- 1School of Nursing, Inner Mongolia Medical University, Hohhot, China
- 2Peking University Cancer Hospital (Inner Mongolia Campus) & Affiliated Cancer Hospital of Inner Mongolia Medical University, Hohhot, China
- 3Department of Spinal Surgery, The Second Affiliated Hospital of Inner Mongolia Medical University, Hohhot, China
- 4Department of Rehabilitation, The Second Affiliated Hospital of Inner Mongolia Medical University, Hohhot, China
- 5Nursing Department, The Second Affiliated Hospital of Inner Mongolia Medical University, Hohhot, China
Objective: To develop a perioperative lower-extremity deep vein thrombosis (DVT) risk prediction model for spinal fracture surgery patients using logistic regression, supporting clinical prevention strategies.
Methods: Clinical data from 249 patients undergoing spinal fracture surgery (July 2019–October 2024) were retrospectively analyzed. Participants were divided into a model group (n = 166) and a validation group (n = 83) in a 2:1 ratio. Univariate and multivariate logistic regression identified independent risk factors for perioperative DVT, and a predictive model was established. Model fit was evaluated using the Hosmer-Lemeshow test, and predictive performance was assessed via receiver operating characteristic (ROC) curve analysis.
Results: Independent risk factors included perioperative blood transfusion, elevated C-reactive protein, D-dimer >500 μg/L, hypertension, age ≥60 years, and prolonged bed rest. The model [P = 1/(1 + e^−Z)] demonstrated a good fit (Hosmer-Lemeshow χ2 = 12.139, P = 0.807). ROC analysis showed AUC values of 0.75 (95% CI: 0.80–0.92) for the model group and 0.81 (95% CI: 0.64–0.98) for the validation group, indicating robust predictive performance.
Conclusion: The identified risk factors are critical predictors of perioperative DVT in spinal fracture patients. The proposed model exhibits strong clinical utility for early risk stratification and intervention guidance.
1 Introduction
Spinal fractures account for approximately 5% to 6% of all fractures (1), with the most common cause being high-energy trauma events. During the perioperative management of patients with spinal fractures, the risk of developing deep vein thrombosis (DVT) in the lower limbs shows a significant upward trend. DVT formation poses significant risks, potentially leading to delayed surgical treatment in the early stages, thereby prolonging the patient's hospital stay and significantly increasing medical costs. More seriously, if DVT is not managed promptly and effectively, it may progress to life-threatening pulmonary embolism (PE) (2). According to literature reports, the overall incidence rate of DVT following spinal surgery ranges from 0.2% to 31% (3). Based on the analysis of collected data, proactive prevention strategies have demonstrated significant clinical benefits in the prevention of DVT during the perioperative period in patients with spinal fractures. Although current orthopedic research on DVT in major surgeries has preliminarily identified some common risk factors (4), there remains a significant gap in research focused on specific risk indicators and targeted intervention measures during the perioperative period in patients with spinal fractures. First, there is a lack of risk indicators that account for dynamic changes during the perioperative period. Existing studies primarily focus on preoperative static factors (such as age, D-dimer levels, comorbidities, etc.), while neglecting the impact of perioperative dynamic indicators (such as surgical approach, intraoperative blood loss, postoperative bedrest duration, timing of anticoagulant initiation, etc.) on DVT occurrence (5); Second, there is no specific risk warning model established for the Chinese population. Existing DVT risk models (such as the Risk Assessment Profile) are primarily designed for Western trauma populations, and some key variables (such as post-discharge follow-up data) are difficult to obtain in real time in clinical practice, making them unsuitable for guiding preoperative decision-making (6). The incidence of DVT in Asian populations is significantly lower than in Western populations, and directly applying Western models may underestimate or overestimate actual risks (7). In particular, there is currently no multi-factor-defined, accurate, and targeted DVT risk warning model. This study employed a retrospective analysis method, systematically collected clinical case data from patients undergoing spinal fracture surgery. Using univariate screening and logistic multivariate regression models, we investigated the significant independent risk factors for DVT formation after spinal fracture surgery. Based on this, we constructed a DVT risk warning model based on logistic risk regression using standardized modeling formulas to achieve rapid and accurate screening of the risk of lower extremity DVT in patients undergoing spinal fracture surgery. The specific findings are reported as follows:
2 Information and methods
2.1 Research data
Clinical data from 249 patients with spinal fractures who underwent surgical treatment at a tertiary orthopedic specialist hospital in Inner Mongolia from July 2019 to October 2024 were retrospectively collected and analyzed. These patients were divided into a model group (n = 166) and a validation group (n = 83) in a 2:1 ratio to ensure the statistical validity of the model construction and validation process through this grouping. The patients were 18–76 years old, with a mean age of 58.65 ± 7.49 years and a BMI of 25.16 ± 4.40 kg/m2. The inclusion criteria were as follows: (1) age ≥18 years; (2) complete clinical data; (3) no history of thrombosis or other related diseases before admission; (4) clear consciousness, normal cognitive function, and ability to cooperate with the investigations and follow-up; and (5) color ultrasound of the venous vessels of the lower limbs. Complete data. The exclusion criteria were as follows: (1) diagnosis of a malignant tumor or spinal fracture caused by osteoporosis or pathological spinal fracture; (2) severe varicose veins, coagulation dysfunction, or a history of oral anticoagulants; (3) orthopedic pathology in parts of the body other than the spine; and (4) any other major stressful events during the period of admission to the hospital. The Declaration of Helsinki and all methods were approved by the Ethics Committee of the Second Affiliated Hospital of Inner Mongolia Medical University (Number: EFY20240059).
