- 1Department of Surgery, University of Kentucky, Med Center Health (Bowling Green), Bowling Green, KY, United States
- 2Department of Surgery - Transplant Division, College of Medicine, University of Kentucky, Lexington, KY, United States
- 3University of Kentucky College of Medicine, Lexington, KY, United States
Neuroendocrine liver metastases (NELM) are commonly observed in patients with advanced neuroendocrine tumors (NETs) and are associated with poor prognosis, primarily due to liver failure and hormone-related complications. While hepatic resection remains the standard surgical approach, orthotopic liver transplantation (OLT) has emerged as a potentially curative treatment in selected patients with unresectable disease. This review summarizes current evidence on the role of OLT in managing NELM, with a focus on patient selection criteria and existing clinical guidelines. Appropriate selection is essential, as improved long-term survival has been consistently demonstrated in patients who meet established eligibility parameters. In conclusion, OLT offers meaningful survival benefits for carefully selected patients with NELM. A multidisciplinary approach and ongoing research into prognostic markers and adjunctive therapies are critical to optimizing outcomes in this challenging clinical setting.
Introduction
Neuroendocrine tumors (NETs) are diagnosed at stage IV in 66%–85% of patients, and liver metastases are the most common location of metastatic disease (1–4). Neuroendocrine liver metastases (NELM) commonly manifest as multiple lesions affecting both lobes of the liver and are associated with a poor prognosis (5–10). Among patients with NELM, liver failure is the most common cause of death (11–13). Additionally, the production of bioactive amines and polypeptides that circumvent hepatic clearance leads to carcinoid syndrome in 8%–45% of patients, significantly reducing the quality of life (14–16).
Surgical management plays a crucial role in improving survival and reducing symptoms in patients with NELM. Surgical options include anatomic hepatic resections, wedge resections, and orthotopic liver transplantation (OLT) in selected patients. Microwave ablation, transarterial embolization, transarterial chemo and radioembolization, chemotherapy and molecular targeted therapies can be used in patients who are not surgical candidates (17, 18). Peptide Receptor Radionuclide Therapy (PRRT) is a therapeutic approach that uses β-emission radiation to induce tumor necrosis. Y-90 and other radiation therapies have shown promising results; however, they carry risks, including the potential for liver failure. Here, we will review the role of OLT in the management of NELM.
Liver resection for the treatment of NELM
Liver resection is the cornerstone in the management of NELM as it leads to significant improvement in survival and decreased endocrine symptoms in selected patients. Liver resection improves survival, even if R0 resection cannot be achieved. Studies have shown that a 70% cytoreduction threshold is sufficient to achieve significant symptom control and survival benefits, and this criterion has now been adopted by the North American Neuroendocrine Tumor Society (NANETS) (19–22).
A meta-analysis published by Yuan et al., analyzing data from seven studies and 696 patients, found that the 2-, 3-, and 5-year survival of those undergoing resection were 82%, 74%, and 50%, respectively, compared to 52%, 40%, and 30% in patients undergoing nonsurgical treatments (23). Similarly, a meta-analysis published by Kaçmaz et al., analyzing data from 11 studies and 1,108 patients, reported that surgical resection leads to the best long-term survival compared to no resection, chemotherapy, or embolization (24).
Frilling et al. described three types of NELM based on their radiologic characteristics. Type I refers to a single metastasis, Type II is an isolated metastatic bulk accompanied by smaller deposits, and Type III refers to disseminated metastatic disease. The authors showed that while patients with type I and type II NELM had a 10-year survival of 100% and 75%, respectively, those with type III tumors had a 10-year survival of only 29%. This observation is important for determining which subgroups of patients with NELM may benefit from OLT (25).
Selection criteria for OLT in patients with NELM
Initially described in 2007, the Milan-NET criteria have proven valuable in identifying patients who may benefit from transplantation. Based on these criteria, the Organ Procurement and Transplantation Network (OPTN) under the United Network for Organ Sharing (UNOS) and the European Society for Medical Oncology (ESMO) have established guidelines to determine transplant eligibility for patients with NELM (Table 1). We will discuss the key factors assessed in patient selection, including tumor histology, resection of the primary tumor and location, absence of extrahepatic disease, extent of liver parenchymal involvement, and age.
