- 1Department of Vascular Surgery, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
- 2Department of Vascular Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 3Department of Interventional Medicine and Vascular, Binzhou Medical University Hospital, Yantai, Shandong, China
- 4Department of Vascular Surgery, Shanghai Fourth People’s Hospital, School of Medicine, Tongji University, Shanghai, China
Objective: To evaluate the feasibility and short-term clinical outcomes of the Kabedon technique-based endovascular aneurysm repair (EVAR) for abdominal aortic aneurysms (AAA) with severe infrarenal neck angulation (angle >60°).
Methods: This retrospective cohort study was based on a single-center database. Between January 2019 and May 2023, 120 patients with AAA of hostile neck angulation underwent endovascular procedures using the Kabedon technique for abdominal aortic remodeling. A standardized protocol was followed to calculate the serial changes in the aneurysmal neck angle. The primary endpoints were proximal type Ia endoleak and stent-graft migration. The secondary endpoints were all-cause and aneurysm-related mortality, proximal neck dilatation, and re-intervention.
Results: The mean age was 71.40 ± 10.69 years, and 95 (79.17%) were male. The mean AAA sac diameter and proximal neck angle were 63.71 ± 17.32 mm and 83.67 ± 18.45°, respectively. All patients underwent the Kabedon-based EVAR, with a technical success rate of 94.17% (113/120). During the operation, 7 cases of endoleak and 2 cases of endograft migration were observed, which were resolved by corresponding measures such as coil embolization and proximal cuff stent salvage. No complications were observed within 30 days. In addition, neck calcification, funnel-shaped aneurysm, intraoperative complications and corresponding treatments may be potential negative factors for technical success, but there was no statistical difference.
Conclusions: Kabedon-based EVAR for AAA with a severely angulated neck provided high technical success, low mortality and complication rates during short-term follow-up. Further studies with larger sample sizes and longer follow-up periods are warranted.
Introduction
Endovascular aneurysm repair (EVAR) has become an effective alternative procedure for open repair of abdominal aortic aneurysm (AAA) owing to a shorter hospital stay and lower postoperative mortality and morbidity (1–4). Upwards of 30% of patients with AAA have unsuitable proximal neck morphology for conventional endovascular repair (5, 6). The most common prognostic factors for poor outcomes include short infrarenal aneurysmal neck, large neck diameter, large aneurysmal sac diameter, neck thrombus, and complex iliac artery anatomy (7–9). Particularly in patients with severe proximal neck angulation (angle >60°), it not only increases the technical difficulty of stent placement but also results in poor short-term outcomes, with a high incidence of type Ia endoleak and graft migration (10–12). Recently, advanced endovascular techniques and devices have been developed to make stent-graft sealing easier by adjusting the neck angulation (13–15). However, procedures using these innovations are still technically demanding and cannot exclude the possibility of endoleaks and migration (16).
In this study, we propose a novel stent-graft deployment technique termed the “Kabedon” technique. Utilizing a stiff guidewire and highly conformable endograft, this method adapts to severe aneurysm neck angulation and conforms to aortic curvature, achieving secure fixation with optimized wall apposition and consequent hemodynamic enhancement. On this basis, we determined the feasibility and short-term clinical outcomes of this novel procedure for AAA with hostile neck anatomy.
Methods
Study design and patient selection
This was a single-center retrospective observational study. From January 2019 to May 2023, 120 consecutive patients underwent EVAR using the Kabedon technique at the Affiliated Hospital of Qingdao University, Shandong, China. They were diagnosed as having AAA with severe proximal neck angulation, by computer tomography angiography (CTA). The inclusion criteria for this study were as follows: (1) infrarenal aortic angulation >60°; (2) proximal neck length >15 mm; (3) distal iliac fixation site diameter <16 mm and >30 mm in length (17); (4) life expectancy >1 years exclusion criteria were as follows: (1) low operative risk for open repair, (2) connective tissue disease, (3) ASA grade IV or V, (4) proximal neck thrombus >50% of circumference, (5) proximal neck calcification >50% of circumference (18); (6) Infected AAA.
Patient demographics, risk factors, operational details, and clinical outcomes were also recorded. Aortic morphology data were obtained using preoperative CTA. The study protocol was approved by the local institutional review board and Ethics Committee (Figure 1).
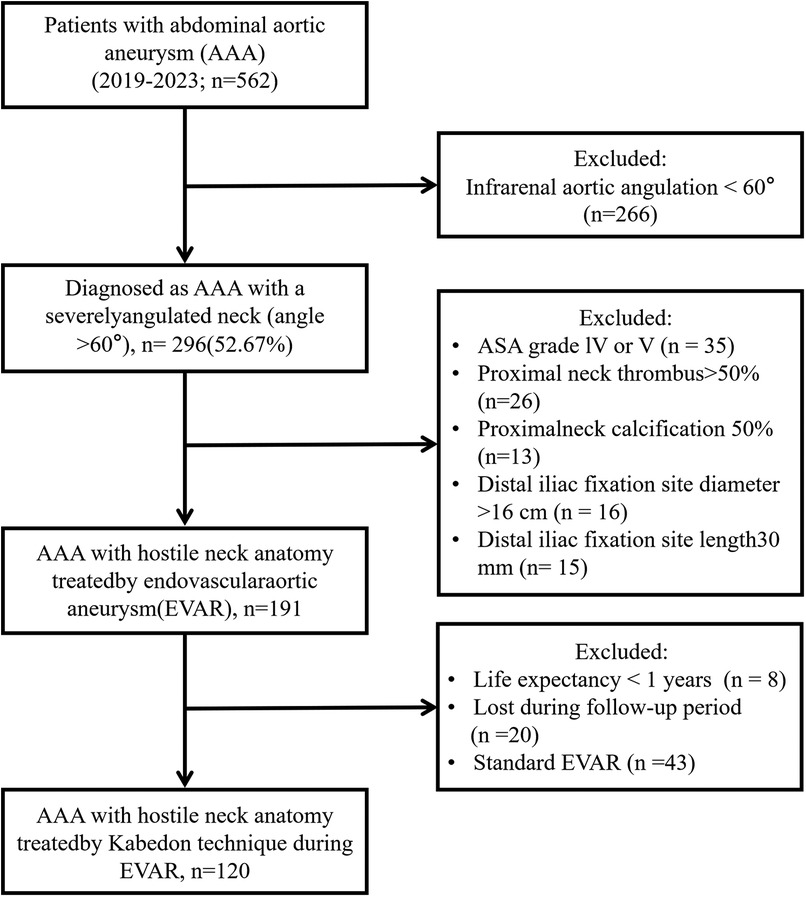
Figure 1. Flow diagram of the enrolled patients of abdominal aortic aneurysm with a severely angulated neck (angle >60°) by Kabedon technique during endovascular aortic repair.
