- Department of Neurosurgery, The Second Xiangya Hospital of Central South University, Changsha, Hunan, China
Post-Traumatic Epilepsy is a common and severe complication following brain trauma. However, there are few reports on intractable epilepsy caused by retained foreign bodies. This article reports a case of a patient with a metal foreign body retained in the cranium for 20 years due to trauma, resulting in drug-resistant epilepsy which type is focal to bilateral tonic-clonic seizure. Through preoperative imaging and neurophysiological techniques, the foreign body and epileptogenic zone were precisely located. Intraoperative cortical electroencephalography monitoring was used to accurately remove the foreign body and resect the epileptogenic focus, which pathological result indicates gliosis and calcification. The intractable epilepsy was effectively controlled, and the patient experienced no seizures postoperatively.
Introduction
Post-Traumatic Epilepsy (PTE) is a common and severe complication following traumatic brain injury, with its incidence varying depending on the type and severity of the trauma. However, there are few reports in the literature on intractable epilepsy caused by retained foreign bodies after cranial trauma (Table 1). We performed operation to remove residual intracranial metal foreign body that had remained in skull for 20 years, curing the patient's refractory epilepsy.
Case presentation
A 39-year-old male was stabbed in the head with a “chisel” near the left eye 20 years ago. Due to limited medical resources at that time, emergency surgery was performed to remove the exposed end of the foreign body, followed by debridement and suturing. Fortunately, the patient did not develop cerebral hemorrhage, infection, brain contusion, or eye injuries. After recovery, the patient returned to normal life. In 2015, the patient experienced sudden loss of consciousness and tonic-clonic seizures of all limbs without obvious triggers, with each episode lasting approximately 3–5 min, followed by gradual recovery of consciousness. Initially, seizures occurred 4–5 times per year. The hospital prescribed sustained-release sodium valproate tablets, but seizure control was poor. In the past 3 years, seizures became more frequent, occurring more than 5 times per month, often preceded by a “premonition”. Multiple hospital visits and treatments with sodium valproate, levetiracetam, and lacosamide failed to significantly reduce seizure frequency.
After admission, a head and skull base three-dimensional CT scan revealed a conical foreign body closely adhering to the lateral orbital wall, penetrating into the cranial cavity toward the temporal base (Figure 1). MRI could not be performed due to the suspected metallic foreign body. Preoperative EEG indicated that abnormal epileptiform discharges originated from the left temporal region, closely matching the site of foreign body penetration (Figure 2). According to the updated epilepsy classification published by the International League Against Epilepsy (ILAE) in 2025 (9), the patient's seizure type is focal to bilateral tonic-clonic seizure. Preoperative patient's MoCA (Beijing Version) and MMSE scores were 24 and 26, respectively. Surgery was scheduled, involving microscopic removal of the intracranial foreign body and epileptic focus resection under cortical EEG monitoring. Preoperative neurophysiological monitoring and intraoperative cortical EEG monitoring were prepared. A left pterional approach was used, and upon separating the temporal muscle to its origin, a black, elongated conical foreign body was observed adhering closely to the left lateral orbital wall, penetrating the zygomatic bone and temporal base bone into the cranial cavity (Figure 3). A craniotomy was performed around the foreign body using a milling cutter, preserving the surrounding bone. The bone around the foreign body was thinned with a drill and gradually removed with bone rongeurs, revealing the foreign body penetrating the dura mater, surrounded by proliferated soft tissue, some of which had hardened and calcified. After incising the dura, microscopic exploration showed the foreign body surrounded by cortical tissue. Adhesions between the cortex and foreign body, caused by gliosis, were carefully dissected. Abnormal proliferative blood vessels were coagulated, and adhesions were sharply released, allowing successful removal of the foreign body. Electrodes were placed at the temporal pole cortex, and cortical EEG showed abnormal epileptiform spike discharges at markers 1, 2, 3 and 4. Cortical mapping of the frontal lobe indicated scattered discharges in the middle and inferior frontal gyri. Under the microscope, based on the abnormal discharge area in the temporal pole and avoiding the language area, resection of the temporal pole cortex was performed, successfully removing both the retained intracranial foreign body and epileptogenic focus. The patient was instructed to continue to take levetiracetam 500 mg q12h regularly after operation. He had a follow-up MRI (Figure 4) and short-term (4-h) dynamic EEG at local hospital 4 months after operation, indicating abnormal sharp waves were not detected. The patient was followed up for over 4 months without any seizure recurrence and has returned to normal work.

