- 1School of Basic Medical Sciences & School of Nursing, Chengdu University, Chengdu, China
- 2Department of Urology, Deyang People’s Hospital, Deyang, China
- 3Department of Proctology, Deyang People’s Hospital, Deyang, China
- 4Department of Urology, The Affiliated Hospital of Chengdu University, Chengdu, China
Background: Ureteroscopic lithotripsy (URSL) is the preferred treatment for urinary tract stones, with urosepsis being its most severe postoperative complication. Although previous studies have investigated risk factors for urosepsis after URSL, significant variations exist in reported risk factors and their associated odds ratios (OR), leading to inconsistent findings across studies. This systematic review and meta-analysis investigated the risk factors for urosepsis after URSL, aiming to establish a scientific foundation for early clinical identification and to reduce the incidence and mortality of this complication.
Methods: Case-control and cohort studies on factors influencing urosepsis after URSL were systematically retrieved from major public medical databases, including PubMed, Web of Science, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang Data, and Chinese Science and Technology Journal Database, up to January 31, 2025. Two researchers independently conducted literature screening, data extraction, quality assessment, and meta-analysis using Stata versions 15.1 and 18.0.
Results: A total of 26 studies were included in this analysis, comprising 12,394 patients, of whom 861 patients developed urosepsis. The influencing factors for urosepsis included stone size[OR = 3.10, 95% CI (1.20,8.00), P = 0.002], number of stones [OR = 7.59, 95% confidence interval (CI): 3.82, 15.08; P < 0.001], history of urinary tract infection (OR = 5.96, 95% CI: 4.12, 8.60; P < 0.001), positive urine culture (OR = 4.95, 95% CI: 3.90, 6.28; P < 0.001), positive urinary nitrite (OR = 7.68, 95% CI: 1.03, 52.27; P = 0.047], C-reactive protein (OR = 4.3, 95% CI: 1.06, 17.49; P = 0.042), diabetes (OR = 3.60, 95% CI: 3.11, 4.16; P < 0.001), operation time (OR = 1.09, 95% CI: 1.07, 1.11; P < 0.001), and stent placement (OR = 3.71, 95% CI: 1.94, 7.09; P < 0.001].
Conclusion: Urosepsis following URSL is associated with a high mortality rate and significantly threatens patient safety and quality of life. Early identification of the factors influencing urosepsis is crucial to reduce its incidence and improve patient outcomes.
Systematic Review Registration: PROSPERO CRD42025641787.
1 Introduction
The global prevalence of urinary stones ranges from 1% to 20% (1), and ureteroscopic lithotripsy (URSL) is the preferred treatment for upper urinary tract stones due to its minimally invasive nature, quick recovery, and high success rate. For stones ≤2 cm, the clearance rate reaches 76%–100% (2–4). However, URSL is associated with a 9%–25% complication rate, including ureteral injury, bleeding, infection, and urosepsis. Urosepsis is the most serious complication, which can progress to septic shock without timely intervention. It may cause multiple organ dysfunction, affecting the brain, heart, and kidneys, with a mortality rate of 30%–40% (5).
Although multiple studies have examined risk factors of urosepsis after URSL, variations in reported factors and odds ratios (OR) have resulted in inconsistent conclusions. This systematic review and meta-analysis evaluated the risk factors for urosepsis following URSL and synthesized current evidence to support the development of early identification and intervention strategies. The findings aim to reduce both the incidence and mortality of postoperative urosepsis.
2 Materials and methods
This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (6).
2.1 Search strategy
Two independent investigators searched PubMed, Web of Science, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang Data, and Viper Information Products databases. The search was conducted using a combination of subject terms and free words. English search terms: “Ureteroscopy/Lithotripsy/Ureteroscopy stone surgery/Ureteroscopic stone removal/Flexible ureteroscopy/Ureterolithotripsy/Ureteroscopic surgery/Ureteroscopic treatment,” “urosepsis/Urinary sepsis/ Septic shock/Urinary tract infection sepsis/Sepsis,” and “Risk factors/Influencing factors/Relevant factors/Associate factors/Predictive factors.” Additionally, references of the retrieved articles were manually reviewed to identify further relevant studies. The search covered the period from the inception of each database to January 31, 2025.
2.2 Inclusion and exclusion criteria
Inclusion criteria were as follows:
• Subjects: Patients undergoing URSL
• Type of study: case-control study or cohort study
• Study: Factors influencing or predictors of urosepsis after URSL
• Outcome metrics: OR and 95% CI for factors influencing urosepsis after URSL
• Diagnostic criteria: Urosepsis diagnosed within 30 days after URSL (7), with concurrent urinary tract infection (UTI) and systemic inflammatory response syndrome (SIRS) (8). SIRS was diagnosed based on ≥2 objective clinical parameters: (a) core hypothermia (<36°C) or hyperthermia (>38°C); (b) sustained tachycardia (>90 bpm); (c) tachypnea (>20 respirations/min) or documented hypocapnia [PaCO₂ < 32 mmHg (4.3 kPa)]; and (d) either leukocytopenia (<4 × 10⁹/L) or leukocytosis (>12 × 10⁹/L).
Exclusion criteria were as follows: duplicate publications, reviews, conference abstracts, or studies without full-text access; reports with incorrect, incomplete, or inaccessible data; and articles not in Chinese or English.
2.3 Data extraction
Two researchers skilled in meta-analysis methods independently removed duplicates using EndNote 20 and then screened the titles and abstracts of the remaining documents to eliminate those that did not meet the eligibility criteria. They reviewed the full text to determine the final inclusion of the selected reports. The screening results were cross-checked, and discrepancies were resolved by discussion; a third researcher adjudicated unresolved disagreements. Information about the relevant literature was extracted, including: first author, year of publication, country, type of study, sample size, number of patients with urosepsis, influencing factors, OR, and 95% confidence (CI).
2.4 Quality assessment of literature
The quality of bias in case-control and cohort studies was assessed using the Newcastle-Ottawa Scale (NOS) (9). This scale evaluates literature quality across three dimensions, comprising a total of eight entries: four entries for participant selection, one for group comparability, and three for outcome assessment. While each standard entry is scored on a 1-point scale, the comparability item allows for a maximum of 2 points, resulting in a total possible score of 9 points across all domains. Studies were categorized as low (0–4 points), medium (5–7 points), and high quality (>7 points). Two researchers independently evaluated the studies, and any differences in scoring were resolved through discussion, with the help of a third researcher.
2.5 Statistical analysis
Stata 18.0 was used for meta-analysis, subgroup analysis, and sensitivity analysis, while Egger's test was performed using Stata 15.1. All data were obtained directly from the original studies without any processing or transformation. The statistical results were presented as OR with 95% CI. Heterogeneity was evaluated using Cochran's Q test and I2 statistics. A fixed-effects model was applied when I2 < 50% and P > 0.10; otherwise, a random-effects model was used when I2 ≥ 50% and P ≤ 0.10. Subgroup analyses and sensitivity analyses were conducted to explore potential sources of heterogeneity. Funnel plots and Egger's test were used to assess publication bias when there were at least 10 studies, and the trim-and-fill method was applied when bias was detected. Statistical significance was set at P < 0.05.