Pre-operative evaluation
Two experienced vascular surgeons used a standardized CT measurement protocol to calculate the serial change in AAA neck angulation using three-dimensional (3D) volume-rendered images generated via post-acquisition software (Endosize, TherenvaSAS, France) before the procedure. The AAA characteristics recorded were the maximum diameter of the AAA and proximal neck anatomy, including diameter, length, angulation, thrombus, and calcification. A center lumen line (CLL) was used to measure diameter and length. The maximum aneurysm diameter and AAA neck length were measured in the axial view (1.0-mm slices) perpendicular to just below the most caudal renal artery. Similarly, the proximal neck angle was measured as described by Chaikof et al. Calcification and thrombus were measured at the level of the lowest renal artery (19). The aortic neck scoring system, according to the Society for Vascular Surgery grading classification for morphological risk, was used.
Selection of endovascular stent grafts
Aortoiliac sizing and endograft planning were performed according to our routine preoperative evaluation practice (20). The selection of aortic stent graft, iliac artery stent, and stiff guidewire type was based on institutional practice and surgeon experience, as were other technical aspects of EVAR procedures performed.
“Kabedon” technique in endovascular procedure
The term “Kabedon” is derived from Japanese popular culture, describing the act of confining someone against a wall with one arm. This metaphorically reflects the technique's core principle: the stiff guidewire forms a supportive “arm” along the aortic curvature (Figure 2B), confining the stent-graft against the greater curvature wall to optimize sealing.All procedures were performed under general anesthesia by experienced vascular surgeons in a hybrid vascular operative room. Systemic heparinization of 100 IU/kg was performed after the percutaneous arterial puncture. The femoral artery was cannulated with an 8 Fr sheath, and a 0.035-inch ultra-sliding guidewire and 5F pigtail catheter were placed. Initial anteroposterior abdominal aortography was performed to inspect the location of the renal or iliac arteries, and the hostile anatomy was characterized by a severely angulated proximal neck. The stiff guidewire was advanced into the ascending aorta to form the “Loop,” then it was kept in tension to establish a through-and-through guidewire along the large curvature of AAA. The stiff guidewire was called “Kabedon.” When the highly conformable endograft was in position, we imposed a certain tension on the through-and-through guidewire to create a stent graft along the aortic curvature, obtaining the ideal aortic neck–endograft alignment. Bilateral iliac arteries were reconstructed using a cross-limb configuration (21). If an iliac aneurysm was observed, concomitant IIA embolization was performed using detachable coils before the reconstruction of the iliac arteries. Final angiography was performed to verify the correct position of the endograft and the absence of an endoleak (Figures 2, 3).
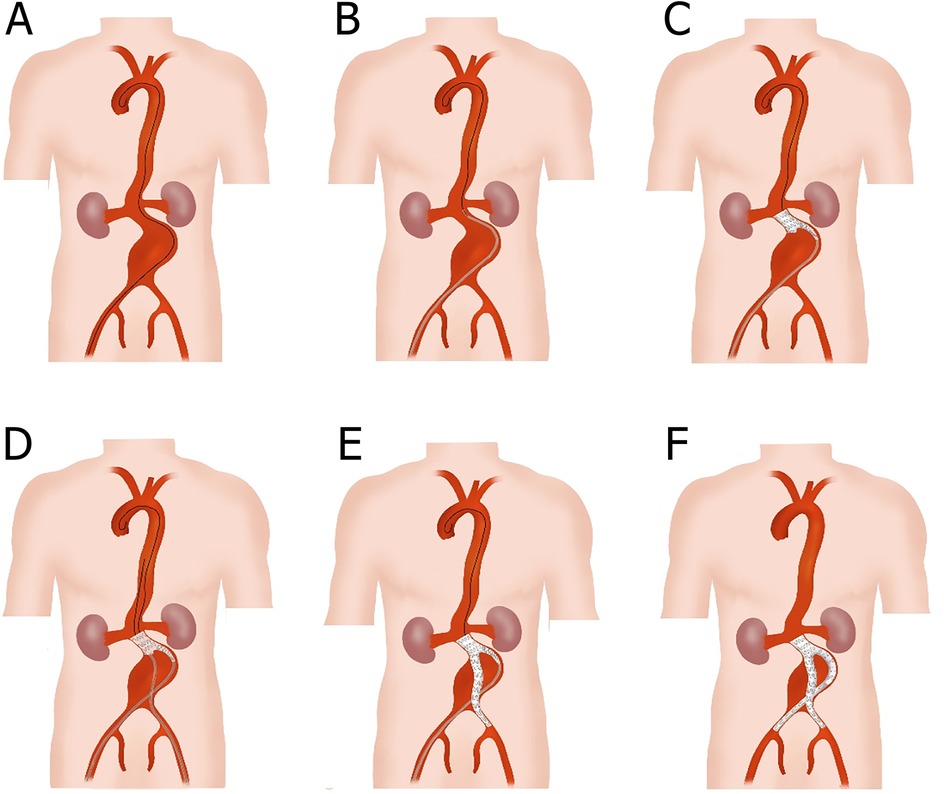
Figure 2. Schematic diagram of the total endovascular treatment with Kabedon technique for abdominal aortic aneurysm with severely angulated neck. (A) Exchange the ultra-sliding guidewire with the stiff guidewire; then, along the large curvature of the AAA, the top end of the stiff guidewire was advanced to the aortic valve and formed a loop. (B) A bifurcated main-body stent graft was placed at the lower edge of the lowest renal artery with the aid of a delivery sheath. (C) The main body of the stent graft was deployed without opening its long limb. (D) Another exchanged stiff guidewire and delivery sheath were advanced to the short limb of the main body stent graft through the contralateral femoral artery. (E) The contralateral common iliac artery was reconstructed with a branch membrane-covered stent, and the end of the branch stent was positioned at the upper edge of the internal iliac artery. (F) The ipsilateral common iliac artery was also reconstructed using a branch membrane-covered stent, and a cross-limb structure was eventually formed.