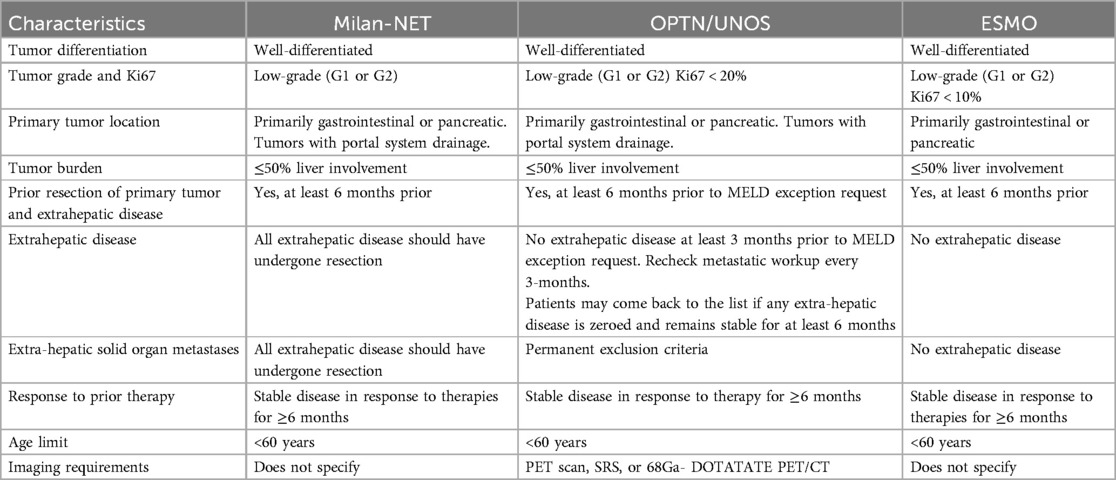
Table 1. Selection criteria for liver transplant consideration in patients with neuroendocrine tumor liver metastases (28–30).
Tumor histology
The tumor's biology is strongly linked to prognosis and must be carefully evaluated when selecting patients for OLT, liver resection, as well as locoregional and systemic therapies. According to the World Health Organization (WHO) NETs can be classified based on their differentiation and proliferation activity. Well-differentiated NETs are G1 (Ki-67 index <2% or less than 2 mitoses per 10HPF), G2 (Ki-67 index 2%–20% or mitotic count 2–20 per 10HPF), or G3 (Ki-67 index >20% or mitotic count >20 per 10HPF). All poorly differentiated tumors are high-grade (G3) with a Ki-67 index >20% or mitotic count >20 per 10HPF (4, 26, 27). The Milan-NET, OPTN/UNOS, and ESMO criteria establish that only patients with G1 or G2 tumor histology should be considered for transplantation (28–30). However, this classification system does not correlate well with liver resection or transplantation outcomes, and the most suitable histologic selection criteria remain a topic of ongoing discussion. In a recent study, Eshmuminov et al. recommended that a 5% cutoff for the Ki-67 index be adopted as a criterion for selecting liver transplantation patients (4). The current recommendation of a 3–6-month observation period after resection of the primary tumor is intertwined with the histologic grading, as it allows the selection of patients with less aggressive tumor biology. In a prior analysis from the OPTN/UNOS database, our group found that longer waiting times were associated with better outcomes and that waiting times greater than 6 months were associated with lower tumor recurrence (25.7% vs. 75.3% (31).
