- 1University Clinic of General, Reconstructive and Cardiovascular Surgery, I.M. Sechenov First Moscow State Medical University of the Ministry of Health of Russia (Sechenov University), Moscow, Russia
- 2University Clinic of General, Reconstructive and Cardiovascular Surgery, Moscow State Budgetary Healthcare Institution “Moscow City Hospital Named After S.S. Yudin, Moscow Healthcare Department”, Moscow, Russia
Celiac artery aneurysms (САА) represent the fourth most common visceral artery aneurysm. Despite its rarity, CAA carries a definite risk of rupture and/or other serious complications, which can be fatal. The reported rupture risk varies in the scientific literature, but it appears to range from 10% to 20%. CAA is often diagnosed at a late stage, after it has ruptured. There is currently no consistent approach to managing patients with CAA or its rupture. The aim of this case report is to present a successful minimally invasive treatment of CAA rupture.
Introduction
Celiac artery aneurysm (CAA) is a rare aneurysm, accounting for 0.1%–2% of all visceral artery aneurysms and representing the fourth most common type (1, 2). CAA is associated with a formidable complication such as rupture, which carries the risk of a fatal outcome. The reported rupture risk varies in the scientific literature, but it appears to range from 10% to 20% (2). Early diagnosis and treatment of the disease is crucial to prevent the development of life-threatening complications. However, to date, there is no uniform strategy for managing patients when CAA or its rupture is detected, which underscores the importance of this issue for both practitioners and researchers in the field of vascular surgery (1, 3, 4). The aim of this case report was to present a successful minimally invasive treatment of CAA rupture.
Case description
A 44-year-old man was admitted to the department of purulent surgery of Moscow State Budgetary Healthcare Institution “Moscow City Hospital named after S.S. Yudin, Moscow Healthcare Department” with an abscess of the left foot and right forearm. The patient underwent surgical intervention, which included opening and draining the abscesses, as well as treating the wounds. The patient also received drug therapy, including empiric antibacterial therapy.
Additionally, the patient had a history of chronic glomerulonephritis (morphologically focal segmental glomerulosclerosis), nephrotic syndrome, and chronic kidney disease (CKD) C1A4 (glomerular filtration rate of 111 ml/min according to the CKD-EPI equation). The patient also had steroid-induced diabetes and a left medial ankle fracture with metal osteosynthesis involving a plate and screws. Trauma and surgery data were unavailable. According to the patient, there was no aggravation of family history, including hereditary diseases. Notably, the patient had no history of abdominal infection or trauma.
On day 6 of the hospitalization, the patient's clinical blood test showed a significant decrease in hemoglobin (Hb) to 82 g/L (Hb was 111 and 96 g/L on admission and on day 5 of the hospitalization, respectively) and hematocrit (Ht) to 24.8% (Ht was 30.2 and 28.4% on admission and on day 5 of the hospitalization, respectively). Upon examination, the patient appeared unstressed and complained of weakness but did not have abdominal pain or tenderness. The heart rate was 92 beats per min, and the blood pressure was 100/60 mmHg. Therefore, the shock index was 0.92, corresponding to a blood loss of 20% of the circulating blood volume. Based on the clinical and laboratory data, internal bleeding was suspected. To confirm the origin of the bleeding, we performed a computed tomography (CT) of the chest, abdomen, and small pelvis with intravenous contrast.
CT angiography revealed a retroperitoneal hematoma and identified an irregularly shaped celiac artery aneurysm as its source. The aneurysm measured 33.5 mm × 24.5 mm × 38 mm (Figure 1). However, there was no evidence of contrast extravasation at the time of the study. The aneurysm was located 5 mm from the celiac artery orifice. A strong accumulation of hyperdense component was detected in the retroperitoneum on the left side, as well as para-aortically. These CT scans, in combination with clinical and laboratory data, were consistent with CAA rupture.
