- 1Urology Department, People’s Hospital of Deyang City, Deyang, Sichuan, China
- 2Pathological Department, People’s Hospital of Deyang City, Deyang, Sichuan, China
- 3Urology Department, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Kidney cancer is the 14th most common cancer worldwide. On the basis of the histological characteristics of kidney cancers, most kidney cancers are renal cell carcinomas. Renal leiomyosarcoma (LMS) is extremely rare and malignant and accounts for less than 1% of all kidney cancer cases. Our study aims to gain deeper insight into the pathological characteristics and synthesized treatment of this uncommon type. Thus, we conducted a retrospective analysis of one patient who was diagnosed with renal leiomyosarcoma and underwent comprehensive treatment at the People’s hospital of Deyang City.
1 Introduction
According to data from the Department of United States Cancer Statistics, kidney cancer has already become the third most common carcinoma of urinary cancers. In 2021, nearly 70,000 new renal-derived cancer cases were reported around the USA (1). Histologically, approximately ninety percent of kidney tumors are renal cell carcinomas (RCCs). Other remaining subtypes, including adenocarcinoma and squamous cell carcinoma, account for less than ten percent of kidney cancers (2). Renal leiomyosarcoma (LMS), first reported in 1,967, is classified as a rare and aggressive mesenchymal tumor that accounts for nearly 0.1% of all renal malignancies and is associated with early invasion and metastasis (3, 4). The published literature reveals that LMS is likely to occur in females with a mean age of 50–60 years (5). Overall, LMS is always correlated with a poor prognosis because of its high tendency for local recurrence and hematogenous spread (6).
2 Patient and observation
2.1 Patient characteristics
A 57-year-old male presented to our hospital with slight pain in the right renal region for more than one year, without other accompanying symptoms such as hematuria, fever or radiating pain. The male did not seek medical attention immediately because the pain was not severe and could be relieved spontaneously after taking nonsteroidal anti-inflammatory drugs (NSAIDs). Approximately one month earlier, the pain worsened, and a palpable firm mass developed under the subhepatic region. The patient was eventually diagnosed with a “right renal tumor” and admitted to our urology department.
2.2 Clinical findings
