- Department of Surgery, Division of Transplant Surgery, University of California San Diego, San Diego, CA, United States
Liver transplantation is increasingly being explored as a treatment option for select patients with metastatic colorectal cancer (mCRC). Historically, transplantation for mCRC was abandoned due to poor long-term outcomes and high recurrence rates. However, recent advancements in patient selection, immunosuppressive strategies, and donor organ availability have led to a renewed interest in this approach. Studies have demonstrated that highly selected patients undergoing liver transplantation can achieve significantly improved survival rates compared to those receiving standard systemic therapies. The implementation of Model for End-Stage Liver Disease (MELD) exception points, improved donor preservation techniques such as machine perfusion, and the growing role of living donor liver transplantation have further supported its feasibility. As research continues, liver transplantation may emerge as a crucial component of a multidisciplinary strategy for treating colorectal liver metastases, offering a select group of patients a chance at prolonged survival and improved quality of life.
1 Introduction
Metastatic colorectal cancer (mCRC) is a significant health concern globally. Colorectal cancer (CRC) is the third most common cancer worldwide, with approximately 1.85 million new cases and 850,000 deaths annually. In the United States, there are nearly 150,000 new cases diagnosed and over 50,000 deaths each year due to CRC (1, 2). Nearly half of patients with colorectal cancer will develop metastatic disease (3). Additionally, 25%–30% of patients present with liver metastases at the time of diagnosis (synchronous liver metastases). Another 20%–25% of patients will develop liver metastases metachronously, or after the initial diagnosis and treatment of the primary tumor (4).
The incidence of colorectal cancer has changed dramatically since the introduction of early screening techniques in the 1990s. Approximately 10%–12% of new CRC diagnoses occur in patients younger than 50 years of age (5). The increased incidence of early onset (individuals <50 years of age) CRC is not yet well understood. However, there are multiple studies that found early onset CRC were associated with later stage distal colon or rectal tumors, poorly differentiated tumors, or mucinous and signet ring tumors that are associated with poor outcomes (6, 7). With the expanding role of liver transplantation in the treatment of mCRC, more patients with mCRC may benefit from liver transplantation.
1.1 Overview of metastatic colorectal cancer: current treatment options and limitations
The prognosis of mCRC remains poor, with a 5-year relative overall survival rate of approximately 15%. Advances in treatment, including systemic therapies and targeted treatments based on molecular profiling, have improved survival outcomes, but cures remain uncommon (1, 2). The current treatment options for colorectal cancer with metastasis to the liver include surgical resection, systemic therapy, and various liver-directed therapies.
Surgical resection remains the only potentially curative treatment for liver-limited metastatic colorectal cancer. Approximately 20%–30% of patients with colorectal cancer with liver metastasis are candidates for surgical resection, which can significantly improve survival outcomes. The American Society of Clinical Oncology (ASCO) recommends neoadjuvant chemotherapy followed by surgery vs. surgery alone for patients who are candidates for potentially curative resection of liver metastases (2).
Systemic therapy is essential for patients with unresectable liver metastases or those with extrahepatic disease. The National Comprehensive Cancer Network (NCCN) guidelines recommend systemic chemotherapy regimens such as FOLFOX (fluorouracil, leucovorin, and oxaliplatin), FOLFIRI (fluorouracil, leucovorin, and irinotecan), or FOLFOXIRI (fluorouracil, leucovorin, oxaliplatin, and irinotecan), often combined with biologic agents like bevacizumab or cetuximab, depending on the molecular profile of the tumor. Pembrolizumab is recommended for patients with microsatellite instability-high or deficient mismatch repair tumors (2, 8).
Liver-directed therapies include ablation techniques (radiofrequency ablation, microwave ablation), transarterial chemoembolization (TACE), selective internal radiation therapy (SIRT), and hepatic arterial infusion (HAI) chemotherapy. These therapies are particularly useful for patients with unresectable liver-confined disease or those who are not candidates for surgery due to comorbidities. The American College of Radiology (ACR) also supports the use of these interventional radiological techniques to address limitations of curative resection (9–12).
Limitations of these treatments include the risk of postoperative liver failure due to insufficient future liver remnant volume, the potential for disease relapse after liver surgery, and the adverse effects associated with systemic and liver-directed therapies. Additionally, the role of stereotactic body radiation therapy (SBRT) and liver transplantation in the management of colorectal cancer with liver metastasis are still being evaluated, with ongoing studies needed to establish definitive recommendations (9, 10, 13).
1.2 Liver transplantation as an alternative treatment approach
The rationale for exploring liver transplantation as a treatment option for metastatic colorectal cancer, specifically in the context of colorectal cancer with metastasis to the liver, is based on several key factors: improved survival outcomes, patient selection, and management of recurrence.
Liver transplantation has demonstrated significantly improved overall survival rates in highly selected patients with nonresectable liver-only metastases. Prospective studies have shown 5-year survival rates of up to 80% in selected patients, which is markedly higher than the approximately 10% 5-year survival seen with palliative chemotherapy alone (14–17).
