- 1Pediatric Surgery Academy, Tor Vergata University of Rome, Rome, Lazio, Italy
- 2Interventional Radiology Unit, Bambino Gesù Children's Hospital- IRCCS, Rome, Lazio, Italy
Introduction: Infantile fibrosarcoma (IFS) represents the most common non-rhabdomyosarcoma soft tissue tumor, with 80% of diagnoses under the first year of life. In contrast with adult fibrosarcoma, IFS has lower risks of metastasis, better long-term survival rate, and higher chemosensitivity. Conservative surgery, in association with chemoradiotherapy in case of metastasis or recurrence, usually represents the gold standard treatment.
Case: We examined the case of a 2-month-old female patient affected by retroperitoneal congenital fibrosarcoma, which had caused high-flow heart failure (HFHF) due to its hypervascularization and multiple arteriovenous fistulas. Given the complexity of the case and its atypical vascularization, after multidisciplinary discussion, we decided to perform an endovascular approach rather than a surgical one, aiming to interrupt pathological flow to this abdominal mass. The procedure was well tolerated with fast improvement in both clinical and ultrasound markers of heart failure.
Conclusion: This is the first instance of arteriographic application for the management of HFHF caused by hypervascularized retroperitoneal IFS that we are aware of. In conclusion, we advise using this approach because of its safety and effectiveness, even though it necessitates a high level of experience.
Introduction
Infantile fibrosarcoma (IFS), a low-grade nonrhabdomyosarcoma soft tissue sarcoma (NRSTS), is the most prevalent soft tissue sarcoma in children under one year of age (1).
It can appear during the first five years of life or be present from birth, particularly in children younger than two (2).
This tumor is typically axial (20%) or limb-related (71%). Other localizations, such as the tongue and oral cavity, ovary, retroperitoneum, chest wall, heart, and bowel, are rarely reported (3).
IFS is distinguished from adult fibrosarcoma by having a higher long-term survival rate (90% at 5 years), a lower incidence of metastasis (<10%), and greater chemosensitivity (2, 4).
The clinical presentation is usually that of a bulging, rapidly growing mass on the extremities or trunk (5). This tumor tends to be locally invasive and metastasizes infrequently (6).
According to the literature, a spontaneous regression of IFS of the left forearm had been described (7).
Current treatment for IFS includes initial biopsy and chemotherapy, followed by conservative resection when the tumor shrinks (8, 9).
A multidisciplinary approach is necessary for approximately 48%–62% of primary tumors that cannot be removed, including local radiotherapy in certain circumstances and preoperative cytoreductive treatment (10).
Conservative resection with negative surgical margins is the key to avoid recurrence (6), although it can lead to significant long-term sequelae due to radical or mutilating surgery (11).
Neoadjuvant chemotherapy with alkylating agents such as vincristine, dactinomycin, and cyclophosphamide (VAC) is used to reduce the volume of the tumor before surgical resection.
It is advised that patients with macroscopic residual disease receive postoperative chemotherapy as their first line of treatment in order to reduce local recurrence (10).
To avoid their cytotoxic effects, new targeted therapies have been proposed considering the molecular biology of the tumor: in particular, the use of tropomyosin-related kinase inhibitors has been described as therapeutic options in neoadjuvant, adjuvant, or metastatic settings (12).
Here, we presented a fascinating case of a 2-month-old patient with congenital fibrosarcoma who came to our attention with cyanosis and respiratory distress as a result of an intralesional venous-arteriosus shunt that was hyperinflowing to the inferior vena cava (IVC).
Case
A 20-day-old female who was born at 38 weeks gestational age was diagnosed with an abdominal mass during pregnancy and brought to our institution.
Clinically, there was no sloping edema or respiratory distress; blood pressure and urine output were both normal. No congenital heart-related conditions were found on the echocardiogram.
Following a multidisciplinary consultation, she underwent thoraco-abdominal CT scan, abdominal ultrasound, and was tested for tumoral markers (Alpha-fetoprotein (AFP), Clinostatic Renin, Human Chorionic Gonadotropin (hCG), Chromogranin, Carcinoembryonic Antigen (CEA), Urinary Vanillylmandelic Acid (VMA) and Homovanillic Acid (HVA), Ferritin and Transferrin).