Figure 1. (a) Axial CT scan showing a foreign body penetrating the lateral orbital wall into the cranial cavity; (b) enhanced coronal CT showing the penetrating foreign body located in the left temporal lobe, at a distance from the sylvian fissure vessels; (e,f) axial CT bone window images showing a conical, elongated foreign body penetrating the temporal bone into the cranial cavity, with a fracture at the left zygomatic bone penetration site; (c,d,g,h) three-dimensional reconstruction of the intracranial foreign body and skull, showing anterior and lateral relationships.
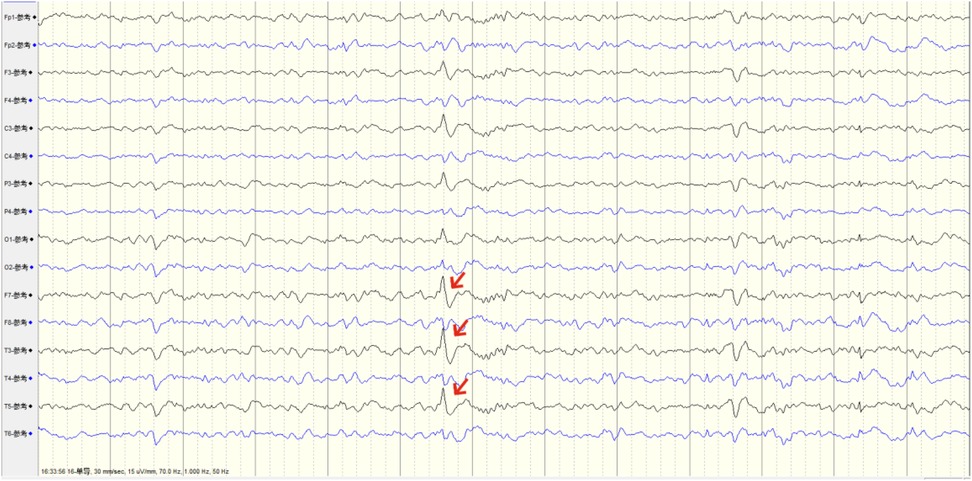
Figure 2. Preoperative EEG capturing abnormal origin discharges from the left temporal pole, consistent with the foreign body penetration site.

Figure 3. (a) After flipping the temporal muscle flap, a metal foreign body (blue arrow) was seen parallel to the lateral orbital wall, penetrating the temporal base bone into the cranial cavity; (b) foreign body penetrating the temporal pole, with cortical EEG monitoring electrodes placed on the temporal lobe; intraoperative EEG mapping identified clustered spikes at markers 2, 3, and 4; (c) after resection of the abnormal epileptogenic focus, repeat cortical EEG mapping around the margin showed disappearance of abnormal spikes; (d) resection of the temporal pole epileptogenic focus exposed the skull base penetration point of the foreign body (blue star).