Advances in patient selection criteria have been crucial. Factors such as carcinoembryonic antigen (CEA) levels, response to chemotherapy, size of liver lesions, and time from primary tumor resection to transplantation have been identified as important prognostic indicators. These criteria help identify patients who are more likely to benefit from liver transplantation (14, 17).
Although recurrence is common, it often occurs as slow-growing metastases, particularly in the lungs, which can be amenable to further curative interventions. This contrasts with the rapid progression typically seen in patients treated with chemotherapy alone (16, 18). The Fong clinical risk score is a preoperative scoring system that was also developed to predict recurrence after hepatic resection for mCRC. Five variables included are nodal-positive primary, disease free interval <12 months, >1 tumor, preoperative CEA > 200 ng/ml, and tumor size >5 cm with one point assigned per criterion (19). The total score was highly predictive of long-term outcomes with scores up to two having a more favorable outcome.
2 Evolution of standard of care and the historical transplant experience
Liver transplantation for colorectal cancer metastasized to the liver has undergone significant evolution, marked by key historical outcomes and advancements in patient selection and perioperative management. Initially, liver transplantation for colorectal cancer with liver metastasis was abandoned in the 1990s due to poor outcomes and high rates of recurrence, with 5-year overall survival rates as low as 18% (15). Liver transplantation for mCRC re-emerged after the landmark SECA I and SECA II studies from Norway. Both studies focused on a highly selective patient population with CRC and unresectable liver-limited metastases. The SECA-I study published in 2013 found that patients who underwent primary tumor resection and at least 6 months of chemotherapy followed by liver transplant demonstrated a 5-year overall survival of 60% (15). This study highlighted the importance of stringent selection criteria, including liver-only metastases, excised primary tumors, and response to chemotherapy.
Subsequently, the SECA-II study further refined these criteria and reported even better outcomes. In this study, patients with CRC and unresectable liver metastases on imaging with favorable prognostic factors, such as low carcinoembryonic antigen (CEA) levels and significant response to chemotherapy, had a 5-year overall survival rate of 83% in patients (14). These findings underscored the potential for liver transplantation to offer long-term survival benefits comparable to other indications for liver transplantation.
A prospective cohort study at Oslo University Hospital evaluated multiple clinical trials on liver transplant for colorectal liver metastases to determine disease recurrence, overall survival and survival after relapse in the modern age. This led to the development of the Oslo scoring system which identified negative predictive factors for overall survival including tumor size greater than 5.5 cm, disease progression on chemotherapy, plasma CEA > 80 μg/L, and time interval between primary tumor resection and liver transplant of less than 2 years (16). Patients with Oslo score of 0 had an overall 5- and 10-year survival of >80% while those with Oslo score of 0–2 had a median 5- and 10-year OS rate of 63% and 45% (16).
A systematic review and meta-analysis by Varley et al. reported pooled 5-year overall survival rates ranging from 50% to 80%, with disease-free survival (DFS) rates between 35% and 56% (20). Another meta-analysis by Dawood et al. confirmed these findings, showing a pooled 5-year overall survival of 53% and highlighting the common recurrence pattern of slow-growing pulmonary metastases (21).
Overall, the historical outcomes of liver transplantation for colorectal cancer with liver metastasis have improved significantly due to better patient selection and perioperative management, making it a viable treatment option for selected patients with nonresectable liver-only metastases.