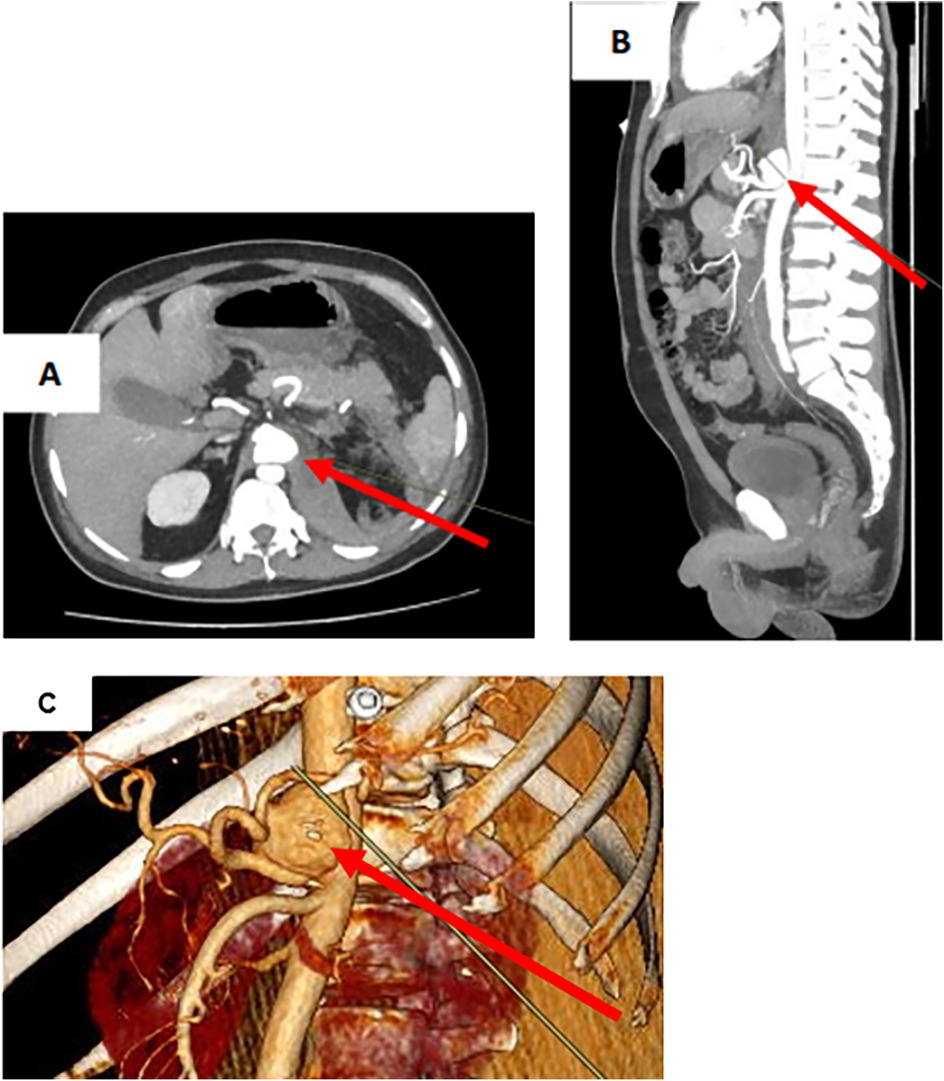
Figure 1. Ct scan of the abdomen with intravenous contrast in CAA rupture. (A) CAA in transverse projection. (B) CAA in sagittal projection. (C) CAA on 3D model. CAA (arrow).
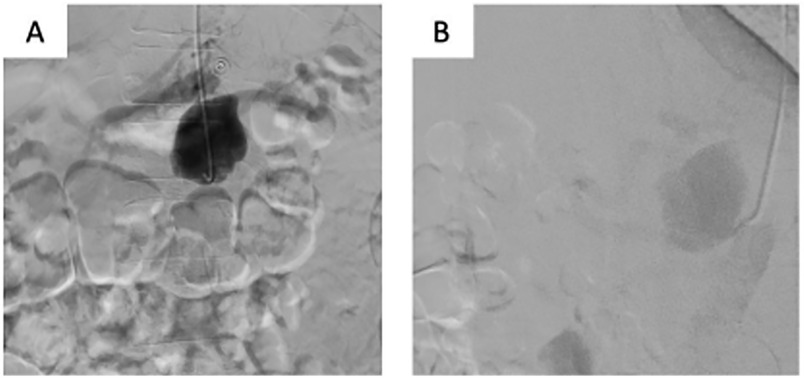
The patient underwent urgent CA angiography performed via a radial access. The CA angiography revealed a 45 mm × 30 mm saccular aneurysm in the proximal third of the artery (Figure 2). The BeGraft stent graft was then implanted using a 7 mm × 37 mm balloon catheter. Control angiography showed aneurysm occlusion and patency of the CA and all its branches.
