- 1Organ Transplantation Center, Koç University Hospital, Istanbul, Türkiye
- 2Department of Surgery, Division of Organ Transplantation, Başkent University, Istanbul Hospital, Istanbul, Türkiye
Introduction: The Milan Criteria (MC) are widely accepted as the standard patient selection criteria for liver transplantation (LT). However, patients who exceed these criteria may still benefit from transplantation. Various extended criteria have been published. This study aimed to evaluate survival outcomes in hepatocellular carcinoma (HCC) patients undergoing LT, comparing those within and beyond MC, and to review the role of extended criteria in LT.
Methods: This retrospective, single-center study included adult patients who underwent LT at Koç University between 2018 and 2024. Pathological data were used to categorize patients into two groups: those within MC and beyond MC. Preoperative data and postoperative overall and disease-free survival rates were compared between these groups. Additionally, a comprehensive literature review of studies evaluating extended criteria for LT in HCC patients was conducted.
Results: A total of 45 adult patients were included in the analysis. There were 23(51.1%) patients within MC, and 22(48.9%) patients in the beyond MC group. Demographics, donor types, graft types, tumor differentiations, Child scores, MELD scores, ischemia times, length of intensive care unit stays, length of hospital stays, and mortality rates were similar (p > 0.05). Tumor count, total tumor diameter, and microvascular invasion rates were statistically higher in patients beyond MC (p < 0.05). Survival analyses revealed no statistically significant differences in 1-year, 3-year, and 5-year survival rates of the patients (p > 0.05).
Conclusion: This study highlights the potential for liver transplantation in HCC patients exceeding the Milan Criteria, with survival outcomes comparable to those within the Milan Criteria in certain cases. Despite numerous studies in the literature, optimal criteria for LT patient selection in beyond MC HCC have not been established. An optimal guideline that will help to better understand tumor behavior, guide the decision-making and timing of liver transplantation (LT), and ultimately improve post-transplant outcomes remains a key objective for future research.
1 Introduction
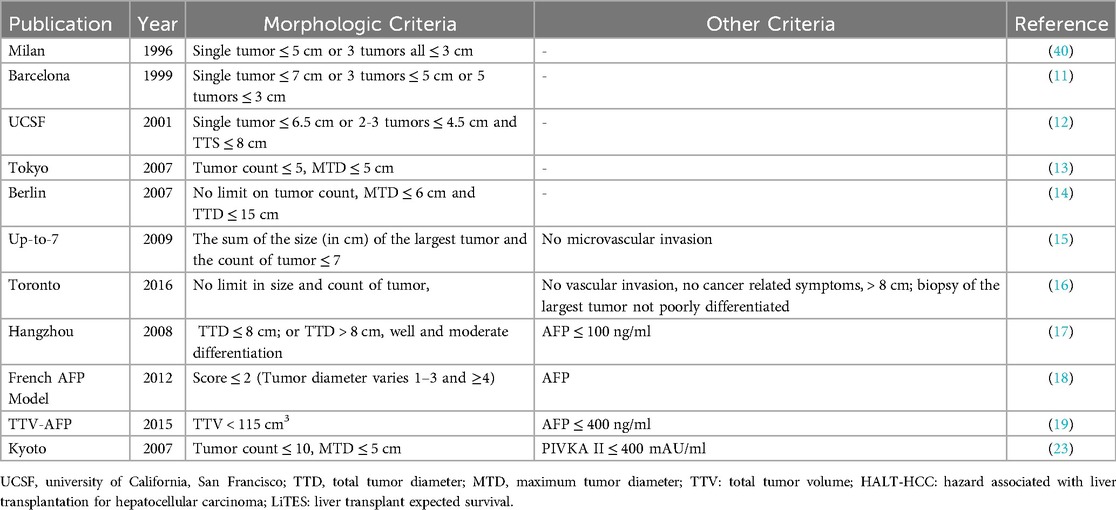
Hepatocellular carcinoma (HCC) is makes up the majority of liver-derived malignant tumors (80%) and is the third most common cause of cancer-related death worldwide (1). Liver resection (LR) and liver transplantation (LT) are the two curative surgical options for the treatment of HCC. LT outperforms LR in treating HCC according to long-term survival rates (2). Liver transplantation (LT) can cure both HCC and underlying liver diseases. However, the first papers reported poor survival and high recurrence rates until Mazzaferro's outstanding, epoch-making report in 1996 (3). Mazzafero and colleagues have proposed the well-known Milan Criteria (MC) to select the candidates for liver transplantation according to these criteria; a single HCC nodule that does not exceed 5 cm in diameter or up to three nodules that do not exceed 3 cm in diameter, without vascular invasion or extrahepatic metastasis (4). The 4-year survival rate was 75%, which was remarkable for its time. Since its publication in 1996, the Milan Criteria have remained the cornerstone for liver transplantation worldwide.
Despite the MC's effectiveness in improving LT outcomes, the demand of the individuals on waiting lists had not been adequately met due to organ shortage. The MC limitations and shortage of organ presentation for deceased donor liver transplantation (DDLT) have restricted the successful outcomes obtained by the MC for LT. Moreover, some studies have shown that approximately 25% of the patients within MC (wMC), according to preoperative imaging techniques, had pathological results that took the patients beyond the MC (bMC) (5). Since the publication of MC with it's attractive outcomes, many other centers have developed numerous extended criteria. Nevertheless, MC remains important since 1996 and continues to be included in many current liver transplantation guidelines worldwide (6–10).
This paper aims to review the current literature related to liver transplantation outcomes for hepatocellular carcinoma within and beyond Milan Criteria and to analyse the Koç University Organ Transplant Center's outcomes on LT for hepatocellular carcinoma retrospectively.
2 History
Following the declaration of the Milan Criteria, novel extended criteria for LT have been reported from many centers. Some extended criteria are purely morphological, whilst others combine some biological parameters along with the morphological findings.
2.1 Morphological criteria
Mazzaferro and colleagues published their trial in 1996 and revealed a remarkable survival benefit for the patient within their criteria (Table 1) (4). It remains the reference for novel criteria based on the tumor burden, which are currently being developed. In chronological order, right after MC, Llovet and colleagues published their trial known as the Barcelona Criteria (11). They have selected the candidates for transplant, with a single tumor ≤7 cm, or 3 tumors ≤5 cm, or 5 tumors ≤3 cm. The 5-year overall survival rates were over 50%.