3 Results
3.1 Literature search
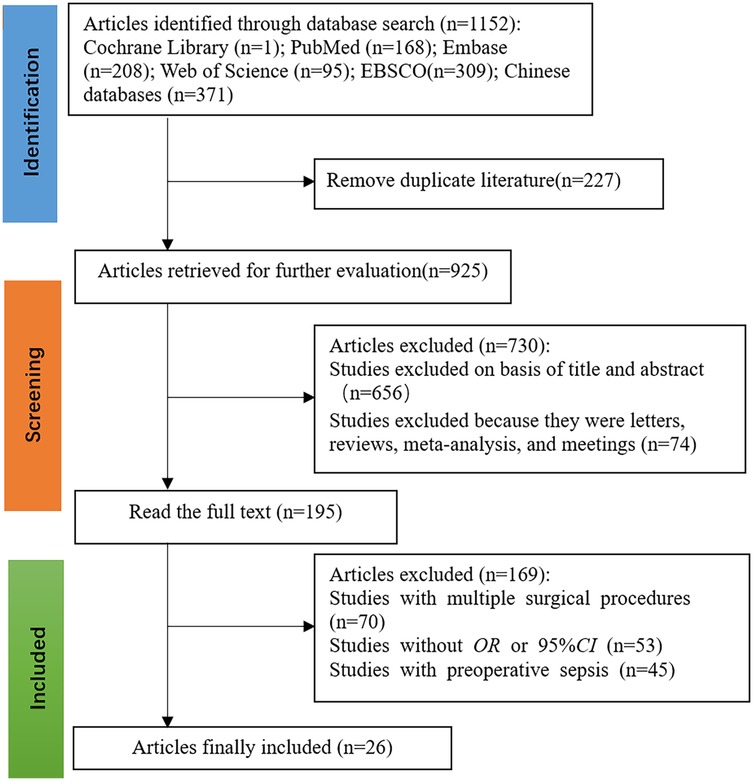
The initial search identified 1,152 potentially relevant articles. After a stepwise screening process, 26 studies (10–27) met the inclusion criteria, consisting of 16 articles published in English and 10 published in Chinese. The combined study population included 12,394 patients, of whom 861 cases were diagnosed with urosepsis. The literature screening process and results are illustrated in Figure 1.
3.2 Characteristics and quality of the included studies
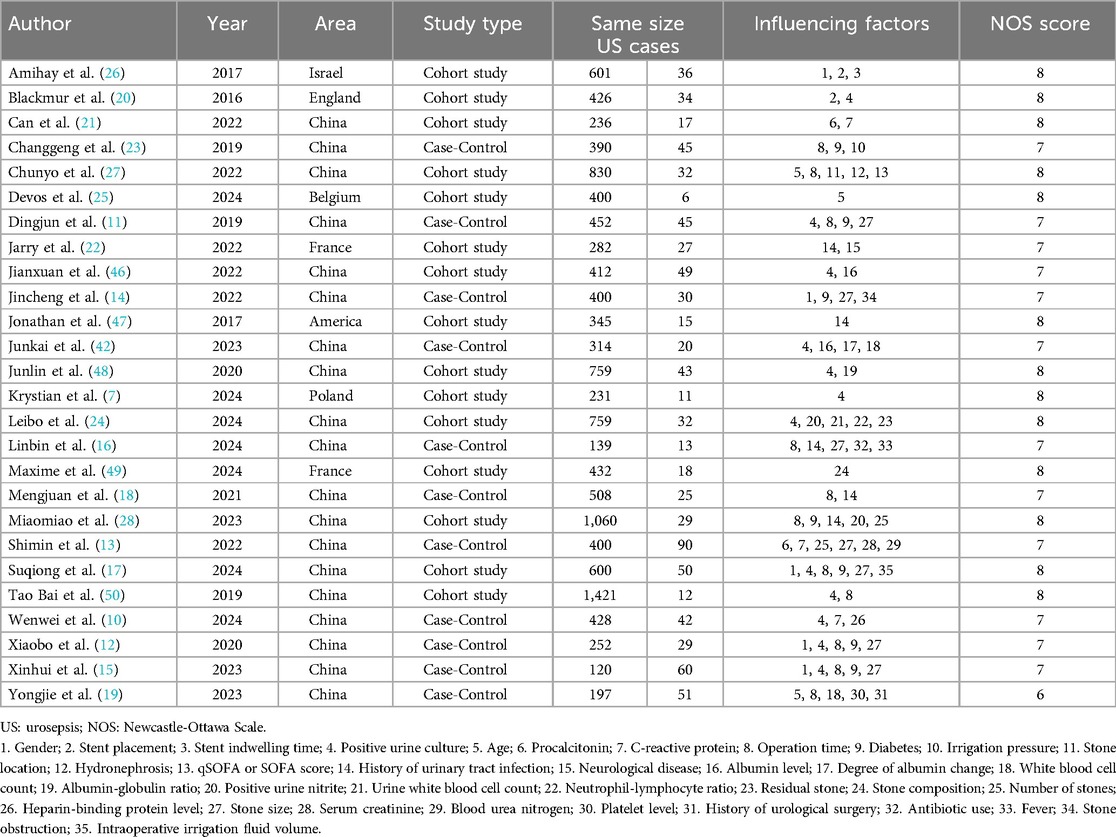
The analysis identified 36 risk factors across all included studies, with 13 factors reported in at least two studies. Based on the NOS quality assessment, 13 studies were rated medium quality and 13 high quality; none of the articles were of low quality. The characteristics of the included studies and the results of the quality assessment are shown in Table 1.
3.3 Meta-analysis
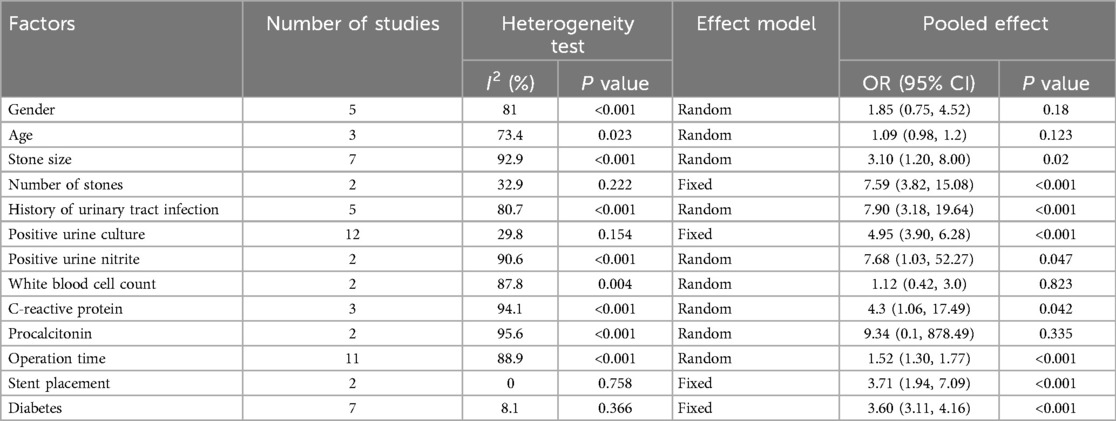
Meta-analysis was conducted for factors reported in at least two studies, including two demographic factors (gender, age), two stone-related factors (stone size, stone number), six infection-related factors [history of UTI, positive urine culture, positive urine nitrite, leukocyte count, C-reactive protein (CRP), procalcitonin], two surgical factors (operation time, stent placement), and one comorbidity-related factor (diabetes mellitus).