2.2 Research methodology
2.2.1 Data collection
A general information questionnaire was developed to record detailed information on the selected patients' demographics, comorbidities, treatments, and major laboratory parameters. Specific information included sex, age, body mass index (BMI), history of preexisting medical conditions, the specific site of the fracture, American Society of Anesthesiologists (ASA) classification, the time interval between fracture and surgery, postoperative bed rest, length of surgical operation, history of smoking, and history of perioperative blood transfusion; intraoperative hypothermia, postoperative drainage, D-dimer (D-D), C-reactive protein (CRP), D-D, and C-reactive protein (CRP) levels were recorded on the first day of the operation; and whether intraoperative hypothermia, postoperative drainage, and D-D levels were recorded on the first day of the operation. D-D, C-reactive protein (CRP), hemoglobin (HGB), cholesterol concentration (CHOL), procalcitonin concentration (PCT), and fibrinogen concentration (FPC).), fibrinogen concentration (FIB), prothrombin time (PT), activated partial thromboplastin time (APTT), and hypoproteinemia (TP concentration ≤ 60 g/L or ALB concentration ≤ 35 g/L).
2.2.2 Research methods
The perioperative course and treatment of the patients were meticulously documented. Based on imaging examination results, such as color Doppler ultrasound and the diagnostic criteria for DVT, the model group (n = 166) was screened for DVT, with positive cases assigned to the DVT group. Univariate analysis was performed to compare the variability in baseline characteristics between the two model group subgroups: DVT group and non-DVT groups. Variables demonstrating statistically significant differences in the univariate analysis were incorporated into a multivariate logistic regression model to identify independent risk factors for lower limb deep vein thrombosis (DVT) in spinal fracture patients during the perioperative period. A perioperative DVT risk prediction model was developed based on the key variables identified in this model. The Hosmer-Lemeshow test was employed to assess the consistency between the model predictions and actual observations to evaluate the prediction model's performance. Additionally, the area under the curve (AUC) was calculated using a receiver operating characteristic (ROC) curve to quantify the model's predictive efficacy and classification ability. Furthermore, venous Doppler ultrasonography, a noninvasive and user-friendly imaging technique, is widely recognized in clinical practice because of its high sensitivity (>90%) and specificity (>90%) for diagnosing DVT (8). Therefore, the preoperative and postoperative venous ultrasonography results were utilized as an objective diagnostic basis for DVT in this study, ensuring the reliability and consistency of the data.
2.3 Statistical methods
The relevant analyses were performed using the SPSS 27.0 software package, customarily distributed measurements were described as (x ± s), and comparisons were made using the independent samples t-test. Data that did not conform to normally distributed measures were described as medians [P25, P75], and the rank sum test comparisons were made. Count data were described by the constitutive ratio (n[%]), and the chi-square test was performed. A logistic regression prediction model was constructed after independent risk factors were identified via multifactorial logistic regression analysis. The risk regression equation was P = 1/(1 + e^–Z). Note: P = probability of occurrence; Z = weighted sum of multiple independent variables,and the known constant e ≈ 2.718. In this study, the Hosmer-Lemeshow test was implemented to assess the calibration performance of the constructed model. With the help of plotting the subjects' work characteristics (ROC) curves and calculating their area under the curve (AUC), the discriminative and predictive efficacies of the model in distinguishing between different categories of objects were systematically assessed. The optimal cut-off value was determined based on the principle of maximization of the Jordon index, which was subsequently applied to set the predictive threshold of the model. This strategy aims to precisely delineate the diagnostic boundaries between sensitivity and specificity to optimize the decision-making accuracy. Statistical significance was indicated by a p-value of 0.05.
3 Results
3.1 Univariate analysis of perioperative lower extremity deep vein thrombosis in spinal fracture surgery patients in the model group
A total of 29 cases of perioperative lower extremity deep vein thrombosis occurred in 166 patients who underwent spinal fracture surgery in the model group. The incidence of DVT was 17.46% (29/166), with the above patients classified in the DVT group (n = 29), and the remaining patients were classified in the non-DVT group (n = 137). Univariate analysis within the model group revealed the differences between the two subgroups of the DVT and non-DVT groups in terms of information (indexes) such as age, duration of surgery, time in bed, history of hypertension, history of coronary artery disease, history of perioperative blood transfusion, amount of postoperative drainage, whether or not hypothermia occurred intraoperatively, and the incidence of hypoproteinemia, D-dimer, and C-reactive protein levels on the first postoperative day was statistically significant (P < 0.05). The details are listed in Table 1.