Two years after the Barcelona Criteria, Yao and colleagues published the University of California, San Francisco (UCSF) Criteria in 2001 (12). Their criteria include patients with a single tumor ≤6.5 cm or ≤3 lesions with the largest one ≤4.5 cm, and total tumor diameter ≤8 cm for LT. Their trial revealed 73% of 5-year overall survival (OS) and 81% of 5-year disease-free survival (DFS) (12).
In 2007, Sugawara et al. published the Tokyo University series (13). Up to 5 nodules with a maximum tumor size of ≤5 cm in diameter were considered acceptable candidates for living donor liver transplantation (LDLT) in their study. 1-year and 3-year survival rates were completely similar to those within MC; 97% and 94%, respectively. In the same year, 2007, Jonas et al. from Germany published their extended criteria outcomes (14). Multiple HCC nodes were considered acceptable up to a diameter of the largest node of 6 cm and a total tumor diameter (TTD) of 15 cm for their study. Rates of 3-year survival and recurrence-free survival were 68% and 64%, respectively.
Mazzaferro and colleagues published their extended criteria “Up-to7” in 2009 (15). Their criteria include the size and number of the tumors. The results were obtained from a retrospective analysis of 1,556 LT patients, showing a group of patients with an estimated 5-year OS in the absence of microvascular invasion (MiVI); seven being the result of the sum of size (in cm) and number of tumors for any given HCC. This included all combinations of a given HCC from one nodule up to 6 cm in size (1 + 6) to many tumors fulfilling seven as the sum of size plus number (2 tumors up to 5 cm, 3 tumors up to 4 cm, 4 tumors up to 3 cm, 5 tumors up to 2 cm). This group of patients had 71.2% 5-year OS rates similar to outcomes of patients wMC. On the other hand, for patients with MiVI, the 5-year overall survival rates decreased to 48.1%. In the analysis for the microvascular invasion rates, the estimated tumor size for no microvascular invasion was 30 mm and 45 mm for patients with microvascular invasion (p < 0.001). This result has proposed a possible relation between tumor size and the presence of MiVI.
DuBay and colleagues from the University of Toronto published the extended Toronto Criteria in 2011 (16). The Toronto criteria do not limit the size or number of the tumor, but it should not have any vascular invasion, extrahepatic disease, or cancer-related symptoms, and the biopsy can not be poorly differentiated. The 5-year survival outcomes revealed 68% rates.
2.2 Combined criteria; AFP
Xu and colleagues revealed the Hangzhou Criteria in 2008 from China (17). They reviewed a total of 6,012 HCC patients; of those, 1,352 cases were bMC but within the extended Hangzhou Criteria. Their criteria were: total tumor diameter (TTD) ≤8 cm; or TTD >8 cm, well and moderate differentiation, and preoperative AFP level ≤100 ng/ml. The reported 1-year, 3-year, and 5-year OS rates were 83.1%, 67%, and 59.8%, respectively. They also had a second study group in their trial with AFP levels of 100–400 ng/ml and the same tumor size. This group of patients had worse overall and DFS rates. This demonstrates the effect of AFP level on predicting the prognosis of LT.
Duvuox and colleagues published the French-AFP model in 2012 (18). The tumor diameters were divided into three groups: , 3–6 cm, >6 cm, and the number of tumors was divided into two groups: 1–3, and ≥4, in the model. The AFP values were also divided into three groups: <100, 100–1,000, and >1,000. The risk assessment was calculated by the points, varying from zero to four. In this study, it was claimed that the French-AFP model could achieve comparable or even better results than MC in predicting recurrence and survival times. It has also been interpreted that increased AFP levels are associated with vascular invasion and loss of differentiation and are a good tool for predicting tumor behavior. Moreover, it has been stated that AFP provides better prognostic information than tumor diameter and number (18).
Toso and colleagues investigated 6,478 patients who underwent LT and published their extended criteria as “total tumor volume and AFP” (TTV-AFP) criteria proposed the following rules: tumor volume <115 cm3 and AFP levels under 400 ng/ml in 2015. They presented 75% of 4-year OS rates for the patients within their criteria (19). Toso et al. have already published the “Total tumor volume” criteria in 2008, and they determined the cut-off values of the tumor volumes from their previous study (20).
The Metroticket 2.0 model includes AFP levels in the algorithm, showing an individualized increase in the risk of death following LT proportional to increased AFP levels (21). Nine years after the publication of the Up-to-7 criteria, Mazzaferro and colleagues published the Metroticket 2.0 in 2018. By the combination of Italian and Chinese data with 1,359 patients, Mazzaferro and colleagues aimed to determine the risk factors affecting the survival rates of patients with HCC. In addition to the Up-to-7 criteria, they prompted AFP levels as a risk factor in their trial. The Milan Criteria's rate of 70% for 5-year overall survival led to determining the extended criteria for Metroticket. In conclusion, they recommended performing LT for patients with HCC with the following features; AFP should be <200 ng/ml and the sum of the number and size of tumors (in centimeters) should not exceed 7; if the level of AFP 200–400 ng/ml, the sum of the number and size of tumors should be ≤5; if the level of AFP 400–1,000 ng/ml, the sum of the number and size of tumors should be ≤4.
The Japanese Liver Transplantation Society proposed the 5-5-500 Model for LT candidates in 2019 (22). The model includes patients with the number of lesions <5, tumor size ≤5 cm, and AFP levels ≤500 ng/ml. Their results proposed a 19% increase in patients to be candidates for LT when compared with MC. The new criteria provided the 7.3% 5-year recurrence rate and +70% 5-year OS rate.
2.3 Combined criteria; alternatives and additions to AFP
Kaido and colleagues published the current Kyoto Criteria results (23). Their criteria involve a combination of tumor number ≤10, maximal diameter of each tumor ≤5 cm, and serum des-gamma-carboxy prothrombin (DCP) levels ≤400 mAU/ml. 198 HCC patients were enrolled in the study. 147 of them were within the Kyoto criteria, and 49 of them exceeded the criteria. The 5-year OS rates were 82% and 42%, respectively. The 5-year recurrence rates were 4% and 51%, respectively. The 5-year overall survival rates were similar for both wMC (n = 118) and bMC (n = 80) groups. The patients were divided into three groups: wMC/Kyoto in (n = 105), bMC/Kyoto in (n = 42), and bMC/Kyoto out (n = 36). The overall survival rates were similar in the wMC/Kyoto in and the bMC/Kyoto in groups. The bMC/Kyoto out group had the worst 5-year survival rates. Also, the same group had the worst 5-year DFS rates.