Stone size, stone number, history of UTI, positive urine culture, positive urine nitrite, CRP levels, operation time, stent placement, and diabetes mellitus were significantly associated with urosepsis following URSL (P < 0.05). However, no significant associations were found for gender, age, leukocyte count, or procalcitonin levels (P > 0.05), as shown in Table 2.
3.4 Descriptive analysis
A total of 22 factors were reported in single studies, including: stone-related factors (stone location, residual stones, stone composition, and stone obstruction); procedure-related factors (stent retention time, irrigation pressure, intraoperative fluid volume, and antibiotic use); laboratory markers (albumin levels, albumin variability, albumin-globulin ratio, urinary leukocyte count, neutrophil-lymphocyte ratio, blood urea nitrogen, platelet count, heparin-binding protein level, and serum creatinine); underlying diseases (neurological disorders and history of urological surgery); and other factors (qSOFA/SOFA score, fever, and hydronephrosis). The clinical significance of these factors requires cautious interpretation due to limited evidence from single studies.
3.5 Subgroup and sensitivity analyses
For factors with I2 > 50%, subgroup analyses were conducted by sample size, study design, and number of urosepsis cases to identify potential sources of heterogeneity. When subgroup analysis was not feasible or significant heterogeneity persisted after subgroup analysis, sensitivity analysis was performed by sequentially excluding individual studies to assess changes in the combined OR values and P values, helping to identify possible sources of heterogeneity.
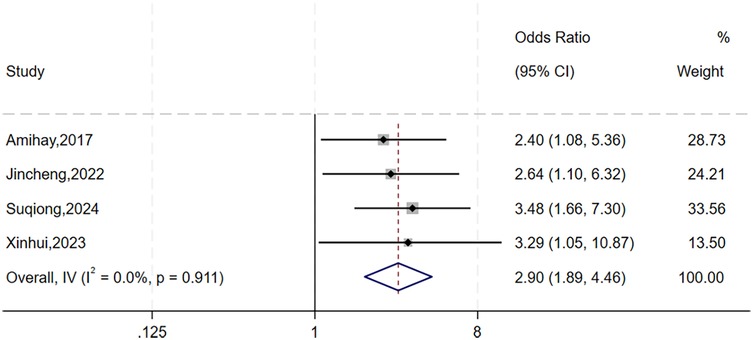
The heterogeneity test for gender showed significant variation across studies (I2 = 81%, P = 0.001). As subgroup analysis was not feasible, we performed sensitivity analysis by sequentially excluding individual studies (Figure 2). The study by Xiaobo et al. (12) was identified as the primary source of heterogeneity. After excluding this study, heterogeneity was significantly reduced (I2 = 0%, P = 0.911), and a fixed-effects model was applied. The analysis revealed that gender was significantly with higher risks of urosepsis following URSL (OR = 2.90, 95% CI: 1.89, 4.46; P < 0.001).
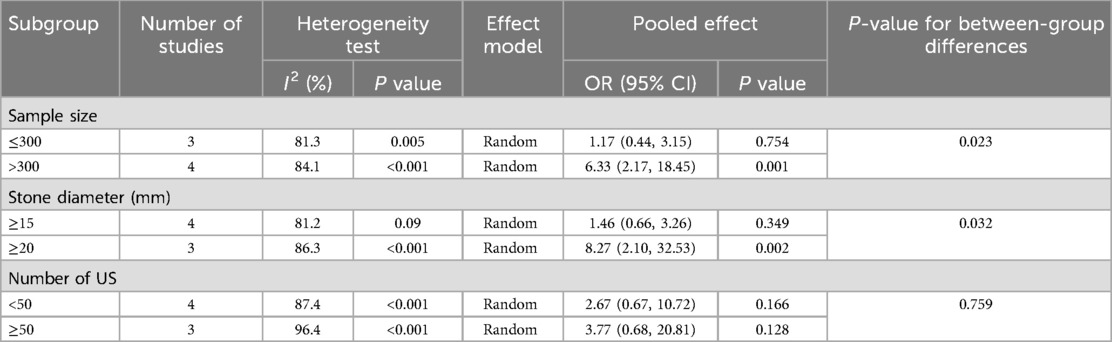
The heterogeneity test for stone size showed significant variation across studies (I2 = 92.9%, P = 0.001). Subgroup analyses by sample size, stone diameter, and number of urosepsis cases (Table 3 and Figure 3) revealed significant between-group differences by sample size (P = 0.001) and stone diameter (P = 0.032), suggesting these factors may influence urosepsis risk after URSL. However, substantial heterogeneity persisted within these subgroups, indicating that other sources of variation remain unaccounted for.
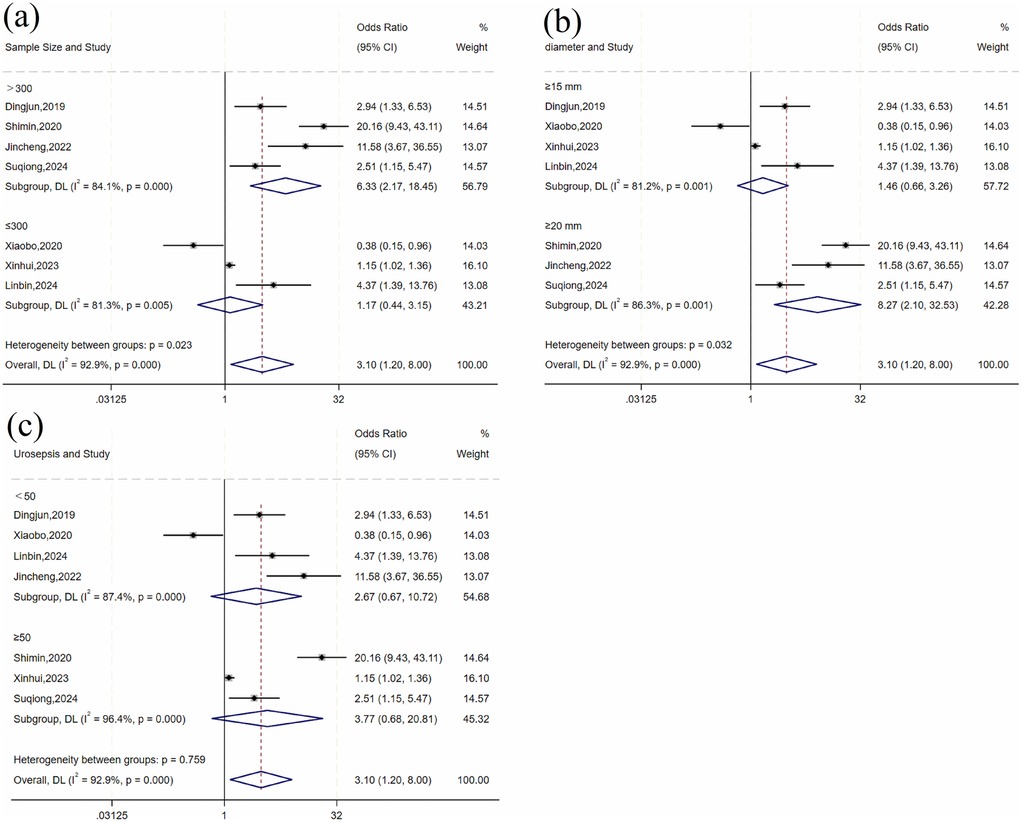
Figure 3. Forest plot of subgroup analysis of stone size: (a) sample size; (b) diameter; (c) number of urosepsis cases.