Abdominal ultrasound revealed a retroperitoneal expansive mass, encasing iliac, renal, and abdominal aortic vessels, with hypervascularization at Doppler-US.
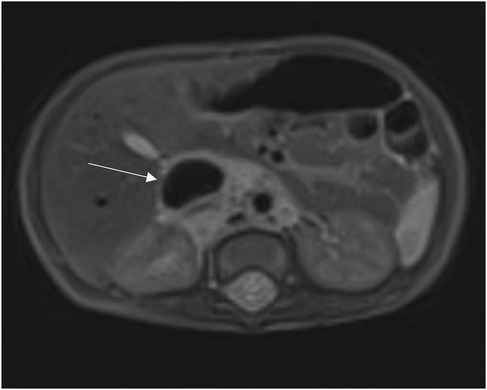
A CT scan confirmed the presence of this bulky mass, measured in 50 × 43 × 27 mm (a total volume of approximately 30.382 mm3), which caused displacement of the right kidney and compression of the inferior vena cava, consequently severely dilated.
A month later, an MRI of the abdomen revealed an enlarged mass (70 × 45 × 30 mm).
Since there were no neurological symptoms like sensory or motor deficits and there were no signs of spinal canal invasion at the CT scan, we did not perform an MRI of the cerebrospinal canal.
It is important to underline that CT scan was carried out before MRI because, using specific contrast agents, it would have provided a faster evaluation of the mass size, shape, and relationship with blood vessels; furthermore, it would have better identified calcifications or necrotic areas within the tumor, which are a common finding in IFS.
With the exception of an increase in TSH and normal FT3 and FT4 values that did not appear to be connected to the lesion, all tumoral markers were negative.
Given the close proximity to great abdominal vessels, a percutaneous bioptical approach was not feasible, necessitating a surgical biopsy of the lesion.
A congenital fibrosarcoma with PRKAR1B::BRAF fusion was found by histological analysis: PRKAR1B encodes a regular subunit of the cyclin AMP-dependent protein kinase A complex, implicated in neurodegenerative dementia but not well associated with cancer, while B-RAF is a well-known proto-oncogene (13).
This implied that Vemurafenib, a B-RAF inhibitor, might be required for targeted treatment.
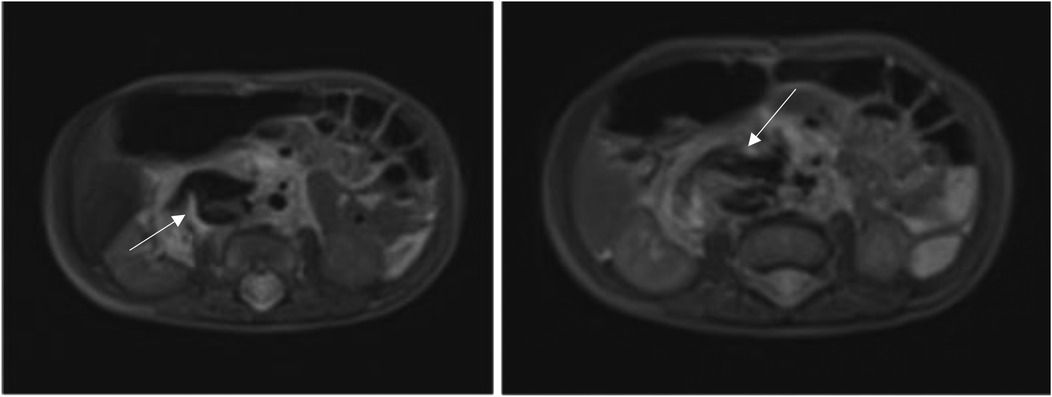
In order to better evaluate the vascularity and vessel proximity of the lesion, our oncologists suggested an abdominal MRI. An intralesional arteriovenous shunt was found in conjunction with a significant IVC dilatation brought on by hyperinflow (Figures 1, 2).
