- Department of General Surgery, Yongjia County Traditional Chinese Medicine Hospital, Wenzhou, Zhejiang, China
Objective: To analyze the clinical efficacy of laparoscopic cholecystectomy (LC) combined with percutaneous transhepatic gallbladder drainage (PTGBD) in patients with acute critical cholecystitis.
Methods: One hundred patients diagnosed with severe acute cholecystitis were retrospectively selected and categorized into two groups based on the surgical approach: the joint group (n = 49, underwent LC combined with PTGBD) and the LC group (n = 51, underwent LC alone).
Results: The joint group demonstrated a significantly shorter surgery duration and lower intraoperative blood loss compared to the LC group (P < 0.05). On the third postoperative day, patients in the joint group exhibited lower levels of WBC and CRP than those in the LC group (P < 0.05). The joint group showed faster recovery of bowel function, earlier ambulation, and shorter time to resume oral intake compared to the LC group (P < 0.05). Additionally, the joint group reported higher satisfaction than the LC group (P < 0.05). However, the joint group incurred higher surgical costs, total hospitalization costs, and medication costs than the LC group (P < 0.05). The independent risk factors for postoperative complications in patients with severe acute cholecystitis included a disease onset longer than 72 h, a surgical approach of LC alone, surgery duration longer than 2 h, intraoperative blood loss >100 ml, and age ≥65 years (P < 0.05).
Conclusion: Compared with LC alone, LC combined with PTGBD is more effective in reducing surgical trauma in patients with severe acute cholecystitis, improving postoperative inflammatory markers, and accelerating recovery. However, this combined approach is associated with significantly higher direct medical costs during hospitalization.
Introduction
Acute cholecystitis is a common acute abdominal condition in general surgery, with its incidence steadily rising. When complications such as gangrene or perforation occur, the condition may progress to severe acute cholecystitis, which can be life-threatening without timely intervention (1, 2). Laparoscopic cholecystectomy (LC) is the gold standard for treating gallbladder diseases due to its minimally invasive nature and rapid recovery (3). However, in case of severe acute cholecystitis, LC poses challenges including increased surgical difficulty, higher complication rates, and a greater likelihood of conversion to open surgery. Moreover, elderly patients or those with comorbidities may not tolerate LC, complicating clinical management (4).
Percutaneous transhepatic gallbladder drainage (PTGBD) offers a minimally invasive means to rapidly alleviate symptoms and reduce gallbladder pressure, thereby facilitating subsequent surgery (5). Recent studies (6, 7) suggest that combining PTGBD with LC can improve outcomes in severe cases by initially controlling inflammation and lowering surgical risk. This approach may also expand treatment options for high-risk patients.
Despite its promise, few studies have systematically analyzed the clinical efficacy of combining LC and PTGBD, particularly in relation to perioperative complications, recovery metrics, patient satisfaction, and cost-effectiveness. Addressing this gap, the present study evaluated key clinical outcomes, such as surgical indicators, inflammatory and liver function markers, complications, and satisfaction, between LC alone and LC combined with PTGBD. Additionally, logistic regression analysis was used to identify independent risk factors for postoperative complications, aiming to inform surgical decision-making and optimize perioperative management.
Materials and methods
Study design and patient selection
This retrospective study was approved by the ethics committee of Yongjia County Traditional Chinese Medicine Hospital. All patients and their families signed the informed consent form prior to the beginning of the treatment. Patient selection was conducted using the hospital's electronic medical record, based on the following inclusion criteria:
Inclusion criteria: (1) Age between 30 and 70 years; (2) Onset of symptoms <2 weeks; (3) Diagnosis of severe acute cholecystitis according to Tokyo Guidelines (8).