To evaluate the patient's condition in the postoperative period, the following examinations were performed: clinical and biochemical blood tests, a coagulogram, an abdominal aortic Doppler ultrasound, a Doppler ultrasound of the lower extremity arteries, and an ultrasound examination of the abdomen and retroperitoneum (Figure 3). According to the results of the laboratory and instrumental examinations, no impairments were observed in the patient. There were no adverse or unanticipated events. On day 16, the patient was discharged under outpatient supervision by the surgeon.
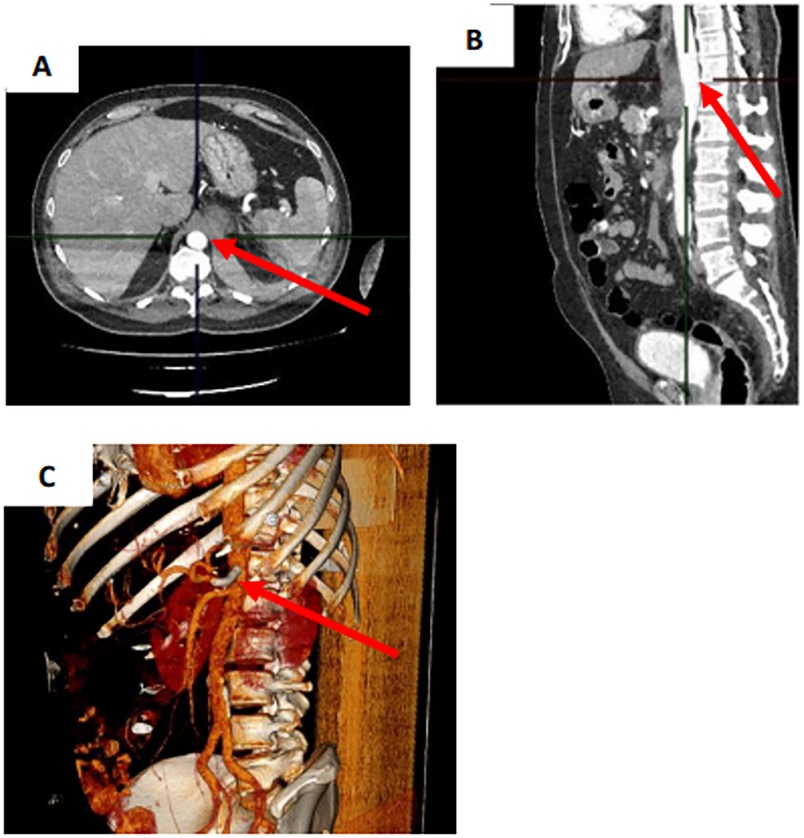
Figure 3. Control CT scan of the abdomen with intravenous contrast on the next day after stent-graft implantation. (A) Transverse projection. (B) Sagittal projection. (C) 3D model. CAA (arrow).
A control CT of the abdomen and a CT angiography of the aorta and its branches with intravenous contrast showed no contrast defects or leakage outside the vascular bed after 3 weeks, while regression of the retroperitoneal hematoma was observed (Figure 4).