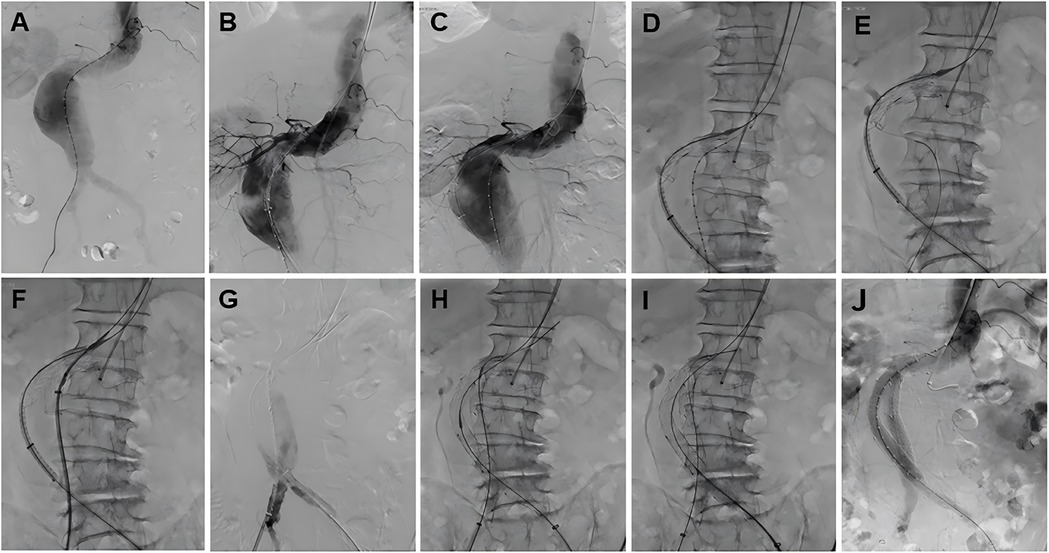
Figure 3. The fluoroscopy procedural steps of the total endovascular treatment with Kabedon technique for treatment of abdominal aortic aneurysm with severely angulated neck. (A) Abdominal aortography. (B) Access the stent to identify the position of the renal arteries. (C) The guidewire provides tension, and the stent delivery system fits the aneurysm. (D) Deploy the stent while maintaining guidewire tension. (E) Select the contralateral iliac branch. (F) Deliver the right iliac artery stent system. (G) Identify the bifurcation position of the right common iliac artery. (H) Deploy the right iliac limb. (I) Deploy the left iliac limb. (J) Angiography after stent deployment. From (D–I), the guidewire is required to provide tension to make the stent fit the aneurysm perfectly, and finally, the iliac limbs on both sides need to reliably provide subsequent stability.
Perioperative monitor and follow-up
Aspirin (100 mg) was administered starting at 1-day post-surgery. Perioperative characteristics were recorded, including adjunctive procedures, procedure and fluoroscopy times, contrast volume, primary technical success, and days of hospitalization. Technical success was defined as the successful introduction and deployment of a stent graft in the absence of stent graft migration, exclusion of the aneurysmal sac on the completion angiogram, and freedom from type Ia or III endoleak or graft limb occlusion. Follow-up visits involved clinical examination and aortic CT angiography (CTA) and were performed at 1, 6, and 12 months and annually after discharge, followed by duplex ultrasound. The clinical course (mortality, morbidity, and incidence of post-implantation syndrome) and follow-up CTA (proximal neck dilatation, endoleak, graft thrombosis, migration, and rupture) were also recorded for each patient. The primary endpoints were proximal type Ia endoleak and stent graft migration. The secondary endpoints were all-cause and aneurysm-related mortality, proximal neck dilatation, aneurysm rupture, and re-intervention.
Statistical analysis
All statistical analyses were performed using the IBM SPSS software (version 23.0; IBM Corp., Armonk, NY, USA). Continuous variables are described as mean ± standard deviation (SD), and the specific data are presented as counts and percentages. For continuous variables, the Student's t-test was conducted if the variable was normally distributed, whereas the Mann–Whitney U-test was used for non-normally distributed variables. The chi-squared test was used for categorical variables. Additionally, logistic regression was employed to identify variables potentially associated with the study endpoint. Statistical p-value < 0.05.
Results
Baseline clinical characteristics
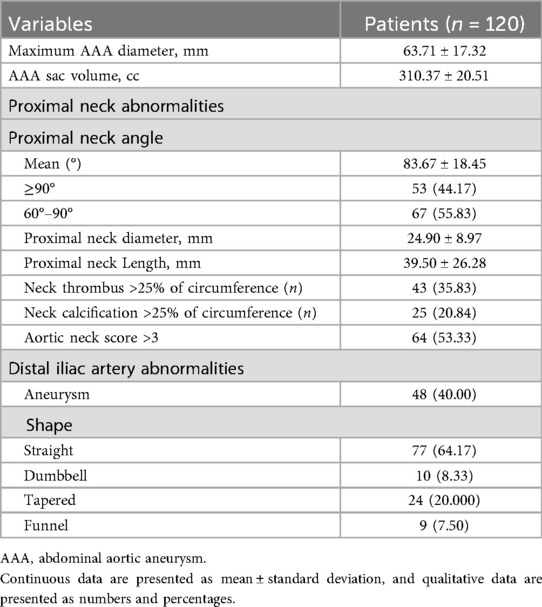
The clinical and anatomical baseline characteristics are presented in Table 1. 120 patients with AAA and severe neck angulation were evaluated for Kabedon-based EVAR at our center. The mean age was 71.40 ± 10.69 years, and 79.17% were male. The top three comorbidities were hypertension (n = 82, 68.33%), smoking history (n = 90, 75.00%), and coronary artery disease (n = 46, 38.33%).