After a month, the patient was readmitted with cyanosis and severe respiratory distress.
Anticoagulant therapy was started after atrio-caval thrombosis diagnosed by Chest-CT.
When our cardiologist examined the patient, he discovered signs of systemic overflow-induced cardiomegaly and high-flow heart failure (Right Ventricle Pressure, RVP = 60 mmHg, 2/3 of Systemic Pressure, SP).
Diuretic and vasodilator therapy with furosemide and milrinone was started in consideration of clinical signs of heart failure and elevated NT-pro-BNP levels (up to 8.385 pg/ml).
Indicators of pulmonary hypertension were monitored.
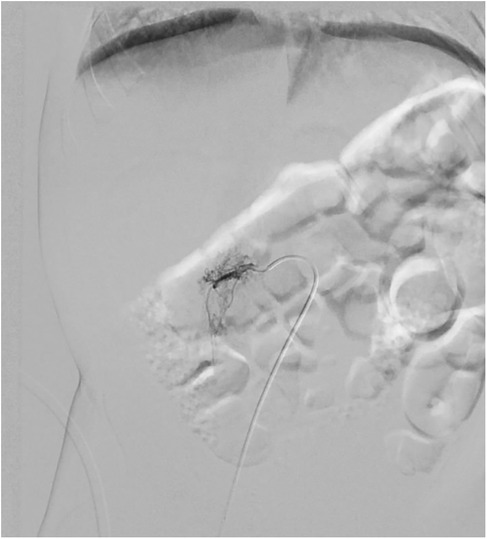
Given the high risk of heart failure persistence, we chose to use digital-subtracted angiography (DSA) to embolize vascular afferences to the lesion: via right femoral access, we performed an arteriography that demonstrated many arteriovenous fistulas inside the lesion with many lumbar and iliac vessels draining into IVC.
A single hypertrophic lumbar vessel was successfully selectively catheterized and embolized using 2 Concerto coils (2 mm × 4 cm and 2 mm × 6 cm, Figure 3).
Three days after the procedure, which was well tolerated, indirect signs of heart failure decreased (NT-pro-BNP dropped from 8.385 pg/ml to 2.068 pg/ml).
US and clinical settings improved: in particular, six days after the procedure, RVP was approximately 50 mmHg (down from 60 mmHg).
Additionally, about two weeks after embolization, she began off-label targeted immunotherapy with oral Vemurafenib at a dose of 10 mg/kg twice a day.
An echocardiogram performed about a month after embolization revealed good heart function and cardiocirculatory compensation (Ejection function of left ventricle 65%), with indirect signs of normal pressure gradient in the pulmonary valve (≤25 mmHg), which was secondary to the prior high right heart preload.
Furthermore, 6 months after embolization, a CT scan revealed that the retroperitoneal mass had significantly shrunk in comparison to previous radiological exams: it measured 30.2 × 14 × 36 mm (a total volume of approximately 7.965 mm3, about 26,2% of the original mass), and it was difficult to determine whether it was still surrounding retroperitoneal vessels (Figure 4).
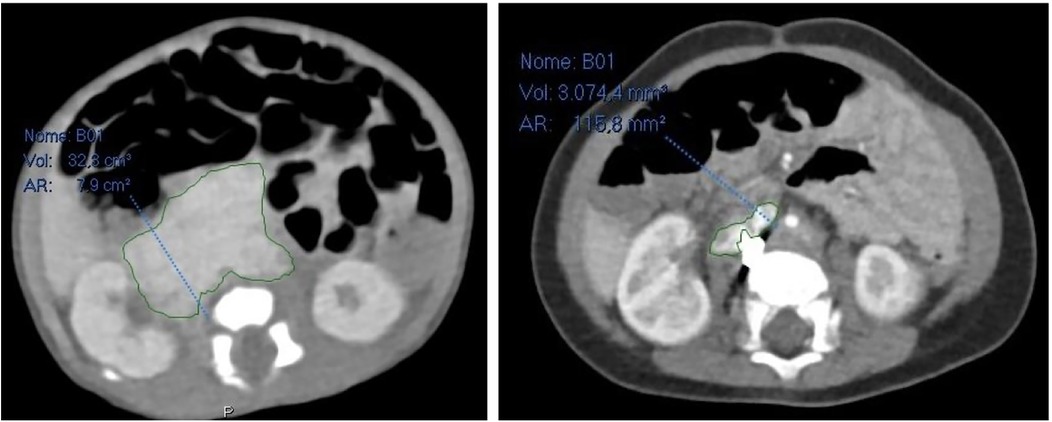
Figure 4. On the left abdominal CT scan at admission, on the right abdominal CT scan 6 months after embolization.
