–66. doi: 10.1056/NEJMra1601333
6. Gill IS, Aron M, Gervais DA, Jewett MA. Small renal mass. N Engl J Med. (2010) 362(7):624–34. doi: 10.1056/NEJMcp0910041
7. Dabestani S, Thorstenson A, Lindblad P, Harmenberg U, Ljungberg B, Lundstam S. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. (2016) 34(8):1081–6. doi: 10.1007/s00345-016-1773-y
8. Rabinovitch RA, Zelefsky MJ, Gaynor JJ, Fuks Z. Patterns of failure following surgical resection of renal cell carcinoma: implications for adjuvant local and systemic therapy. J Clin Oncol. (1994) 12(1):206–12. doi: 10.1200/JCO.1994.12.1.206
9. Sandock DS, Seftel AD, Resnick MI. A new protocol for the followup of renal cell carcinoma based on pathological stage. J Urol. (1995) 154(1):28–31. doi: 10.1016/S0022-5347(01)67215-X
10. Capitanio U, Montorsi F. Renal cancer. Lancet. (2016) 387(10021):894–906. doi: 10.1016/S0140-6736(15)00046-X
11. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. (2015) 373(19):1803–13. doi: 10.1056/NEJMoa1510665
12. Motzer RJ, Tannir NM, McDermott DF, Frontera OA, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. (2018) 378:1277–1290. doi: 10.1056/NEJMoa1712126
13. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. (2007) 356(2):115–24. doi: 10.1056/NEJMoa065044
14. Sternberg CN, Davis ID, Mardiak J, Szczylik C, Wagstaff J, Salman P, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. (2010) 28:1061–8. doi: 10.1200/JCO.2009.23.9764
15. Escudier B, Bellmunt J, Négrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. (2010) 28(13):2144–50. doi: 10.1200/JCO.2009.26.7849
16. Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. (2011) 378(9807):1931–9. doi: 10.1016/S0140-6736(11)61613-9
17. Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol. (2017) 35(6):591. doi: 10.1200/JCO.2016.70.7398
18. Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. (2015) 16(15):1473–82. doi: 10.1016/S1470-2045(15)00290-9
19. Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. (2006) 24(35):5601–8. doi: 10.1200/JCO.2006.08.5415
20. Vijithananda SM, Jayatilake ML, Hewavithana B, Gonçalves T, Rato LM, Weerakoon BS, et al. Feature extraction from MRI ADC images for brain tumor classification using machine learning techniques. Biomed Eng Online. (2022) 21(1):52. doi: 10.1186/s12938-022-01022-6
21. Fan Z, Huang Z, Huang X. Bone metastasis in renal cell carcinoma patients: risk and prognostic factors and nomograms. J Oncol. (2021) 2021:5575295. doi: 10.1155/2021/5575295
22. Tong Y, Huang Z, Hu C, Chi C, Lv M, Song Y. Construction and validation of a convenient clinical nomogram to predict the risk of brain metastasis in renal cell carcinoma patients. BioMed Res Int. (2020) 2020:9501760. doi: 10.1155/2020/9501760
23. Sheng X, Lu X, Wu J, Chen L, Cao H. A nomogram predicting the prognosis of renal cell carcinoma patients with lung metastases. BioMed Res Int. (2021) 2021:6627562. doi: 10.1155/2021/6627562
24. Shinder BM, Rhee K, Farrell D, Farber NJ, Stein MN, Jang TL, et al. Surgical management of advanced and metastatic renal cell carcinoma: a multidisciplinary approach. Front Oncol. (2017) 7:107. doi: 10.3389/fonc.2017.00107