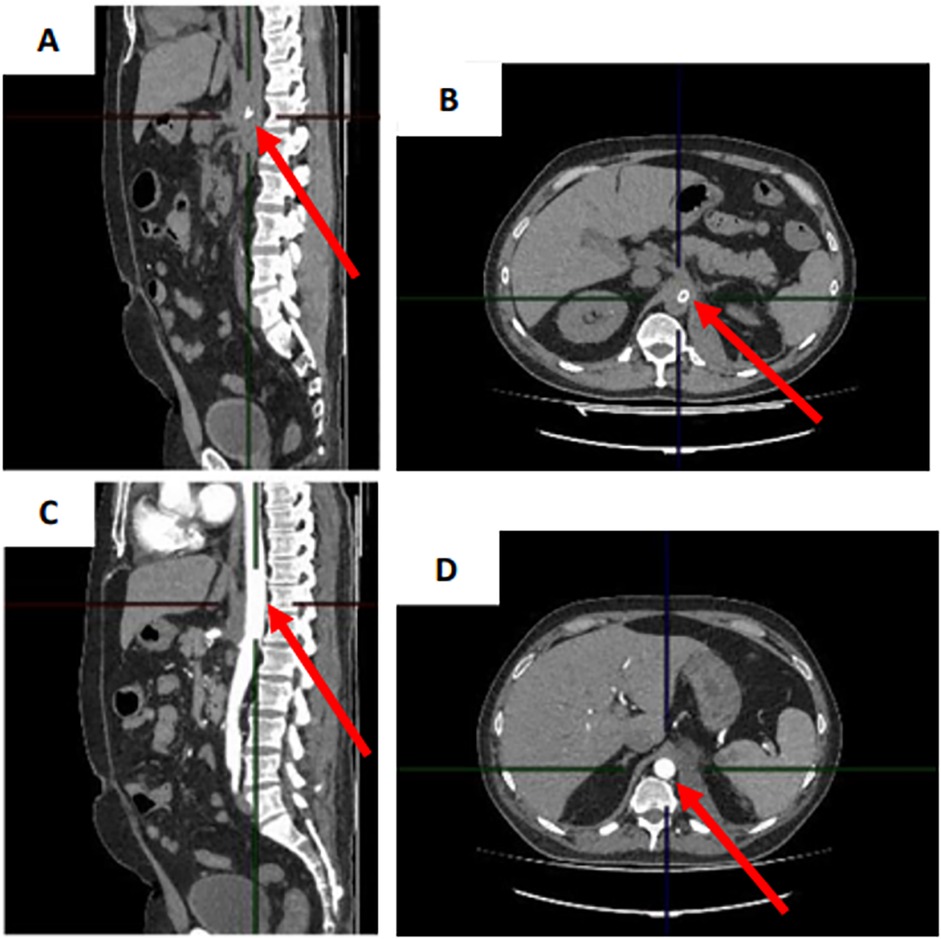
Figure 4. (A,B) CT scan of the abdomen with intravenous contrast in sagittal and transverse projection and (C,D) CT angiography scan of aorta and its branches with intravenous contrast in sagittal and transverse projection 3 weeks after stent graft implantation.
Discussion
The most common cause of CAA is atherosclerosis (1). However, other causes, such as infections, congenital diseases, trauma, and hereditary diseases associated with connective tissue weakness (e.g., Ehlers-Danlos syndrome), are also important (5).
CAA is classified into two types based on its anatomical location. Type I is an aneurysm of the CAA, and type II is an aneurysm of its branches. Each type is further subdivided into spindle-shaped and saccular aneurysms, according to their shape (6). In the case of our patient, type Ib CAA was present.
The high mortality rate among CAA patients is associated with late diagnosis and subsequent rupture, so early detection and treatment are crucial (7, 8). It is important to examine the vessels for an aneurysm, especially CAA, during a CT scan of the abdomen with intravenous contrast. Additionally, a differential diagnosis should be performed when examining patients, taking into account the presence of this rare pathology (1, 9).
The scientific literature describes three following treatments for CAA: conservative therapy, endovascular treatment with the possibility of using stent grafts or embolization, and open surgical treatment with ligation of the CA with or without revascularization (1, 3). However, there are no clear indications for choosing one treatment over another. However, the scientific literature discusses the following approaches: surgical treatment is recommended in cases of clinical symptoms, CAA > 2 cm, growth of CAA > 0.5 cm per year, and CAA in pregnant women or women of reproductive age, as these patients are most susceptible to aneurysm rupture (4, 10, 11). When a patient has indications for surgical treatment, the choice of treatment is strictly based on individual factors. It should be noted that open surgery is associated with a high mortality rate due to frequent complications. However, open surgery is a relevant treatment option when endovascular treatment is ineffective or inapplicable (9).