The oncological follow-up is still ongoing.
Discussion
We presented the case of a rare localization of congenital fibrosarcoma in the retroperitoneum.
This anatomical specific localization represents an overall challenge, both clinical and surgical, because chemotherapeutic drugs have difficulty reaching it and it could not be surgically approached due to its close proximity to the great abdominal vessels.
The EpSSG recommends conservative tumor resection for localized disease and vincristine-actinomycin (VA) chemotherapy as the first-line option for patients with unresectable disease (14).
Neoadjuvant chemotherapy has been recommended in some cases in order to minimize the need for mutilating resections (15, 16).
Recent years have seen the development of novel targeted therapies, particularly tropomyosin receptor kinase (TRK) inhibitors like Larotrectinib, which have been successfully used to stop or prevent tumor growth thanks to their ability to block the tyrosine kinase domain of TRK protein that is constitutively activated in IFS and other sarcomas with overall response rates above 90% (17–19).
70% of cases carry the ETV6-NTRK3 gene fusion as an oncogenic driver (20, 21).
Patients with TRK fusion sarcomas might proceed to surgery after treatment with this medication, thereby avoiding demolitive surgery in case of insufficient response to chemotherapy or in case of metastatic disease (14, 17).
However, the use of this medication was not feasible in this patient due to a PRKAR1B::BRAF fusion.
This kind of mutation is uncommon in IFS but had been described by Charo et al. in 2018 in a pregnant 32-year-old woman affected by a mid-jejunal gastrointestinal stromal tumor (GIST), which was resected, but, considering its teratogenic potential, a therapy with a B-RAF inhibitor like Imatinib or Vemurafenib was not recommended (13).
In this instance, however, given the patient's age, our oncologists determined there were no contraindications to using Vemurafenib off-label. Its safety and efficacy should be assessed during her oncological follow-up in the upcoming months.
The additional issues in this case were represented by the hypervascularization of this abdominal mass and, more importantly, by the arteriovenous fistulas between the arteries feeding the lesion and the IVC with subsequent right heart failure due to overflow.
IFS is usually fed by arteries of irregular caliber in a disordered branching pattern; the venous phase shows several tortuous and slightly enlarged veins, while the capillary phase is characterized by a dense but inhomogeneous tumor blush (22).
In order to treat this condition, in accordance with neonatal surgeons and oncologists, we decided to perform an abdominal arteriography in order to embolize the pathological hypertrophic vessels inside the fibrosarcoma, blocking its blood supply and causing its shrinkage before eventual surgery.
To the best of our knowledge, this is the first description of embolization of a retroperitoneal fibrosarcoma in an infant: in 2021 Filho et al. described a successful transarterial chemoembolization (TACE) in a 71-year-old patient with an inoperable retroperitoneal soft tissue sarcoma (23).
Significant tumor necrosis and symptomatic relief were the outcomes of this intervention, underscoring the potential use of interventional radiology as a bridging or palliative treatment in situations where surgery is not practical.
Embolization offers several advantages: firstly, it targets the tumor precisely, reducing damage to surrounding structures. Secondly, by cutting off the tumor's blood supply, debulking of the tumor are expected, making surgery easier.
Even if the endovascular approach is a relatively safe technique, it presents some limitations: it may not always be feasible and may require great technical expertise, so it should be managed by dedicated pediatric interventional radiologists.