In 2016, Lee and colleagues developed a model to predict tumor recurrence after LDLT for HCC, with the well-known name, MoRAL (24). They used the serum AFP and serum protein induced by vitamin K absence-II (PIVKA-II) levels to calculate risk stratifications for HCC recurrence. They showed that the serum AFP levels were significantly associated with the maximal tumor size. In this study, serum AFP and serum PIVKA levels were used to calculate the MoRAL score. The higher MoRAL score (>314,8) has been shown to be a risk factor for higher tumor recurrence. Interestingly, the patients wMC with a high MoRAL revealed a significantly higher risk of recurrence than those bMC with a low MoRAL score. At a cutoff value of 314,8, the MoRAL score provided significant discrimination functions on tumor recurrence in both the wMC and the bMC cohorts (24).
In 2017, Halazun and colleagues updated their previously presented MORAL study (25). A total of 339 HCC patients were enrolled in the study. The neutrophil to lymphocyte ratio (NLR) was measured from the immediate routine pretransplant labs by dividing the neutrophil count by the lymphocyte count. NLR 5 was considered the cutoff level; a level of 5 and above was considered elevated. The AFP 200 level was considered the cutoff level. In the multivariate analysis, the largest tumor size >3 cm, >200 AFP levels, and NLR >5 were detected as preoperative risk factors for DFS rates. Patients were examined in 3 separate risk groups according to their MORAL scores. The 5-year DFS rate of the low-risk group was found to be 98.6%. The medium-risk group reached 69.8% and the high-risk group reached 55.8% DFS values.
Sasaki and colleagues from the Cleveland Clinic developed the Hazard Associated with Liver Transplantation for Hepatocellular Carcinoma (HALT-HCC) score in 2017 (26). Unlike other models, the general health status of the recipient was also included in the calculation. This study showed that the tumor morphology (TBS), AFP levels, and the recipient MELD-Na were significantly associated with overall survival by the multivariable analysis. The TBS was a tumor morphological score, consisting of maximum tumor diameter and tumor number, but it represents tumor morphology in a single, continuous fashion, similar to the total tumor volume for this trial.
In 2021, Goldberg and colleagues published the Liver Transplant Expected Survival (LiTES) HHC score (27). There were 6,502 HCC patients in this cohort, and 6,224 (95,7%) were wMC. The 1-, 3-, 5-, and 10-year survival rates were 92.5%, 83.8%, 76.2%, and 60.1% respectively. The patients were divided into three groups: the top 25% LiTES score, 25%–75% LiTES score, and the bottom 25% LiTES score, and compared with other well-known risk models. The LiTES score was calculated by the following variables: bilirubin levels, international normalized ratios, estimated glomerular filtration rate, chronic kidney disease, age, diabetes, and the etiology of the LT.
2.4 Other variables
2.4.1 Microvascular invasion
Microvascular invasion (MiVI) has consistently been shown to negatively affect survival outcomes after LT for HCC. Studies by Mazzaferro et al. (2009) and Dudek et al. (2009) demonstrated its significant impact on survival rates post-LT, a finding further emphasized by Alim et al. and Yankol et al. in 2021 (15, 27–31). It is not always possible to detect the presence of MiVI preoperatively. Therefore, several studies have been published to predict MiVI during the preoperative period. Esnaola et al. proposed that the likelihood of MiVI significantly increases in HCCs larger than 4 cm in diameter (32). In their study, Chandarana et al. demonstrated that the risk of MiVI is significantly higher in multifocal HCC lesions (33). Si et al. suggested that the presence of MiVI could be predicted preoperatively using AFP and PIVKA-II levels (34). Zhang et al. from China published their study in 2023, in which they used a prediction model based on AFP levels, tumor borders, cirrhosis, and fibrinogen to preoperatively detect the presence of MiVI (35). Despite numerous studies in the literature, an ideal marker for this purpose has yet to be identified. On the other hand, Sun and colleagues in their study examining the assessment of recurrence risk following liver transplantation in patients with no MiVI (M0), suggested that some factors increase the risk of recurrence development even if M0 is detected in explant pathology (35). Using the Eastern Hepatobiliary Surgery Hospital (EHBH) scoring system they developed in this study, they recommend that high-risk patients without MiVI should be candidates for adjuvant treatment as if there were a MiVI. In the scoring system, the following were emphasized as risk factors: AFP ≥400 ng/ml, tumor diameter ≥5 cm, total bilirubin ≥17,1 µmol/L, aspartate aminotransferase ≥40 U/L, albumin level <35 g/L, and presence of cirrhosis (36).
2.4.2 Tumor differentiation
Poor tumor differentiation is another indicator of tumor aggressiveness and is typically associated with a poor prognosis in patients with HCC. Tumor differentiation is one of the most important factors in determining the survival rates of HCC patients (37). Molecular studies have identified mature hepatocytes as the origin cells of HCC (38). These cells dedifferentiate into hepatocyte precursor cells and then become HCC cells that express progenitor cell markers (39). As it is known that dedifferentiation is a process of HCC development, so the therapeutic strategies aiming to reverse tumor dedifferentiation may be promising (40). Various molecules such as Hepatocyte nuclear factor 1α (HNF1α), HNF1β, HNF3γ, HNF4, HNF6, and C/EBPα were already determined in the molecular pathway of HCC development (40). Experimental studies showed exogenous expression of some molecules, such as FOX3, and inducing the intracellular pathways may be promoting the transformation of HCC cells into hepatocyte-like cells (41).
Poor differentiation of HCC has been shown to be an independent poor prognostic factor by various trials (37, 42). The Toronto HCC series highlighted that the presence of a large tumor alongside poor differentiation serves as a contraindication for LT. Additionally, liver biopsy is recommended for all patients who have exceeded MC during the preoperative evaluation. They further emphasized that a tumor with poor differentiation found in a liver biopsy is a contraindication for LT (43). Yılmaz et al. demonstrated that having more than four nodules and a platelet volume <8.6 fL are risk factors for poor tumor differentiation (44).