Primary tumor resection and location
The Milan-NET, OPTN/UNOS, and ESMO criteria emphasize that surgical resection of the primary tumor must occur before liver transplant consideration. This criterion ensures adequate staging and assessment of the tumor's histologic grade before pursuing OLT. It also guarantees that the primary tumor has been located and that resection with curative intent has been pursued. Mazzaferro et al. also suggested that neuroendocrine tumors (NET) originating from the gastrointestinal tract with portal vein drainage have a higher incidence of liver-only metastatic disease compared to other primaries with hematogenous spread and liver metastases. Accordingly, patients with primary tumors located in the pharynx, esophagus, distal rectum, or extra-gastrointestinal sites are now excluded from OLT by most centers (28–30).
Absence of extrahepatic disease
The OPTN/UNOS, Milan-NET, and ESMO criteria recommend that there should not be extrahepatic disease at the time of transplantation. The resection of the primary tumor and all extrahepatic disease should occur at least six months before OLT consideration. Only the OPTN/UNOS guidelines are explicit about extrahepatic solid organ metastases as permanent exclusion criteria for transplantation (Table 1). There is a paucity of data reporting outcomes of OLT in patients who, at their initial presentation, had evidence of extrahepatic disease (32). However, it has been demonstrated that transplanting outside the Milan-NET criteria does not offer significant survival benefits when compared to liver resection (4). In addition, simultaneous resection of the primary tumor at the time of transplantation has been associated with lower overall survival (33).
Common radiologic workup includes multiphasic CT scan, MRI, and different modalities of scintigraphy. Given that 80%–90% of NETs overexpress somatostatin receptors (except for insulinomas), scintigraphy is a key diagnostic tool before transplantation. The OPTN/UNOS selection criteria requires that all patients must undergo PET-scan, somatostatin receptor scintigraphy (SRS), or 68Ga- DOTATATE PET/CT before being considered for transplantation. Etchebehere et al. reported that 68Ga- DOTATATE PET/CT outperforms SRS for detecting well-differentiated NET lesions with a sensitivity of 96% and specificity of 97% compared to 60% and 97% for SRS, respectively. In this series, DOTATE PET/CT was also more sensitive for detecting unknown primary lesions (34). Supporting the OPTN/UNOS recommendation to include some modality of scintigraphy, the study by Albanus et al. found that 68Ga-DOTATATE PET/CT had a sensitivity of 100% for detecting extrahepatic metastases, including bone and lymph node lesions, compared to 47% for CT scan alone (35).
Extent of liver parenchymal involvement
The Milan-NET, OPT/UNOS, and ESMO selection criteria exclude patients with liver tumor involvement greater than 50%. As described by Mazzaferro et al., this cutoff is “pragmatical” and has been proven valuable when combined with the other criteria. Supporting data for a cutoff of 50% includes the report by Touzios et al. where patients with NELM affecting more than 50% of the liver had significantly lower 5-year survival (8% vs. 67%, p < 0.001) (36). Conversely, the study by Le Treut et al. did not find significant differences in patients transplanted above or below a cut-off of 40% liver involvement. In this study, the authors found that patients with “hepatomegaly” (defined as the enlargement of the explanted liver by 20% or more beyond the patient's normal liver volume determined by Heinemann's formula) had worse 5-year survival (34% vs. 72%, p 0.0037) (37). The lack of data comparing outcomes for OLT above and below a cut-off of 50% raises the question of whether there are patients who could benefit from OLT that are being excluded under current criteria.
Age
The age range for patients diagnosed with NELM typically spans 47–77 years, with an average diagnosis age of 62 years. Patients undergoing liver transplantation for NELM are, on average, between the ages of 45 and 49 years. In addition to showing improved long-term survival after OLT compared to non-transplant strategies, Mazzaferro et al. analyzed the relationship between patient age and mortality. Patients older than 54 years old were found to have a higher mortality risk associated with comorbidities compared to those who were younger (28). Similarly, our group found that age 45 or younger was associated with significantly better survival compared with those >45 years (70.9% vs. 60% p = 0.03) (31). Currently, most guidelines recommend favoring OLT in patients younger than 60 years (Table 1).
OLT for the treatment of NELM
The role of OLT for the treatment of NELM is still a matter of debate (38). The North American Neuroendocrine Tumor Society (NANETS) guidelines emphasize that, while transplantation yields favorable outcomes in carefully selected patients, its application is limited by the scarcity of donor organs and the requirement for patients to exhibit favorable tumor biology (22).