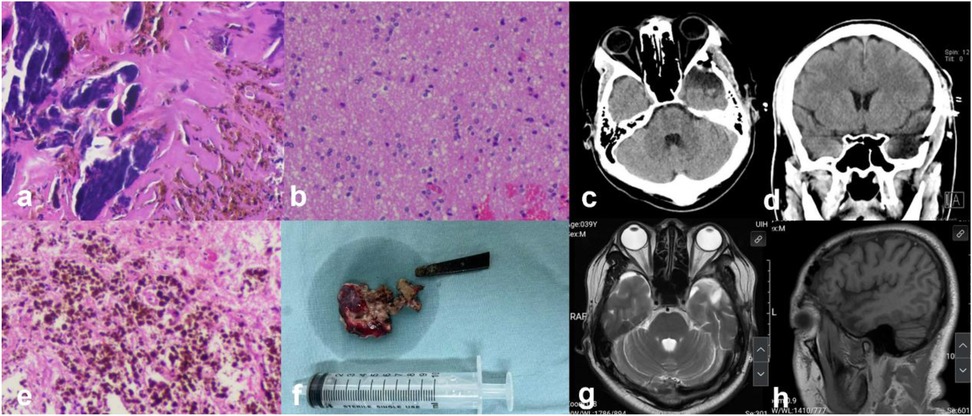
Figure 4. (a,b,e) Histopathology (HE*100) showing focal gliosis and calcification, with some areas containing hemosiderin deposits; (f) gross view of the removed intracranial metal foreign body and resected epileptogenic focus; (c,d) postoperative CT scans (axial and coronal) showing clear removal of the foreign body and postoperative changes after operation; (g,h) postoperative 4 month MR showing changes in the temporal pole.
Discussion
PTE varies in incidence from 5% to 30% to the complexity of trauma severity, type, and associated conditions (10). Studies have shown that up to 22% of severe traumatic brain injury patients exhibit subclinical epileptic abnormalities on EEG (11). Retained intracranial foreign bodies represent a special type of cranial trauma, which are relatively rare. Previous literature reported that the proportion of seizures in such cases increased (12).
Retained intracranial foreign bodies typically result from penetrating brain injuries or iatrogenic retention during craniotomy. They can lead to various neurological complications, including headaches, focal neurological deficits, intracranial infections and abscesses, or mass effects from inflammatory proliferation and scarring. In this case, the metal foreign body had remained in the cranium for over 20 years, with no significant epileptic symptoms initially. However, over the past decade, seizures became increasingly frequent, progressing focal to bilateral tonic-clonic seizure. Preoperative EEG confirmed that abnormal epileptiform discharges originated from the cortex surrounding the foreign body. After confirming the diagnosis and surgical indications, treatment with epileptic focus resection and foreign body removal under cortical EEG monitoring resulted in significant postoperative seizure control.
The mechanisms by which intracranial foreign bodies cause epilepsy are multifaceted, including mechanical stimulation, inflammatory responses, and scar formation (13). Mechanical stimulation may directly damage brain tissue, triggering abnormal neuronal discharges. Studies suggest that increased neuronal excitability after traumatic brain injury is a key factor in epilepsy development (14). In this patient, the long-term presence of a metal foreign body likely triggered local inflammatory responses and metal ion release, stimulating surrounding glial cell proliferation and focal calcification or fibrosis, forming granuloma-like proliferation. This local gliosis and granuloma formation disrupted normal neuronal network conduction, leading to an imbalance between local excitability and inhibition or the formation of abnormal discharge networks, resulting in drug-resistant epilepsy. Penetrating brain injuries from foreign bodies can also disrupt the blood-brain barrier locally, promoting the aggregation of inflammatory chemokines and further recruiting and migrating inflammatory cells (15). Previous studies have identified CXCL8 and CCL2 as key chemokines mediating neutrophil and monocyte migration to injury sites (16).