25. Bousquet C, Beltramin D. Machine learning in medicine: to explain, or not to explain, that is the question. Stud Health Technol Inform. (2022) 294:114–5. doi: 10.3233/SHTI220407
26. Verma AA, Murray J, Greiner R, Cohen JP, Shojania KG, Ghassemi M, et al. Implementing machine learning in medicine. Can Med Assoc J. (2021) 193(34):E1351–7. doi: 10.1503/cmaj.202434
27. Eminaga O, Shkolyar E, Breil B, Semjonow A, Boegemann M, Xing L, et al. Artificial intelligence-based prognostic model for urologic cancers: a SEER-based study. Cancers (Basel). (2022) 14(13):3135. doi: 10.3390/cancers14133135
28. Kim SP, Thompson RH, Boorjian SA, Weight CJ, Han LC, Murad MH, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol. (2012) 188:51–7. doi: 10.1016/j.juro.2012.03.006
29. Yue G, Deyu L, Lianyuan T, Fengmin S, Mei G, Yajun H, et al. Clinical features and prognostic factors of patients with metastatic renal cell carcinoma stratified by age. Aging (Albany NY). (2021) 13(6):8290. doi: 10.18632/aging.202637
30. Marchioni M, Martel T, Bandini M, Pompe RS, Tian Z, Kapoor A, et al. Marital status and gender affect stage, tumor grade, treatment type and cancer specific mortality in T1–2 N0 M0 renal cell carcinoma. World J Urol. (2017) 35(12):1899–905. doi: 10.1007/s00345-017-2082-9
31. Li Y, Zhu M-x, Qi S-h. Marital status and survival in patients with renal cell carcinoma. Medicine (Baltimore). (2018) 97(16):e0385. doi: 10.1097/MD.0000000000010385
32. Wang H, Wang L, Kabirov I, Peng L, Chen G, Yang Y. Impact of marital status on renal cancer patient survival. Oncotarget. (2017) 8(41):70204. doi: 10.18632/oncotarget.19600
33. Rosiello G, Knipper S, Palumbo C, Dzyuba-Negrean C, Pecoraro A, Mazzone E, et al. Unmarried status is a barrier for access to treatment in patients with metastatic renal cell carcinoma. Int Urol Nephrol. (2019) 51(12):2181–8. doi: 10.1007/s11255-019-02266-3
34. Chen J, Cao N, Li S, Wang Y. Identification of a risk stratification model to predict overall survival and surgical benefit in clear cell renal cell carcinoma with distant metastasis. Front Oncol. (2021) 11:630842. doi: 10.3389/fonc.2021.630842
35. Haley WE. Family caregivers of elderly patients with cancer: understanding and minimizing the burden of care. J Support Oncol. (2003) 1(4 Suppl 2):25–9. PMID: 1534699715346997
36. Hemminki K, Chen B. Lifestyle and cancer: effect of parental divorce. Eur J Cancer Prev. (2006) 15:524–30. doi: 10.1097/01.cej.0000220633.93104.64
37. Kaiser NC, Hartoonian N, Owen JE. Toward a cancer-specific model of psychological distress: population data from the 2003–2005 national health interview surveys. J Cancer Surviv. (2010) 4(4):291–302. doi: 10.1007/s11764-010-0120-3
38. Daugherty M, Sedaghatpour D, Shapiro O, Vourganti S, Kutikov A, Bratslavsky G. The metastatic potential of renal tumors: influence of histologic subtypes on definition of small renal masses, risk stratification, and future active surveillance protocols. In: Maranchie J, editor. Urologic Oncology: Seminars and Original Investigations: 2017. New York, NY: Elsevier (2017). p. 153.e115–e120.