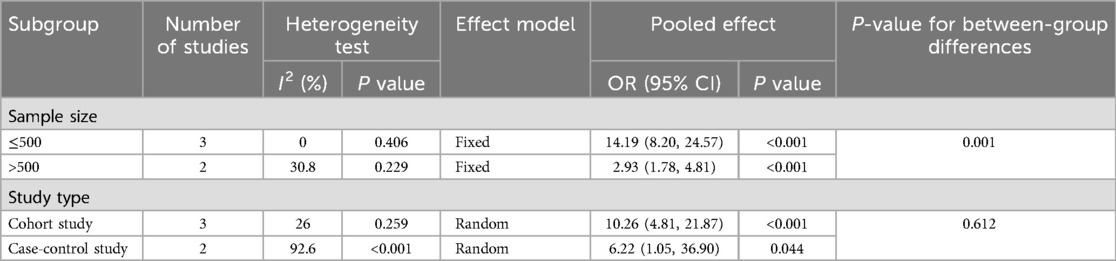
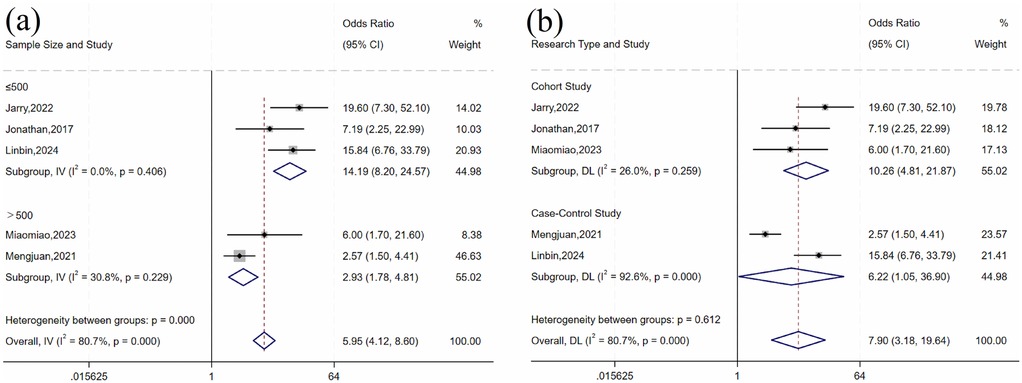
For history of UTI, there was heterogeneity across studies (I2 = 80.7%, P = 0.001). Subgroup analysis by sample size and study design (Table 4 and Figure 4) showed statistically significant differences by sample size subgroups (P = 0.032). Heterogeneity was significantly reduced in both subgroups (≤500 group: I2 = 0%, P = 0.406; >500 group: I2 = 30.8%, P = 0.229), suggesting that sample size may influence the association between UTI history and risk of urosepsis after URSL. Fixed-effects model analysis showed a statistically significant pooled effect (OR = 5.96, 95% CI: 4.12, 8.60; P < 0.001).
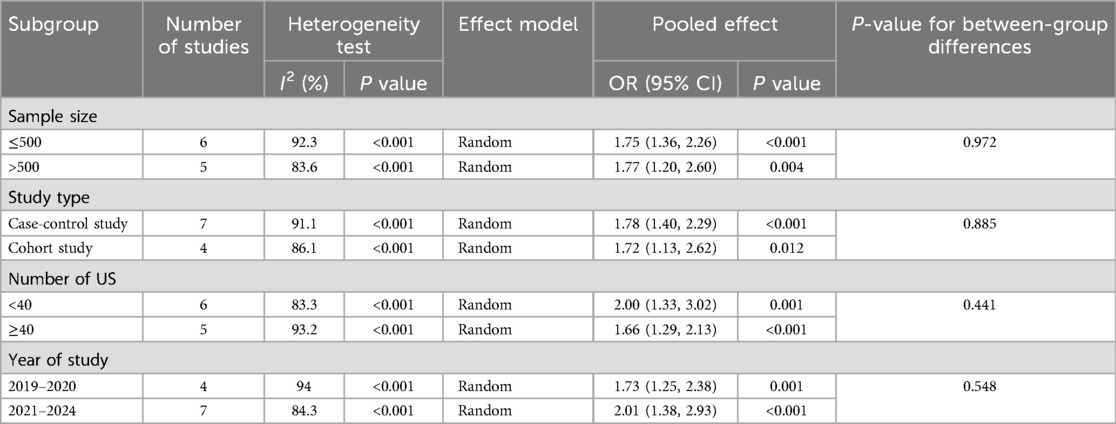
For operative time, there was significant variation across studies (I2 = 88.9%, P < 0.001). Subgroup analyses by sample size, study design, number of urosepsis cases, and publication year did not significantly reduce heterogeneity (Table 5 and Figure 5). Sensitivity analysis by sequentially excluding individual studies identified five studies (11, 12, 16, 17, 28) as the primary sources of heterogeneity. After excluding these studies, heterogeneity was significantly reduced (I2 = 43.8%, P = 0.113). Fixed-effects meta-analysis demonstrated a statistically significant pooled effect (OR = 1.09, 95% CI: 1.07, 1.11; P < 0.001).