Preoperative EEG monitoring to localize epileptogenic foci typically involves standard EEG and invasive stereo-EEG (SEEG). EEG to identify the origin of epileptic discharges is closely related to surgical planning and postoperative seizure recurrence rates (17). Similar to this case, when preoperative EEG and imaging findings are consistent, resection of the lesion or removal of the foreign body can effectively control secondary epilepsy. Intraoperative ElectroCorticoGraphy (ECoG) involves real-time recording of cortical electrical activity during surgery to identify cortical epileptogenic foci and map functional areas such as language and motor regions to avoid damage. In complex, non-standard epilepsy surgeries, studies show that 77.5% of patients using ECoG were seizure-free one year postoperatively, compared to only 37.5% in those without ECoG, indicating that ECoG significantly improves surgical outcomes (18, 19). In this case, after exposing the left temporal lobe cortex, cortical EEG mapping around the temporal pole identified the primary abnormal discharge sites. Microsurgery was used to remove the penetrating foreign body, clear surrounding inflammatory lesions, and resect the abnormal discharge cortex while protecting the left temporal lobe language area. Post-resection cortical EEG mapping confirmed the disappearance of abnormal spikes. Previous reports suggest that confirming the absence of abnormal spike-and-wave signals at the surgical margin via cortical mapping can verify surgical success and predict long-term seizure freedom postoperatively (20). Early studies by the Cleveland Functional Neurosurgery Team on 47 patients with structural brain lesions and drug-resistant epilepsy demonstrated that precise resection of the epileptogenic focus is closely related to seizure control, and even partial, targeted resections can improve postoperative outcomes (21).
Conclusion
Retained intracranial foreign bodies can lead to secondary intractable epilepsy through complex pathophysiological mechanisms, involving mechanical stimulation, local inflammatory responses, and gliosis or granuloma formation, resulting in heterogeneous brain electrical networks, increased local discharge circuits, and drug-resistant seizures. When preoperative imaging and EEG diagnosis confirm anatomical consistency between the foreign body and the origin of abnormal electrical potentials, combined with intraoperative ECoG, safe removal of the foreign body and resection of the epileptogenic focus can effectively control seizures.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Second Xiangya Hospital Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZT: Writing – original draft, Funding acquisition. WM: Formal analysis, Writing – original draft, Data curation. JW: Investigation, Data curation, Writing – original draft. XL: Writing – original draft, Data curation. YC: Resources, Funding acquisition, Writing – review & editing, Supervision, Validation, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by: (1) Natural Science Foundation of Hunan Province of China (2023JJ40849); (2) Health Commission of Hunan province of China (B202304047281).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ullah Z, Ur Rehman M, Akbar A, Tasneem S, Jadoon SK. Unidentified intracranial foreign body in an epileptic child: infanticide or child abuse? Cureus. (2023) 15:e47167. doi: 10.7759/cureus.47167
2. Saeidiborojeni HR, Fakheri T, Iizadi B. Intracranial foreign body granuloma simulating brain tumor: a case report. J Res Med Sci. (2011) 16:358–60.22091258
3. Wang CB, Wang H, Zhao JS, Wu ZJ, Liu HD, Wang CJ, et al. Right-to-left displacement of an airgun lead bullet after transorbital entry into the skull complicated by posttraumatic epilepsy: a case report. J Korean Neurosurg Soc. (2023) 66:598–604. doi: 10.3340/jkns.2022.0234
4. Inci S, Karakoc E, Saygi S, Ozgen T. Unrecognized intracerebral glass particle mimicking cavernoma: case report. Neurosurgery. (2006) 58:E203; discussion E203. doi: 10.1227/01.NEU.0000192387.03428.E1