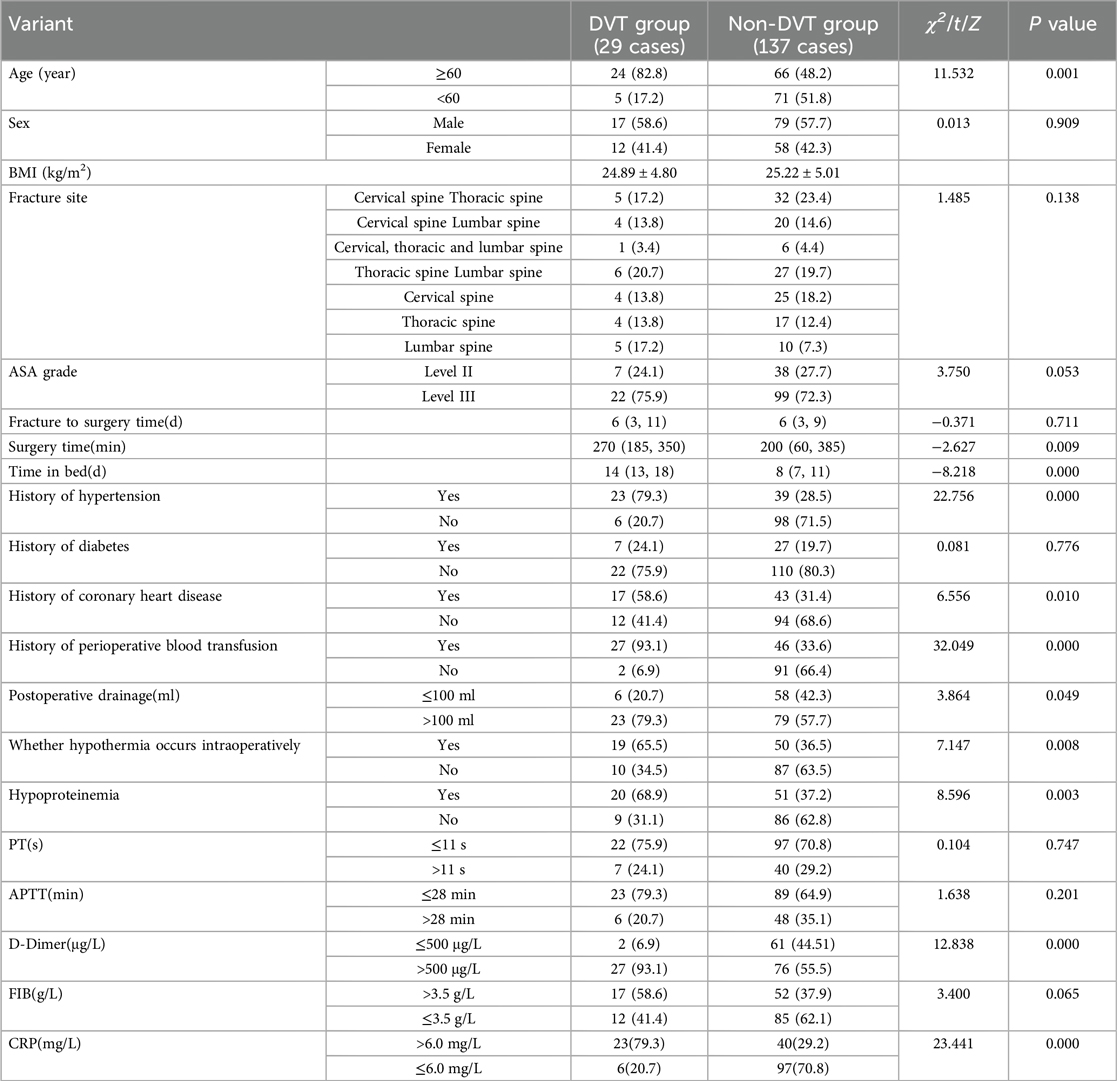
Table 1. Univariate analysis of perioperative lower extremity deep vein thrombosis in patients in the model group.
3.2 Multifactorial analysis of perioperative lower-limb deep vein thrombosis in spinal fracture surgery patients in the model group
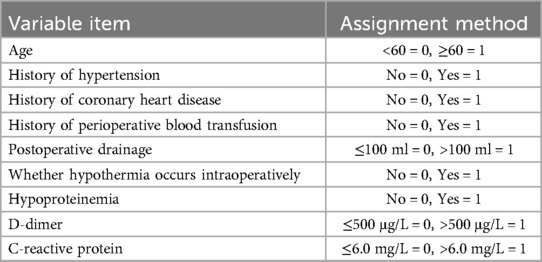
In this study, variables showing statistical significance in univariate analyses, including age, length of surgery, bed rest cycle, history of hypertension and coronary heart disease, perioperative blood transfusion, postoperative drainage, intraoperative hypothermic events, hypoproteinemia status, D-dimer and C-reactive protein levels on the first postoperative day were entered into logistic multivariate regression models as covariates to identify the independent risk factors for lower extremity deep vein thrombosis (DVT) after spinal fracture. (The categorical variables in the above indicators were first assigned values when used as independent variables; the assignment methods are specified in Table 2.) The results of the logistic regression analysis revealed that a history of perioperative blood transfusion, a high level of C-reactive protein (CRP), a D-dimer concentration >500 μg/L, a history of hypertension, an age ≥60 years old, and a prolonged bed rest period were independent risk factors for perioperative lower-extremity DVT in spine fracture patients. Detailed results are presented in Table 3.