39. Pichler R, Comperat E, Klatte T, Pichler M, Loidl W, Lusuardi L. Renal cell carcinoma with sarcomatoid features: finally new therapeutic hope? Cancers (Basel). (2019) 11:422. doi: 10.3390/cancers11030422
40. Porfyris O, Alexandrou P, Masaoutis C, Nikolakakos F. Ovarian metastasis of renal cell carcinoma: clinical and pathological presentation of a case. Turk J Urol. (2019) 45(2):150. doi: 10.5152/tud.2018.96237
41. Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. (2011) 135(1):92–109. doi: 10.5858/2010-0478-RAR.1
42. Li Y, Chen P, Chen Z. A population-based study to predict distant metastasis in patients with renal cell carcinoma. Ann Palliat Med. (2021) 10:4273–88. doi: 10.21037/apm-20-2481
43. Tadayoni A, Paschall AK, Malayeri AA. Assessing lymph node status in patients with kidney cancer. Transl Androl Urol. (2018) 7(5):766. doi: 10.21037/tau.2018.07.19
44. Zastrow S, Phuong A, von Bar I, Novotny V, Hakenberg OW, Wirth MP. Primary tumor size in renal cell cancer in relation to the occurrence of synchronous metastatic disease. Urol Int. (2014) 92(4):462–7. doi: 10.1159/000356325
45. Smaldone MC, Kutikov A, Egleston BL, Canter DJ, Viterbo R, Chen DY, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer. (2012) 118(4):997–1006. doi: 10.1002/cncr.26369
46. Li W, Wang J, Liu W, Xu C, Li W, Zhang K, et al. Machine learning applications for the prediction of bone cement leakage in percutaneous vertebroplasty. Front Public Health. (2021) 9:812023. doi: 10.3389/fpubh.2021.812023
47. Zhuang W, Li Y, Chen P, Wang J, Liu W, Chen J. Do renal cell carcinoma patients with brain metastases still need nephrectomy? Int Urol Nephrol. (2019) 51(6):941–9. doi: 10.1007/s11255-019-02139-9
48. Bianchi M, Sun M, Jeldres C, Shariat S, Trinh Q-D, Briganti A, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. (2012) 23(4):973–80. doi: 10.1093/annonc/mdr362
49. Hu J, Guan W, Liu P, Dai J, Tang K, Xiao H, et al. Endoglin is essential for the maintenance of self-renewal and chemoresistance in renal cancer stem cells. Stem Cell Rep. (2017) 9(2):464–77. doi: 10.1016/j.stemcr.2017.07.009
50. Bowman IA, Bent A, Le T, Christie A, Wardak Z, Arriaga Y, et al. Improved survival outcomes for kidney cancer patients with brain metastases. Clin Genitourin Cancer. (2019) 17(2):e263–72. doi: 10.1016/j.clgc.2018.11.007
51. Hua K-C, Hu Y-C. Establishment of predictive model for patients with kidney cancer bone metastasis: a study based on SEER database. Transl Androl Urol. (2020) 9(2):523. doi: 10.21037/tau.2020.01.24
52. Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. (2011) 79(4):965–76. doi: 10.1016/j.ijrobp.2010.11.026
53. Zelefsky MJ, Greco C, Motzer R, Magsanoc JM, Pei X, Lovelock M, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys. (2012) 82(5):1744–8. doi: 10.1016/j.ijrobp.2011.02.040
54. Chen W-K, Wu Z-G, Xiao Y-B, Wang Q-Q, Yu D-D, Cai J, et al. Prognostic value of site-specific metastases and therapeutic roles of surgery and chemotherapy for patients with metastatic renal pelvis cancer: a SEER based study. Technol Cancer Res Treat. (2021) 20:15330338211004914. doi: 10.1177/15330338211004914
Keywords: kidney cancer, distal metastasis, nomogram, machine learning, predictive model
Citation: Zhang T, Tian T, Zhang Y, Teng Q, Zhang Y, Pang J, Wang Y and Yang C (2025) Clinical decision system for renal cell carcinoma integrating interpretable machine learning algorithms. Front. Surg. 12:1588208. doi: 10.3389/fsurg.2025.1588208
Received: 5 March 2025; Accepted: 24 September 2025;
Published: 27 November 2025.
Edited by:
Shailesh Tripathi, Rajendra Institute of Medical Sciences, IndiaReviewed by:
Wenle Li, Xiamen University, ChinaJinxiang Shang, The Affiliated Hospital of Shaoxing University, China
Copyright: © 2025 Zhang, Tian, Zhang, Teng, Zhang, Pang, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Pang, cGFuZ2p1bjJAbWFpbC5zeXN1LmVkdS5jbg==; Ya Wang, NDk4MzAzNDAzQHFxLmNvbQ==; Chao Yang, Njc1ODcxNjIzQHFxLmNvbQ==
†These authors have contributed equally to this work
 Tianhong Zhang1,†
Tianhong Zhang1,† Yifan Zhang
Yifan Zhang Jun Pang
Jun Pang