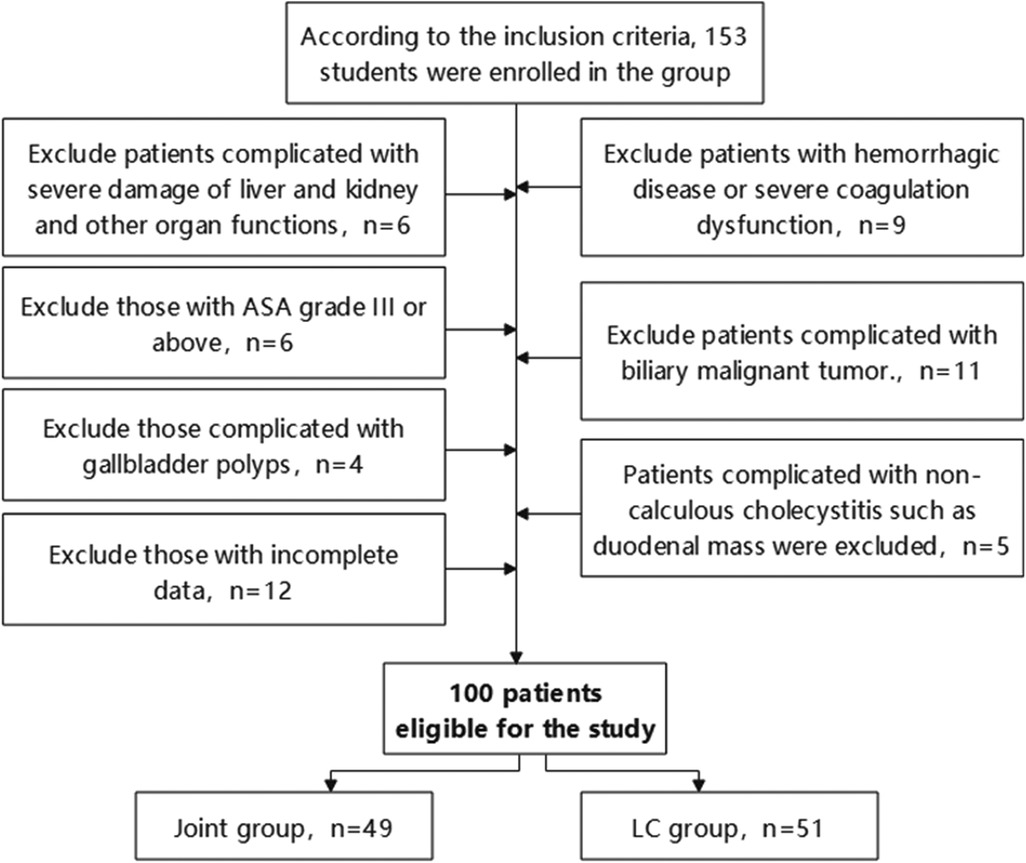
Initially, 153 patients met these criteria. Subsequently, a second screening based on the exclusion criteria was performed:
Exclusion criteria: (1) Severe dysfunction of liver, kidney, or other organs; (2) Concurrent hemorrhagic disorders or severe coagulation dysfunction; (3) ASA classification of III or higher; (4) Concurrent biliary malignancies; (5) Presence of gallbladder polyps; (6) Non-calculous cholecystitis with duodenal space-occupying mass or other complications; (7) Incomplete data.
After screening, 100 patients were included in the study (Figure 1). Based on surgical approach, they were categorized into two groups: the joint group (n = 49, LC combined with PTGBD) and the LC group (n = 51, LC alone).
Scheduling of combined procedures
Patients in the joint group (n = 49) first underwent PTGBD to control acute inflammatory response. Following PTGBD, patients entered an observation phase. Once clinical symptoms improved, inflammatory markers such as WBC and CRP declined, and ultrasound confirmed resolution of gallbladder wall edema, LC was performed. The average interval between PTGBD and LC was 7–14 days, determined based on the degree of inflammation resolution and results of preoperative evaluation. The primary criteria for proceeding with LC included: (1) Normalization of body temperature and either complete resolution or significant relief of abdominal pain; (2) Normalization or near-normalization of WBC counts; (3) A marked decrease in CRP levels compared with pre-PTGBD values; (4) Ultrasonographic evidence indicating substantial resolution of gallbladder wall edema. All LC procedures were performed by the same surgical team using standardized surgical techniques and protocols. Intraoperatively, the drain was removed concurrently with cholecystectomy in all patients. Adhesions between the gallbladder and the drain were carefully dissected first. The drain was then clamped, and the gallbladder was completely resected. No additional postoperative drain removal was required.
Data collection
Data were collected from the hospital information system and included: (1) Baseline characteristics: sex, age, BMI, LYM, RBC, PLT, blood pressure, surgical history, alcohol consumption, hypertension, diabetes, etc.; (2) Surgical parameters: surgery duration, intraoperative blood loss, total hospital stay and postoperative stay; (3) Laboratory indicators: preoperative and 3-day postoperative inflammatory markers (WBC count and CRP levels) and liver function indicators (AST and ALT levels); (4) Perioperative complications: bile leaks, incision infections, etc.; (5) Postoperative recovery indicators: bowel function recovery, time to ambulation, and time to resume oral intake; (6) Patient satisfaction: assessed using a self-developed scale with a maximum score of 100, with higher scores indicating greater satisfaction; (7) Economic indicators: surgical costs, total hospitalization expenses, and medication costs.
Outcome measures
Main outcome measures
The preoperative and postoperative inflammatory markers, liver function indicators, postoperative recovery, and perioperative complications were compared between the two patient groups. Logistic regression analysis was employed to identify independent risk factors influencing the occurrence of perioperative complications in patients with severe acute cholecystitis.
Secondary outcome measures
The basic surgical parameters, patient satisfaction, and economic indicators were compared between the two groups.
Application of results
In this study, a data separation approach was employed to minimize analytical bias. Data collection and data analysis were assigned to different personnel. Specifically, patient information was extracted from the electronic medical record system by designated data collectors and then anonymized using coded labels (e.g., Patient 1, Patient 2). The dataset was then reviewed by the project leader to ensure completeness before being transferred to independent data analysts for statistical analysis. Strict confidentiality measures were implemented throughout the process. All research team members received prior training on the appropriate use of clinical data to ensure compliance with relevant data protection and ethical standards.