2.4.3 Downstaging hepatocellular carcinoma
Downstaging patients who are beyond the Milan Criteria (bMC) to within criteria (wMC) has become an effective and reliable strategy for selecting appropriate candidates for LT (45). Techniques such as transarterial chemoembolization (TACE), transarterial radioembolization (TARE), radiofrequency ablation (RFA) and microwave ablation (MWA), and stereotactic body radiation therapy (SBRT) are commonly used to achieve downstaging (46). When evaluating 5-year overall survival after LT, patients who were successfully downstaged to within Milan Criteria have been reported to achieve comparable outcomes to those who initially met the criteria (47). A favorable response to downstaging may offer tumor biology–based insight into the potential for improved outcomes following the planned LT (48). Tabrizian and colleagues reported that patients initially classified as bMC, who were successfully downstaged to wMC and underwent LT, achieved a 10-year post-transplant survival rate of 52%, which is comparable to the 62% observed in patients who met the wMC from the outset (49). Another important finding of this study was that even among patients who remained beyond the Milan Criteria (bMC) after downstaging, the 10-year post-transplant survival rate was approximately 43% (49).
3 Current status of extended criteria
In 2017, Haberal and colleagues published their experience of LT for the patients bMC (50). There were 36 pediatric and adult LT patients bMC in that trial, and overall 5-year and 10-year survival rates were reported as 71.7% and 62.7%, respectively. Moreover, Haberal's team published their long-term survey of LT in 2022 and reported that 8 of their 13 bMC patients (61.5%) with HCC had more than 10-year survival rates (51).
In 2020, Victor and colleagues divided their LT patients into three groups: wMC, within USCFC, and beyond UCSFC (52). In this trial, the patients had loco-regional therapy (LRT) and/or systemic chemotherapy followed by at least 9-month oncologically stable periods before LT. They reported the 1- and 5-year DFS rates as 100% and 92% for LT wMC, 95.5% and 88.6% within UCSFC, and 91.1% and 85.4% for beyond UCSFC consecutively, in their trial.
In 2021, Alim and colleagues from İstanbul revealed their high-volume single-center experience. 202 HCC patients who underwent LT during the 2004–2019 period were studied (30). 121 of them were wMC, while the rest 81 were bMC. In patients within MC, the 1-,3-, and 5-year survival rates were 91.9%, 81.6%, and 76.3% consecutively. In patients bMC, the 1-,3-, and 5-year survival rates were 88.8%, 81.8%, and 72.3% consecutively, and these outcomes were statistically similar in both groups (p = 0.41). Secondly, the DFS rates were also comparable. In patients wMC, the 1-,3-, and 5-year DFS rates were 89.3%, 79.3%, and 73.2% consecutively. In patients bMC, the 1-,3-, and 5-year DFS rates were 81.4%, 76.2%, and 66.3% consecutively, and these outcomes were statistically similar in both groups (p = 0.21). This report was one of the largest series from a single center.
In 2023, Wang and colleagues included 437 patients with HCC in their study from China Database and compared these three models; AFP, Metroticket 2.0, and up-to 7 criteria. They found that the modified AFP level before LT for patients with multiple tumors is predictive in estimating the recurrence risk for the post-LT period (53).
Pauley and colleagues have just emphasized that although most pre-LT criteria focused on the tumors' morphologic characteristics, tumors that contain the radiographic response to therapies and a variety of tumor markers have been increasingly recognized as a crucial factor predicting the post-LT survival rates (54).
In 2024, Bekki and his colleagues from Japan examined the national registry of US data in their study (55). In this study, DDLT cases were examined between 2010 and 2014, and the participants were divided into 4 groups: 1- within MC/5-5-500, 2- beyond MC/within 5-5-500, 3- within MC/beyond 5-5-500, 4- beyond MC/5-5-500. The highest 5-year recurrence rate was detected in the within MC/beyond 5-5-500 group, and a recurrence rate of 25.4% was reported. The lowest 5-year recurrence was in the MC/5-5-500 group (7.4%). Additionally, participants were divided into 4 groups according to AFP level: <100, 101–300, 301–500, and >500, and a statistically significant relationship was shown between 5-year survival times and AFP level (p < 0.01).
4 Koç University Organ Transplantation Center experience
4.1 Materials and methods
In this study, liver transplant patients who underwent transplantation at Koç University Organ Transplantation Center between September 2018 and July 2024 were retrospectively analyzed. The study included patients in the adult age group who were diagnosed with hepatocellular carcinoma based on definitive pathology reports. Patients with HCC-CCC mixed tumors and those in the pediatric age group with HCC were excluded from the study. Patients were divided into two groups based on their pathological results: within the Milan Criteria (wMC) and beyond the Milan Criteria (bMC). Preoperative features, intraoperative and postoperative outcomes were compared. Overall survival and disease-free survival rates were compared between the groups. This study was approved by the Institutional Review Board (Project No: KA25/48-18.02.2025).
Statistical analysis was performed using SPSS statistical software (Version 25.0, SPSS Inc., Chicago, IL, United States). Continuous variables that were normally distributed were described as the mean ± standard deviation (p > 0.05) based on the Kolmogorov–Smirnov or Shapiro–Wilk tests (n < 30). Non-normally distributed continuous variables were described as the median. Comparisons between groups were evaluated using the Mann–Whitney U test. The categorical variables between groups were analyzed using the Chi-square test or Fisher's Exact Test. Kaplan–Meier survival curves were generated to compare overall and disease-free survival rates between the wMC and bMC groups. The log-rank test was used to compare survival distributions between groups. The association of the variables with overall survival and disease-free survival was analyzed using the Cox proportional hazard model. A retrospective power analysis was performed to examine the reliability of the results.