Preoperative morphological characteristic
The mean maximum diameter of AAA was 63.71 ± 17.32 mm, with a mean aneurysm volume of 310.37 ± 20.51 cc and proximal neck angle of 83.67 ± 18.45°. The neck angulation was >60° in all cases (100.00%) and ≥90° in 53 (44.17%). The mean proximal neck length was 39.50 ± 26.28 mm, and the mean proximal neck diameter was 24.90 ± 8.97 mm. 25 (20.84%) and 43 patients (35.83%) were respectively observed with neck thrombus and calcification >25% of the circumference. 64 patients (53.33%) had aortic neck scores were >3 points. The rate of aneurysm at the distal iliac artery was 40.00% (48/120). In terms of AAA shape, most are Straight or Tapered, but there are still 9 cases (7.50%) showing Funnel. All the data are presented in Table 2.
Details and outcomes of the surgery
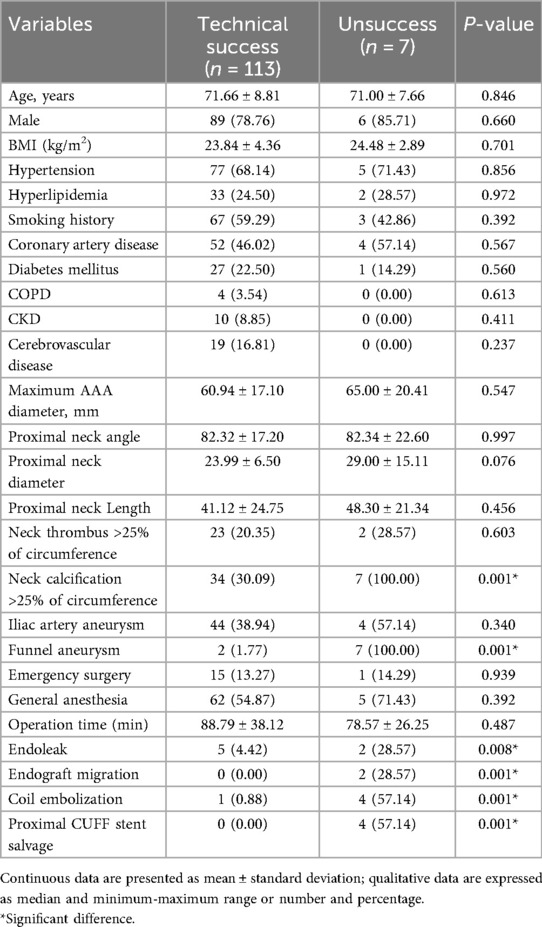
The intraoperative details of the 120 patients treated with Kabedon-based EVAR are presented in Table 3. Among all patients, 16 (13.33%) underwent emergency EVAR due to unstable AAA. The overall operational time was 105.00 ± 30.13 min. The mean contrast volume and fluoroscopic time were 97.31 ± 22.44 ml and 30.25 ± 4.31 min. The median oversize of the proximal aorta was 21.42 ± 8.55%. The mean diameter of the aortic stent graft was 28.12 ± 11.33 mm. The mean centerline length of the stent graft in the proximal landing zone was 36.21 ± 14.27 mm and 16.78 ± 12.13 mm in the modeled and actual seal zones, respectively. Comfortable aortic stent grafts were used to reconstruct the AAA, including those using Excluder (Gore C3, n = 87) and Minos (MicroPort, n = 33). The bridge endograft for the common iliac artery was the Iliac Branch Endoprosthesis of corresponding brands (W.L. Gore & Associates, USA, n = 87; (Shanghai MicroPort Endovascular MedTech Co., Shanghai, PRC, n = 33). In terms of surgical outcomes, the technical success rate reached 94.17% (113/120). 2 (1.67%) patients required conversion to open surgery due to AAA rupture, and 1 (0.83%) patient with ruptured AAA died intraoperatively due to hemorrhagic shock. Regarding complications, 7 (5.83%) patients experienced immediate endograft migration and endoleak formation. They underwent coil embolization and proximal cuff stent bridging. A patients received chimney procedure for renal artery reconstruction.
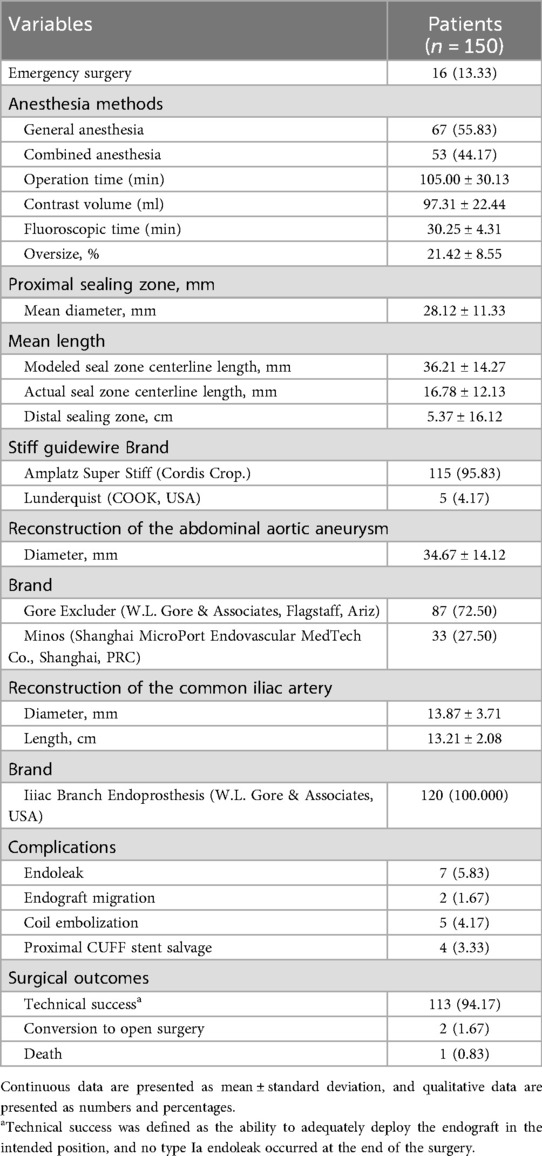
Table 3. Operative procedures of 120 patients treated by Kabedon-based endovascular aneurysm repair for abdominal aortic aneurysm with a severely angulated neck.