Fourteen clinical cases of CAA have been presented in the scientific literature published in the last 5 years, highlighting the importance of this issue (1, 3, 5, 7–9, 12–17). We have summarized the data and treatment strategies for these cases in Table 1. These articles propose different approaches to treating CAA patients. In 8 cases, open surgical interventions with aneurysm ligation and, in some cases, additional reconstructive procedures were performed. In one case, a palliative bleeding area package was performed due to an inability to stop the bleeding (1, 5, 8, 9, 12).
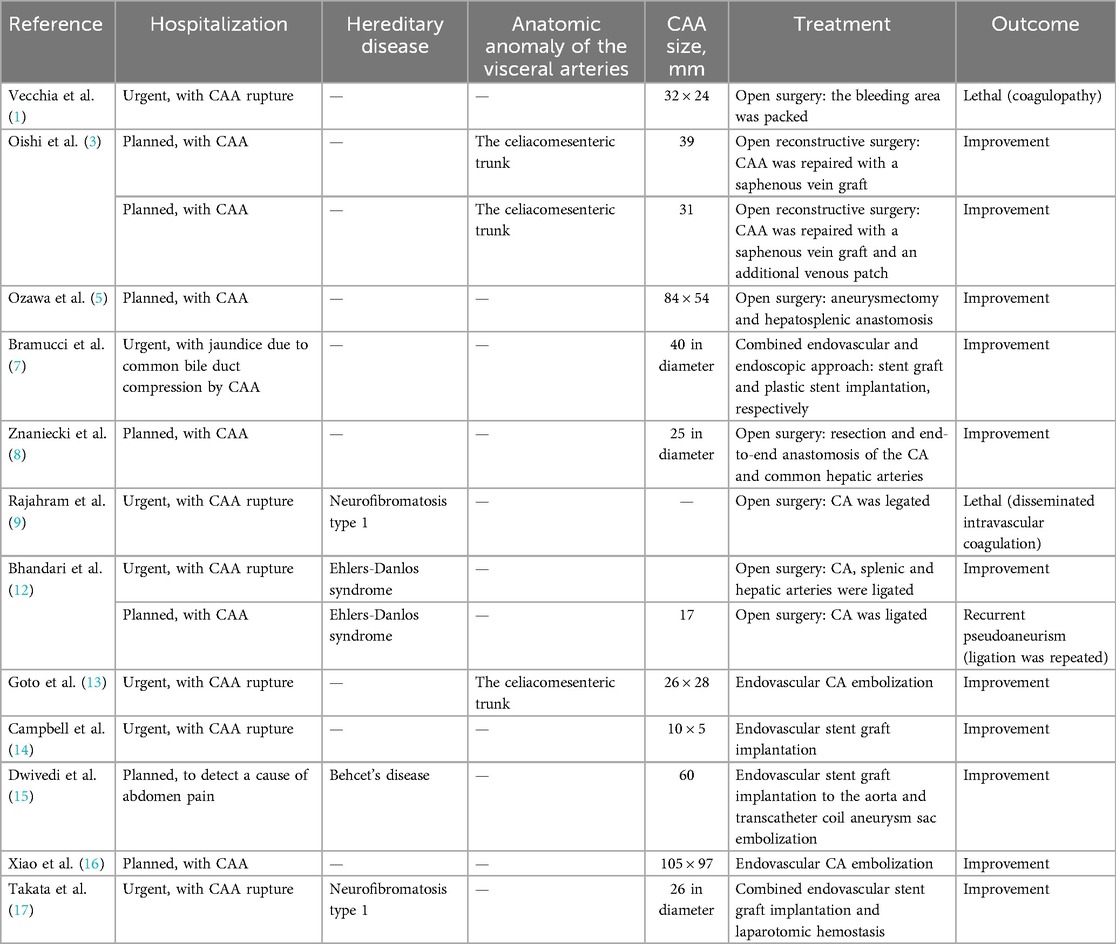
Table 1. Data from 14 clinical cases of CAA published in the scientific literature in the last 5 years.