4.2 Results
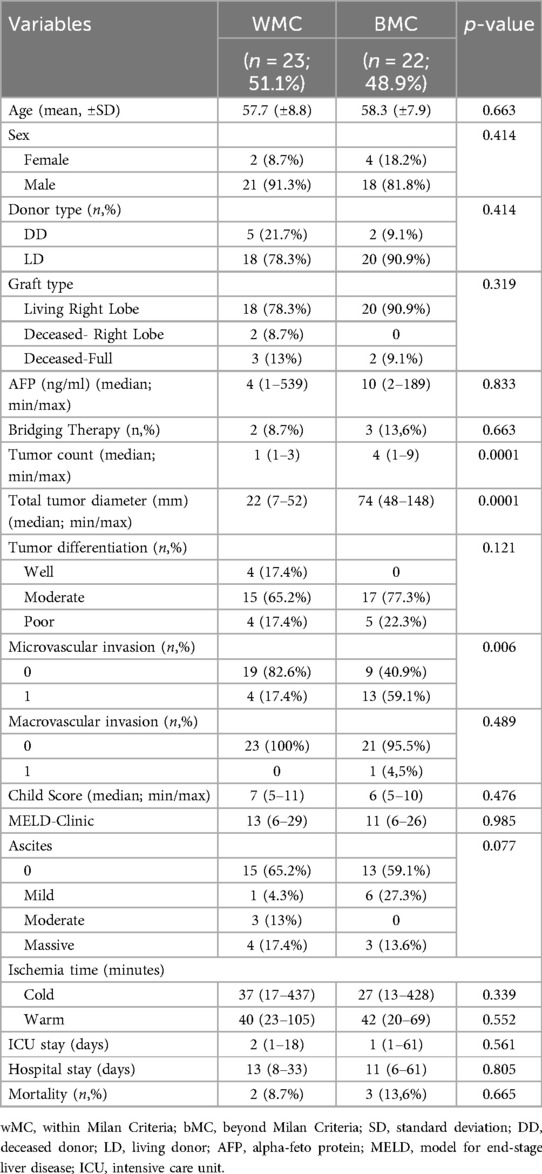
A total of 279 liver transplants (LT) were performed at Koç University Hospital Organ Transplant Center during the study period. According to pathological results, hepatocellular carcinoma (HCC) was diagnosed in 54 patients. Six patients had mixed hepatocellular carcinoma-cholangiocellular carcinoma (HCC-CCC) tumors. Three patients were in the pediatric age group. Patients with HCC-CCC mixed tumors and those in the pediatric age group with HCC were excluded from the study. The median follow-up time was 49 (2-76) months. The mean age was 57.9 ± 15.5 years. Among the 45 HCC patients, 23 (51.1%) were within the Milan Criteria, with a mean age of 57.7 ± 8.8 years. The remaining 22 patients (48.9%) were beyond the Milan Criteria, with a mean age of 58.3 ± 7.9 years (p = 0.663). The mean body mass index (BMI) was 23.7 ± 6.2. Among the 45 adult patients with HCC, 39 were male, and 6 were female. In the wMC group, 21 (91.3%) patients were male, while 18 (81.8%) male patients were in the bMC group (p = 0.414). Thirty-eight cases underwent living donor liver transplant (LDLT), and 7 received deceased donor liver transplant (DDLT). A total of 38 patients received a right lobe graft from living donors, while 7 patients received deceased donor grafts, including 2 with a split right graft and 5 with a full graft. The distributions of donor and graft types were similar between the groups. Although AFP levels appeared higher in the bMC group, no statistically significant difference was found between the two groups (Table 2). The tumor count and total tumor diameter were statistically higher in the bMC group, as expected. No statistically significant difference was found in the distribution of tumor differentiation.
Microvascular invasion (MiVI) was detected in 17 patients, with 4 patients (17.4%) in the wMC group and 13 patients (59.1%) in the bMC group showing MiVI (p = 0.006). Among all patients, macrovascular invasion (MaVI) was observed in only one patient, who was in the bMC group (p = 0.489).
The MELD scores were similar in both groups, with the wMC group having a median MELD score of 13 (range 6–29) and the bMC group having a median MELD score of 11 (range 6–26) (p = 0.985). Child scores were also comparable. The median Child score for the wMC group was 7 (range 5–11), while for the bMC group it was 6 (range 5–10) (p = 0.476). The distribution of preoperative ascites presence and severity was also similar between the two groups. Warm and cold ischemia times, ICU length of stay, total length of stay, and mortality rates were also comparable between the two groups (Table 2).
Bridging procedures were performed before liver transplantation in five patients. Of these, one patient underwent RFA, three underwent TACE, and one patient received both TACE and TARE.
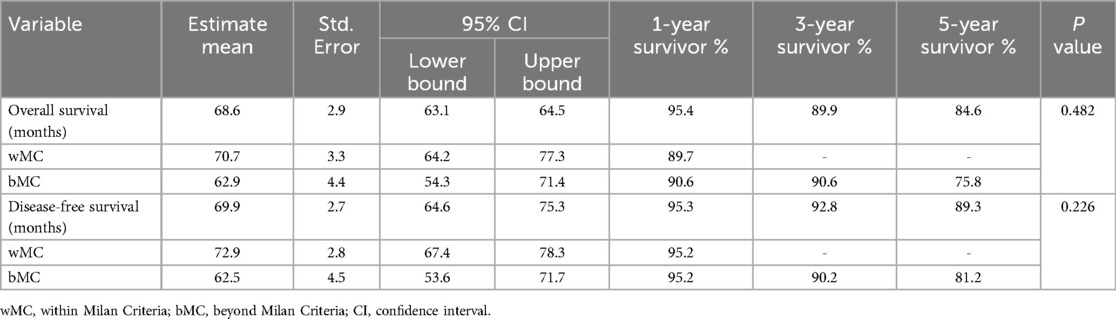
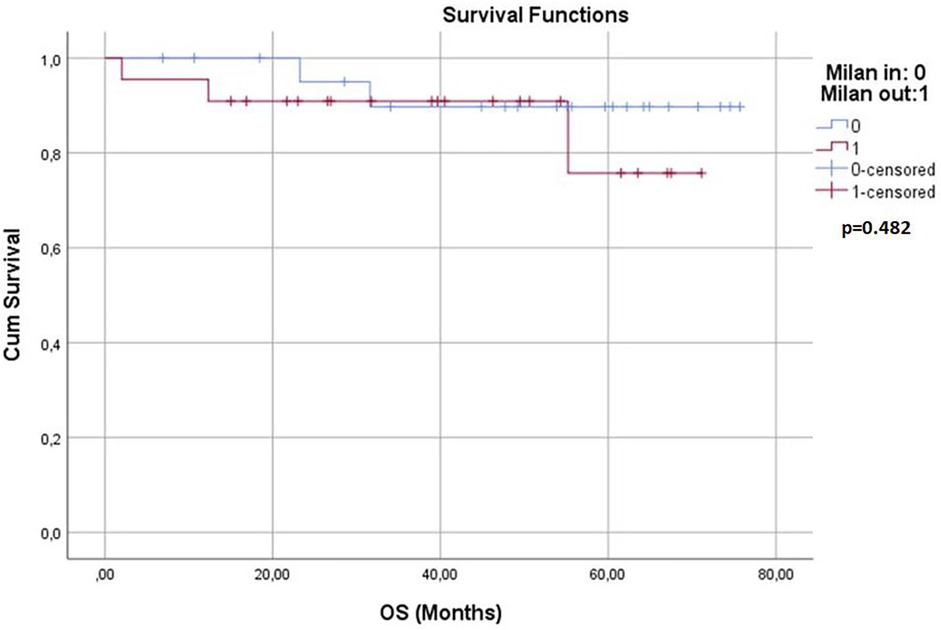
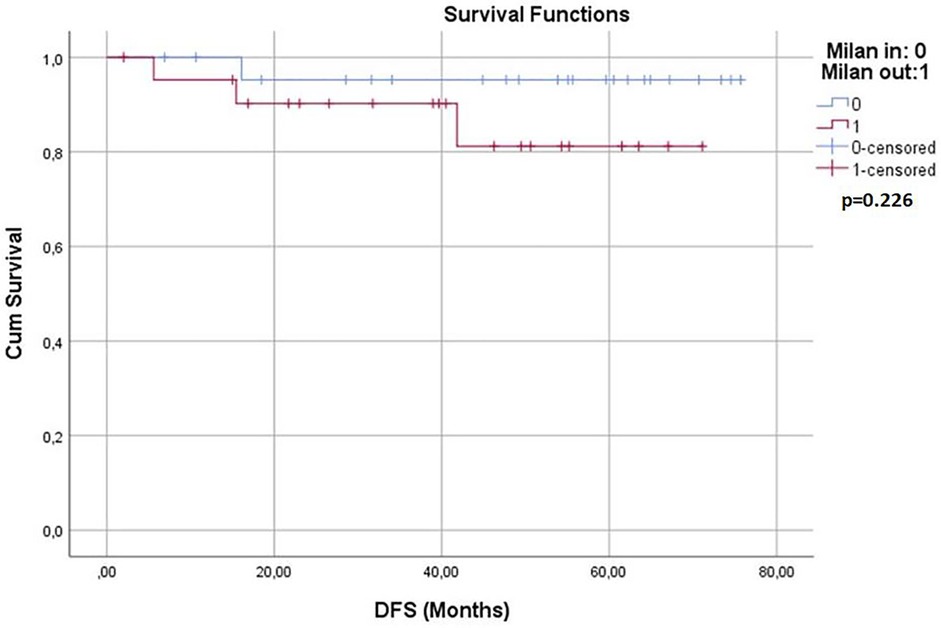
Survival analyses were conducted for both groups. The 1-year, 3-year, and 5-year overall survival (OS) rates and disease-free survival (DFS) rates were statistically similar between the two groups (p > 0.05) (Table 3). Kaplan–Meier survival curves are shown in Figures 1, 2. Perioperative mortality was observed in one patient in the bMC group due to intra-abdominal sepsis. The second mortality in the bMC group was due to disease progression on postoperative day (POD) 375. The third mortality in the bMC group occurred due to cardiac decompensation on POD 1,679. The first mortality in the wMC group was due to disease progression on POD 707. The second mortality in the wMC group was due to chronic rejection on POD 961.
Recurrence occurred in four patients. In the wMC group, one patient developed lung metastasis on POD 463. In the bMC group, one patient developed vertebral metastasis on POD 168, and two patients had liver recurrences on PODs 483 and 1,254. Pathological examination of the explanted specimens from the four patients who developed recurrence revealed no evidence of microvascular invasion.
Post-hoc power analysis based on tumor count and total tumor diameter revealed a study power of 89% within a 95% confidence interval. Moreover, the post-hoc power calculated based on the survival analyisis results was to be found 71%.
5 Discussion
In our study, the retrospective analysis of a single-center experience demonstrated that post-transplant survival rates of HCC patients beyond the Milan Criteria (bMC) were comparable to those of patients within the Milan Criteria (wMC), which is consistent with current literature. The majority of the transplant cases at our center involved living donor liver transplantation (84.4%), which represents one of the key differences between our study and those commonly found in Western literature. It is evident that drawing universally applicable conclusions is difficult due to the limited number of patients, and that retrospective analyses inherently do not provide high levels of evidence.