Risk factors of technical success in Kabedon-EVAR
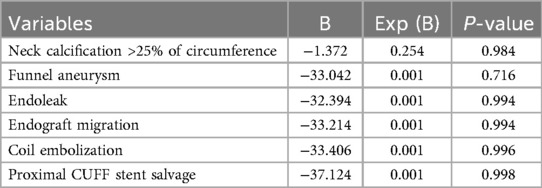
To evaluate the application value of the Kabedon technique, we analyzed the factors that may be related to its technical success rate. Univariate analysis showed that the shape of the aneurysm was associated with technical success, with patients having funnel-shaped aneurysms showing a lower technical success rate. In addition, patients with neck calcification were associated with technical failure. From the details of the surgery, complications such as endoleaks and endograft migration occurring during the operation, as well as procedures like coil embolization and proximal CUFF stent salvage, may all indicate a lower technical success rate. According to the research results, no other factors showed a potential correlation with the technical success rate (Table 4).
Based on the results of the univariate analysis and clinical relevance, we selected 6 variables for multivariate regression analysis. Although all the aforementioned factors suggested that they might be negative influencing factors of the Kabedon technique, the current data analysis indicated that there were no statistically significant differences (Table 5).
Early and short-term follow-up outcomes
The early and short-term outcomes are summarized in Table 6. The duration of hospitalization was 13.50 ± 4.03 days. In the one-month follow-up, the patients were in stable condition, and no one developed complications such as endograft migration or endoleak. The mean follow-up was 14.00 ± 2.59 months. None of the patients were lost to follow-up. During the follow-up period, 5 patients (4.17%) were found to have sac expansion, among whom 2 (1.67%) had type Ib endoleaks and received active treatment. One (0.83%) patient developed iliac limb occlusion, which was considered possibly due to thrombosis (Table 6). No cases of significant endograft migration were detected in the latest follow-up CTA. The complete imaging treatment and follow-up periods are shown in Figure 4.
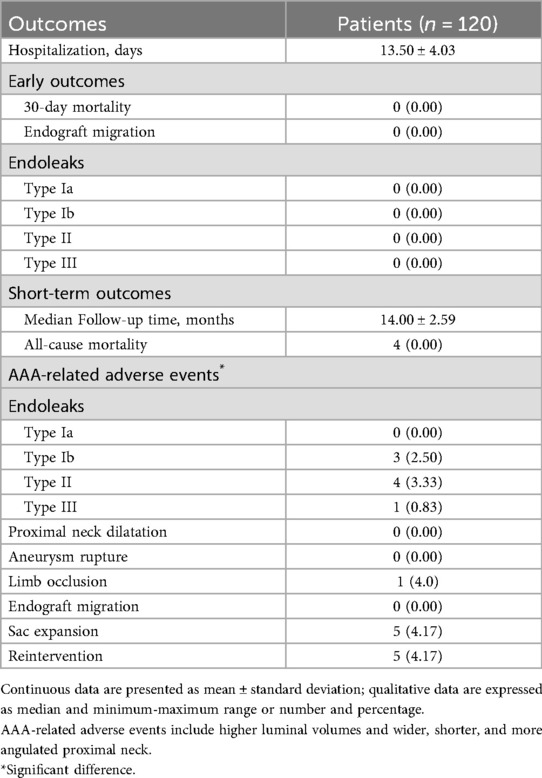
Table 6. Early and short-term clinical outcomes in 120 cases of abdominal aortic aneurysm with a severely angulated neck treated with Kabedon-based endovascular aneurysm repair.
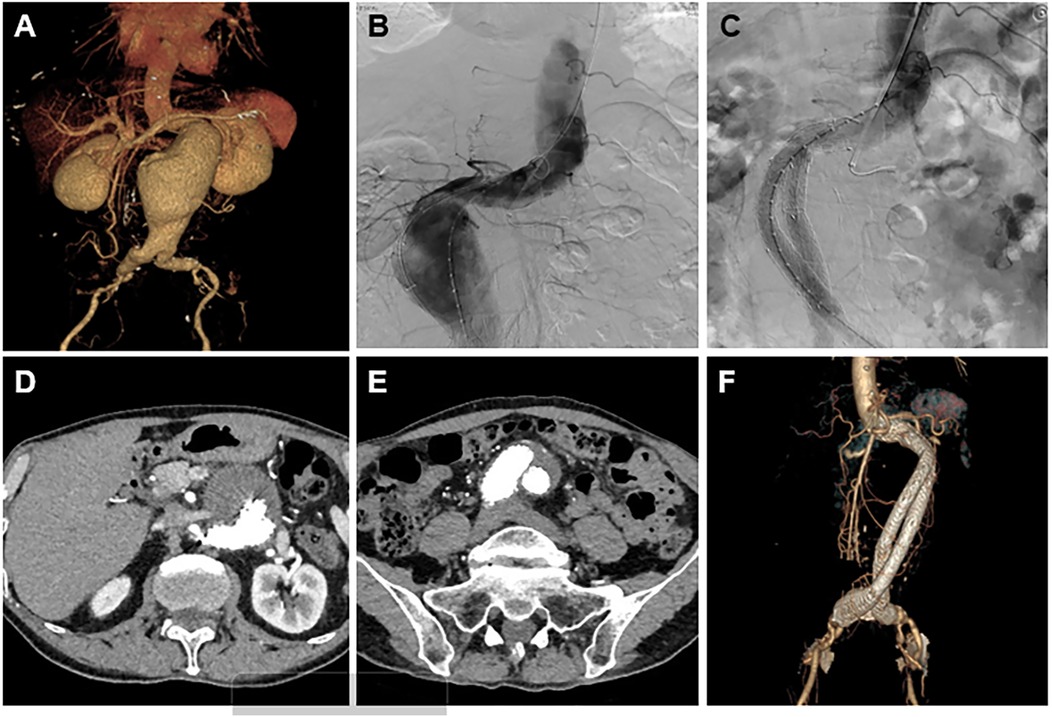
Figure 4. A result of the treatment and follow-up using the Kabedon technique for endovascular management of severely angulated neck infrarenal abdominal aortic aneurysms. (A) Preoperative CTA 3-dimensional volume-rendering reconstruction showing a proximally angulated neck, infrarenal aneurysmal neck, and abdominal aortic aneurysm sac. (B) Intraoperative angiography showing the stiff guidewire status, herein called “Kabedon.” Tension was maintained through the guidewire along the large curvature of the AAA. (C) Completion angiography demonstrates abdominal aorta and limb reconstruction without endoleaks. (D,E) The latest follow-up CTA showed no endoleaks, and migration occurred at the proximal and distal stent grafts. (F) A CTA scan 1 year after endovascular repair showing exclusion of the aneurysmal sac with no evidence of type Ia or III endoleak or graft limb occlusion.