Statistical methods
The data were processed with SPSS 21.0. The normality of continuous variables was assessed using the Shapiro–Wilk test. The results indicated that all continuous variables were normally distributed (P > 0.05); therefore, data were presented as mean ± standard deviation, and comparisons between groups were conducted using the independent samples t-test. In cases where data did not meet the assumption of normality, results were planned to be expressed as median (interquartile range, IQR), and comparisons between groups would be performed using the non-parametric Mann–Whitney U test. Categorical variables were expressed as percentages (%), and comparisons between groups were conducted using the chi-square (χ2) test or Fisher's exact test, as appropriate. Risk factor analysis was performed using logistic regression. To minimize the exclusion of potential risk factors, variables with P < 0.1 in the univariate analysis were included in the multivariable analysis. All graphs in this study were generated using GraphPad Prism 8.0. P < 0.05 was considered statistically significant.
Results
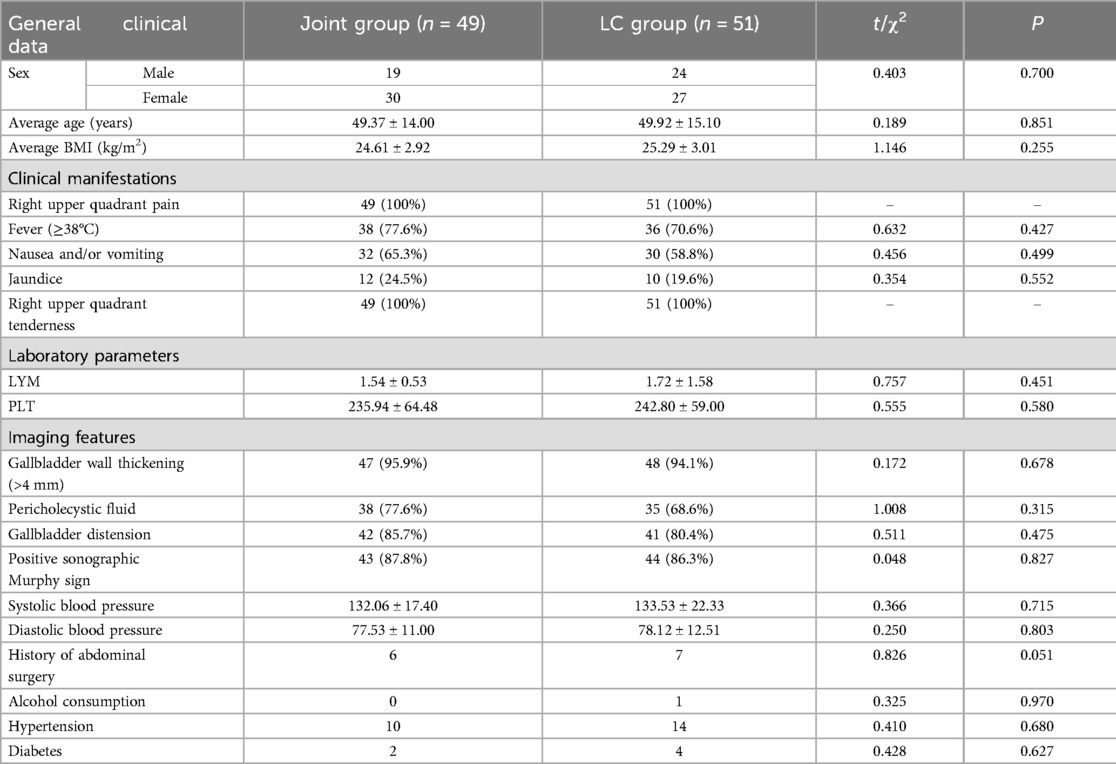
Comparison of baseline clinical data
Baseline characteristics, including sex, age, BMI, and medical history, showed no statistically significant differences between the two groups (P > 0.05). However, the proportion of patients in the joint group with a disease onset longer than 72 h was significantly higher than that in the LC group (P < 0.05, Table 1).
Comparison of surgical parameters
The joint group had a significantly shorter surgery duration and less intraoperative blood loss compared to the LC group (P < 0.05). No significant differences were observed in total hospital stay and postoperative stay (P > 0.05, Figure 2).