Conclusion
Tumoral embolization is a promising approach for congenital fibrosarcoma; however, more research and cooperation between a multidisciplinary team of surgeons, interventional radiologists, and oncologists are required to determine its feasibility and medium- and long-term efficacy in pediatric age.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
MB: Writing – original draft, Writing – review & editing. GC: Conceptualization, Data curation, Methodology, Supervision, Writing – review & editing, Validation. GN: Conceptualization, Data curation, Methodology, Project administration, Supervision, Writing – review & editing, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This work was supported by the Italian Ministry of Health with “Current Research Funds”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kurkchubasche AG, Halvorson EG, Forman EN, Terek RM, Ferguson WS. The role of preoperative chemotherapy in the treatment of infantile fibrosarcoma. J Pediatr Surg. (2000) 35:880–3. doi: 10.1053/jpsu.2000.6871
2. Parida L, Parida L, Fernandez-Pineda I, Uffman JK, Davidoff AM, Krasin MJ, et al. Clinical management of infantile fibrosarcoma: a retrospective single-institution review. Pediatr Surg Int. (2013) 29:703–8. doi: 10.1007/s00383-013-3326-4
3. Scirè G, Mantovani A, Zampieri N, Guerriero VA, Segala D, Pecori S, et al. Transumbilical laparoscopic treatment of congenital infantile fibrosarcoma of the ileum. Pediatr Med Chir. (2014) 36:173–6. doi: 10.4081/pmc.2014.93
4. Grier HE, Perez-Atayde AR, Weinstein HJ. Chemotherapy for inoperable infantile fibrosarcoma. Cancer. (1985) 56:1507–10. doi: 10.1002/1097-0142(19851001)56:7%3C1507::AID-CNCR2820560705%3E3.0.CO;2-7
5. Sulkowski JP, Raval MV, Browne M. Margin status and multimodal therapy in infantile fibrosarcoma. Pediatr Surg Int. (2013) 29:771–6. doi: 10.1007/s00383-013-3318-4
6. Hicks J, Mierau G. The spectrum of pediatric fibroblastic and myofibroblastic tumors. Ultrastruct Pathol. (2004) 28:265–81. doi: 10.1080/019131290882105
7. Madden NP, Spicer RD, Allibone EB, Lewis IJ. Spontaneous regression of neonatal fibrosarcoma. Br J Cancer. (1992) 66:S72–5.PMID: 1503930 and PMCID: PMC2149666
8. Han Y, Lian K, Zhang D. Treatment of infantile fibrosarcoma: a tertiary care center experience. Front Pediatr. (2022) 10:1015185. doi: 10.3389/fped.2022.1015185
9. Cecchetto G, Carli M, Alaggio R, Dall'Igna P, Bisogno G, Scarzello G, et al. Fibrosarcoma in pediatric patients: results of the Italian cooperative group studies (1979–1995). J Surg Oncol. (2001) 78:225–31. doi: 10.1002/jso.1157
10. Sait SF, Danzer E, Ramirez D, LaQuaglia MP, Paul M. Spontaneous regression in a patient with infantile fibrosarcoma. J Pediatr Hematol Oncol. (2018) 40:e253–5. doi: 10.1097/MPH.0000000000001013
11. Akyüz C, Küpeli S, Varan A, Gedikoglu G, Yalçin B, Kutluk T, et al. Infantile fibrosarcoma: retrospective analysis of eleven patients. Tumori. (2011) 97:166–9. doi: 10.1177/030089161109700206
12. Yoshihara H, Yoshimoto Y, Hosoya Y, Hasegawa D, Kawano T, Sakoda A, et al. Infantile fibrosarcoma treated with postoperative vincristine and dactinomycin. Pediatr Int. (2017) 59:371–4. doi: 10.1111/ped.13229
13. Charo LM, Burgoyne AM, Fanta PT, Patel H, Chmielecki J, Sicklick JK, et al. A novel PRKAR1B-BRAF fusion in gastrointestinal stromal tumor guides adjuvant treatment decision-making during pregnancy. J Natl Compr Canc Netw. (2018) 16:238–42. doi: 10.6004/jnccn.2017.7039