3 Current literature review: examining recent data from Europe, Canada, and the US
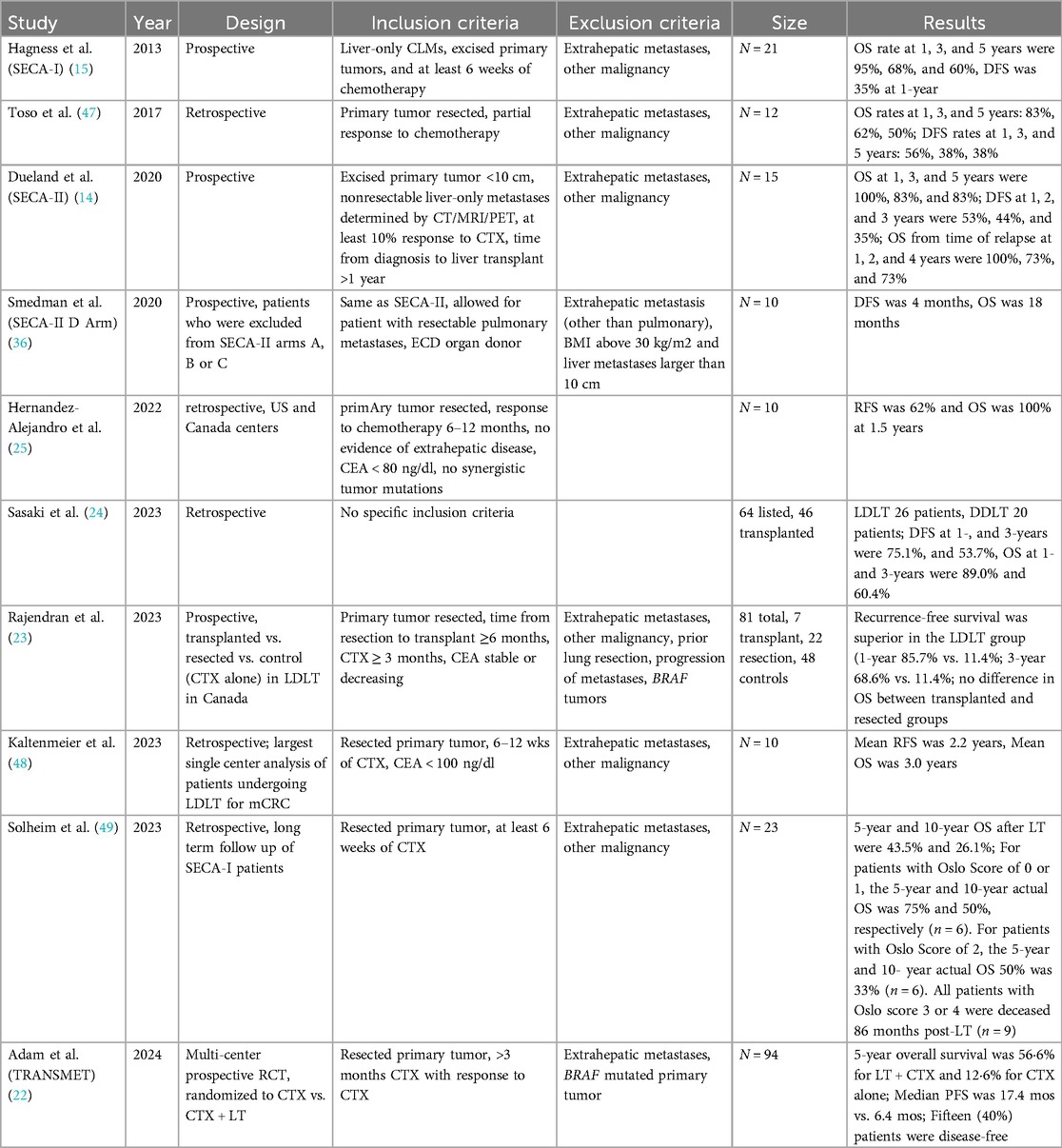
Recent studies from Europe, Canada, and the United States have provided compelling evidence supporting the use of liver transplantation as a treatment for metastatic colorectal cancer in highly selected patients (Table 1).
A multicenter, randomized controlled trial from Europe, the TransMet study, demonstrated that liver transplantation plus chemotherapy significantly improved 5-year overall survival to 56.6% compared to 12.6% with chemotherapy alone in patients with permanently unresectable colorectal cancer with liver metastasis (22). This study underscores the potential of liver transplantation to offer a survival benefit in this patient population.
In Canada, the Toronto Living Donor Liver Transplant Program reported promising outcomes with living donor liver transplantation (LDLT) for unresectable colorectal cancer with liver metastasis. This prospective study recruited patients with unresectable CRLM (defined as bilobar disease) receiving systemic chemotherapy. The participants were then divided into three groups: patients who underwent LDLT, patients who underwent chemotherapy alone, and patients who underwent chemotherapy, converted to resectable disease, and underwent liver resection only. The study found that LDLT provided superior recurrence-free survival compared to resection, with a 3-year recurrence-free survival of 68.6% vs. 11.4% (23). This highlights the potential of LDLT as a viable option for selected patients.
In the United States, a systematic review and meta-analysis by Dawood et al. reported pooled 5-year overall survival rates of 53% for liver transplantation in patients with nonresectable colorectal cancer with liver metastasis, with the lungs being the most common site of recurrence (21). This meta-analysis supports the notion that liver transplantation can offer substantial survival benefits.
Sasaki et al. identified 46 patients with mCRC who underwent liver transplant in the US. 21% of patients experienced disease recurrence. 1- and 3-year disease free survival rates were 75% and 53%, and 1- and 3-year overall survival rates were 89% and 60%, respectively (24). Hernandez-Alejandro et al. identified 10 patients with mCRC who underwent LDLT. 3 patients had recurrence, disease free survival and overall survival rates were 62% and 100%, respectively, with a median follow up period of 1.5 years (25). While recent studies have a small cohort, they highlight the improvement in patient outcomes and need for stringent selection criteria. In 2021, the International Hepato-Pancreato-Biliary Association published consensus guidelines to identify and select patients with non-resectable mCRC who benefit from liver transplantation (26). These guidelines are standardized nomenclature focused on patient selection, with strict clinic-patho-radiological criteria, evaluation of biological behavior, graft selection and organ allocation, and recipient immunosuppression and prevention or management of recurrent disease.