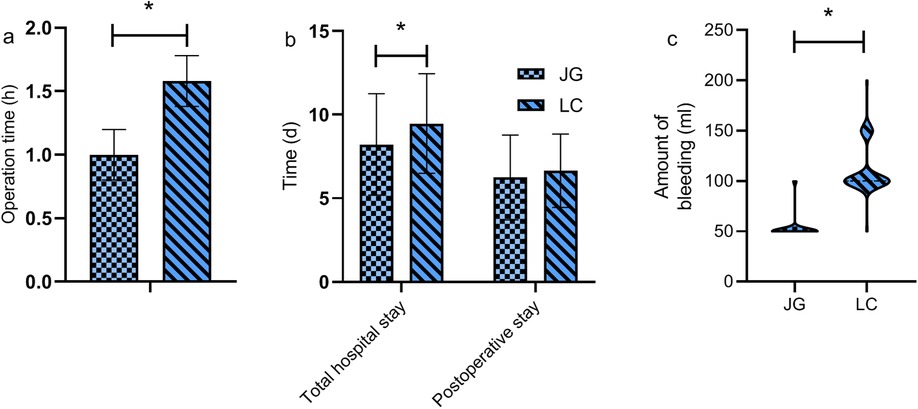
Figure 2. Comparison of surgical parameters between the two groups. The joint group demonstrated a significantly shorter surgery duration (a) and lower intraoperative blood loss (c) compared to the LC group (P < 0.05). However, there were no statistically significant differences between the groups in terms of total hospital stay and postoperative stay (P > 0.05) (b). Note: JG, joint group; LC, LC group. * indicates a statistically significant difference between groups.
Comparison of preoperative and 3-day postoperative inflammatory markers
No significant differences were observed in the levels of preoperative WBC and CRP between the two groups (P > 0.05). On postoperative day 3, both markers were significantly lower in the joint group (P < 0.05, Figure 3).
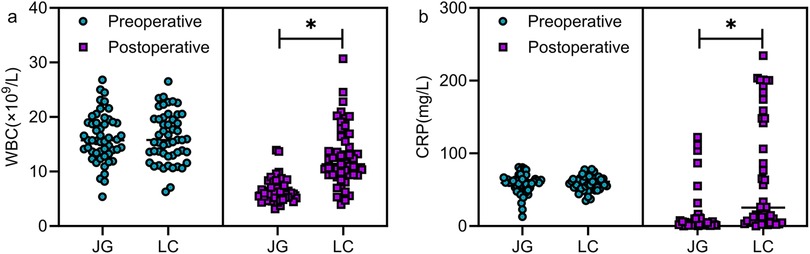
Figure 3. Comparison of preoperative and 3-day postoperative inflammatory markers between the two groups. Preoperative comparison revealed no statistically significant differences in the levels of WBC (a) and CRP (b) between the two groups (P > 0.05). On the third postoperative day, patients in the joint group exhibited lower levels of WBC and CRP than those in the LC group (P < 0.05). Note: JG, joint group; LC, LC group. * indicates a statistically significant difference between groups.
Comparison of preoperative and postoperative liver function indicators
No significant differences were found in AST and ALT levels between the two groups either preoperatively or on postoperative day 3 (P > 0.05, Figure 4).
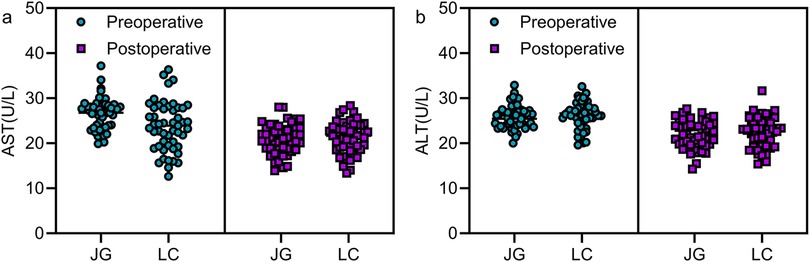
Figure 4. Comparison of preoperative and postoperative liver function indicators between the two groups. No statistically significant differences were observed in AST and ALT levels between the two groups preoperatively and 3 days postoperatively (P > 0.05). Note: JG, joint group; LC, LC group.
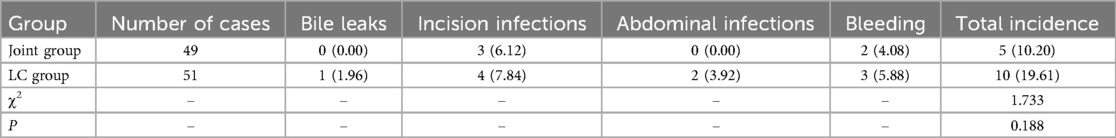
Comparison of postoperative complications
The joint group had slightly lower incidences of bile leaks, abdominal infections, incision infections, and bleeding compared to the LC group, but none reached statistical significance (P > 0.05, Table 2).
Comparison of postoperative recovery indicators
The joint group achieved earlier bowel function recovery, earlier ambulation, and shorter time to resume oral intake compared to the LC group, with all differences reaching statistical significance (P < 0.05, Figure 5).