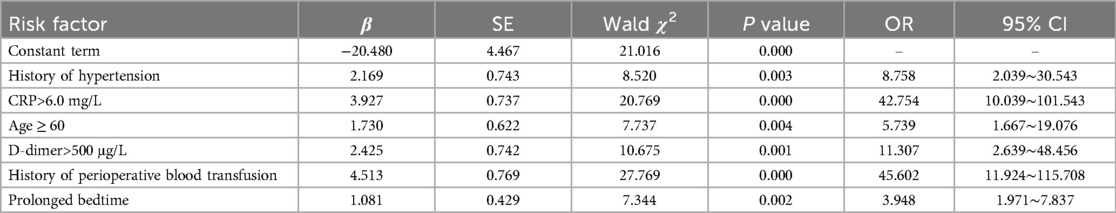
Table 3. Logistic regression analysis of perioperative lower-limb deep vein thrombosis in the model group.
3.3 Construction and testing of an early warning model for perioperative DVT risk in patients undergoing spinal fracture surgery
3.3.1 Establishment of early warning model
Based on the independent risk factors established by logistic risk regression analysis, namely, a history of perioperative blood transfusion, a high level of C-reactive protein (CRP), a D-dimer concentration >500 μg/L, a history of hypertension, an age ≥60 years, and prolonged bed rest, a total of six items were given the sequential numbers of X1–X6 to complete the extraction of partial regression coefficients of the predictors, which described the perioperative DVT risk warning model for patients who underwent spinal fracture. The logistic regression linear equation of the early warning model for perioperative DVT risk was as follows: Logit(p) = −20.480 + 4.513 × X1 + 3.927 × X2 + 2.425 × X3 + 2.169 × X4 + 1.730 × X5 + 1.081 × X6.
3.3.2 Goodness-of-fit test of the early warning model
In this study, the Hosmer-Lemeshow goodness-of-fit test was used to judge the fit of the model; the results showed χ2 = 12.139, P = 0.807, which revealed that the difference between the observed values and the predicted values of the model was not significant, suggesting that there was no significant bias in the model, and that the fit was good.
3.4.2 Differentiation test of early warning model
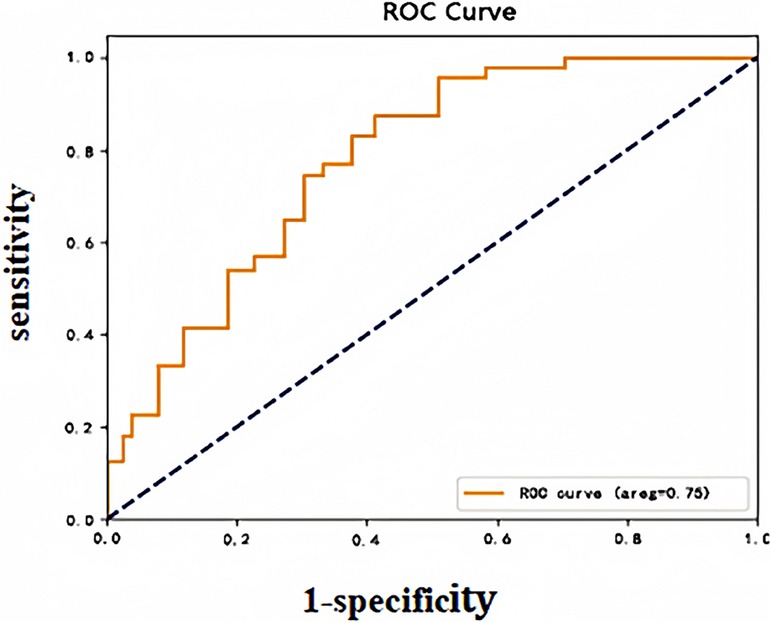
In this study, after importing the clinical case data from the model group into the prediction model, the predictive probability value of each sample was calculated and this probability was used as a predictor variable. The occurrence status of DVT was defined as a dichotomous response variable. The ROC curves of the subjects were plotted, and the model's classification performance was quantitatively assessed by calculating the area under the curve (AUC). The Yoden index maximization criterion was further used to determine the optimal cut-off value for the model prediction probability, which corresponded to a model prediction threshold of 0.076, an AUC of 0.75 (95% CI: 0.80–0.92), a sensitivity of 82.75%, and a specificity of 75.9%, which confirms that the model had a good predictive ability. The ROC curves of the model group are plotted in Figure 1.