4 BRAF, RAS, and other genetic mutations
CRC primary tumors in the left colon have better outcomes than primary right colon tumors. This is because BRAF mutations are more likely to originate from the right colon and sporadic mutations from the left colon (27–30). BRAF mutations occur in about 10% of CRC and patients with CRC from BRAF mutations have increased risk of recurrence. Multiple retrospective studies found that patients with BRAF mutations, who underwent curative-intent resection with hepatectomy, were a predictor of worse overall survival and recurrence free survival (27, 29–33). In addition, those with BRAF mutations lack response to current anti-EGFR systemic chemotherapy treatments. Thus, patients with V600E BRAF mutations are not considered candidates for liver transplantation due to poor prognosis, risk of recurrence, and the decreased benefit of transplant. RAS mutations occur in about 50% of colorectal tumors, and while it is a negative prognostic factor, is not an absolute contraindication to liver transplantation.
Mismatch repair deficiency in mCRC occurs in about 5% of patients and results in high mutation burden and microsatellite instability (MSI-H). The KEYNOTE-177 study demonstrated that Pembrolizumab, a PD-1 blockade immunotherapy, had superior progression free survival and no difference in overall survival in patients with MSI-H mCRC (34). However, liver transplantation in patients with prior immune check point inhibitor therapy remains in flux. Multiple studies in patients with HCC who underwent immune check point inhibitor therapy prior to liver transplantation experienced an acute allograft rejection rate of 16%–37% (34, 35). Of note, patients with MSI-H and BRAF mutations mCRC tumors are contraindicated and often excluded in the current studies.
The follow up study for patients with extended criteria, the SECA-II arm D study, included patients with poorly differentiated or signet ring cell primary tumors as well as patients with primary tumors in the ascending colon and patients with extensive liver tumor burden. Median disease-free survival was 4 months, with the most common recurrence being pulmonary metastasis, and median OS was 18 months which was significantly lower compared to patients in the SECA-I and SECA-II trials (36). Thus, poorly differentiated mCRC tumors are contraindication to liver transplantation.
Overall, recent and current studies in mCRC and liver metastases aim to refine strict patient selection criteria for candidates of liver transplant to improve outcomes.
4.1 Future directions in liver transplantation for mCRC: MELD exception points
Model for End-Stage Liver Disease (MELD) exception points are used to prioritize patients with metastatic colorectal cancer for liver transplantation by assigning them a higher allocation MELD (aMELD) score. This is done to reflect the patient's increased risk of waitlist dropout and to ensure equitable access to transplantation.
Patients with colorectal cancer with liver metastasis often have a lower laboratory MELD (lMELD) score, which may not accurately represent their urgency for transplantation. MELD exception points are granted to these patients to adjust their priority on the transplant waitlist, thereby increasing their chances of receiving a liver transplant. This approach is supported by studies showing that patients with non-HCC MELD exceptions, including those with colorectal cancer with liver metastasis, have a higher likelihood of undergoing liver transplantation and a reduced risk of waitlist dropout when adjusted for aMELD (37, 38).
The allocation of MELD exception points are all adjudicated via the National Liver Transplant Oncology Review Board and United Network for Organ Sharing (UNOS). These boards evaluate the medical urgency and potential benefit of transplantation for each patient, ensuring that those with colorectal cancer with liver metastasis who meet specific criteria receive appropriate prioritization (37, 39).
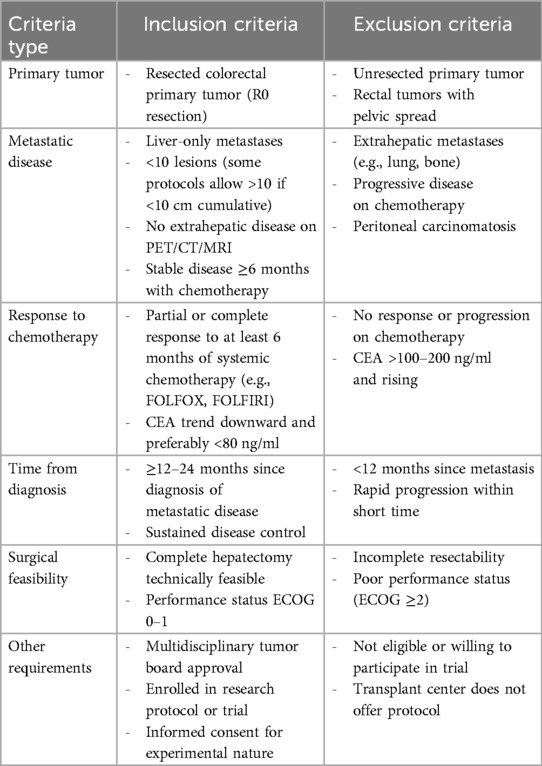
In June 2024, the OPTN and UNOS approved updates to the transplant oncology allocation policy to include MELD exception points for patients with mCRC with unresectable liver metastases. Criteria for MELD exception points include resection of primary tumor with negative margins, no extrahepatic metastatic disease or local recurrence and stable disease on systemic chemotherapy. Exclusion criteria include extrahepatic disease after primary tumor resection, local relapse of primary disease, or CEA > 80 μg/L. A table summarizing these inclusion and exclusion criteria is included as Table 2 (40).