Several groups have investigated the utility of liver transplantation in patients with liver metastases without extrahepatic disease. Mazzaferro et al. reported a 10-year survival of 88.8% after OLT in patients with NELM fulfilling the Milan-NET criteria compared to 22.4% in the non-transplant group (28). In a subsequent study, they compared OLT vs. liver resection among patients within Milan-NET criteria, finding that OLT was associated with improved 10-year survival (93% vs. 75%, p = 0.007) and 10-year disease-free survival (52% vs. 18%, p < 0.001) (39). Living donor liver transplantation (LDLT) has been performed for neuroendocrine liver metastases (NELM), though data remain limited. However, emerging evidence suggests that with meticulous patient selection and integration of multimodal therapies, LDLT may offer favorable outcomes in the management of unresectable NELM (40).
Although patients undergoing liver transplants for NELM have shown decreased tumor recurrence compared to those undergoing liver resection, recurrence is still frequent, and it follows a different pattern. Maspero et al. observed that patients undergoing OLT for NELM experienced a higher incidence of multisite recurrences (48%) compared to the resection group (12%), whereas the majority of recurrences in the resection group were intra-hepatic (88%) compared to only 8% in the transplantation group. Sposito et al. studied post-recurrence survival after transplantation and found a median time between transplantation and recurrence of 82.9 months. The most common sites of metastasis were abdominal lymph nodes (59.4%), peritoneum (6.3%), and lungs (6.3%). The only factor associated with decreased post-recurrence 5-year survival in this series was the time from OLT of less than 2 years (0% vs. 89.5%, p = 0.001) (41). In an analysis of the OPTN/UNOS dataset from October 1998 to June 2018, our group identified a recurrence rate of 34% among 258 patients transplanted for NELM, with significantly lower recurrence rates observed in those who waited over 6 months for transplantation (31).
Conclusion
Patients with NELM who undergo OLT demonstrate longer survival rates and extended intervals before tumor recurrence compared to those undergoing resection. The long-term benefits of liver transplantation for NELM are evident primarily in patients who meet the UNOS or Milan criteria. Patients with low-grade NET draining through the portal system, who have had resection of the primary tumor, less than 50% liver involvement, and stable disease for at least 6 months, exhibit a higher likelihood of success. Patients outside these criteria may benefit from other treatment options such as surgical resection, locoregional or systemic therapies. Although numerous studies have found that younger patients experience longer survival rates compared to older patients with similar tumor histology, this age cut-off has varied from 45 to 55 years. Further research should be conducted to study other prognostic factors that benefit or adversely impact long-term outcomes after OLT.
Currently, research has shown that patients who had tumor recurrence after 2 years post-transplant had significantly higher 5-year survival compared to those who experienced early recurrence. Given the complexities in managing these patients, a multidisciplinary approach is recommended to identify appropriate treatment options. The role of liver transplantation for palliation in selected patients with advanced disease should be investigated. Further research is required to determine the most effective combinations of therapies and immunosuppressants that will not only enhance survival rates but also improve patients’ quality of life.