3.5 Validation of early warning model
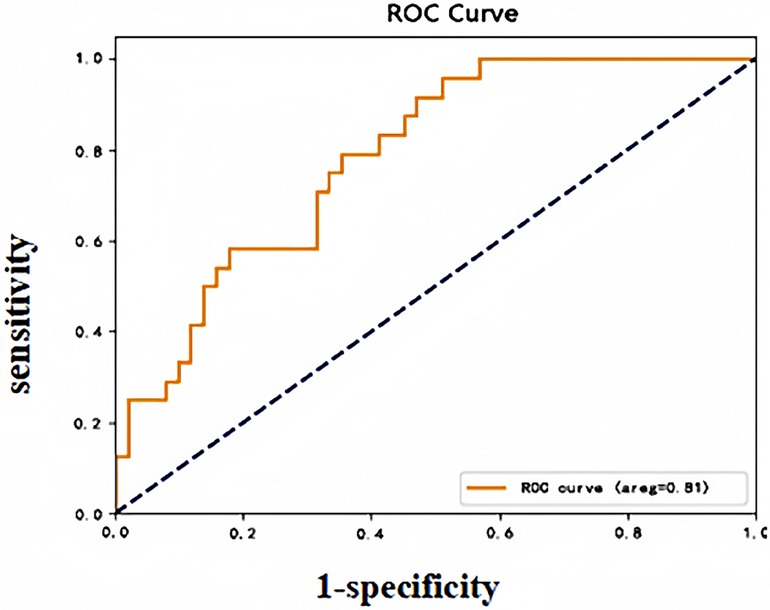
DVT occurred in 11 of 83 patients in the validation group during the perioperative period, and the early warning results revealed a sensitivity of 72.73%, a specificity of 84.72%, and an accuracy of 83.13%. The validation group early warning model subject operating characteristic (ROC) curve (see Figure 2), AUC: 0.81 (95% CI: 0.64–0.98), indicated that the model risk warning ability was good.
4 Discussion
The results of this study revealed that the incidence of perioperative lower extremity deep vein thrombosis in patients with spinal fractures was 17.46%, which is an approximate or slightly lower incidence than the epidemiological data reported in the literature (0.2%–31%) (9, 10). However, this result still suggests that the potential risk of perioperative DVT in such patients needs to be given due attention in the clinic. Notably, perioperative hemodynamic and venous haemodynamic abnormalities in patients with thoracolumbar spine fractures caused by high-energy injuries are more pronounced because of the complex mechanism of trauma and the high demand for tissue repair, further exacerbating the formation of DVTs. In clinical therapeutic interventions for spinal fractures, establishing a risk warning model to quickly and accurately assess the risk of DVT and provide patients with individualized preventive and therapeutic measures is an urgent problem. We conducted a retrospective study of patients admitted for spinal fracture surgery in recent years to systematically assess a series of potential high-risk factors associated with postoperative DVT formation. Univariate and logistic multifactorial analyses were performed on all suspected high-risk factors closely related to perioperative DVT, based on which a risk warning system was constructed via logistic regression modeling to provide a more precise and effective strategy for preventing DVT in major orthopedic surgeries.
In this study, a history of perioperative blood transfusion, high levels of C-reactive protein (CRP), D-dimer concentrations >500 μg/L, a history of hypertension, age ≥60 years, and prolonged bed rest were identified by univariate and multivariate logistic regression analyses as independent risk factors for perioperative lower-extremity DVT in patients with spinal fractures. Previous studies have shown that perioperative blood transfusion during surgery is strongly associated with thrombosis (11, 12). A multicenter study conducted by a national scholar (13) confirmed that intraoperative and/or postoperative blood transfusion, as well as postoperative blood transfusion alone, were significantly associated with the development of DVT in patients with traumatic spinal fractures, and the results of the present study are consistent with this finding. The mechanisms by which perioperative blood transfusion increases the risk of DVT may involve a hypercoagulable state of the blood, vascular endothelial injury, and abnormalities in immune regulation (14). When red blood cells (RBCs) or plasma are transfused, coagulation factors in blood products may lead to increased erythrocyte aggregation and decreased cell membrane deformity, thereby triggering a hypercoagulable state in the blood (15). In addition, the transfusion process may trigger an inflammatory response, leading to vascular endothelial damage and hemodynamic alterations. It may affect the body's immunomodulatory function, increasing the risk of thrombosis. Findings have shown that C-reactive protein (CRP), an acute temporal response protein with low serum levels in healthy individuals, is significantly elevated to reflect the presence of a systemic sensitively, but not specific, inflammatory response state in the organism. Studies have shown (16) that the expression level of CRP is significantly correlated with the degree of activity of the inflammatory response, especially in the state of trauma or infection, and its dynamic changes can provide an objective basis for the quantitative assessment of the inflammatory state. Previous retrospective cohort studies have shown that the inflammatory response is one of the most important factors in thrombosis and can be regarded as a chronic, low-level sterile inflammatory process (17). Individuals with high C-reactive protein levels have a significantly increased risk of developing DVT during the perioperative period, suggesting that C-reactive protein (CRP) stimulates the production of tissue factor (TF) by monocytes, which directly triggers abnormalities in coagulation mechanisms and promotes platelet adhesion and aggregation, ultimately leading to thrombosis. In addition, elevated CRP levels are positively correlated with the degree of complement system activation, further exacerbating vascular endothelial damage and promoting thrombus formation and development (18–20). Therefore, continuous monitoring of C-reactive protein concentration is highly valuable for the early identification of patients at high risk of DVT, especially those undergoing different orthopedic surgery.