In summary, MELD exception points are used to prioritize patients with metastatic colorectal cancer for liver transplantation by assigning them a higher MELD score, reflecting their increased risk and ensuring equitable access to transplantation.
The study by Sjule et al. utilized a discrete event simulation model to explore the effects of expanding liver transplantation eligibility to include CRLM patients in Norway (41). The model predicted that for every additional CRLM patient listed per year, the overall median wait-list time increased by 8% to 11%. Adding two additional CRLM patients under restrictive eligibility criteria increased the CRLM patients’ average life expectancy by 10.64 years and resulted in a net gain of 149.61 life-years over a 10-year period. This suggests a modest but significant increase in the number of liver transplants performed annually for CRLM patients. The study by Ueberroth et al. highlighted advances in donor organ preservation, including machine perfusion technology, have expanded organ availability, allowing for the inclusion of CRLM patients in liver transplantation programs (42). This expansion is supported by data showing comparable overall survival for liver transplantation in CRLM patients to other liver transplantation indications.
Additionally, the study by Adam et al. in the TransMet trial demonstrated that liver transplantation plus chemotherapy significantly improved 5-year overall survival compared to chemotherapy alone, supporting the validation of liver transplantation as a new standard option for patients with permanently unresectable liver-only metastases (22). This trial's findings suggest that incorporating MELD exception points and utilizing marginal livers through machine perfusion technology could further increase the number of liver transplants performed annually for CRLM patients.
4.2 Utilizing marginal or historically discarded livers with machine perfusion technology
Marginal or historically discarded livers can be utilized for liver transplantation in patients with metastatic colorectal cancer through the application of machine perfusion technology, specifically normothermic machine perfusion (NMP). NMP allows for the ex vivo preservation and functional assessment of livers that would otherwise be discarded due to concerns about their viability. This technology maintains the liver at physiological temperatures, providing oxygen and nutrients, which helps to reduce ischemia-reperfusion injury and allows for real-time evaluation of liver function.
Studies have demonstrated that NMP can successfully rescue a considerable proportion of marginal livers. For instance, the RESTORE trial reported that 72.7% of declined livers treated with NMP were successfully transplanted, with no graft-related deaths or primary nonfunction (43). Similarly, another study found that 71.5% of discarded livers subjected to NMP were deemed suitable for transplantation, with good post-transplant outcomes (44).
NMP has expanded the donor pool, increased the utilization of marginal allografts, and has been increasingly adopted in transplant centers across the US. UNOS reported the use of NMP rose to over 11% in 2022 compared to 3.5% in 2021 (45). The use of NMP can be particularly beneficial for colorectal cancer with liver metastasis patients, who often have lower MELD scores and may face longer wait times for transplantation. By expanding the donor pool with marginal livers, NMP can increase the availability of suitable grafts for these patients, potentially improving their survival outcomes (43, 44, 46). The adoption of NMP and other novel perfusion techniques is an important tool for increasing the number of liver allografts available as the indications for liver transplantation in the setting of transplant oncology continue to evolve.
5 Conclusion
Liver transplantation is emerging as a viable treatment option for select patients with metastatic colorectal cancer. Studies from Europe, Canada, and the United States demonstrate that liver transplantation can achieve 5-year survival rates exceeding those of standard systemic therapies, particularly when strict eligibility criteria are applied. The implementation of MELD exception points, advances in donor organ preservation through machine perfusion technology, and the growing acceptance of living donor liver transplantation further support the feasibility of expanding liver transplantation for colorectal liver metastases. However, ongoing research is needed to refine patient selection criteria, optimize immunosuppressive strategies, and address long-term outcomes, including disease recurrence. As the field continues to evolve, liver transplantation is likely to become an integral component of the multidisciplinary approach to managing metastatic colorectal cancer, offering select patients a potential for long-term survival and improved quality of life.