A painless 10–15 cm mass was palpable in his right subcostal margin with a local projection of the skin upon clinical examination, accompanied by percussion pain in the right kidney region. Other abnormalities were not revealed.
2.3 Imaging examination
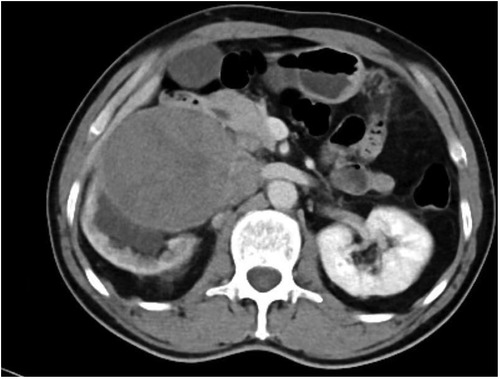
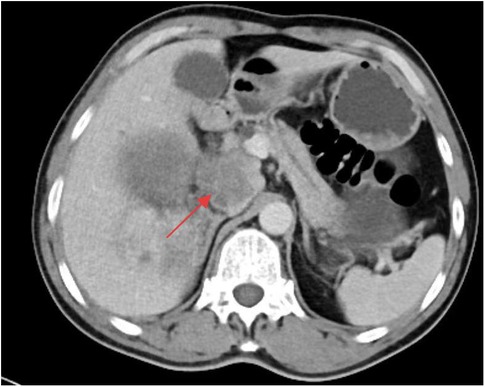
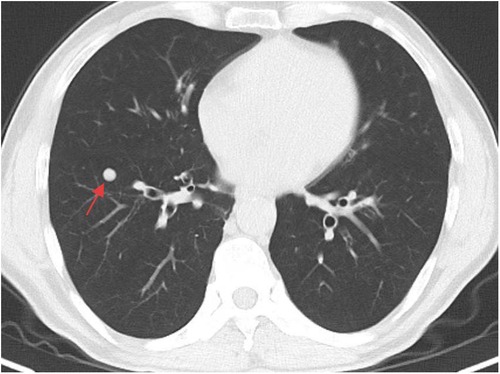
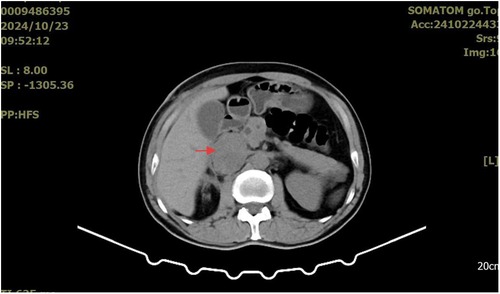
A whole-abdominal plain CT scan revealed a tumor with an abundant blood supply located in the region near the caudate lobe and right kidney. The enhanced scan demonstrated delayed enhancement (Figure 1). A tumor embolus was found in the inferior vena cava (Figure 2). In addition, perioperative renal dynamic imaging and glomerular filtration rate (GFR) measurements revealed decreased blood perfusion and glomerular filtration function in the right kidney. Moreover, a chest CT scan revealed scattered solid nodules in both lungs, which were suspected to be metastatic tumors (Figure 3).
2.4 Therapeutic intervention
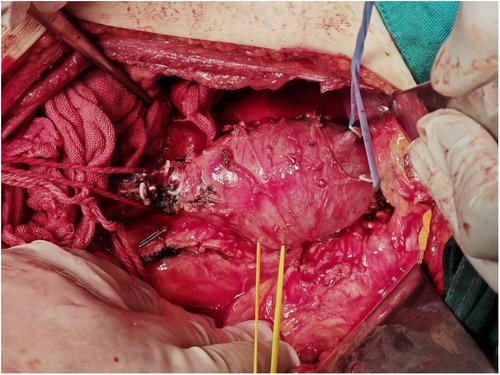
To reduce cancer-related complications, the patient underwent laparoscopic radical resection of the right renal cancer under general anesthesia. Laparoscopic radical nephrectomy was forced to be converted to open radical nephrectomy because of severe adhesions. In addition, the tumor embolus in the inferior vena cava was resected as much as possible (Figure 4). Local abdominal lymph nodes were also dissected. All the removed samples were routinely sent for pathological examination.
2.5 Follow-up
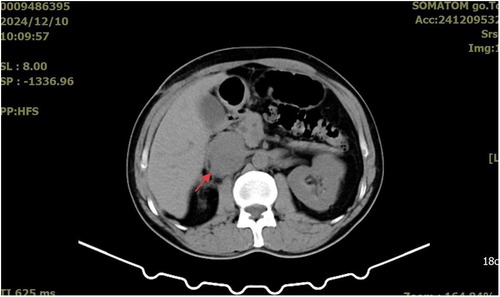
The patient returned to the oncology center for postoperative follow-up one month later. An abdominal computed tomography (CT) scan revealed a 3.5*3.0 cm low-density mass around and within the inferior vena cava (Figure 5). He received adjuvant chemotherapy every three weeks with ifosfamide (2,400 mg, days 1–4) and epirubicin (120 mg, day 1). Unfortunately, three months after the last abdominal CT scan, a larger mass (5.1*4.2 cm) was detected (Figure 6). In addition, no significant changes were found in the metastatic lesions in the lungs of the patient.
2.6 Patient's perspective
During the diagnostic process, the patient was informed of all diagnostic possibilities and prognoses depending on the ancillary test results. Moreover, all possible situations of surgical and chemical intervention were also reported before our treatment. To date, all the doubts of this patient were resolved during his follow-up period.