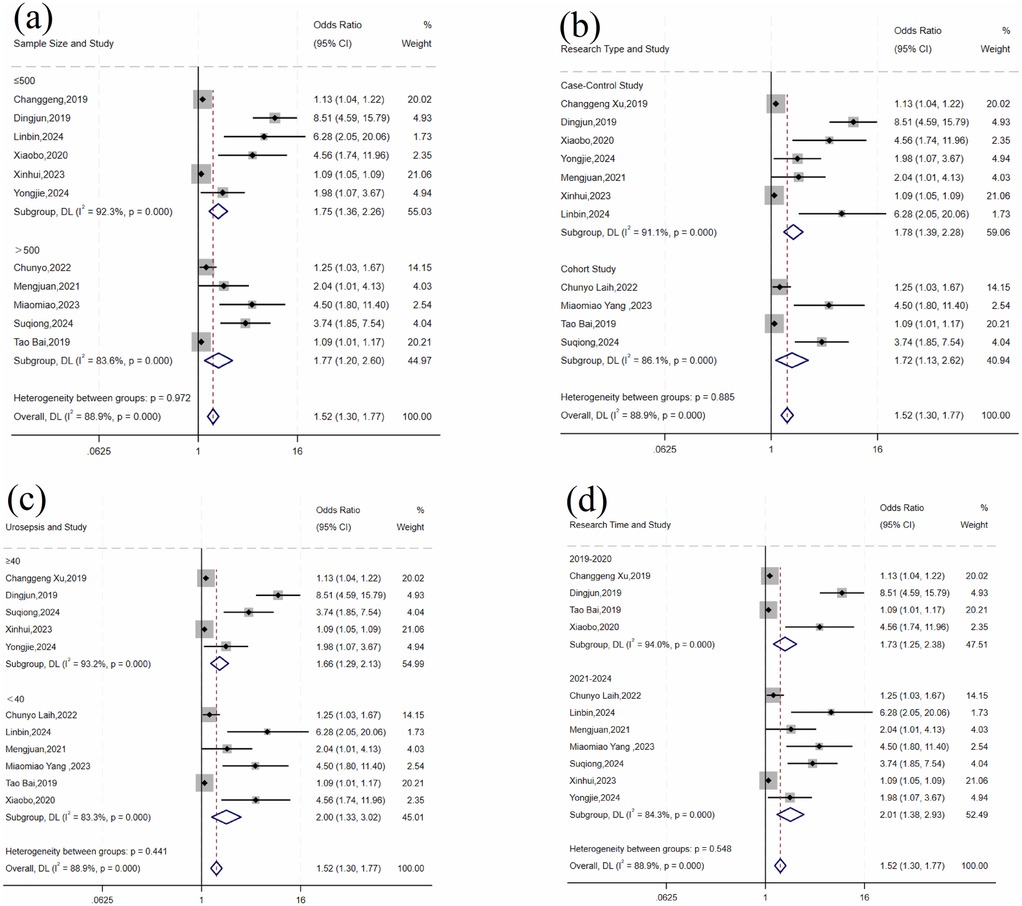
Figure 5. Forest plot of subgroup analysis of operative time: (a) sample size; (b) research type; (c) number of urosepsis cases; (d) research time.
3.6 Publication bias
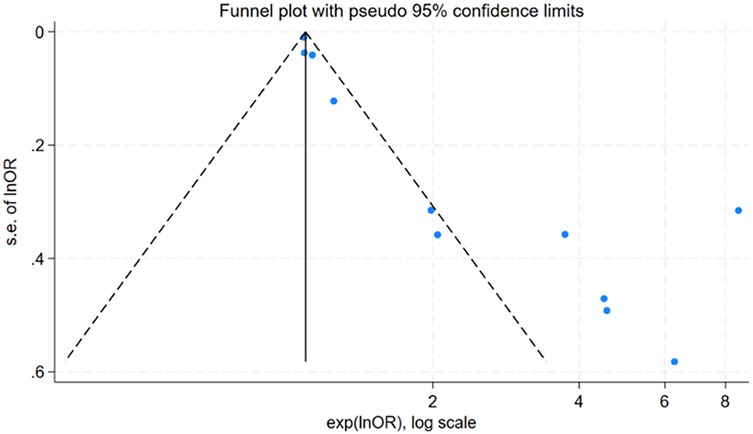
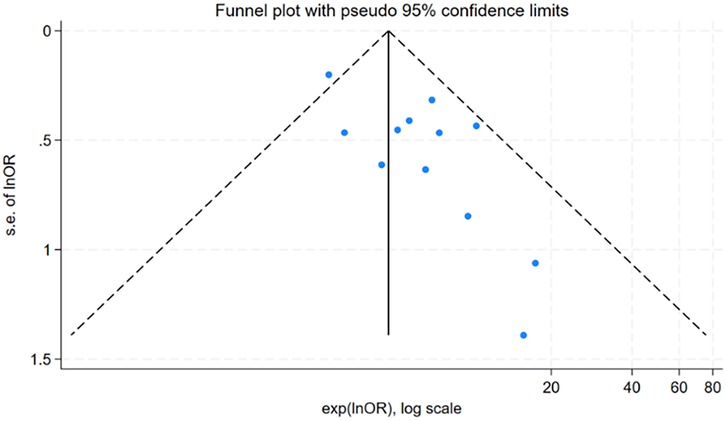
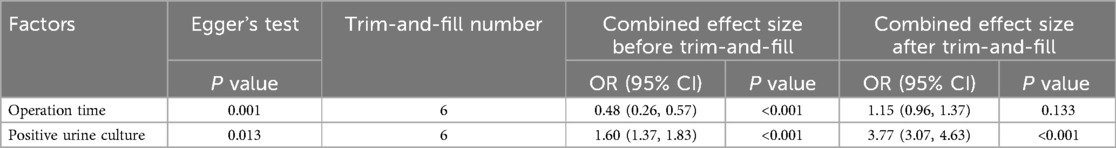
For factors reported in more than 10 studies, publication bias was assessed using funnel plots (Figure 6 and Figure 7) and Egger's test (Table 6). The findings revealed potential publication bias for operation time (P = 0.001) and positive urine culture (P = 0.013). The trim-and-fill method was applied using Stata 15.0 to correct for potential bias.
For surgical time, the adjusted effect size (OR = 1.15; 95% CI: 0.96, 1.37; P = 0.133), differing substantially from the original estimate (OR = 0.48, 95% CI: 0.26, 0.57); P < 0.001), suggesting significant publication bias, indicating that the original results may have been affected by unpublished studies. In contrast, the adjusted effect size for positive urine culture (OR = 3.77, 95% CI: 3.07, 4.63; P < 0.001) was relatively consistent with the original findings (OR = 1.60 95% CI: 1.37, 1.83; P < 0.001), suggesting the initial results were robust despite some publication bias.
4 Discussion
4.1 General demographic and disease-related factors
The findings of this meta-analysis demonstrated that diabetes mellitus was significantly associated with urosepsis post-URSL, consistent with the findings of Sun et al. (29), which revealed an association between diabetes mellitus and the risk of infection. Hyperglycemia in diabetes mellitus not only provides an energy source for bacterial growth but also significantly dysregulates both innate and adaptive immune responses, increasing susceptibility to infections. Innate immune dysfunction includes impaired neutrophil chemotaxis, phagocytosis, and oxidative burst, reduced complement activation, and NK cell cytotoxicity. Adaptive immunity is suppressed by defective antigen-presenting cell (APC) maturation, diminished cytokine production, CD4+ T-cell dysfunction, and disrupted B-cell antibody production (30, 31). These immune and metabolic alterations account for the increased infection risk in diabetic patients following URSL.