Author contributions
GO: Conceptualization, Writing – original draft, Writing – review & editing. DR: Writing – original draft, Writing – review & editing. AC: Writing – original draft, Writing – review & editing. SD: Writing – original draft, Writing – review & editing. RG: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Grammar.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Loosen SH, Kostev K, Jann H, Tetzlaff F, Tacke F, Krieg S, et al. Distribution of gastrointestinal neuroendocrine tumors in Europe: results from a retrospective cross-sectional study. J Cancer Res Clin Oncol. (2023) 149(4):1411–6. doi: 10.1007/s00432-022-04003-3
2. Loosen SH, Kostev K, Eschrich J, Krieg S, Krieg A, Luedde T, et al. Clinical characteristics of 662 patients with pancreatic neuroendocrine tumors receiving antitumoral therapy. Medicine (Baltimore). (2022) 101(50):e32044. doi: 10.1097/MD.0000000000032044
3. Jann H, Roll S, Couvelard A, Hentic O, Pavel M, Muller-Nordhorn J, et al. Neuroendocrine tumors of midgut and hindgut origin: tumor-node-metastasis classification determines clinical outcome. Cancer. (2011) 117(15):3332–41. doi: 10.1002/cncr.25855
4. Eshmuminov D, Studer DJ, Lopez Lopez V, Schneider MA, Lerut J, Lo M, et al. Controversy over liver transplantation or resection for neuroendocrine liver metastasis: tumor biology cuts the deal. Ann Surg. (2023) 277(5):e1063–e71. doi: 10.1097/SLA.0000000000005663
5. Jiao X, Luan W, Peng X, Liu L, Zhang L, Zhou L. Effects of tumor origins and therapeutic options on the prognosis of hepatic neuroendocrine tumors: a retrospective study. Medicine (Baltimore). (2020) 99(51):e23655. doi: 10.1097/MD.0000000000023655
6. Gaujoux S, Gonen M, Tang L, Klimstra D, Brennan MF, D'Angelica M, et al. Synchronous resection of primary and liver metastases for neuroendocrine tumors. Ann Surg Oncol. (2012) 19(13):4270–7. doi: 10.1245/s10434-012-2462-8
7. Ramdhani K, Braat AJAT. The evolving role of radioembolization in the treatment of neuroendocrine liver metastases. Cancers. (2022) 14(14):3415. doi: 10.3390/cancers14143415
8. Fairweather M, Swanson R, Wang J, Brais LK, Dutton T, Kulke MH, et al. Management of neuroendocrine tumor liver metastases: long-term outcomes and prognostic factors from a large prospective database. Ann Surg Oncol. (2017) 24(8):2319–25. doi: 10.1245/s10434-017-5839-x
9. Ruzzenente A, Bagante F, Bertuzzo F, Aldrighetti L, Ercolani G, Giuliante F, et al. A novel nomogram to predict the prognosis of patients undergoing liver resection for neuroendocrine liver metastasis: an analysis of the Italian neuroendocrine liver metastasis database. J Gastrointest Surg. (2017) 21(1):41–8. doi: 10.1007/s11605-016-3228-6
10. Tierney JF, Poirier J, Chivukula S, Pappas SG, Hertl M, Schadde E, et al. Primary tumor site affects survival in patients with gastroenteropancreatic and neuroendocrine liver metastases. Int J Endocrinol. (2019) 2019:9871319. doi: 10.1155/2019/9871319
11. Boudreaux JP, Klimstra DS, Hassan MM, Woltering EA, Jensen RT, Goldsmith SJ, et al. The nanets consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the Jejunum, ileum, appendix, and cecum. Pancreas. (2010) 39(6):753–66. doi: 10.1097/MPA.0b013e3181ebb2a5
12. Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. (2013) 42(4):557–77. doi: 10.1097/MPA.0b013e31828e34a4
13. Keutgen XM, Schadde E, Pommier RF, Halfdanarson TR, Howe JR, Kebebew E. Metastatic neuroendocrine tumors of the gastrointestinal tract and pancreas: a surgeon’s plea to centering attention on the liver. Semin Oncol. (2018) 45(4):232–5. doi: 10.1053/j.seminoncol.2018.07.002