2.7 Pathological results
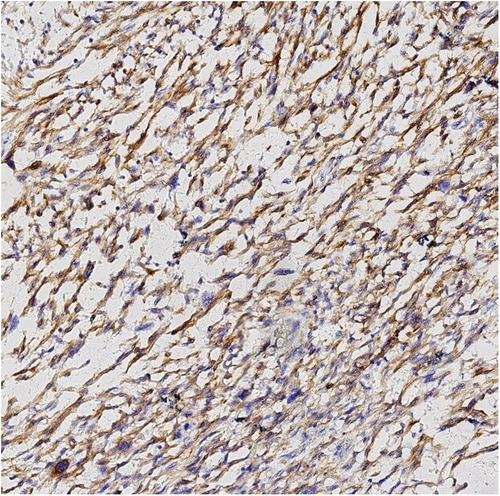
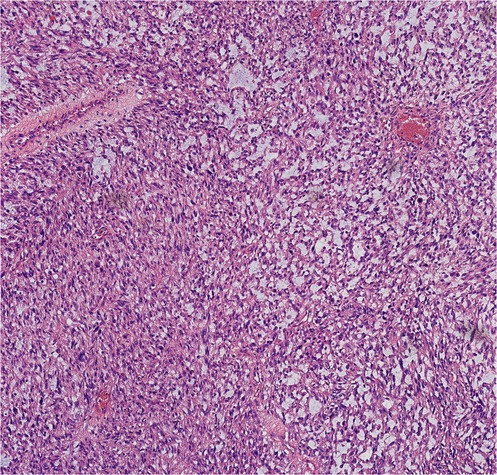
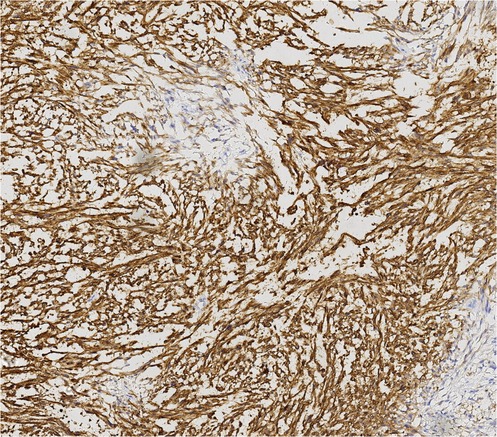
Finally, pathological results confirmed that the tumor was renal LMS. According to the pathological result of Hematoxylin and Eosin (HE) stainning, renal LMS performed a significant cellular pleomorphism with foci of tumor necrosis. In addition, most of the composition in the field of view consists of spindle cells, combined with smooth muscle bundles. Immunohistochemical staining revealed varying degrees of positivity for several markers, including Desmin, EMA, Caldemson, SMA, SMARCA4/Brgl and CA IX. Other non-muscle origin markers like HMB-45 and S-100 were not observed (Figures 7–9).
3 Discussion
LMS are malignant neoplasms that arise from the mesenchyme in the smooth muscle of various organs and account for approximately 10%–20% of all types of soft tissue sarcomas (STSs) (7). Renal LMS is likely to arise from intrarenal blood vessels and the renal pelvis (5). According to etiologic research, few predisposing factors for renal LMS have been recognized to date (8). Moreover, compared with other commonly diagnosed solid tumors, almost all categories of LMS exhibit a lower sensitivity, with a less than 30% overall rate of medical therapeutic reactions (9).
Currently, among all kinds of screening tools, magnetic resonance imagingmagnetic (MRI) may have the greatest potential in diagnosing LMS. According to the published literature, low apparent diffusion coefficient (ADC) values, irregular margins and intermediate/hyperintensity in T2-weighted images may be common characteristics of LMS (10). However, only histopathological examination could be the gold standard for diagnosing renal LMS. The characteristics of renal LMS are consistent with those of other locations, which exhibit cellular pleomorphism accompanied by foci of tumor necrosis. The typical microscopic features are characterized by an interlacing pattern of spindle cells and smooth muscle cells. In addition, immunohistochemistry results always reveal positive markers of muscle origin, which is consistent with the findings in our case (5).