Author contributions
VB: Writing – review & editing, Writing – original draft. DO: Writing – original draft, Writing – review & editing. GS: Conceptualization, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
mCRC, Metastatic colorectal cancer; CRC, colorectal cancer; ASCO, American Society of Clinical Oncology; NCCN, National Comprehensive Cancer Network; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; FOLFIRI, fluorouracil, leucovorin, and irinotecan; FOLFOXIRI, fluorouracil, leucovorin, oxaliplatin, and irinotecan; TACE, transarterial chemoembolization; SIRT, selective internal radiation therapy; HAI, hepatic arterial infusion; ACR, American College of Radiology; SBRT, stereotactic body radiation therapy; CTX, chemotherapy; CEA, carcinoembryonic antigen; DFS, disease-free survival; OS, overall survival; RFS, recurrence free survival; LT, liver transplantation; LDLT, living donor liver transplantation; DDLT, deceased donor liver transplantation; EGFR, epidermal growth factor receptor; MSI-H, microsatellite instability; MELD, Model for End-Stage Liver Disease; UNOS, United Network for Organ Sharing; OPTN, organ procurement and transplantation network; CRLM, colorectal liver metastases; NMP, normothermic machine perfusion.
References
1. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. (2021) 325(7):669–85. doi: 10.1001/jama.2021.0106
2. Morris VK, Kennedy EB, Baxter NN, Benson AB 3rd, Cercek A, Cho M, Ciombor KK, Cremolini C, Treatment of metastatic colorectal cancer: aSCO guideline. J Clin Oncol. 2023;41(3):678–700. doi: 10.1200/JCO.22.01690
3. Kindler HL, Shulman KL. Metastatic colorectal cancer. Curr Treat Options Oncol. (2001) 2(6):459–71. doi: 10.1007/s11864-001-0068-7
4. Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. (2018) 18(1):78. doi: 10.1186/s12885-017-3925-x
5. Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology. (2020) 158(2):341–53. doi: 10.1053/j.gastro.2019.07.055
6. Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J, et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. (2014) 89(2):216–24. doi: 10.1016/j.mayocp.2013.09.006
7. Fairley TL, Cardinez CJ, Martin J, Alley L, Friedman C, Edwards B, et al. Colorectal cancer in U.S. Adults younger than 50 years of age, 1998–2001. Cancer. (2006) 107(5 Suppl):1153–61. doi: 10.1002/cncr.22012
8. Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19(5):541–65. doi: 10.6004/jnccn.2021.0022
9. Birrer DL, Tschuor C, Reiner C, Fritsch R, Pfammatter T, Garcia Schüler H, et al. Multimodal treatment strategies for colorectal liver metastases. Swiss Med Wkly. (2021) 151:w20390. doi: 10.4414/smw.2021.20390
10. Zampino MG, Magni E, Ravenda PS, Cella CA, Bonomo G, Della Vigna P, et al. Treatments for colorectal liver metastases: a new focus on a familiar concept. Crit Rev Oncol Hematol. (2016) 108:154–63. doi: 10.1016/j.critrevonc.2016.11.005
11. Vulasala SSR, Sutphin PD, Kethu S, Onteddu NK, Kalva SP. Interventional radiological therapies in colorectal hepatic metastases. Front Oncol. (2023) 13:963966. doi: 10.3389/fonc.2023.963966
12. Expert Panel on Interventional Radiology, Knavel Koepsel EM, Smolock AR, Pinchot JW, Kim CY, Ahmed O, et al. ACR Appropriateness criteria® management of liver cancer: 2022 update. J Am Coll Radiol. (2022) 19(11S):S390–408. doi: 10.1016/j.jacr.2022.09.005
13. Chow FCL, Chok KSH. Colorectal liver metastases: an update on multidisciplinary approach. World J Hepatol. (2019) 11(2):150–72. doi: 10.4254/wjh.v11.i2.150
14. Dueland S, Syversveen T, Solheim JM, Solberg S, Grut H, Bjørnbeth BA, et al. Survival following liver transplantation for patients with nonresectable liver-only colorectal metastases. Ann Surg. (2020) 271(2):212–8. doi: 10.1097/SLA.0000000000003404
15. Hagness M, Foss A, Line PD, Scholz T, Jørgensen PF, Fosby B, et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg. (2013) 257(5):800–6. doi: 10.1097/SLA.0b013e3182823957
16. Dueland S, Smedman TM, Syversveen T, Grut H, Hagness M, Line PD. Long-term survival, prognostic factors, and selection of patients with colorectal cancer for liver transplant: a nonrandomized controlled trial. JAMA Surg. (2023) 158(9):e232932. doi: 10.1001/jamasurg.2023.2932
17. Dueland S, Guren TK, Hagness M, Glimelius B, Line PD, Pfeiffer P, et al. Chemotherapy or liver transplantation for nonresectable liver metastases from colorectal cancer? Ann Surg. (2015) 261(5):956–60. doi: 10.1097/SLA.0000000000000786