14. Kulke MH, Shah MH, Benson AB 3rd, Bergsland E, Berlin JD, Blaszkowsky LS, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. (2015) 13(1):78–108. doi: 10.6004/jnccn.2015.0011
15. Halperin DM, Shen C, Dasari A, Xu Y, Chu Y, Zhou S, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol. (2017) 18(4):525–34. doi: 10.1016/S1470-2045(17)30110-9
16. Kostiainen I, Karppinen N, Simonen P, Rosengard-Barlund M, Linden R, Tarkkanen M, et al. Arterial function, biomarkers, carcinoid syndrome and carcinoid heart disease in patients with small intestinal neuroendocrine tumours. Endocrine. (2022) 77(1):177–87. doi: 10.1007/s12020-022-03065-0
17. Harrelson A, Wang R, Stewart A, Ingram C, Gillis A, Rose JB, et al. Management of neuroendocrine tumor liver metastases. Am J Surg. (2023) 226(5):623–30. doi: 10.1016/j.amjsurg.2023.08.011
18. Fernandes ESM, Kyt CVG, de Mello FPT, Pimentel LS, Andrade RO, Girao C, et al. Liver transplantation in gastroenteropancreatic neuroendocrine tumors. Front Oncol. (2022) 12:1001163. doi: 10.3389/fonc.2022.1001163
19. Graff-Baker AN, Sauer DA, Pommier SJ, Pommier RF. Expanded criteria for carcinoid liver debulking: maintaining survival and increasing the number of eligible patients. Surgery. (2014) 156(6):1369–76. doi: 10.1016/j.surg.2014.08.009
20. Morgan RE, Pommier SJ, Pommier RF. Expanded criteria for debulking of liver metastasis also apply to pancreatic neuroendocrine tumors. Surgery. (2018) 163(1):218–25. doi: 10.1016/j.surg.2017.05.030
21. Maxwell JE, Sherman SK, O'Dorisio TM, Bellizzi AM, Howe JR. Liver-directed surgery of neuroendocrine metastases: what is the optimal strategy? Surgery. (2016) 159(1):320–33. doi: 10.1016/j.surg.2015.05.040
22. Howe JR, Cardona K, Fraker DL, Kebebew E, Untch BR, Wang YZ, et al. The surgical management of small bowel neuroendocrine tumors: consensus guidelines of the north American neuroendocrine tumor society. Pancreas. (2017) 46(6):715–31. doi: 10.1097/MPA.0000000000000846
23. Yuan CH, Wang J, Xiu DR, Tao M, Ma ZL, Jiang B, et al. Meta-analysis of liver resection versus nonsurgical treatments for pancreatic neuroendocrine tumors with liver metastases. Ann Surg Oncol. (2016) 23(1):244–9. doi: 10.1245/s10434-015-4654-5
24. Kaçmaz E, Heidsma CM, Besselink MGH, Dreijerink KMA, Klumpen HJ, Nieveen van Dijkum EJM, et al. Treatment of liver metastases from midgut neuroendocrine tumours: a systematic review and meta-analysis. J Clin Med. (2019) 8(3):403. doi: 10.3390/jcm8030403
25. Frilling A, Li J, Malamutmann E, Schmid KW, Bockisch A, Broelsch CE. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br J Surg. (2009) 96(2):175–84. doi: 10.1002/bjs.6468
26. Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for research on cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. (2018) 31(12):1770–86. doi: 10.1038/s41379-018-0110-y
27. Kloppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. (2011) 18(Suppl 1):S1–16. doi: 10.1530/ERC-11-0013
28. Mazzaferro V, Sposito C, Coppa J, Miceli R, Bhoori S, Bongini M, et al. The long-term benefit of liver transplantation for hepatic metastases from neuroendocrine tumors. Am J Transplant. (2016) 16(10):2892–902. doi: 10.1111/ajt.13831
29. (OPTN) OPTN. Guidance to Liver Transplant Programs and the National Liver Review Board For: Adult Meld Exception Review (2020) Available online at: https://optn.transplant.hrsa.gov/media/esdjnjok/20200804_nlrb_adult_other_guidance.pdf (Accessed April 25, 2022).