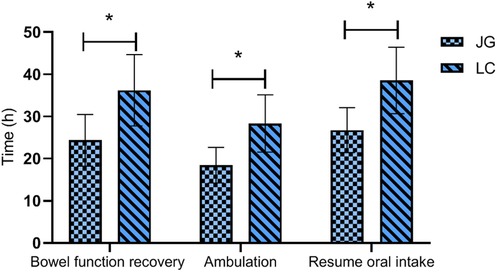
Figure 5. Comparison of postoperative recovery indicators between the two groups. The joint group showed faster recovery of bowel function, earlier ambulation, and shorter time to resume oral intake compared to the LC group postoperatively (P < 0.05). Note: JG, joint group; LC, LC group. * indicates a statistically significant difference between groups.
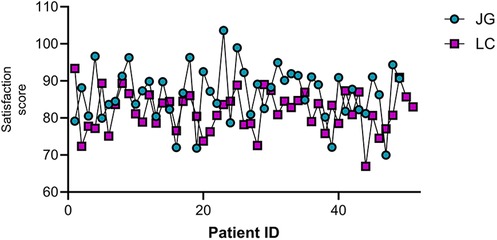
Comparison of patient satisfaction
The joint group reported significantly higher satisfaction scores (86.53 ± 7.20) compared to the LC group (81.84 ± 5.40) (P < 0.05, Figure 6).
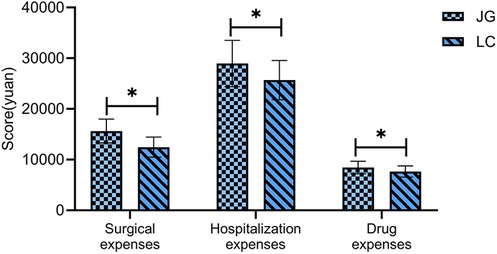
Comparison of economic indicators
The joint group incurred significantly higher surgical costs, total hospitalization costs, and medication costs than the LC group (P < 0.05, Figure 7).
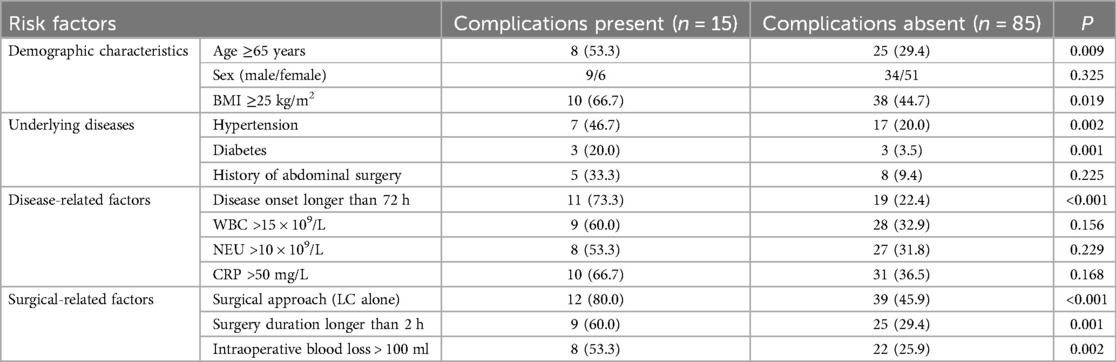
Univariate and multivariable analysis of risk factors for complications
The presence of postoperative complications was considered the dependent variable (complications present = 0, complications absent = 1), while the independent variables included demographic characteristics (such as sex, age, BMI), underlying diseases (hypertension, diabetes, history of abdominal surgery), disease-related factors (disease onset longer than 72 h, preoperative WBC levels), and surgical-related factors (surgical approach, surgery duration, intraoperative blood loss). Univariate analysis identified age ≥65 years, BMI ≥25 kg/m2, hypertension, diabetes, disease onset longer than 72 h, surgical approach (LC alone), surgery duration longer than 2 h, and intraoperative blood loss >100 ml as significant factors of risk factors for postoperative complications (P < 0.1, Table 3). Multivariable logistic regression identified disease onset longer than 72 h, surgical approach (LC alone), surgery duration longer than 2 h, intraoperative blood loss >100 ml, and age ≥65 years as independent risk factors for postoperative complications (P < 0.05, Table 4, Figure 8).
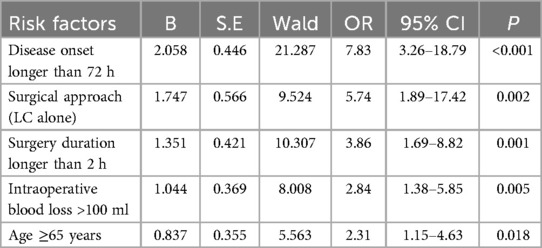
Table 4. Multivariable logistic regression analysis results of risk factors for postoperative complications.
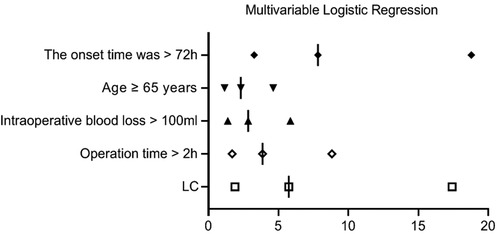
Figure 8. Multivariable logistic regression analysis results of risk factors for postoperative complications.