Although gender was not statistically significant in this meta-analysis, Dan et al. (32) have reported that gender significantly impacts the risk of infection following URSL. Potential reasons for women's increased susceptibility to urosepsis after URSL may include genetic factors and effects of sex hormones (29). Genetic differences can affect immune system function and infection resistance by altering how immune-related genes work. Female sex hormones like estrogen and progesterone may also influence inflammation and immune responses by changing how immune cells function and produce cytokines, which could affect the body's ability to fight infections. Assessment of preoperative infection risk is essential in female patients and patients with diabetes undergoing URSL. Appropriate preventive measures, such as glycemic control, should be implemented to reduce the risk of postoperative urosepsis.
4.2 Stone-related factors
The meta-analysis found that stone size and stone number were significantly associated with the risk of urosepsis following URSL. Although flexible ureteroscopy has been demonstrated to be effective for renal calculi >2 cm (33), Jeong et al. (34) found that stone diameter ≥1.5 cm is associated with increased infection risk. The duration of lithotripsy is significantly prolonged in cases involving larger or multiple urinary stones. Continuous laser energy and mechanical forces break down the stone structure, releasing trapped endotoxins and bacteria (35). During the procedure, the necessary high-pressure irrigation increases the permeability of small blood vessels and lymph channels of the kidney, creating pathways for bacteria to enter the bloodstream. Additionally, larger stones can cause urinary tract obstruction (36), impairing urine drainage and promoting bacterial growth. These physiological changes allow bacteria and their toxic products to spread throughout the body, significantly increasing the risk of serious infection. For patients with large or multiple stones, preoperative infection risk assessment is crucial, and lithotripsy time should be minimized to reduce postoperative infections.
4.3 Infection-related factors
This study found that a history of UTI, positive urine culture, positive urinary nitrite, and elevated CRP levels were significantly associated with urosepsis following URSL. Persistent fever typically indicates the presence of pro-inflammatory factors and uncontrolled infection. Inflammation in the urinary tract induces mucosal edema, vasodilation, and compromised vascular barrier function, thereby disrupting the natural mucosal defense and increasing the risk of bacterial and toxin translocation into the circulation. Francesco et al. (37) demonstrated that prolonged URSL operation time is significantly associated with an elevated risk of postoperative UTI. Urine culture positivity is the gold standard for diagnosing UTI, providing direct evidence of viable bacteria in the urine. A meta-analysis by Naeem et al. (38) demonstrated that a positive urine culture is a risk factor for sepsis, with microbiological analysis identifying Escherichia coli as the predominant uropathogen (39). A positive nitrite result indicates the reduction of nitrate to nitrite by Gram-negative bacteria in urine. This biochemical conversion typically requires a high bacterial load, and such significant bacteriuria frequently triggers pronounced inflammatory responses. CRP serves as a sensitive systemic inflammation marker that can increase up to 1,000-fold at infection or inflammation sites (40). The non-significant association for procalcitonin in this study may be attributed to the limited number of included studies. Villanueva et al. (41) reported that leukocyte counts typically increase during bacterial UTIs. The inconsistent findings in this study may be because severe infections can show an initial decrease followed by an increase due to endotoxin-mediated leukocyte depletion (42). Notably, the associations for both procalcitonin (OR = 9.34, 95% CI: 0.1–878.49; P = 0.335) and positive urine nitrite (OR = 7.68, 95% CI: 1.03–52.27; P < 0.001) had markedly wide confidence intervals, reflecting population heterogeneity and methodological differences. Thus, we strongly recommend cautious interpretation of these biomarkers in clinical practice. Therefore, UTIs should be treated before lithotripsy. Antibiotic therapy should be guided by preoperative urine culture (43), and prophylactic antibiotics may also help reduce infection risk in patients with negative urine cultures.
4.4 Surgery-related factors
We found that procedure duration and stent placement were significantly associated with urosepsis post-URSL. Ozgor et al. (44) reported that procedures lasting more than one hour doubled the risk of infectious complications, as prolonged exposure increases the likelihood of pathogenic bacteria and endotoxins entering the bloodstream. Preoperative double J stent placement improves stone clearance in patients undergoing URSL. However, a meta-analysis by Corrales et al. (45) demonstrated that stent retention exceeding 30 days increases sepsis rates. Prolonged stenting may induce chronic inflammation, causing local tissue damage and persistent release of inflammatory factors, thereby elevating sepsis risk. Therefore, although preoperative stent placement is recommended, its duration should be carefully monitored. Additionally, controlled procedure times are essential during surgery.
4.5 Study limitations
This study has several limitations: (1) The inclusion of only Chinese and English literature may introduce language bias; (2) Most included studies were of moderate quality, highlighting the need for additional high-quality research; (3) Significant heterogeneity was observed in some influencing factors—although subgroup analyses identified partial sources, other heterogeneity factors remain to be elucidated; (4) Some risk factors were reported in single studies, limiting the reliability of these findings due to insufficient data.
5 Conclusion
Urosepsis, although relatively rare, represents the most severe complication following URSL and is associated with significant mortality rates. This study employed meta-analysis to identify risk factors for urosepsis after URSL. The analysis revealed that stone size, stone number, history of UTI, positive urine culture, positive urinary nitrites, elevated CRP levels, prolonged operative time, stent placement, and diabetes mellitus were significantly associated with postoperative US. These findings provide valuable insights for developing early identification and treatment strategies. Future research should focus on creating predictive models and web-based calculators to improve clinical risk assessment, which may enhance patient outcomes and optimize healthcare resource utilization.
Author contributions
LD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. JX: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing. XL: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Software, Validation, Writing – original draft, Writing – review & editing. XW: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing. LT: Data curation, Project administration, Validation, Writing – review & editing. JZ: Data curation, Formal analysis, Project administration, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by the Key Research and Development Project of Sichuan Provincial Department of Science and Technology (Grant No. 2022YFS0109), the “Xinglin Scholar” Hospital Special Fund from Chengdu University of Traditional Chinese Medicine (Grant No. YYZX2022054), and the Research Project of Sichuan Medical Association (Grant No. S22049).