18. Maspero M, Sposito C, Virdis M, Citterio D, Pietrantonio F, Bhoori S, et al. Liver transplantation for hepatic metastases from colorectal cancer: current knowledge and open issues. Cancers (Basel). (2023) 15(2):345. doi: 10.3390/cancers15020345
19. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. (1999) 230(3):309–18. discussion 318-321. doi: 10.1097/00000658-199909000-00004
20. Varley R, Tarazi M, Davé M, Mobarak S, Stott MC, Baltatzis M, et al. Liver transplantation for non-resectable liver metastases from colorectal cancer: a systematic review and meta-analysis. World J Surg. (2021) 45(11):3404–13. doi: 10.1007/s00268-021-06248-4
21. Dawood ZS, Brown ZJ, Munir MM, Waqar U, Rawicz-Pruszynski K, Endo Y, et al. Outcomes of liver transplant for colorectal liver metastasis: a systematic review and meta-analysis. J Gastrointest Surg. (2024) 28(11):1943–50. doi: 10.1016/j.gassur.2024.09.006
22. Adam R, Piedvache C, Chiche L, Adam JP, Salamé E, Bucur P, et al. Liver transplantation plus chemotherapy versus chemotherapy alone in patients with permanently unresectable colorectal liver metastases (TransMet): results from a multicentre, open-label, prospective, randomised controlled trial. Lancet. (2024) 404(10458):1107–18. doi: 10.1016/S0140-6736(24)01595-2
23. Rajendran L, Claasen MP, McGilvray ID, Cattral MS, Ghanekar A, Selzner N, et al. Toronto Management of initially unresectable liver metastasis from colorectal cancer in a living donor liver transplant program. J Am Coll Surg. (2023) 237(2):231–42. doi: 10.1097/XCS.0000000000000734
24. Sasaki K, Ruffolo LI, Kim MH, Fujiki M, Hashimoto K, Imaoka Y, et al. The current state of liver transplantation for colorectal liver metastases in the United States: a call for standardized reporting. Ann Surg Oncol. (2023) 30(5):2769–77. doi: 10.1245/s10434-023-13147-6
25. Hernandez-Alejandro R, Ruffolo LI, Sasaki K, Tomiyama K, Orloff MS, Pineda-Solis K, et al. Recipient and donor outcomes after living-donor liver transplant for unresectable colorectal liver metastases. JAMA Surg. (2022) 157(6):524–30. doi: 10.1001/jamasurg.2022.0300. Erratum in: JAMA Surg. 2022 November 1;157(11):1067. doi: 10.1001/jamasurg.2022.4936.35353121
26. Bonney GK, Chew CA, Lodge P, Hubbard J, Halazun KJ, Trunecka P, et al. Liver transplantation for non-resectable colorectal liver metastases: the international hepato-pancreato-biliary association consensus guidelines. Lancet Gastroenterol Hepatol. (2021) 6(11):933–46. doi: 10.1016/S2468-1253(21)00219-3. Epub 2021 September 8. Erratum in: Lancet Gastroenterol Hepatol. 2021 Nov;6(11):e7. doi: 10.1016/S2468-1253(21)00345-9.34506756
27. Teng HW, Huang YC, Lin JK, Chen WS, Lin TC, Jiang JK, et al. BRAF Mutation is a prognostic biomarker for colorectal liver metastasectomy. J Surg Oncol. (2012) 106(2):123–9. doi: 10.1002/jso.23063
28. Karagkounis G, Torbenson MS, Daniel HD, Azad NS, Jr DL, Donehower RC, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. (2013) 119(23):4137–44. doi: 10.1002/cncr.28347
29. Yaeger R, Cercek A, Chou JF, Sylvester BE, Kemeny NE, Hechtman JF, et al. BRAF Mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer. (2014) 120(15):2316–24. doi: 10.1002/cncr.28729
30. Schirripa M, Bergamo F, Cremolini C, Casagrande M, Lonardi S, Aprile G, et al. BRAF And RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br J Cancer. (2015) 112(12):1921–8. doi: 10.1038/bjc.2015.142
31. Pikoulis E, Margonis GA, Andreatos N, Sasaki K, Angelou A, Polychronidis G, et al. Prognostic role of BRAF mutations in colorectal cancer liver metastases. Anticancer Res. (2016) 36(9):4805–11. doi: 10.21873/anticanres.11040
32. Margonis GA, Buettner S, Andreatos N, Kim Y, Wagner D, Sasaki K, et al. Association of BRAF mutations with survival and recurrence in surgically treated patients with metastatic colorectal liver cancer. JAMA Surg. (2018) 153(7):e180996. doi: 10.1001/jamasurg.2018.0996
33. Umeda Y, Nagasaka T, Mori Y, Sadamori H, Sun DS, Shinoura S, et al. Poor prognosis of KRAS or BRAF mutant colorectal liver metastasis without microsatellite instability. J Hepatobiliary Pancreat Sci. (2013) 20(2):223–33. doi: 10.1007/s00534-012-0531-9
34. Wassmer CH, El Hajji S, Papazarkadas X, Compagnon P, Tabrizian P, Lacotte S, et al. Immunotherapy and liver transplantation: a narrative review of basic and clinical data. Cancers (Basel). (2023) 15(18):4574. doi: 10.3390/cancers15184574