Discussion
This study compared the clinical outcomes of LC combined with PTGBD vs. LC alone in treating severe acute cholecystitis. The results showed that the joint group had clear advantages in surgical parameters, including shorter surgery duration and reduced intraoperative blood loss. Additionally, patients in the joint group experienced faster postoperative recovery, greater improvements in inflammatory markers, and higher satisfaction. However, the combined approach was associated with higher hospitalization costs. Several independent risk factors for postoperative complications were identified, including onset of symptoms longer than 72 h, LC alone, surgery duration longer than 2 h, intraoperative blood loss >100 ml, and age ≥65 years.
The observed reductions in surgery duration and blood loss in the joint group are consistent with previous studies. Nassar et al. (9) reviewed 14 studies involving 1,347 cases and found that PTGBD reduced complications, biliary tract injuries, and hospital stay. Similarly, Wang et al. (10) reviewed 12 studies involving 4,379 patients, reporting that the combined approach led to less bleeding, reduced tissue damage, and faster recovery. These findings may be attributable to preoperative drainage through PTGBD, which decreases gallbladder pressure, alleviates inflammation, and improves surgical exposure, ultimately simplifying the procedure (11, 12). Despite these benefits, patients in the joint group had a slightly longer total hospital stay, likely due to the interval required between PTGBD and LC. Subramani et al. (13) highlighted a similar observation in patients with obstructive jaundice, noting that surgery after inflammation resolution minimized complications. This underscores the importance of early anti-inflammatory treatment upon admission, which may explain the longer hospital stay in the joint group.
On postoperative day 3, WBC and CRP levels were significantly lower in the joint group, indicating better control of the inflammatory response. Kang et al. (14) observed similar benefits in high-risk patients treated with PTGD, with a complication rate of about 10.8%. Jin et al. (15) also reported that PTGD led to reduced inflammatory markers, faster recovery, and fewer complications in patients with severe cholecystitis. These improvements likely stem from reduced gallbladder inflammation before surgery, leading to less surgical trauma and a milder postoperative inflammatory response (16, 17). However, our study did not observe significant differences in liver function indicators, possibly due to differences in patient selection or timing of measurements. PTGBD may offer greater benefits for patients with impaired liver function, suggesting the need for individualized surgical planning.
Although the total incidence of complications was slightly lower in the joint group, the difference was not statistically significant. This contrasts with findings from Niiya et al. (18), who reported high technical success and low complication rates with PTGBD. While our study did not show a significant difference, there was a trend toward fewer severe complications, such as bile leaks and abdominal infections, in the joint group. This suggests a potential safety advantage of the combined approach (19), which warrants further validation through large-scale studies.
In terms of postoperative recovery, the joint group showed faster recovery time of bowel function, earlier time to ambulation, and shorter time to resume oral intake. These findings align with Ábrahám et al. (20), who advocated for a staged treatment strategy tailored to individual patient conditions. The reduced surgical trauma in the joint group likely contributed to their quicker recovery. Patient satisfaction scores were also higher in the joint group, probably due to the combination of reduced pain and faster improvement (21).
The study also analyzed the risk factors for postoperative complications. Key predictors included a disease onset longer than 72 h, LC alone, surgery duration longer than 2 h, intraoperative blood loss >100 ml, and age ≥65 years. Notably, a disease onset longer than 72 h emerged as the most significant risk factor (OR = 7.83), emphasizing the need for early intervention. Ie et al. (22) found that a longer interval between disease onset and surgical procedure was associated with higher intraoperative bleeding volume and incidence of postoperative complications (such as wound infection and liver abscess), concluding that prompt surgical intervention significantly improved patient outcomes. LC alone was the second most significant risk factor (OR = 5.74), supporting the value of PTGBD in patients with severe acute cholecystitis. Similar risk indicators were identified by Yamazaki et al. (23), who highlighted blood loss ≥45 ml (OS = 12.14), age ≥75 years, and an ASA-PS score ≥3 (OS = 9.85) as independent risk factors for major perioperative complications. Monitoring these risk factors can help clinicians refine perioperative strategies and develop individualized treatment plans (24).
In terms of economic evaluation, the combined surgical approach was associated with significantly higher surgical, hospitalization, and medication costs. While earlier studies (25) have suggested that the combined approach may reduce long-term healthcare costs by lowering complication and readmission rates, our study lacked long-term follow-up and economic analysis. As such, it is not possible to evaluate the long-term cost-effectiveness of the two surgical strategies. The current findings only demonstrate that the combined procedure is associated with higher direct medical costs during hospitalization. Whether it offers long-term economic benefits remains to be determined through well-designed, prospective studies with adequate follow-up. Clinical decision-making should take into account both the potential clinical benefits and the financial burden on patients, particularly those with limited economic resources.