As a specific marker of the fibrinolytic process, an increase in D-dimer concentration directly reflects the presence of hypercoagulability and the subsequent enhancement of fibrinolytic activity in vivo, which is closely related to thrombosis and its induced dysregulation of the dynamic balance between coagulation and fibrinolysis (21). According to the available literature (22), activation of the fibrinolytic system is triggered when a thrombus is present in the body, a process in which cross-linked fibrin is broken down to produce derivatives such as D-dimer. Hui et al. (23) reported that the sensitivity and specificity for predicting DVT with an elevated D-dimer level on day 3 after spinal surgery were 72.7% and 76.5%, respectively, and the D-dimer cut-off level was 5.82 μg/ml. Joseph et al. (24) reported that surgery increased D-dimer levels in patients with spinal fractures combined with spinal cord injury. When D-dimer concentrations were ≥2 times the normal's upper limit, their DVT risk was 3.5 times greater than that of patients with typical D-dimer values. The present study revealed that D-dimer levels fluctuate and increase after surgery in patients with spinal fractures. Elevated D-dimer levels can reflect the status of thrombosis in the body. A D-dimer level >500 μg/L may indicate the presence of DVT, and the results of the present study are basically in agreement with those of the above studies. This study confirmed that there is a close relationship between a history of hypertension and the risk of DVT in patients undergoing spinal fracture surgery. Most scholars believe prolonged elevated blood pressure damages vascular endothelial cells, an important factor in developing DVT (25). Second, hypertension is often associated with increased red blood cell aggregation and increased plasma viscosity, coupled with decreased vascular elasticity and luminal narrowing, which trigger the body's coagulation cascade response, which in turn significantly elevates the risk of DVT (26). The present study revealed that age greater than or equal to sixty years and prolonged bed rest were independent risk factors for DVT in patients with spinal fractures and that these factors can be used together as indicators to assess the risk of DVT. Prolonged bed rest increases the risk of DVT after orthopedic surgery. Reduced activity after orthopedic surgery, decreased cardiac output, decreased pump function of the lower limb muscles, increased resistance to venous blood return in the lower limbs, and activation of the coagulation state are more likely to lead to DVT (27). Spinal fractures are unique compared with other fractures, especially when combined with spinal cord and nerve damage, sensory and motor dysfunction of the lower limbs, and prolonged bed rest is more common in these patients after surgery, which can further exacerbate venous blood flow stagnation and weaken muscle pump function, and therefore the incidence of DVT is even greater. Previous studies have established advanced age as a risk factor for DVT in patients undergoing major orthopedic surgery and noted that this risk tends to increase with age (28). This is mainly because changes in vascular fragility are positively correlated with age, and the older the patient is, the poorer the cardiac function, the greater the fibrin activity, the greater the platelet aggregation, and the more severe the intima-media damage and blood flow abnormalities in his/her vessels (29). In addition, advanced age usually coexists with other DVT risk factors (e.g., obesity, hypertension, and prolonged bed rest), and there is a synergistic effect between these risk factors, which can further increase the risk of DVT.
5 Conclusions
In this study, based on the results of a multivariate regression analysis model (OR values and their influence weights), an early warning model for early risk identification of perioperative DVT in patients undergoing spinal fracture surgery (incorporating a total of six independent risk factors) was further developed through data processing with logistic regression formulas. Using the Hosmer-Lemeshow test, we validated the predictive efficacy of this predictive model in terms of the number of perioperative DVT cases, and the results showed consistency between its predictive values and the actual number of observed cases. A good fit (χ2 = 12.139, P = 0.807) suggests that the early warning ability of the model has some credibility and is clinically beneficial. This study further validated the logistic regression model internally and externally. The AUC, sensitivity, and specificity of the model for predicting the perioperative occurrence of DVT in patients who underwent spinal fracture surgery in the model group, as plotted by the subjects' operating characteristic (ROC) curves, were 0.75%, 82.75%, and 75.9%, respectively. The validation group's values were 0.75%, 82.75%, and 75.9%, respectively. Moreover, specificity was 0.81, 72.73%, and 84.72%, respectively, and the best diagnostic value was obtained according to the maximum principle of Jordon's index, with a p-value of 0.075, that is, when the predictive probability was ≥0.075, suggesting that perioperative patients are at risk of developing DVT. The above data revealed that the sensitivity and specificity were significantly more significant than the threshold of 70% in the constructed model and validation phase, which indicated that the developed model demonstrated a strong differentiation ability to identify risk factors and that the probability of appearing against false-negative and false-positive cases was low. These results suggested that the early warning model had good clinical predictive efficacy and applicability, which is beneficial for clinical operation and application.