An endovascular approach with embolization and/or stent-graft implantation was applied in 5 cases (3, 7, 13–16). One case involved a combined endovascular stent graft implantation and laparotomy hemostasis procedure (17). The type of surgical intervention was chosen based on CAA size, distance from the CA orifice, patient's comorbidities, and urgency (1, 3, 5, 7–9, 12–17). It should be noted that 2 cases had lethal outcomes due to late hospitalization and the development of irreversible and uncorrectable coagulation disorders (1, 9). One case presented with a recurrent pseudoaneurysm one year later, requiring repeated surgery (12).
We decided to perform an endovascular intervention with stent graft implantation because it is the fastest and least traumatic treatment. We also consider this method to be the best option for patients with comorbidities or emaciating conditions. Lastly, despite the saccular shape of the CAA, the patient had no signs of a current abdominal infection and no history of a previous one. The treatment was successful due to the timely diagnosis of CAA rupture. The endovascular intervention effectively repaired the CAA while preserving the patency of the CA, thereby minimizing risks to the patient.
Our study has the following limitations: there are no clear criteria for determining the optimal treatment for CAA. This makes it difficult to decide on a case-by-case basis. Long-term follow-up of patients is necessary to evaluate the stability of the results achieved and exclude late complications.
Conclusion
Our report highlights endovascular intervention in a CAA patient to be an effective treatment. In addition, we consider stent-graft implantation to be the method of choice in comorbid and emaciated CAA patients.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: We present a case report. Requests to access these datasets should be directed to Valentina Yumasheva,dmFsZW50aW5hLWp1bWFzaGV2YUByYW1ibGVyLnJ1.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
IS: Writing – original draft, Writing – review & editing. VY: Methodology, Formal analysis, Data curation, Supervision, Writing – original draft, Conceptualization, Writing – review & editing. NA: Writing – original draft, Visualization. NY: Supervision, Writing – review & editing. VS: Writing – original draft, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vecchia JD, Blazar E. A missed celiac artery aneurysm leading to rupture: a case report. Clin Pract Cases Emerg Med. (2020) 4(3):440–2. doi: 10.5811/cpcem.2020.6.46511
2. Chaer RA, Abularrage CJ, Coleman DM, Eslami MH, Kashyap VS, Rockman C, et al. The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. J Vasc Surg. (2020) 72(1S):3S–39S. doi: 10.1016/j.jvs.2020.01.039
3. Oishi A, Yamamoto T, Kajimoto K, Amano A. Surgical treatment of celiacomesenteric trunk aneurysm: report of 2 cases. Am J Case Rep. (2020) 21:e927077. doi: 10.12659/AJCR.927077
4. Azimi-Ghomi O, Khan K, Ulloa K. Celiac artery aneurysm diagnosis and repair in the postpartum female. J Surg Case Rep. (2017) 2:rjx010. doi: 10.1093/jscr/rjx010
5. Ozawa H, Kaneko K, Momose M, Hirayama S, Ohki T. Open surgical repair of a giant celiac artery aneurysm with complex anatomy using a retrograde balloon occlusion technique. J Vasc Surg Cases Innov Tech. (2023) 9(4):101112. doi: 10.1016/j.jvscit.2023.101112
6. Li X, Zhang W, Zhou M, Ding Y, Wang Y, Xie T, et al. A new classification and strategies for endovascular treatment of celiac artery aneurysms. Vascular. (2022) 30(5):834–41. doi: 10.1177/17085381211032768