Acknowledgments
We sincerely thank all members of our research team for their valuable contributions. We are particularly grateful to the faculty members of Chengdu University and the urology department staff at Deyang People's Hospital for their expert guidance in study design and manuscript preparation. Their insightful suggestions significantly improved the quality of this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1603311/full#supplementary-material
References
1. Cook J, Lamb BW, Lettin JE, Graham SJ. The epidemiology of urolithiasis in an ethnically diverse population living in the same area. Urol J. (2016) 13(4):2754–8. doi: 10.22037/uj.v13i4.3336
2. De S, Autorino R, Kim FJ, Zargar H, Laydner H, Balsamo R, et al. Percutaneous nephrolithotomy versus retrograde intrarenal surgery: a systematic review and meta-analysis. Eur Urol. (2015) 67(1):125–37. doi: 10.1016/j.eururo.2014.07.003
3. Doizi S, Traxer O. Flexible ureteroscopy: technique, tips and tricks. Urolithiasis. (2018) 46(1):47–58. doi: 10.1007/s00240-017-1030-x
4. Tang QL, Liang P, Ding YF, Zhou XZ, Tao RZ. Comparative efficacy between retrograde intrarenal surgery with vacuum-assisted ureteral access sheath and minimally invasive percutaneous nephrolithotomy for 1–2 cm infectious upper ureteral stones: a prospective, randomized controlled study. Front Surg. (2023) 10:1200717. doi: 10.3389/fsurg.2023.1200717
5. Inoue T, Okada S, Hamamoto S, Fujisawa M. Retrograde intrarenal surgery: past, present, and future. Investig Clin Urol. (2021) 62(2):121–35. doi: 10.4111/icu.20200526
6. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
7. Kaczmarek K, Jankowska M, Kalembkiewicz J, Kienitz J, Chukwu O, Lemiński A, et al. Assessment of the incidence and risk factors of postoperative urosepsis in patients undergoing ureteroscopic lithotripsy. Cent European J Urol. (2024) 77(1):122. doi: 10.5173/ceju.2023.167
8. Guliciuc M, Maier AC, Maier IM, Kraft A, Cucuruzac RR, Marinescu M, et al. The urosepsis-a literature review. Medicina (Kaunas). (2021) 57(9):872. doi: 10.3390/medicina57090872
9. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
10. Chen W, He Y, Lu K, Liu C, Jiang T, Zhang H, et al. Construction of a back propagation neural network model for predicting urosepsis after flexible ureteroscopic lithotripsy. J Zhejiang Univ Med Sci. (2025) 54(01):1–9. doi: 10.3724/zdxbyxb-2024-0128
11. Ding J, Zhou C, Li G, Chen G, Zhu X, Liu J, et al. Influencing factors for postoperative urine-induced sepsis in flexible ureteroscope lithotripsy patients. Chinese J Nosocomiol. (2019) 29(14):2134–7+42. doi: 10.11816/cn.ni.2019-181950
12. Hu X, Tang Q. Risk factors of urosepsis after flexible ureteroscopic holmium Laser lithotripsy. Chinese Gen Pract. (2020) 23(S1):86–8. https://kns.cnki.net/kcms2/article/abstract?v=6WbqZcVy3rg_sY-78zNPpR7Xa1DaxaEb-dpnr60ohIb4ZXEdxO59bxYCbLuxBQ5bkS6dcw0i3OFfRb6-eNThqHRbGvcKrXqFwPoBuruyZ7PCv0Hok91AlBTVb6hKJQ4amEFcMvd56bjzrNORcuXYmXAzeISXYE5jGGSPp4kkvuA=&uniplatform=NZKPT
13. Ma S, Huang Y, Yang M. Analysis of influencing factor of urinary sepsis in patients undergoing retrograde intrarenal surgery under flexible ureteroscope. China Med Herald. (2022) 19(27):76–9. doi: 10.20047/j.issn1673-7210.2022.27.17
14. Yin J, Gu Y, Tang X, Guo Z, Liu H. Risk factors for urosepsis after retrograde intrarenal surgery and construction of a nomogram prediction model. J Minimally Invasive Urol. (2022) 11(04):239–45. doi: 10.19558/j.cnki.10-1020/r.2022.04.006
15. Xinhui W, Liangzhi Y, Yanhua Y. Study on the influencing factors of urinary sepsis after ureteral flexible lithotripsy. Shenzhen J Integr Traditional Chinese Western Med. (2023) 33(12):15–8. doi: 10.16458/j.cnki.1007-0893.2023.12.005
16. Wu L, Chen W, Yang J, Chen D. Analysis of risk factors for urosepsis after flexible ureteroscopic lithotripsy. Zhejiang J Clin Med. (2024) 26(04):530–1. doi: 10.3969/j.issn.1008-7664.2024.4.zjlcyx202404018
17. Zhang S, He D, Zhao X. Construction and validation of a risk prediction model for postoperative urogenic sepsis in patients with ureteral calculi. Evid Based Nurs. (2024) 10(16):2913–8. doi: 10.12102/j.issn.2095-8668.2024.16.013
18. Meng J, Cai H, Zhang X, Yang H, Huang Y. Influencing factors of urinary sepsis in patients with ureteral calculi and diagnostic value of peripheral blood Nlr, Plr And Pct. Chinese J Nosocomiol. (2021) 31(20):3082–5. doi: 10.11816/cn.ni.2021-203764
19. Ma Y, Deng S, Wei X, Wu Z, Wei W. Risk factors for postoperative urogenic sepsis in patients undergoing ureteroscopy with holmium Laser lithotripsy and changes of Tlr4/nf-Κb signaling pathways. Chinese J Nosocomiol. (2024) 34(02):235–8. doi: 10.11816/cn.ni.2024-230795
20. Blackmur JP, Maitra NU, Marri RR, Housami F, Malki M, McIlhenny C. Analysis of factors’ association with risk of postoperative urosepsis in patients undergoing ureteroscopy for treatment of stone disease. J Endourol. (2016) 30(9):963–9. doi: 10.1089/end.2016.0300
21. Wang C, Xu R, Zhang Y, Wu Y, Zhang T, Dong X, et al. Nomograms for predicting the risk of sirs and urosepsis after uroscopic minimally invasive lithotripsy. Biomed Res Int. (2022) 2022:6808239. doi: 10.1155/2022/6808239
22. Jarry É, Garot M, Marlière F, Fantoni J-C, Villers A, Lebuffe G, et al. Predictive factors of postoperative septic complications after flexible ureteroscopy for urinary stones. Prog Urol. (2022) 32(2):85–91. doi: 10.1016/j.purol.2021.07.010
23. Xu CG, Guo YL. Diagnostic and prognostic values of bmper in patients with urosepsis following ureteroscopic lithotripsy. BioMed Res Int. (2019) 2019(1):8078139. doi: 10.1155/2019/8078139
24. Wang L, Yu X, Qiu Z, Liu P, Tian W, He W, et al. Influence of preoperative urine culture and bacterial Species on urogenital sepsis after ureteral flexible lithotripsy in patients with upper urinary tract stones. Front Med (Lausanne). (2024) 11:1393734. doi: 10.3389/fmed.2024.1393734
25. Devos B, Vanderbruggen W, Claessens M, Duchateau A, Hente R, Keller EX, et al. Risk factors of early infectious complications after ureterorenoscopy for stone disease: a prospective study. World J Urol. (2024) 42(1):277. doi: 10.1007/s00345-024-04983-6