In this study, unifactorial and multifactorial logistic regression analyses were used to identify and quantify risk variables that contribute significantly and independently to the development of DVT as a clinical event in patients undergoing spinal fracture surgery during postoperative recovery. The model considers a variety of factors that may influence the development of DVT, including a history of blood transfusion, medical history, age, coagulation, and inflammatory markers. The study's results revealed that the constructed early warning model had a high AUC in both the model and validation groups, and the evaluation indices, such as sensitivity and specificity, were within the acceptable range. This study suggested that the model demonstrated excellent predictive performance and accurately quantified the likelihood of patients undergoing spinal fracture surgery to develop lower-limb DVT, thus providing strong support for decision-making in clinical practice. Notably, although this study achieved its intended goals and produced valuable findings, its results are subject to several limitations that need to be fully considered when interpreting the research results are interpreted. For example, the sample sizes included in the retrospective analyses tend to be more limited, which may limit the ability of the constructed models to be generalized, that is, the efficacy of the model's performance in the face of unseen data. Notably, there is significant heterogeneity in treatment protocols and patient composition across different healthcare organizations. This implies that the existing models' scope of application and generalizability must be more fully explored and confirmed through subsequent studies. In the future, we will continue to monitor the research progress in this area and continuously improve and update such risk warning models.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Second Affiliated Hospital of Inner Mongolia Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SYZ: Conceptualization, Investigation, Writing – original draft, Data curation, Methodology, Project administration, Writing – review & editing. JWa: Writing – original draft, Writing – review & editing, Methodology, Project administration PD: Conceptualization, Data curation, Methodology, Project administration, Resources, Writing – review & editing. SHD: Investigation, Software, Writing – review & editing. JWu: Investigation, Software, Writing – review & editing. DLL: Investigation, Software, Writing – review & editing. YTZ: Conceptualization, Formal analysis, Methodology, Project administration, Writing – review & editing. LL: Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank all the participants in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DVT, deep vein thrombosis; CRP, C-reactive protein; BMI, body mass index; PE, pulmonary embolism.
References
1. Geriatric Spine Surgery Perioperative Multidisciplinary Management Guidelines Working Group, Cervical Spine Study Group of Chinese Orthopaedic Association, Spine Medicine Branch of China International Exchange and Promotive Association for Medical and Health Care, Spine and Spinal Cord Committee of Chinese Association of Rehabilitation Medicine, Chen F, Meng H, et al. Multidisciplinary management guidelines for common perioperative problems in elderly spine surgery patients. Chin J Bone Joint Surg. (2023) 16(11):961–80. doi: 10.3969/j.issn.2095-9958.2023.11.01
2. Maheshati A, Yi Y, Habulihan H, Jin GL. Incidence and risk factors of preoperative lower-extremity deep vein thrombosis in patients with spinal fractures. Zhongguo Gu Shang. (2022) 35(08):717–23. doi: 10.12200/j.issn.1003-0034.2022.08.004
3. Solaru S, Alluri RK, Wang JC, Hah RJ. Venous thromboembolism prophylaxis in elective spine surgery. Global Spine J. (2021) 11(7):1148–55. doi: 10.1177/2192568220962439
4. Lin S, Alepuz A, Tritsch T, Schwartz G. Deep vein thrombosis prophylaxis in orthopedic surgery. Cureus. (2024) 16(2):e53726. doi: 10.7759/cureus.53726
5. Yuncan Z, Keyi Y, Hengyan Z. Consensus and controversies in the prevention of venous thromboembolism after spinal surgery. Chin J Bone Joint Surg. (2019) 12(02):145–9. doi: 10.3969/j.issn.2095-9958.2019.02.14
6. Ma J, Tian M, Zhu Y, Hu J, Zhang Y, Li X. Development and validation of a predictive model for preoperative deep vein thrombosis following traumatic thoracolumbar fractures. Sci Rep. (2024) 14(1):19547. doi: 10.1038/s41598-024-70464-w
7. Eissa AT, Alanagari A, Alrowaili F, Aleissa S. The incidence and risk factors of thromboembolism in patients with traumatic spine fractures in a tertiary care hospital. J Musculoskelet Surg Res. (2022) 6(1):94–100. doi: 10.25259/JMSR_133_2021
8. Blitzer RR, Eisenstein S. Venous thromboembolism and pulmonary embolism: strategies for prevention and management. Surg Clin N Am. (2021) 101(5):925–38. doi: 10.1016/j.suc.2021.06.015
9. Lai J, Wu S, Fan Z, Jia M, Yuan Z, Yan X, et al. Comparative study of two models predicting the risk of deep vein thrombosis progression in spinal trauma patients after operation. Clin Neurol Neurosurg. (2024) 236:108072–76. doi: 10.1016/j.clineuro.2023.108072
10. Ma J, Du P, Qin J, Zhou Y, Liang N, Hu J, et al. Incidence and risk factors predicting deep venous thrombosis of lower extremity following spinal fractures. Sci Rep. (2021) 11(1):2441. doi: 10.1038/s41598-021-82147-x
11. Kraaijpoel N, van Es N, Porreca E, Büller HR, Di Nisio M. The diagnostic management of upper extremity deep vein thrombosis: a review of the literature. J Thromb Haemostasis. (2017) 15(1):6–73. doi: 10.1016/j.thromres.2017.05.035
12. Grits D, Kuo A, Acuña AJ. The association between perioperative blood transfusions and venous thromboembolism risk following surgical management of hip fractures. J Orthop. (2022) 34:123–31. doi: 10.1016/j.jor.2022.08.016
13. Zhang J, Shao Y, Zhou H, Li R, Xu J, Xiao Z, et al. Prediction model of deep vein thrombosis risk after lower extremity orthopedic surgery. Heliyon. (2024) 10(9):e29517. doi: 10.1016/j.heliyon.2024.e29517
14. Jiang PF, Yang DF. Efficacy of anterior-posterior decompression on thoracolumbar spine fracture with spinal cord injury and analysis of risk factors for postoperative deep vein thrombosis. Am J Transl Res. (2022) 14(6):4033–41.35836875
15. Weihao C, Zhongwei L, Khan HH, Jin GL. Analysis of risk factors for deep vein thrombosis after spinal surgery. J Pract Orthop. (2021) 27(6):488–91, 511.