7. Bramucci A, Miceli F, Fontana A, Tusini N, Sereni G, Sassatelli R. Successful endovascular and endoscopic treatment of a symptomatic celiac artery aneurysm for obstructive jaundice: a clinical case report. Ann Vasc Surg. (2022) 80:395.e1–e7. doi: 10.1016/j.avsg.2021.10.057
8. Znaniecki Ł, Tarnawski J, Żegleń B, Dymecki M, Gniedziejko M, Wojciechowski J. Surgical repair of a symptomatic celiac artery aneurysm with resection and end-to-end anastomosis. J Vasc Surg Cases Innov Tech. (2023) 9(2):101197. doi: 10.1016/j.jvscit.2023.101197
9. Rajahram D, Satchithanantham V, Veerasingam S, Tharmalingam T. Rare cause of fatal acute abdomen-celiac artery aneurysm. Int J Surg Case Rep. (2023) 109:108546. doi: 10.1016/j.ijscr.2023.108546
10. Zhu F, Zhang L, Shang D. The management of spontaneous isolated celiac artery dissection: a case report and literature review. Vascular. (2024) 32(6):1314–21. doi: 10.1177/17085381231197931
11. Ratner M, Hartwell CA, Zhang J, Johnson W, Nwachukwu C, Garg K, et al. The natural history of celiac artery aneurysms. Vascular. (2024) 16:17085381241245142. doi: 10.1177/17085381241245142
12. Bhandari A, Siu V, Duncan AA. Spontaneous celiac artery aneurysms in 13-year-old and 10-year-old brothers with PLOD1-related kyphoscoliotic Ehlers-Danlos syndrome. J Vasc Surg Cases Innov Tech. (2024) 10(3):101465. doi: 10.1016/j.jvscit.2024.10146
13. Goto T, Fujimura H, Shintani T, Shibuya T, Miyagawa S. Use of selective visceral angiography in surgical strategy planning for celiac artery aneurysm in the celiacomesenteric trunk. J Cardiothorac Surg. (2024) 19(1):11. doi: 10.1186/s13019-024-02483-7
14. Campbell D, Tamaska W, Medlenov S, Espinosa J, Lucerna A. Celiac artery aneurysm: a rare cause of abdominal pain. Cureus. (2023) 15(11):e48494. doi: 10.7759/cureus.48494
15. Dwivedi A, Wayne E, Sangroula D, Sigdel A. Endovascular treatment of giant celiac artery aneurysm in Behcet’s disease. Vasc Endovascular Surg. (2021) 55(4):398–401. doi: 10.1177/1538574420975906
16. Xiao N, Mansukhani NA, Resnick SA, Eskandari MK. Giant celiac artery aneurysm. J Vasc Surg Cases Innov Tech. (2019) 5(4):447–51. doi: 10.1016/j.jvscit.2019.05.003
17. Takata Y, Katayama K, Shimizu H, Inoue R, Takasaki T, Takahashi S. Treatment of celiac artery rupture with a hybrid procedure involving aortic stent grafting and open surgery in a patient with neurofibromatosis type 1. J Vasc Surg Cases Innov Tech. (2022) 8(4):625–8. doi: 10.1016/j.jvscit.2022.07.020
Keywords: aneurysm, celiac artery, rupture, endovascular, stent graft
Citation: Semenenko IA, Yumasheva VA, Alieva N, Yasnopolskaya NV and Sysoev VM (2025) Case report of successful treatment of patient with ruptured celiac artery aneurysm. Front. Surg. 12:1602499. doi: 10.3389/fsurg.2025.1602499
Received: 29 March 2025; Accepted: 17 June 2025;
Published: 10 July 2025.
Edited by:
Mounir J. Haurani, East Carolina University, United StatesReviewed by:
Georgios I. Karaolanis, University Hospital of Ioannina, GreeceEgan Kalmykov, Brandenburg Medical School Theodor Fontane, Germany
Copyright: © 2025 Semenenko, Yumasheva, Alieva, Yasnopolskaya and Sysoev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: V. A. Yumasheva, dmFsZW50aW5hLWp1bWFzaGV2YUByYW1ibGVyLnJ1
†These authors have contributed equally to this work and share first authorship
‡ORCID:
N. V. Yasnopolskaya
orcid.org/0000-0002-4388-7890
 I. A. Semenenko
I. A. Semenenko V. A. Yumasheva
V. A. Yumasheva N. Alieva1
N. Alieva1