35. Ros J, Salva F, Dopazo C, López D, Saoudi N, Baraibar I, et al. Liver transplantation in metastatic colorectal cancer: are we ready for it? Br J Cancer. (2023) 128(10):1797–806. doi: 10.1038/s41416-023-02213-1. Erratum in: Br J Cancer. 2023 Sep;129(4):733. doi: 10.1038/s41416-023-02370-3.36879000
36. Smedman TM, Line PD, Hagness M, Syversveen T, Grut H, Dueland S. Liver transplantation for unresectable colorectal liver metastases in patients and donors with extended criteria (SECA-II arm D study). BJS Open. (2020) 4(3):467–77. doi: 10.1002/bjs5.50278
37. Cannon RM, Davis EG, Goldberg DS, Lynch RJ, Shah MB, Locke JE, et al. Regional variation in appropriateness of non-hepatocellular carcinoma model for End-stage liver disease exception. J Am Coll Surg. (2020) 230(4):503–512.e8. doi: 10.1016/j.jamcollsurg.2019.12.022
38. Dirchwolf M, Becchetti C, Gschwend SG, Toso C, Dutkowski P, Immer F, et al. The MELD upgrade exception: a successful strategy to optimize access to liver transplantation for patients with high waiting list mortality. HPB (Oxford). (2022) 24(7):1168–76. doi: 10.1016/j.hpb.2021.12.009
39. Ahn DJ, Kwong AJ, Wall AE, Parker WF. Association of nonstandardized model for end-stage liver disease score exceptions with waitlist mortality in adult liver transplant candidates. Am J Transplant. (2025) 25(2):385–95. doi: 10.1016/j.ajt.2024.09.028
40. National Liver Review Board (NLRB) Updates Related to Transplant Oncology. Health Resources and Services Administration. Rockford, MD: OPTN Liver & Intestinal Organ Transplantation Committee with HRSA (2024).
41. Sjule HM, Vinter CN, Dueland S, Line PD, Burger EA, Bjørnelv GMW. The spillover effects of extending liver transplantation to patients with colorectal liver metastases: a discrete event simulation analysis. Med Decis Making. (2024) 44(5):529–42. doi: 10.1177/0272989X241249154
42. Ueberroth BE, Kriss M, Burton JR, Messersmith WA. Liver transplantation for colorectal cancer with liver metastases. Oncologist. (2025) 30(1):oyae367. doi: 10.1093/oncolo/oyae367
43. Olumba FC, Zhou F, Park Y, Chapman WC, RESTORE Investigators Group. Normothermic machine perfusion for declined livers: a strategy to rescue marginal livers for transplantation. J Am Coll Surg. (2023) 236(4):614–25. doi: 10.1097/XCS.0000000000000555
44. Quintini C, Del Prete L, Simioni A, Del Angel L, Diago Uso T, D'Amico G, et al. Transplantation of declined livers after normothermic perfusion. Surgery. (2022) 171(3):747–56. doi: 10.1016/j.surg.2021.10.056
45. Abu-Gazala S, Tang H, Abt P, Mahmud N. National trends in utilization of normothermic machine perfusion in DCD liver transplantation. Transplant Direct. (2024) 10(5):e1596. doi: 10.1097/TXD.0000000000001596
46. Mergental H, Laing RW, Kirkham AJ, Perera MTPR, Boteon YL, Attard J, et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun. (2020) 11(1):2939. doi: 10.1038/s41467-020-16251-3
47. Toso C, Pinto Marques H, Andres A, Castro Sousa F, Adam R, Kalil A, et al. Liver transplantation for colorectal liver metastasis: survival without recurrence can be achieved. Liver Transpl. (2017) 23(8):1073–6. doi: 10.1002/lt.24791
48. Kaltenmeier C, Geller DA, Ganesh S, Tohme S, Molinari M, Tevar A, et al. Living donor liver transplantation for colorectal cancer liver metastases: midterm outcomes at a single center in North America. Am J Transplant. (2024) 24(4):681–7. doi: 10.1016/j.ajt.2023.09.001
Keywords: metastatic colorectal cancer, hepatic metastasis, liver transplantation, MELD exception, machine perfusion
Citation: Bendersky VA, Olaso DG and Schnickel GT (2025) The evolving role of liver transplantation for metastatic colorectal cancer: current perspectives and future directions. Front. Surg. 12:1608467. doi: 10.3389/fsurg.2025.1608467
Received: 9 April 2025; Accepted: 25 June 2025;
Published: 17 July 2025.
Edited by:
Derek DuBay, Prisma Health, United StatesReviewed by:
Malay Shah, University of Kentucky, United StatesAltan Alim, Koç University Hospital, Türkiye
Copyright: © 2025 Bendersky, Olaso and Schnickel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriel T. Schnickel, Z3NjaG5pY2tlbEBoZWFsdGgudWNzZC5lZHU=
 Victoria A. Bendersky
Victoria A. Bendersky Danae G. Olaso
Danae G. Olaso Gabriel T. Schnickel
Gabriel T. Schnickel