Discussion
Since the first report of EVAR by Parodi et al. in 1991, this procedure has been introduced as an alternative to open surgery, although it has become a popular treatment associated with low perioperative mortality and short hospital stays for AAA patients (22).
However, the incidence rate of complications and overall and aneurysm-related mortality in the long term is higher after EVAR than after open surgical repair (23). Adverse morphological features of the proximal aortic neck are considered major factors for EVAR failure related to proximal endoleaks and migration (24). In particular, a severely angulated proximal aortic neck is associated with an increased risk of adverse aneurysm-related events (25). Currently, there is no consensus on the optimal management of AAA with a severely angulated neck during EVAR.
Currently, the main solutions for tortuous aortic necks primarily involve applying external forces to correct the tortuous angle. One approach is to use an ultra-stiff guidewire to correct the tortuous neck before stent deployment. However, the tension generated after forced correction of the neck may cause stent migration once the deployment guidewire is withdrawn. Moreover, the stent struggles to resist neck retraction, resulting in unstable fit between the stent and the arterial wall, which can lead to endoleaks. Taneva et al. reported a multicenter study showing that the incidence rates of early and late type I endoleaks using this modified technique could reach 16.4% and 12.1%, respectively (26). In addition, placing a CUFF stent at the neck first can correct the neck angulation to a certain extent, increase the effective length of the neck to obtain a longer anchoring zone, and allow better fixation of the main stent. Similarly, this technique still cannot completely relieve the stress of the stent on the aortic wall, which can easily lead to complications such as stent migration, endoleak, and occlusion. Hobo et al. found that the incidence rates of early and late type I endoleaks after applying this technique were 3.2%–6.5%, and the incidence rate of stent migration was 4.3%–5.9% (27, 28).
In the present study, we report a single-center experience of 120 consecutive patients with hostile anatomy of the proximal neck managed by the novel Kabedon technique. It is a novel technique for complying with aneurysmal morphology, with a flexible proximal configuration and proximal active control system to fit a severely angulated neck. According to our experience, in a severely angulated neck, the initial deployment of the endograft should be performed above the lowest renal artery ostium to reposition the endograft by pulling it downstream rather than pushing it cranially. In short, the shape of the neck can be maximized to increase the contact area between the stent and artery wall.
However, stent-graft stiffness is recognized as another major risk factor for early type Ia endoleaks and is caused by small gaps formed between the severely angulated neck and proximal stent-graft (11). Greater graft oversizing is needed to seal the proximal attachment zone, leading to potential graft migration associated with neck dilation due to negative remodeling over time (29). Therefore, a highly comfortable aortic endograft combined with a mean oversize effectively decreased this gap effectively and not have negative effects on aortic dilatation. Given that the present series had a high prevalence of more severe neck angulation on pre-EVAR CTA, the primary technical success rate was still excellent, which may be related to an increase in neck length via the tension of stiff guidewire and the enhanced anchoring effect of the stent-grafts, preventing acute endograft migration during the procedure.
Generally, the Amplatz guidewire is used to establish the “Kabedon” pathway. Compared to the Amplatz (Boston Scientific, Hemel Hempstead, UK) guidewire, the Lunderquist (Cook Aortic Interventions, Bloomington, USA) guidewire has a stiffer body and is not easy to bend (30). It may have resulted in the rebound of the stent-grafts, and the neck was forced to straighten the aneurysm morphology and then cause upward displacement of the stent-grafts. Once the stent-grafts is released, it is difficult to change its release position. A patient who used the Lunderquist guidewire experienced upward displacement of the stent-grafts. Chimney grafts were released to remedy the double renal arteries and prevent the renal arteries from being covered, and no adverse events occurred after the operation.
Distal attachment sites are of great importance for the feasibility and durability of EVAR. The “hypogastric snorkel” technique similarly involves the placement of a covered stent-grafts parallel to an iliac limb device to preserve perfusion to the hypogastric artery to extend the distal sealing zone in short common iliac arteries or cases of common iliac artery aneurysms (31). However, Wang et al. reported that the cross-limb procedure in EVAR was more advantageous than the standard limb configuration, particularly in patients with large aneurysm sacs or tortuous iliac arteries. The Pericles registry suggested that type Ia endoleaks can be minimized by utilizing at least 20 mm of the landing zone (32). In this study, bilateral iliac arteries were reconstructed using a cross-limb configuration to increase the distal sealing zone.
In this study, we observed that complications such as neck calcification, funnel-shaped aneurysm sac, type I endoleak, and procedures like proximal cuff stent salvage may all be negative predictors of the success of the Kabedon-EVAR technique. Interestingly, these multiple factors actually represent the potential dilemmas encountered during surgery for aneurysms with complex necks. Calcified necks and irregular aneurysm bodies have poor compliance, leading to inadequate adherence and greater difficulty in stent fixation, which increases the likelihood of endograft migration and type I endoleak. For this reason, remedial measures such as coil embolization and proximal cuff stent salvage need to be adopted. Therefore, the degree of neck calcification and the morphology of the aneurysm body should be features of key concern. Although there was no statistical difference in the multivariate analysis, this may be related to the small number of positive outcomes, and longer follow-up and further analysis are required.