In conclusion, compared with LC alone, LC combined with PTGBD is more effective in reducing surgical trauma in patients with severe acute cholecystitis, improving postoperative inflammatory markers, and accelerating recovery. However, this combined approach is associated with significantly higher direct medical costs during hospitalization. It is recommended to intensify perioperative intervention for patients of advanced age, those undergoing LC alone, and those with prolonged surgery duration and blood loss, to reduce the incidence of perioperative complications. The strength of this study lies in its systematic evaluation of combined surgery and identification of independent risk factors using multivariable analysis. However, this study has several limitations. First, as a single-center retrospective study, the sample size was relatively limited. Second, the follow-up period was short and restricted to the duration of hospitalization; important outcomes such as 30-day readmission rates and late postoperative complications were not assessed, which may limit the clinical generalizability of the findings. Third, some economic indicators, such as long-term healthcare costs, were not included in the analysis. Future studies should adopt a prospective design, increase the sample size, and extend the follow-up beyond 30 days to allow for a more comprehensive evaluation of the long-term outcomes of the two surgical strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study is a retrospective analysis approved by the ethics committee of Yongjia County Traditional Chinese Medicine Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LC: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft. MH: Data curation, Formal analysis, Validation, Writing – original draft. SH: Data curation, Formal analysis, Writing – review & editing. YS: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fu Y, Pang L, Dai W, Wu S, Kong J. Advances in the study of acute acalculous cholecystitis: a comprehensive review. Dig Dis. (2022) 40:468–78. doi: 10.1159/000520025
2. Kurihara H, Binda C, Cimino MM, Manta R, Manfredi G, Anderloni A. Acute cholecystitis: which flow-chart for the most appropriate management? Dig Liver Dis. (2023) 55:1169–77. doi: 10.1016/j.dld.2023.02.005
3. Kalata S, Thumma JR, Norton EC, Dimick JB, Sheetz KH. Comparative safety of robotic-assisted vs. laparoscopic cholecystectomy. JAMA Surg. (2023) 158:1303–10. doi: 10.1001/jamasurg.2023.4389
4. Jiang B, Ye S. Pharmacotherapeutic pain management in patients undergoing laparoscopic cholecystectomy: a review. Adv Clin Exp Med. (2022) 31:1275–88. doi: 10.17219/acem/151995
5. Guzmán-Calderón E, Chacaltana A, Díaz-Arocutipa C, Díaz R, Arcana R, Ramón Aparicio J. EUS-guided gallbladder drainage vs. percutaneous transhepatic drainage in patients with acute cholecystitis: a systematic review and meta-analysis. Rev Gastroenterol Peru. (2022) 42:163–70. doi: 10.47892/rgp.2022.423.1375
6. Nakamura Y, Kuwahara M, Ito K, Inagaki F, Mihara F, Takemura N, et al. Percutaneous transhepatic gallbladder intervention as a bridge to cholecystectomy: aspiration or drainage? World J Surg. (2023) 47:1721–8. doi: 10.1007/s00268-023-06987-6
7. Cirocchi R, Amato L, Ungania S, Buononato M, Tebala GD, Cirillo B, et al. Management of acute cholecystitis in high-risk patients: percutaneous gallbladder drainage as a definitive treatment vs. emergency cholecystectomy-systematic review and meta-analysis. J Clin Med. (2023) 12:4903. doi: 10.3390/jcm12154903
8. Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. (2018) 25:41–54. doi: 10.1002/jhbp.515
9. Nassar A, Elshahat I, Forsyth K, Shaikh S, Ghazanfar M. Outcome of early cholecystectomy compared to percutaneous drainage of gallbladder and delayed cholecystectomy for patients with acute cholecystitis: systematic review and meta-analysis. HPB (Oxford). (2022) 24:1622–33. doi: 10.1016/j.hpb.2022.04.010
10. Wang X, Niu X, Tao P, Zhang Y, Su H, Wang X. Comparison of the safety and effectiveness of different surgical timing for acute cholecystitis after percutaneous transhepatic gallbladder drainage: a systematic review and meta-analysis. Langenbecks Arch Surg. (2023) 408:125. doi: 10.1007/s00423-023-02861-0
11. Iwamoto T, Suda T, Inoue T, Nozaki Y, Mizumoto R, Arimoto Y, et al. Postoperative bleeding after percutaneous transhepatic gallbladder drainage and aspiration in patients receiving antithrombotic therapy. PLoS One. (2023) 18:e0288463. doi: 10.1371/journal.pone.0288463