At present, the most useful treatment for early renal LMS is surgical intervention with negative margins. Owing to the high local recurrence rate of LMS, radical nephrectomy should first be considered rather than simple partial nephrectomy unless the renal LMS has been identified as low grade through pathological examination (11). For primary renal LMS with a large tumor size, local invasive metastasis, or medium- or high-pathologic grade, radical or extended radical nephrectomy combined with perioperative and postoperative neoadjuvant treatments (including radiotherapy and chemotherapy) can be selected, which needs coordination by the surgeon and the radiation oncologist. Published studies have shown that R0 or R1 resection after neoadjuvant radiotherapy in patients diagnosed with intermediate/high-grade retroperitoneal soft tissue sarcoma (RSTS) can lead to favorable 5-year survival (12). Other studies revealed that for retroperitoneal/intra-abdominal STS where local recurrence can cause undue morbidity, radiotherapy (RT), including intensity-modulated RT and protons, might improve the comprehensive therapeutic effect. However, according to data from the STRASS trial (one of the randomized studies that evaluated neoadjuvant radiotherapy for retroperitoneal STS), whether neoadjuvant radiotherapy could become a standard application for STS treatment is still controversial (13, 14). However, although an updated meta-analysis involving 1,953 participants revealed that adjuvant chemotherapy has advantages in decreasing the overall recurrence rate of overall resectable localized STS (15), available comprehensive evaluations of neoadjuvant therapy for simple resectable renal LMS are still lacking. Neoadjuvant chemotherapy with doxorubicin and dacarbazine may have a positive effect on retroperitoneal LMS according to an unfinished prospective randomized controlled trial, but the final analysis of prognostic outcomes is still ongoing (16). In addition, chemotherapy with single agents or anthracycline-based combination regimens has been widely used among patients with unresectable or metastatic STS (17). For various histologic subtypes of unresectable or metastatic STS, gemcitabine in combination with docetaxel, vinorelbine, or dacarbazine has been confirmed to be effective. In addition, a new DNA-binding agent, trabectedin, has been shown to lead to greater progression-free survival than dacarbazine in the treatment of patients with LMS (18, 19). Moreover, according to the literature, targeted therapy could also delay the progression of LMS. A multitarget tyrosine kinase inhibitor, pazopanib, has already been demonstrated to be effective in prolonging the median progression-free survival (PFS) of patients with multiple advanced STS subtypes (20–22). However, the overall prognosis of advanced STS patients is still poor (17). Owing to the limited sample size, the comprehensive efficacy of renal LMS treatment still needs long-term research.
The progression and prognosis of renal LMS have been demonstrated to be associated with genetic factors. Studies have reported that the expression of the p16, p53, MED12, PRUNE2 and c-Myc tumor suppressor proteins might hold potential value in the prognostic assessment of renal LMS (23–25). In addition, a recent study revealed that TRPV4 may significantly promote the progression of leiomyosarcoma. TRPV4 is a nonselective cation channel that allows the passage of Ca2+. It contributes to the progression of LMS by directly stimulating the FAK pathway and indirectly activating the FAK/PI3K/AKT/GSK3β signaling pathway by promoting ECM1 expression and secretion, which may provide new ideas and targets for the clinical treatment of renal LMS in the future (26).
Currently, the diagnosis of various types of renal tumors primarily relies on imaging evaluations, such as contrast-enhanced CT and renal MRI. Totally, it is difficult for surgeons to distinguish between RCCs and renal LMS based solely on imaging examinations. Possible features that differentiate renal LMS from RCCs on CT scans are that LMS may expand to large sizes without lymphadenopathy, and tumor calcification rarely occurs (27). Moreover, the typical contrast-enhanced CT feature of RCCs demonstrates a “fast-in and fast-outfast-in and fast-out” pattern, which performs a marked enhancement in the arterial phase and a rapid washout in the delayed phase. By contrast, due to the less tumor vasculature, LMS may demonstrates a different venous phase with gradual enhancement of contrast agent (28, 29). Unfortunately, specific imaging characteristics has not been observed in renal LMS due to its rarity (6). Thus, image-guided percutaneous renal mass biopsy is still a significant and safe perioperative tool for renal LMS diagnosing (30). In addition, intraoperative frozen section examination may also guide the selection of surgical approaches.