6 Conclusion
Surgical options remain the cornerstone of current treatment for hepatocellular carcinoma (HCC). Despite the availability of surgical resection and liver transplantation (LT), no effective treatment method has yet been established. Although limited organ availability and complications related to immunosuppressive therapy are major drawbacks of LT, its ability to address underlying liver diseases remains one of its most significant advantages. Recent advances have shown that various biochemical and radiological parameters can predict LT outcomes, yet no universally accepted gold standard guideline has been established. It is well known, however, that parameters such as tumor burden, plasma AFP levels, the presence of microvascular invasion, and tumor differentiation significantly impact survival rates following liver transplantation. Building on the promising developments from Mazzaferro's work on patient selection, it is clear that definitive conclusions have yet to be reached, and we believe that future research will offer deeper insights into organ and patient selection.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Başkent University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
İT: Data curation, Formal analysis, Investigation, Writing – review & editing, Methodology, Writing – original draft. TK: Writing – review & editing, Conceptualization, Methodology, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to express their sincere gratitude to Çağla Sarıtürk from the Department of Statistics at Başkent University for her tremendous efforts in analyzing the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Drefs M, Schoenberg MB, Börner N, Koliogiannis D, Koch DT, Schirren MJ, et al. Changes of long-term survival of resection and liver transplantation in hepatocellular carcinoma throughout the years: a meta-analysis. Eur J Surg Oncol. (2024) 50(3):107952. doi: 10.1016/j.ejso.2024.107952
3. Höckerstedt K. Liver transplantation: present results and problems. Ann Med. (1992) 24(5):325–8. doi: 10.3109/07853899209147831
4. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. (1996) 334(11):693–9. doi: 10.1056/NEJM199603143341104
5. Pavel MC, Fuster J. Expansion of the hepatocellular carcinoma Milan criteria in liver transplantation: future directions. World J Gastroenterol. (2018) 24(32):3626–36. doi: 10.3748/wjg.v24.i32.3626
6. Bruix J, Sherman M. Practice guidelines committee, American association for the study of liver diseases. Management of hepatocellular carcinoma. Hepatology. (2005) 42:1208–36. doi: 10.1002/hep.20933
7. Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. (2008) 100:698–711. doi: 10.1093/jnci/djn134
8. Bruix J, Sherman M. American association for the study of liver diseases. Management of hepatocellular carcinoma: an update. Hepatology. (2011) 53:1020–2. doi: 10.1002/hep.24199
9. Cabibbo G, Daniele B, Borzio M, Casadei-Gardini A, Cillo U, Colli A, et al. Multidisciplinary treatment of hepatocellular carcinoma in 2023: Italian practice treatment guidelines of the Italian association for the study of the liver (AISF), Italian association of medical oncology (AIOM), Italian association of hepato-bilio-pancreatic surgery (AICEP), Italian association of hospital gastroenterologists (AIGO), Italian association of radiology and clinical oncology (AIRO), Italian society of pathological anatomy and diagnostic cytology (SIAPeC-IAP), Italian society of surgery (SIC), Italian society of gastroenterology (SIGE), Italian society of medical and interventional radiology (SIRM), Italian organ transplant society (SITO), and association of patients with hepatitis and liver disease (EpaC)—part I—surgical treatments. Dig Liver Dis. (2024) 56(2):223–34. doi: 10.1016/j.dld.2023.10.029
10. European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASLEORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. (2012) 56:908–43. doi: 10.1016/j.jhep.2011.12.001
11. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. (1999) 19(3):329–38. doi: 10.1055/s-2007-1007122
12. Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. (2001) 33(6):1394–403. doi: 10.1053/jhep.2001.24563
13. Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: tokyo university series. Dig Dis. (2007) 25(4):310–2. doi: 10.1159/000106910
14. Jonas S, Mittler J, Pascher A, Schumacher G, Theruvath T, Benckert C, et al. Living donor liver transplantation of the right lobe for hepatocellular carcinoma in cirrhosis in a European center. Liver Transpl. (2007) 13(6):896–903. doi: 10.1002/lt.21189
15. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. (2009) 10(1):35–43. doi: 10.1016/S1470-2045(08)70284-5
16. DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. (2011) 253(1):166–72. doi: 10.1097/SLA.0b013e31820508f1
17. Xu X, Lu D, Ling Q, Wei X, Wu J, Zhou L, et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut. (2016) 65(6):1035–41. doi: 10.1136/gutjnl-2014-308513
18. Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including a-fetoprotein improves the performance of Milan criteria. Gastroenterology. (2012) 143(4):986–94. doi: 10.1053/j.gastro.2012.05.052
19. Toso C, Asthana S, Bigam DL, Shapiro AJ, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the scientific registry of transplant recipients database. Hepatology. (2009) 49:832–8. doi: 10.1002/hep.22693
20. Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, et al. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. (2008) 14(8):1107–15. doi: 10.1002/lt.21484
21. Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. (2018) 154(1):128–39. doi: 10.1053/j.gastro.2017.09.025
22. Shimamura T, Akamatsu N, Fujiyoshi M, Kawaguchi A, Morita S, Kawasaki S, et al. Expanded living-donor liver transplantation criteria for patients with hepatocellular carcinoma based on the Japanese nationwide survey: the 5-5-500 rule—a retrospective study. Transpl Int. (2019) 32(4):356–68. doi: 10.1111/tri.13391
23. Kaido T, Ogawa K, Mori A, Fujimoto Y, Ito T, Tomiyama K, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery. (2013) 154(5):1053–60. doi: 10.1016/j.surg.2013.04.056
24. Lee JH, Cho Y, Kim HY, Cho EJ, Lee DH, Yu SJ, et al. Serum tumor markers provide refined prognostication in selecting liver transplantation candidate for hepatocellular carcinoma patients beyond the Milan criteria. Ann Surg. (2016) 263(5):842–50. doi: 10.1097/SLA.0000000000001578
25. Halazun KJ, Najjar M, Abdelmessih RM, Samstein B, Griesemer AD, Guarrera JV, et al. Recurrence after liver transplantation for hepatocellular carcinoma: a new MORAL to the story. Ann Surg. (2017) 265(3):557–64. doi: 10.1097/SLA.0000000000001966
26. Sasaki K, Firl DJ, Hashimoto K, Fujiki M, Diago-Uso T, Quintini C, et al. Development and validation of the HALT-HCC score to predict mortality in liver transplant recipients with hepatocellular carcinoma: a retrospective cohort analysis. Lancet Gastroenterol Hepatol. (2017) 2(8):595–603. doi: 10.1016/S2468-1253(17)30106-1
27. Alim A, Karataş C. Prognostic factors of liver transplantation for HCC: comparative literature review. J Gastrointest Cancer. (2021) 52(4):1223–31. doi: 10.1007/s12029-021-00730-x
28. Goldberg D, Mantero A, Newcomb C, Delgado C, Forde KA, Kaplan DE, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma using the LiTES-HCC score. J Hepatol. (2021) 74(6):1398–406. doi: 10.1016/j.jhep.2020.12.021
29. Dudek K, Kornasiewicz O, Remiszewski P, Kobryń K, Ziarkiewicz-Wróblewska B, Górnicka B, et al. Impact of tumor characteristic on the outcome of liver transplantation in patients with hepatocellular carcinoma. Transplant Proc. (2009) 41(8):3135–7. doi: 10.1016/j.transproceed.2009.08.016
30. Alim A, Erdogan Y, Dayangac M, Yuzer Y, Tokat Y, Oezcelik A. Living donor liver transplantation: the optimal curative treatment for hepatocellular carcinoma even beyond milan criteria. Cancer Cont. (2021) 28:10732748211011960. doi: 10.1177/10732748211011960
31. Yankol Y, Hoş G, Kanmaz T, Mecit N, Çakaloğlu Y, Kalayoğlu M, et al. Are the criteria always right? Assessment of hepatocellular carcinoma cases in living donor liver transplantation at a high-volume center. Turk J Med Sci. (2021) 51(5):2383–95. doi: 10.3906/sag-2101-51
32. Esnaola NF, Lauwers GY, Mirza NQ, Nagorney DM, Doherty D, Ikai I, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. (2002) 6(2):224–32. doi: 10.1016/S1091-255X(01)00015-4
33. Chandarana H, Robinson E, Hajdu CH, Drozhinin L, Babb JS, Taouli B. Microvascular invasion in hepatocellular carcinoma: is it predictable with pretransplant MRI? AJR Am J Roentgenol. (2011) 196(5):1083–9. doi: 10.2214/AJR.10.4720
34. Si YQ, Wang XQ, Fan G, Wang CY, Zheng YW, Song X, et al. Value of AFP and PIVKA-II in diagnosis of HBV-related hepatocellular carcinoma and prediction of vascular invasion and tumor differentiation. Infect Agent Cancer. (2020) 15(1):70. doi: 10.1186/s13027-020-00337-0
35. Zhang J, Zeng F, Jiang S, Tang H, Zhang J. Preoperative prediction model of microvascular invasion in patients with hepatocellular carcinoma. HPB (Oxford). (2023) 25(1):45–53. doi: 10.1016/j.hpb.2022.08.007
36. Sun JX, Yang Z, Wu JY, Shi J, Yu HM, Yan ML, et al. A new scoring system for predicting the outcome of hepatocellular carcinoma patients without microvascular invasion-a large-scale multicentre study. HPB (Oxford). (2024) 26(6):741–52. doi: 10.1016/j.hpb.2024.02.007