14. Ferrari A, Brennan B, Casanova M, Corradini N, Berlanga P, Schoot RA, et al. Pediatric non-rhabdomyosarcoma soft tissue sarcomas: standard of care and treatment recommendations from the European paediatric soft tissue sarcoma study group (EpSSG). Cancer Manag Res. (2022) 14:2885–902. doi: 10.2147/CMAR.S368381
15. Sulkowski JP, Nicol K, Raval MV, Yeager N, Setty B, Groner JI, et al. Infantile fibrosarcoma of the intestine: a report of two cases and literature review. J Pediatr Surg Case Rep. (2014) 2:290–3. doi: 10.1016/j.epsc.2014.06.003
16. Corral Sánchez MD, Jiménez Carrascoso R, Rubio Aparicio P, Plaza López de Sabando D, Sastre Urgelles A, Pozo-Kreilinger JJ, et al. Therapeutic strategies and clinical evolution of patients with infantile fibrosarcoma: a unique paediatric case series. Clin Transl Oncol. (2023) 25:3307–11. doi: 10.1007/s12094-023-03175-9
17. DuBois SG, Laetsch TW, Federman N, Turpin BK, Albert CM, Nagasubramanian R, et al. The use of neoadjuvant larotrectinib in the management of children with locally advanced TRK fusion sarcomas. Cancer. (2018) 124:4241–7. doi: 10.1002/cncr.31701
18. Orbach D, Sparber-Sauer M, Laetsch TW, Minard-Colin V, Bielack SS, Casanova M, et al. Spotlight on the treatment of infantile fibrosarcoma in the era of neurotrophic tropomyosin receptor kinase inhibitors: international consensus and remaining controversies. Eur J Cancer. (2020) 137:183–92. doi: 10.1016/j.ejca.2020.06.028
19. Sahni S, Rastogi S, Yadav R, Barwad A. Limb salvage of an infant with infantile fibrosarcoma using TRK inhibitor larotrectinib. Ecancermedicalscience. (2023) 17:1575. doi: 10.3332/ecancer.2023.1575
20. Bielack SS, Cox MC, Nathrath M, Apel K, Blattmann C, Holl T, et al. Rapid, complete and sustained tumour response to the TRK inhibitor larotrectinib in an infant with recurrent, chemotherapy-refractory infantile fibrosarcoma carrying the characteristic ETV6-NTRK3 gene fusion. Ann Oncol. (2019) 30:viii31–5. doi: 10.1093/annonc/mdz382
21. Wang D, Zhang F, Feng W, Pan J, Yuan T. Larotrectinib treatment for infantile fibrosarcoma in newborns: a case report and literature review. Front Oncol. (2023) 13:1206833. doi: 10.3389/fonc.2023.1206833
22. Konez O, Burrows P, Mulliken J, Fishman S, Kozakewich H. Angiographic features of rapidly involuting congenital hemangioma (RICH). Pediatr Radiol. (2003) 33:15–9. doi: 10.1007/s00247-002-0726-3
Keywords: infantile fibrosarcoma (IFS), high-flow heart failure (HFHF), arteriography, embolization, case report
Citation: Bartoli ME, Cassanelli G and Natali GL (2025) The strategic use of embolization in treating infantile fibrosarcoma-related heart failure: a case report. Front. Surg. 12:1638718. doi: 10.3389/fsurg.2025.1638718
Received: 31 May 2025; Accepted: 16 October 2025;
Published: 30 October 2025.
Edited by:
Tomasz Szczepanski, Medical University of Silesia, PolandReviewed by:
Teresa Stachowicz-Stencel, University Medical Centre Gdansk, PolandMichał Dobrakowski, Medical University of Silesia in Katowice, Poland
Copyright: © 2025 Bartoli, Cassanelli and Natali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. E. Bartoli, YmFydG9saS5tYXJpYWVsaXNhYmV0dGFAZ21haWwuY29t
†ORCID:
M. E. Bartoli
orcid.org/0009-0004-8930-0452
G. Cassanelli
orcid.org/0000-0003-3162-6690
G. L. Natali
orcid.org/0000-0002-0758-0699
 M. E. Bartoli
M. E. Bartoli G. Cassanelli2,†
G. Cassanelli2,†