The limitations of this study are its small sample size and the fact that only two brands of aortic stent graft device were used. In addition, we retrospectively analyzed the outcomes of EVAR using one technique (the Kabedon technique) and did not directly compare the outcomes of several techniques. Finally, multicenter, prospective, and large-sized studies with long-term follow-up are warranted to fully gauge the effects of Kabedon-based EVAR for AAA with infrarenal neck angulation.
Conclusion
Kabedon-based EVAR provided a high technical success rate and no mortality or complication rates during short-term follow-up for AAA with a severely hostile neck. This technique appears to contribute to enhanced technical and clinical success rates, even when the neck angle is more hostile. Future studies with long-term follow-up and larger sample sizes are required to evaluate the durability and risk of the late complications associated with this technique.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Boards of Qingdao University Health Science Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XS: Writing – review & editing, Writing – original draft. WH: Writing – review & editing, Writing – original draft. HM: Writing – review & editing, Writing – original draft. HQ: Writing – review & editing, Writing – original draft. HZ: Writing – review & editing. JY: Writing – review & editing. XJ: Writing – review & editing. JL: Writing – original draft, Writing – review & editing. MG: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82270518), the Qingdao West Coast New Area Science and Technology Project (No. 2022-57), and the Natural Science Foundation of Shandong Province (No. ZR2022MH031). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ullery BW, Hallett RL, Fleischmann D. Epidemiology and contemporary management of abdominal aortic aneurysms. Abdom Radiol (NY). (2018) 43(5):1032–43. doi: 10.1007/s00261-017-1450-7
2. Hensley SE, Upchurch GR Jr. Repair of abdominal aortic aneurysms: JACC focus seminar, part 1. J Am Coll Cardiol. (2022) 80(8):821–31. doi: 10.1016/j.jacc.2022.04.066
3. Kansagra K, Kang J, Taon MC, Ganguli S, Gandhi R, Vatakencherry G, et al. Advanced endografting techniques: snorkels, chimneys, periscopes, fenestrations, and branched endografts. Cardiovasc Diagn Ther. (2018) 8(Suppl 1):S175–83. doi: 10.21037/cdt.2017.08.17
4. Li B, Khan S, Salata K, Hussain MA, de Mestral C, Greco E, et al. A systematic review and meta-analysis of the long-term outcomes of endovascular versus open repair of abdominal aortic aneurysm. J Vasc Surg. (2019) 70(3):954–69.e930. doi: 10.1016/j.jvs.2019.01.076
5. Chinsakchai K, Sirivech T, Moll FL, Tongsai S, Hongku K. The correlation of aortic neck angle and length in abdominal aortic aneurysm with severe neck angulation for prediction of intraoperative neck complications and postoperative outcomes after endovascular aneurysm repair. J Clin Med. (2023) 12(18):5797. doi: 10.3390/jcm12185797
6. Liu Y, Qing M, Zhao J, Huang B, Yang Y, Zheng T, et al. Influence of severe neck angulation on hemodynamic and clinical outcomes following endovascular aneurysm repair: a hemodynamic analysis and a retrospective cohort study. Chin Med J (Engl). (2022) 135(21):2577–84. doi: 10.1097/CM9.0000000000002280
7. Gallitto E, Gargiulo M, Faggioli G, Pini R, Mascoli C, Freyrie A, et al. Impact of iliac artery anatomy on the outcome of fenestrated and branched endovascular aortic repair. J Vasc Surg. (2017) 66(6):1659–67. doi: 10.1016/j.jvs.2017.04.063
8. Oliveira-Pinto J, Ferreira RS, Oliveira NFG, Hoeks S, Van Rijn MJ, Raa ST, et al. Total luminal volume predicts risk after endovascular aneurysm repair. Eur J Vasc Endovasc Surg. (2020) 59(6):918–27. doi: 10.1016/j.ejvs.2020.02.011
9. Figueroa AV, Tanenbaum MT, Costa Filho JE, Gonzalez MS, Coronel NI, Baig MS, et al. Long-term outcomes of staged iliofemoral endoconduits prior to complex endovascular aortic aneurysm repair. J Vasc Surg. (2024) 80(1):45–52. doi: 10.1016/j.jvs.2024.02.001
10. Zuidema R, van der Riet C, El Moumni M, Schuurmann RCL, Ünlü Ç, de Vries JPM. Pre-operative aortic neck characteristics and post-operative sealing zone as predictors of type 1a endoleak and migration after endovascular aneurysm repair: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. (2022) 64(5):475–88. doi: 10.1016/j.ejvs.2022.08.017
11. Özdemir-van Brunschot DMD, Holzhey D, Botsios S. Sex-related differences in proximal neck anatomy and their consequences in patients after EVAR: a matched cohort analysis. J Clin Med. (2023) 12(15):4929. doi: 10.3390/jcm12154929
12. Morisaki K, Matsubara Y, Kurose S, Yoshino S, Furuyama T. Effect of abdominal aortic aneurysm sac shrinkage after endovascular repair on long-term outcomes between favorable and hostile neck anatomy. J Vasc Surg. (2022) 76(4):916–22. doi: 10.1016/j.jvs.2022.03.011
13. Mascoli C, Faggioli G, Goretti M, Gallitto E, Pini R, Logiacco AM, et al. Endovascular treatment of abdominal aortic aneurysm with severe angulation of infrarenal aortic neck by gore conformable endograft. J Endovasc Ther. (2023) 30(3):410–8. doi: 10.1177/15266028221083461
14. Bonvini S, Spadoni N, Frigatti P, Antonello M, Irsara S, Veraldi GF, et al. Early outcomes of the conformable endograft in severe neck angulation from the triveneto conformable registry. J Vasc Surg. (2023) 78(4):954–962.e2. doi: 10.1016/j.jvs.2023.06.006