12. Tanaka K, Takano Y, Kigawa G, Shiozawa T, Takahashi Y, Nagahama M. Percutaneous transhepatic gallbladder drainage versus endoscopic gallbladder stenting for managing acute cholecystitis until laparoscopic cholecystectomy. Asian J Endosc Surg. (2024) 17:e13253. doi: 10.1111/ases.13253
13. Subramani VN, Avudaiappan M, Yadav TD, Kumar H, Sharma V, Mandavdhare H, et al. Outcome following percutaneous transhepatic biliary drainage (PTBD) in carcinoma gallbladder: a prospective observational study. J Gastrointest Cancer. (2022) 53:543–8. doi: 10.1007/s12029-021-00655-5
14. Kang C, Zhang J, Hou S, Wang J, Li X, Li X, et al. The efficacy of percutaneous transhepatic gallbladder drainage combined with gallbladder-preserving cholecystolithotomy in high-risk patients with acute calculous cholecystitis. J Inflamm Res. (2022) 15:2901–10. doi: 10.2147/jir.S363610
15. Jin X, Jiang Y, Tang J. Ultrasound-guided percutaneous transhepatic gallbladder drainage improves the prognosis of patients with severe acute cholecystitis. Evid Based Complement Alternat Med. (2022) 2022:5045869. doi: 10.1155/2022/5045869
16. Han J, Xue D, Tuo H, Liang Z, Wang C, Peng Y. Percutaneous transhepatic gallbladder drainage combined with laparoscopic cholecystectomy versus emergency laparoscopic cholecystectomy for the treatment of moderate acute cholecystitis: a meta-analysis. J Laparoendosc Adv Surg Tech A. (2022) 32:733–9. doi: 10.1089/lap.2021.0579
17. Pan YL, Wu PS, Chen JH, Chen LY, Fang WL, Chau GY, et al. Early cholecystectomy following percutaneous transhepatic gallbladder drainage is effective for moderate to severe acute cholecystitis in the octogenarians. Arch Gerontol Geriatr. (2023) 106:104881. doi: 10.1016/j.archger.2022.104881
18. Niiya F, Tamai N, Yamawaki M, Noda J, Azami T, Takano Y, et al. Benefits of endoscopic gallbladder stenting following percutaneous transhepatic gallbladder drainage. World J Gastrointest Surg. (2024) 16:2902–9. doi: 10.4240/wjgs.v16.i9.2902
19. Katsura M, Matsushima K, Kato T, Bent CI, Kubota T, Kubota T, et al. Interval cholecystectomy following drainage procedures for acute cholecystitis: percutaneous transhepatic vs. endoscopic transpapillary gallbladder drainage. J Gastrointest Surg. (2024) 28:1912–4. doi: 10.1016/j.gassur.2024.08.002
20. Ábrahám S, Tóth I, Benkő R, Matuz M, Kovács G, Morvay Z, et al. Surgical outcome of percutaneous transhepatic gallbladder drainage in acute cholecystitis: ten years’ experience at a tertiary care centre. Surg Endosc. (2022) 36:2850–60. doi: 10.1007/s00464-021-08573-0
21. Matsumoto M, Abe K, Futagawa Y, Furukawa K, Haruki K, Onda S, et al. New scoring system for prediction of surgical difficulty during laparoscopic cholecystectomy after percutaneous transhepatic gallbladder drainage. Ann Gastroenterol Surg. (2022) 6:296–306. doi: 10.1002/ags3.12522
22. Ie M, Katsura M, Kanda Y, Kato T, Sunagawa K, Mototake H. Laparoscopic subtotal cholecystectomy after percutaneous transhepatic gallbladder drainage for grade II or III acute cholecystitis. BMC Surg. (2021) 21:386. doi: 10.1186/s12893-021-01387-w
23. Yamazaki S, Shimizu A, Kubota K, Notake T, Yoshizawa T, Masuo H, et al. Urgent versus elective laparoscopic cholecystectomy following percutaneous transhepatic gallbladder drainage for high-risk grade II acute cholecystitis. Asian J Surg. (2023) 46:431–7. doi: 10.1016/j.asjsur.2022.05.046
24. Han JW, Choi YH, Lee IS, Chun HJ, Choi HJ, Hong TH, et al. Early laparoscopic cholecystectomy following percutaneous transhepatic gallbladder drainage is feasible in low-risk patients with acute cholecystitis. J Hepatobiliary Pancreat Sci. (2021) 28:515–23. doi: 10.1002/jhbp.921
Keywords: severe acute cholecystitis, laparoscopic cholecystectomy, percutaneous transhepatic gallbladder drainage, clinical efficacy, prognosis
Citation: Chen L, Hu M, Huang S and Sun Y (2025) Clinical efficacy of laparoscopic cholecystectomy combined with percutaneous transhepatic gallbladder drainage in severe acute cholecystitis: an analysis of prognostic risk factors. Front. Surg. 12:1609327. doi: 10.3389/fsurg.2025.1609327
Received: 10 April 2025; Accepted: 12 June 2025;
Published: 26 June 2025.
Edited by:
Paolo Bruzzone, Sapienza University of Rome, ItalyReviewed by:
Attila Szijarto, Semmelweis University, HungaryMohamed Wael, Alexandria University, Egypt
Copyright: © 2025 Chen, Hu, Huang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Sun, MTM2MDA2Njc1MjhAMTYzLmNvbQ==
 Lexiang Chen
Lexiang Chen Yi Sun
Yi Sun