30. Pavel M, Oberg K, Falconi M, Krenning EP, Sundin A, Perren A, et al. Gastroenteropancreatic neuroendocrine neoplasms: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2020) 31(7):844–60. doi: 10.1016/j.annonc.2020.03.304
31. Valvi D, Mei X, Gupta M, Shah MB, Ancheta A, Marti F, et al. Younger age is associated with improved survival in patients undergoing liver transplantation alone for metastatic neuroendocrine tumors. J Gastrointest Surg. (2021) 25(6):1487–93. doi: 10.1007/s11605-020-04708-1
32. Lehnert T. Liver transplantation for metastatic neuroendocrine carcinoma: an analysis of 103 patients. Transplantation. (1998) 66(10):1307–12. doi: 10.1097/00007890-199811270-00007
33. Lai Q, Coppola A, Mrzljak A, Cigrovski Berkovic M. Liver transplantation for the cure of neuroendocrine liver metastasis: a systematic review with particular attention to the risk factors of death and recurrence. Biomedicines. (2024) 12(11):2419. doi: 10.3390/biomedicines12112419
34. Etchebehere EC, de Oliveira Santos A, Gumz B, Vicente A, Hoff PG, Corradi G, et al. 68ga-dotatate pet/ct, 99mtc-hynic-octreotide spect/ct, and whole-body mr imaging in detection of neuroendocrine tumors: a prospective trial. J Nucl Med. (2014) 55(10):1598–604. doi: 10.2967/jnumed.114.144543
35. Albanus DR, Apitzsch J, Erdem Z, Erdem O, Verburg FA, Behrendt FF, et al. Clinical value of (6)(8)Ga-dotatate-pet/ct compared to stand-alone contrast enhanced ct for the detection of extra-hepatic metastases in patients with neuroendocrine tumours (net). Eur J Radiol. (2015) 84(10):1866–72. doi: 10.1016/j.ejrad.2015.06.024
36. Touzios JG, Kiely JM, Pitt SC, Rilling WS, Quebbeman EJ, Wilson SD, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. (2005) 241(5):776–83. doi: 10.1097/01.sla.0000161981.58631.ab
37. Le Treut YP, Gregoire E, Belghiti J, Boillot O, Soubrane O, Mantion G, et al. Predictors of long-term survival after liver transplantation for metastatic endocrine tumors: an 85-case French multicentric report. Am J Transplant. (2008) 8(6):1205–13. doi: 10.1111/j.1600-6143.2008.02233.x
38. Muller PC, Pfister M, Eshmuminov D, Lehmann K. Liver transplantation as an alternative for the treatment of neuroendocrine liver metastasis: appraisal of the current evidence. Hepatobiliary Pancreat Dis Int. (2024) 23(2):146–53. doi: 10.1016/j.hbpd.2023.08.007
39. Maspero M, Rossi RE, Sposito C, Coppa J, Citterio D, Mazzaferro V. Long-term outcomes of resection versus transplantation for neuroendocrine liver metastases meeting the Milan criteria. Am J Transplant. (2022) 22(11):2598–607. doi: 10.1111/ajt.17156
40. Kasai Y, Ito T, Masui T, Nagai K, Anazawa T, Uchida Y, et al. Liver transplantation for gastroenteropancreatic neuroendocrine liver metastasis: optimal patient selection and perioperative management in the era of multimodal treatments. J Gastroenterol. (2025) 60(1):1–9. doi: 10.1007/s00535-024-02166-z
Keywords: neuroendocrine neoplasia, liver metastasases, liver transplant, liver resection, neuroendocrine (D018278)
Citation: Orozco G, Ramaiah D, Cracco A, Desai S and Gedaly R (2025) Liver transplantation for the treatment of neuroendocrine liver metastases. Front. Surg. 12:1603704. doi: 10.3389/fsurg.2025.1603704
Received: 31 March 2025; Accepted: 9 June 2025;
Published: 1 July 2025.
Edited by:
Krzysztof Zieniewicz, Medical University of Warsaw, PolandReviewed by:
Altan Alim, Koç University Hospital, TürkiyeIsmail Tirnova, Başkent University, Türkiye
Copyright: © 2025 Orozco, Ramaiah, Cracco, Desai and Gedaly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Gedaly, cmdlZGEyQHVreS5lZHU=
 Gabriel Orozco1
Gabriel Orozco1 Siddharth Desai
Siddharth Desai Roberto Gedaly
Roberto Gedaly