15. Dohi S, Yokoyama Y, Yamamoto T, Kuwaki K, Hariya A, Kajimoto K, et al. Push-up technique and anatomical deployment with the endurant stent-graft system for severely angulated aneurysm necks. J Endovasc Ther. (2017) 24(3):435–9. doi: 10.1177/1526602817692790
16. George JM, Hatzis CM, Choinski KN, Tadros RO, Faries PL, Marin ML. Technological advances to address the challenging abdominal aortic aneurysm neck. Rev Cardiovasc Med. (2023) 24(3):70. doi: 10.31083/j.rcm2403070
17. Lee JH, Choi JH, Kim EJ. The influence of unfavorable aortoiliac anatomy on short-term outcomes after endovascular aortic repair. Korean J Thorac Cardiovasc Surg. (2018) 51(3):180–6. doi: 10.5090/kjtcs.2018.51.3.180
18. van Rijswijk RE, Jebbink EG, Zeebregts CJ, Reijnen MMPJ. A systematic review of anatomic predictors of abdominal aortic aneurysm remodeling after endovascular repair. J Vasc Surg. (2022) 75(5):1777–85. doi: 10.1016/j.jvs.2021.11.071
19. Chaikof EL, Fillinger MF, Matsumura JS, Rutherford RB, White GH, Blankensteijn JD, et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. (2002) 35(5):1061–6. doi: 10.1067/mva.2002.123991
20. Gallitto E, Gargiulo M, Freyrie A, et al. Results of standard suprarenal fixation endografts for abdominal aortic aneurysms with neck length </=10 mm in high-risk patients unfit for open repair and fenestrated endograft. J Vasc Surg. (2016) 64(3):563–70.e127183854
21. Wang J, Zhao J, Ma Y, Huang B, Yang Y, Yuan D, et al. Editor’s choice—mid term outcomes of crossed limb vs. Standard limb configuration in endovascular abdominal aortic aneurysm repair: a propensity score analysis. Eur J Vasc Endovasc Surg. (2021) 61(4):579–88. doi: 10.1016/j.ejvs.2021.01.018
22. Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. (1991) 5(6):491–9. doi: 10.1007/BF02015271
23. Antoniou GA, Antoniou SA, Torella F. Editor’s choice. Endovascular vs. open repair for abdominal aortic aneurysm: systematic review and meta-analysis of updated peri-operative and long term data of randomised controlled trials. Eur J Vasc Endovasc Surg. (2020) 59(3):385–97. doi: 10.1016/j.ejvs.2019.11.030
24. Bernardini G, Litterscheid S, Torsello GB, Torsello GF, Beropoulis E, Özdemir-van Brunschot D. A meta-analysis of safety and efficacy of endovascular aneurysm repair in aneurysm patients with severe angulated infrarenal neck. PLoS One. (2022) 17(2):e0264327. doi: 10.1371/journal.pone.0264327
25. Mathlouthi A, Abdelkarim A, Elsayed N, Ramakrishnan G, Naazie I, Malas MB. Novel risk score calculator for perioperative mortality after EVAR with incorporation of anatomical factors. Ann Vasc Surg. (2023) 94:289–95. doi: 10.1016/j.avsg.2023.02.020
26. Taneva GT, Criado FJ, Torsello G, Veith F, Scali ST, Kubilis P, et al. Results of chimney endovascular aneurysm repair as used in the PERICLES registry to treat patients with suprarenal aortic pathologies. J Vasc Surg. (2020) 71(5):1521–7.e1. doi: 10.1016/j.jvs.2019.08.228
27. Hobo R, Kievit J, Leurs LJ, Buth J, EUROSTAR Collaborators. Influence of severe infrarenal aortic neck angulation on complications at the proximal neck following endovascular AAA repair: a EUROSTAR study. J Endovasc Ther. (2007) 14(1):1–11. doi: 10.1583/06-1914.1
28. Yao C, Ning J, Li Z, Wang M, Wu R, Wang S, et al. Parallel covered stents technique in the treatment of abdominal aortic diseases. J Vasc Interv Radiol. (2020) 31(5):771–7. doi: 10.1016/j.jvir.2019.09.022
29. Ma H, Wang X, Liu Y, Li Y, Guo M. The impact of endovascular stents types on perioperative outcomes of ruptured abdominal aortic aneurysms: a single-center experience. Front Cardiovasc Med. (2024) 11:1272389. doi: 10.3389/fcvm.2024.1272389
30. Arindam C, Frederic H, Nabil C. Are all wires created the same? A quality assurance study of the stiffness of wires typically employed during endovascular surgery using tension dynamometry. EJVES Vasc Forum. (2021) 52:20–4. doi: 10.1016/j.ejvsvf.2021.06.006
31. Forsyth A, Carlson S, Martin M, Raffetto J, Alfson D, McPhee J. Late type III endoleaks are common in early generation endologix AFX stent grafts. J Vasc Surg. (2022) 76(3):680–7. doi: 10.1016/j.jvs.2022.02.020
Keywords: abdominal aortic aneurysm, severe infrarenal neck angulation, endovascular aneurysm repair, Kabedon technique, short-term outcomes
Citation: Sun X, Huang W, Ma H, Qu H, Zhang H, Yan J, Jiao X, Liu J and Guo M (2025) The “Kabedon” technique for treatment of abdominal aortic aneurysm with severe angulation of infrarenal aortic neck. Front. Surg. 12:1593437. doi: 10.3389/fsurg.2025.1593437
Received: 17 March 2025; Accepted: 20 August 2025;
Published: 8 September 2025.
Edited by:
Apostolos Tassiopoulos, Stony Brook University, United StatesReviewed by:
Xiangchen Dai, Tianjin Medical University General Hospital, ChinaNikolaos Schoretsanitis, University General Hospital of Thessaloniki AHEPA, Greece
Copyright: © 2025 Sun, Huang, Ma, Qu, Zhang, Yan, Jiao, Liu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingjin Guo, cWR1YWh2YXNjQDE2My5jb20=; Junjun Liu, NTAzNzc3MzQ0QHFxLmNvbQ==
†These authors have contributed equally to this work
 Xiaozhi Sun
Xiaozhi Sun Wenjing Huang
Wenjing Huang Huibo Ma
Huibo Ma Hongyu Qu3,†
Hongyu Qu3,† Heng Zhang
Heng Zhang Junwei Yan
Junwei Yan Junjun Liu
Junjun Liu Mingjin Guo
Mingjin Guo