16. Wang G, Wu BF, Zhao WJ, Hu WP, Wang JY, Gao HZ. C-reactive protein is a predictor for lower-extremity deep venous thrombosis in patients with primary intracerebral hemorrhage. Eur J Med Res. (2024) 29(1):311. doi: 10.1186/s40001-024-01842-3
17. Jianfei E, Zheng M, Wei W, He S, Yuan C, Chen Z. Diagnostic value of thromboelastogram and thrombus molecular markers in early detection of deep vein thrombosis in patients with lower extremity fracture. Eur J Med Res. (2024) 29(1):592. doi: 10.1186/s40001-024-02190-y
18. Zhao Y, Kong X, Song K, Liu Z, Zhang Y, Cheng L. Analysis of risk factors and establishment of prediction model for lower extremity deep vein thrombosis after lumbar fusion surgery. BMC Surg. (2024) 24(1):392. doi: 10.1186/s12893-024-02689-5
19. Zhang J, Zhao K, Li J, Meng H, Zhu Y, Zhang Y. Age over 65 years and high levels of C-reactive protein are associated with the risk of preoperative deep vein thrombosis following closed distal femur fractures: a prospective cohort study. J Orthop Surg Res. (2020) 15(1):559. doi: 10.1186/s13018-020-02089-4
20. Rolling CC, Barrett TJ, Berger JS. Platelet-monocyte aggregates: molecular mediators of thromboinflammation. Front Cardiovasc Med. (2023) 10:960398. doi: 10.3389/fcvm.2023.960398
21. Zhang W, Liu BH, Xia CD, Qiu JH, Lou HP, Di JD, et al. Predictive value of D-dimer for deep venous thrombosis of lower extremity in adult burn patients. Chin J Burn. (2022) 38(4):335–40. doi: 10.3760/cma.j.cn501120-20201021-00444
22. Hvas CL, Larsen JB. The fibrinolytic system and its measurement: history, current uses and future directions for diagnosis and treatment. Int J Mol Sci. (2023) 24(18):14179. doi: 10.3390/ijms241814179
23. Hui Z, Bo D, Ani M, Wulin K, Deyu L. Research progress of D-dimer in the diagnosis of perioperative lower limb deep vein thrombosis in fracture. Acad J Med Health Sci. (2023) 4(5):1–5. doi: 10.25236/AJMHS.2023.040501
24. Joseph L. Redox dysregulation in vascular pathobiology. Free Radic Biol Med. (2014) 75(S1):S2. doi: 10.1016/j.freeradbiomed.2014.10.597
25. Fang C, Huang F, Yao M, Wang Z, Ma J, Wu D, et al. Advances in microRNA regulation of deep vein thrombosis through venous vascular endothelial cells. Mol Med Rep. (2024) 29(6):1–9. doi: 10.3892/mmr.2024.13220
26. Miyamoto S, Hayakawa M, Tsuruta W, Shirakawa M, Beppu M, Sakai N, et al. Antiplatelets before or during endovascular therapy after intravenous thrombolysis for atherothrombotic large vessel occlusion. J Clin Neurosci. (2025) 133:111014. doi: 10.1016/j.jocn.2024.111014
27. Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. (2019) 133(6):511–20. doi: 10.1182/blood-2018-07-818211
28. Tan Z, Hu H, Deng X, Zhu J, Zhu Y, Ye D, et al. Incidence and risk factors for deep venous thrombosis of lower extremity after surgical treatment of isolated patella fractures. J Orthop Surg Res. (2021) 16(1):90. doi: 10.1186/s13018-021-02240-9
Keywords: spinal fracture, deep vein thrombosis, risk factors, predictive model, lower limb
Citation: Zhuang SY, Wang J, Du P, Dong SH, Wu J, Li DL, Zang YT and Li L (2025) A clinical risk prediction model for perioperative lower extremity DVT in patients undergoing spinal fracture surgery. Front. Surg. 12:1597101. doi: 10.3389/fsurg.2025.1597101
Received: 20 March 2025; Accepted: 13 August 2025;
Published: 18 September 2025.
Edited by:
Panagiotis G. Korovessis, AIMIS (American Institute of Minimal Invassive Surgery), CyprusReviewed by:
Anita Sahu, National Jewish Health, United StatesSoukaina Amniouel, National Center for Advancing Translational Sciences (NIH), United States
Copyright: © 2025 Zhuang, Wang, Du, Dong, Wu, Li, Zang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Li, MTM0NzQ3Mzc5NTVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 ShuYuan Zhuang
ShuYuan Zhuang Jing Wang2,†
Jing Wang2,†