37. Guerrini GP, Pinelli D, Di Benedetto F, Marini E, Corno V, Guizzetti M, et al. Predictive value of nodule size and differentiation in HCC recurrence after liver transplantation. Surg Oncol. (2016) 25:419–28. doi: 10.1016/j.suronc.2015.09.003
38. Song J, Zhou H, Gu D, Xu Y. Hepatocellular carcinoma differentiation: research progress in mechanism and treatment. Front Oncol. (2022) 11:790358. doi: 10.3389/fonc.2021.790358
39. Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. (2017) 152:745–61. doi: 10.1053/j.gastro.2016.11.048
40. Hayashi Y, Wang W, Ninomiya T, Nagano H, Ohta K, Itoh H. Liver enriched transcription factors and differentiation of hepatocellular carcinoma. Mol Pathol. (1999) 52:19–24. doi: 10.1136/mp.52.1.19
41. Cheng Z, He Z, Cai Y, Zhang C, Fu G, Li H, et al. Conversion of hepatoma cells to hepatocyte-like cells by defined hepatocyte nuclear factors. Cell Res. (2019) 29:124–35. doi: 10.1038/s41422-018-0111-x
42. Shinkawa H, Tanaka S, Kabata D, Takemura S, Amano R, Kimura K, et al. The prognostic impact of tumor differentiation on recurrence and survival after resection of hepatocellular carcinoma is dependent on tumor size. Liver Cancer. (2021) 10(5):461–72. doi: 10.1159/000517992
43. Sapisochin G, Goldaracena N, Laurence JM, Dib M, Barbas A, Ghanekar A, et al. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: a prospective validation study. Hepatology. (2016) 64(6):2077–88. doi: 10.1002/hep.28643
44. Kılcı BM, İnce V, Carr BI, Usta S, Bağ HG, Şamdancı E, et al. Parameters predicting microvascular invasion and poor differentiation in hepatocellular carcinoma patients with normal alpha-fetoprotein level before liver transplantation. Turk J Gastroenterol. (2023) 34(7):753–9. doi: 10.5152/tjg.2023.22538
45. Yilma M, Mehta N. Optimal liver transplantation criteria for hepatocellular carcinoma. Surg Oncol Clin N Am. (2024) 33(1):133–42. doi: 10.1016/j.soc.2023.06.011
46. Matevish L, Patel MS, Vagefi PA. Downstaging techniques for hepatocellular carcinoma in candidates awaiting liver transplantation. Surg Clin North Am. (2024) 104(1):145–62. doi: 10.1016/j.suc.2023.07.004
47. Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. (2015) 61(6):1968–77. doi: 10.1002/hep.27752
48. Yao FY, Fidelman N. Reassessing the boundaries of liver transplantation for hepatocellular carcinoma: where do we stand with tumor down-staging? Hepatology. (2016) 63(3):1014–25. doi: 10.1002/hep.28139
49. Tabrizian P, Holzner ML, Mehta N, Halazun K, Agopian VG, Yao F, et al. Ten-year outcomes of liver transplant and downstaging for hepatocellular carcinoma. JAMA Surg. (2022) 157(9):779–88. doi: 10.1001/jamasurg.2022.2800
50. Haberal M, Akdur A, Moray G, Arslan G, Özçay F, Selçuk H, et al. Expanded criteria for hepatocellular carcinoma in liver transplant. Exp Clin Transplant. (2017) 15(Suppl 2):55–8. doi: 10.6002/ect.TOND16.L14
51. Ayvazoğlu Soy EH, Akdur A, Karakaya E, Moray G, Haberal M. Liver transplant recipients who survive for more than 10 years: a long-term survey. Exp Clin Transplant. (2022) 20(Suppl 1):20–3. doi: 10.6002/ect.MESOT2021.O8
52. Victor DW, Monsour HP Jr, Boktour M, Lunsford K, Balogh J, Graviss EA, et al. Outcomes of liver transplantation for hepatocellular carcinoma beyond the university of California San Francisco criteria: a single-center experience. Transplantation. (2020) 104(1):113–21. doi: 10.1097/TP.0000000000002835
53. Wang J, Bao J, Wang R, Hong J, Zhang L, Que Q, et al. The predictive value of the modified AFP model for liver transplantation outcomes in multinodular hepatocellular carcinoma patients. World J Surg Oncol. (2023) 21(1):104. doi: 10.1186/s12957-023-02994-y
54. Jones-Pauley M, Victor DW, Kodali S. Pushing the limits of treatment for hepatocellular carcinoma. Curr Opin Organ Transplant. (2024) 29(1):3–9. doi: 10.1097/MOT.0000000000001123
Keywords: liver transplantation, Milan Criteria, extended criteria, hepatocellular carcinoma, HCC
Citation: Tırnova İ and Kanmaz T (2025) Liver transplantation for HCC within and beyond Milan Criteria: single center experience with literature review. Front. Surg. 12:1594361. doi: 10.3389/fsurg.2025.1594361
Received: 15 March 2025; Accepted: 25 June 2025;
Published: 10 July 2025.
Edited by:
Derek DuBay, Prisma Health, United StatesReviewed by:
Alessandro Dario Mazzotta, Sapienza University of Rome, ItalySenol Emre, Istanbul University Cerrahpasa, Türkiye
Copyright: © 2025 Tırnova and Kanmaz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: İ. Tırnova, dGlybm92YTc3QGdtYWlsLmNvbQ==
†ORCID:
İ. Tırnova
orcid.org/0000-0003-4488-1607
T. Kanmaz
orcid.org/0000-0003-1886-7721
 İ. Tırnova
İ. Tırnova T. Kanmaz1,†
T. Kanmaz1,†