5. Brawanski N, Baumgarten P, Konczalla J, Seifert V, Senft C. Cerebral foreign body granuloma in brain triggering generalized seizures without obvious craniocerebral injury: a case report and review of the literature. Surg Neurol Int. (2016) 7:S775–8. doi: 10.4103/2152-7806.193924
6. Gopaul R, Xiao WS, Yan J, Wei DZ. Intracranial foreign body through the sagittal sinus: case report and review of literature. Chin Neurosurg J. (2016) 2:16. doi: 10.1186/s41016-016-0029-4
7. Chunhua Q, Qun W. A late-onset seizure due to a retained intracranial foreign body–pencil lead: a case report and review. J Craniofac Surg. (2014) 25:e109–10. doi: 10.1097/SCS.0000000000000439
8. Yürekli VA, Doğan M, Kutluhan S, Koyuncuoglu HR. Status epilepticus in a 52-year-old woman due to intracranial needle. Seizure. (2012) 21:652–4. doi: 10.1016/j.seizure.2012.05.006
9. Beniczky S, Trinka E, Wirrell E, Abdulla F, Al Baradie R, Alonso Vanegas M, et al. Updated classification of epileptic seizures: position paper of the international league against epilepsy. Epilepsia. (2025). doi: 10.1111/epi.18338
10. Pease M, Gupta K, MOSHÉ SL, Correa DJ, Galanopoulou AS, Okonkwo DO, et al. Insights into epileptogenesis from post-traumatic epilepsy. Nat Rev Neurol. (2024) 20:298–312. doi: 10.1038/s41582-024-00954-y
11. Kazis D, Chatzikonstantinou S, Ciobica A, Kamal FZ, Burlui V, Calin G, et al. Epidemiology, risk factors, and biomarkers of post-traumatic epilepsy: a comprehensive overview. Biomedicines. (2024) 12(2):410. doi: 10.3390/biomedicines12020410
12. Akhaddar A, Turgut AT, Turgut M. Foreign body granuloma after cranial surgery: a systematic review of reported cases. World Neurosurg. (2018) 120:457–75. doi: 10.1016/j.wneu.2018.09.143
13. Golub VM, Reddy DS. Post-traumatic epilepsy and comorbidities: advanced models, molecular mechanisms, biomarkers, and novel therapeutic interventions. Pharmacol Rev. (2022) 74:387–438. doi: 10.1124/pharmrev.121.000375
14. Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. (2018) 15:144. doi: 10.1186/s12974-018-1192-7
15. Webster KM, Sun M, Crack P, O'brien TJ, Shultz SR, Semple BD. Inflammation in epileptogenesis after traumatic brain injury. J Neuroinflammation. (2017) 14:10. doi: 10.1186/s12974-016-0786-1
16. Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice. J Cereb Blood Flow Metab. (2010) 30:769–82. doi: 10.1038/jcbfm.2009.262
17. Plummer C, Vogrin SJ, Woods WP, Murphy MA, Cook MJ, Liley DTJ. Interictal and ictal source localization for epilepsy surgery using high-density EEG with MEG: a prospective long-term study. Brain. (2019) 142:932–51. doi: 10.1093/brain/awz015
18. Hussain I, Kocharian G, Tosi U, Schwartz TH, Hoffman CE. Foundations of the diagnosis and surgical treatment of epilepsy. World Neurosurg. (2020) 139:750–61. doi: 10.1016/j.wneu.2020.03.033
19. King-Stephens D. The value of localizing subclinical seizures. Epilepsy Curr. (2020) 20:147–8. doi: 10.1177/1535759720917401
20. Choi HY, Koh EJ. Long-term outcome of surgical treatment of patients with intractable epilepsy associated with schizencephaly. Acta Neurochir (Wien). (2013) 155:1717–24. doi: 10.1007/s00701-013-1791-0
Keywords: epilepsy, retained intracranial foreign body, EEG, neurosurgery, post traumatic epilepsy
Citation: Tan Z, Wang M, Wan J, Li X and Cui Y (2025) Surgical cure of intractable epilepsy caused by retained intracranial foreign body under cortical electroencephalography monitoring: case report and literature review. Front. Surg. 12:1614564. doi: 10.3389/fsurg.2025.1614564
Received: 19 April 2025; Accepted: 26 May 2025;
Published: 6 June 2025.
Edited by:
Roberto Colasanti, Maurizio Bufalini Hospital, ItalyReviewed by:
Syed Sarmad Bukhari, Harvard University, United StatesCarlos Silvado, Federal University of Paraná, Brazil
Copyright: © 2025 Tan, Wang, Wan, Li and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Cui, Y3VpeWFuQGNzdS5lZHUuY24=
 Zhigang Tan
Zhigang Tan Ming Wang
Ming Wang Yan Cui
Yan Cui