26. Nevo A, Mano R, Baniel J, Lifshitz DA. Ureteric stent dwelling time: a risk factor for post-ureteroscopy sepsis. BJU Int. (2017) 120(1):117–22. doi: 10.1111/bju.13796
27. Laih C-Y, Hsiao P-J, Hsieh P-F, Wang Y-D, Lai C-M, Yang C-T, et al. Qsofa and Sofa scores are valuable tools for predicting postoperative sepsis resulting from ureteroscopic lithotripsy (ursl). Medicine (Baltimore). (2022) 101(50):e31765. doi: 10.1097/MD.0000000000031765
28. Yang M, Li Y, Huang F. A nomogram for predicting postoperative urosepsis following retrograde intrarenal surgery in upper urinary calculi patients with negative preoperative urine culture. Sci Rep. (2023) 13(1):2123. doi: 10.1038/s41598-023-29352-y
29. Sun J, Xu J, OuYang J. Risk factors of infectious complications following ureteroscopy: a systematic review and meta-analysis. Urol Int. (2020) 104(1-2):113–24. doi: 10.1159/000504326
30. Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. (2020) 16(5):442–9. doi: 10.2174/1573399815666191024085838
31. Velloso LA, Eizirik DL, Cnop M. Type 2 diabetes mellitus–an autoimmune disease? Nat Rev Endocrinol. (2013) 9(12):750–5. doi: 10.1038/nrendo.2013.131
32. Tan D, Wu F, Huo W. Clinical characteristics and risk factors of systemic inflammatory response syndrome after flexible ureteroscopic lithotripsy. Arch Esp Urol. (2022) 75(7):618–23. doi: 10.56434/j.arch.esp.urol.20227507.89
33. Cosmin C, Georgescu DA, Geavlete P, Popescu RI, Geavlete B. Comparison between retrograde flexible ureteroscopy and percutaneous nephrolithotomy for the treatment of renal stones of 2–4 cm. Medicina (Kaunas). (2023) 59(1):124. doi: 10.3390/medicina59010124
34. Yoo JW, Lee KS, Chung BH, Kwon SY, Seo YJ, Lee KS, et al. Optimal duration of preoperative antibiotic treatment prior to ureteroscopic lithotripsy to prevent postoperative systemic inflammatory response syndrome in patients presenting with urolithiasis-induced obstructive acute pyelonephritis. Investig Clin Urol. (2021) 62(6):681–9. doi: 10.4111/icu.20210160
35. Ye S, Wang W, Yu Z, Luo J. Risk factors for systemic inflammatory response syndrome after endoscopic lithotripsy for upper urinary calculi. BMC Urol. (2023) 23(1):59. doi: 10.1186/s12894-023-01230-9
36. Heijkoop B, Galiabovitch E, York N, Webb D. Consensus of multiple national guidelines: agreed strategies for initial stone management during COVID-19. World J Urol. (2021) 39(9):3161–74. doi: 10.1007/s00345-020-03491-7
37. Prata F, Cacciatore L, Salerno A, Tedesco F, Ragusa A, Basile S, et al. Urinary tract infection predictors in patients undergoing retrograde intrarenal surgery for renal stones: does the instrument make the difference? J Clin Med. (2024) 13(10):2758. doi: 10.3390/jcm13102758
38. Bhojani N, Miller LE, Bhattacharyya S, Cutone B, Chew BH. Risk factors for urosepsis after ureteroscopy for stone disease: a systematic review with meta-analysis. J Endourol. (2021) 35(7):991–1000. doi: 10.1089/end.2020.1133
39. Scotland KB, Lange D. Prevention and management of urosepsis triggered by ureteroscopy. Res Rep Urol. (2018) 10:43–9. doi: 10.2147/rru.S128071
40. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. (2018) 9:754. doi: 10.3389/fimmu.2018.00754
41. Villanueva-Congote J, Hinojosa-Gonzalez D, Segall M, Eisner BH. The relationship between neutrophil/lymphocyte ratio, platelet/neutrophil ratio, and risk of urosepsis in patients who present with ureteral stones and suspected urinary tract infection. World J Urol. (2024) 42(1):596. doi: 10.1007/s00345-024-05229-1
42. Huang J, Xie L, Yang Y, Xie H, Liu C. Hypoalbuminemia within one hour after surgery as a predictor of post-operative urosepsis in patients undergoing flexible ureteroscopy lithotripsy: a retrospective study. Surg Infect (Larchmt). (2023) 24(1):75–81. doi: 10.1089/sur.2022.290
43. Khusid JA, Hordines JC, Sadiq AS, Atallah WM, Gupta M. Prevention and management of infectious complications of retrograde intrarenal surgery. Front Surg. (2021) 8:718583. doi: 10.3389/fsurg.2021.718583
44. Ozgor F, Sahan M, Cubuk A, Ortac M, Ayranci A, Sarilar O. Factors affecting infectious complications following flexible ureterorenoscopy. Urolithiasis. (2019) 47(5):481–6. doi: 10.1007/s00240-018-1098-y
45. Corrales M, Sierra A, Doizi S, Traxer O. Risk of sepsis in retrograde intrarenal surgery: a systematic review of the literature. Eur Urol Open Sci. (2022) 44:84–91. doi: 10.1016/j.euros.2022.08.008
46. Sun J-X, Xu J-Z, Liu C-Q, Xun Y, Lu J-l, Xu M-Y, et al. A novel nomogram for predicting post-operative sepsis for patients with solitary, unilateral and proximal ureteral stones after treatment using percutaneous nephrolithotomy or flexible ureteroscopy. Front Surg. (2022) 9:814293. doi: 10.3389/fsurg.2022.814293
47. Bloom J, Fox C, Fullerton S, Matthews G, Phillips J. Sepsis after elective ureteroscopy. Can J Urol. (2017) 24(5):9017–23. https://pubmed.ncbi.nlm.nih.gov/28971790/28971790
48. Lu J, Xun Y, Yu X, Liu Z, Cui L, Zhang J, et al. Albumin-globulin ratio: a novel predictor of sepsis after flexible ureteroscopy in patients with solitary proximal ureteral stones. Transl Androl Urol. (2020) 9(5):1980–9. doi: 10.21037/tau-20-823
49. Pattou M, Yonneau L, de Gouvello A, Almeras C, Saussine C, Hoznek A, et al. Urosepsis after ureterorenoscopy, intraoperative recognition of type-iv stones could change clinical practice. World J Urol. (2024) 42(1):534. doi: 10.1007/s00345-024-05251-3
Keywords: lithotripsy, sepsis, risk factors, disease prevention and control, meta-analysis
Citation: Dai L, Xiang J, Liu X, Wen X, Tan L and Zhang J (2025) Risk factors for urosepsis following ureteroscopic lithotripsy: a systematic review and meta-analysis. Front. Surg. 12:1603311. doi: 10.3389/fsurg.2025.1603311
Received: 31 March 2025; Accepted: 6 June 2025;
Published: 19 June 2025.
Edited by:
Francesco Prata, Campus Bio-Medico University Hospital, ItalyReviewed by:
Hsiang Ying Lee, Kaohsiung Medical University Hospital, TaiwanMun Su Chung, Davos Hospital, Republic of Korea
Copyright: © 2025 Dai, Xiang, Liu, Wen, Tan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junlian Xiang, MTg5MDgxMDM4NjdAMTYzLmNvbQ==; Xiaoli Liu, eGlhb2xpX2xpdTEyMTdAMTYzLmNvbQ==
 Lifei Dai
Lifei Dai Junlian Xiang
Junlian Xiang Xiaoli Liu3*
Xiaoli Liu3* Jiali Zhang
Jiali Zhang