4 Conclusion
Renal LMS is extremely rare and lethal. To date, the most effective treatment remains radical surgical intervention. Even if LMS has metastasized locally or to distant sites, although radiotherapy, chemotherapy or targeted therapies may delay its progression, the overall prognosis remains unfavorable. Thus, further research on renal LMS is warranted in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PW: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. JL: Writing – review & editing. QL: Resources, Writing – review & editing. DL: Resources, Supervision, Writing – review & editing. LL: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank all the authors of the included articles and all the editors who reviewed this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
LMS, leiomyosarcoma; RCCs, renal cell carcinomas; NSAIDs, nonsteroidal anti-inflammatory drugs; GFR, glomerular filtration rate; CT, computed tomography; STSs, soft tissue sarcomas; RSTS, retroperitoneal soft tissue sarcoma; ADC, apparent diffusion coefficient; HE, Hematoxylin and Eosin; MRI, magnetic resonance imagingmagnetic; PFS, progression-free survival; RT, radiotherapy.
References
1. U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute (2024). Available online at: https://www.cdc.gov/cancer/dataviz (Accessed January 06, 2025).
2. Bukavina L, Bensalah K, Bray F, Carlo M, Challacombe B, Karam JA, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol. (2022) 82(5):529–42. doi: 10.1016/j.eururo.2022.08.019
3. Lopez Varela EA, Pereira Garro C. Leiomyosarcoma of the renal vein. Int Surg. (1967) 47:340–3.6033910
4. Dhawan S, Chopra P, Dhawan S. Primary renal leiomyosarcoma: a diagnostic challenge. Urol Ann. (2012) 4(1):48–50. doi: 10.4103/0974-7796.91623
5. Miller JS, Zhou M, Brimo F, Guo CC, Epstein JI. Primary leiomyosarcoma of the kidney: a clinicopathologic study of 27 cases. Am J Surg Pathol. (2010) 34(2):238–42. doi: 10.1097/PAS.0b013e3181cad8c9
6. Zafar R, Manthri S, Shurbaji MS. Renal leiomyosarcoma. In: StatPearls. StatPearls Publishing (2023). p. 1–4.
7. George S, Serrano C, Hensley ML, Ray-Coquard I. Soft tissue and uterine leiomyosarcoma. J Clin Oncol. (2018) 36(2):144–50. doi: 10.1200/JCO.2017.75.9845
8. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. (2013) 27(5):957–74. doi: 10.1016/j.hoc.2013.07.002
9. Roberts ME, Aynardi JT, Chu CS. Uterine leiomyosarcoma: a review of the literature and update on management options. Gynecol Oncol. (2018) 151(3):562–72. doi: 10.1016/j.ygyno.2018.09.010
10. Tong A, Kang SK, Huang C, Huang K, Slevin A, Hindman N. MRI Screening for uterine leiomyosarcoma. J Magn Reson Imaging. (2019) 49:e282–294. doi: 10.1002/jmri.26630
11. Demir A, Yazici CM, Eren F, Türkeri L. Case report: good prognosis in leiomyosarcoma of the kidney. Int Urol Nephrol. (2007) 39(1):7–10. doi: 10.1007/s11255-005-4025-4
12. Pawlik TM, Pisters PWT, Mikula L, Feig BW, Hunt KK, Cormier JN, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. (2006) 13:508–17. doi: 10.1245/ASO.2006.05.035
13. Albertsmeier M, Rauch A, Roeder F, Hasenhütl S, Pratschke S, Kirschneck M, et al. External beam radiation therapy for resectable soft tissue sarcoma: a systematic review and meta-analysis. Ann Surg Oncol. (2018) 25:754–67. doi: 10.1245/s10434-017-6081-2
14. Bonvalot S, Gronchi A, Le Péchoux C, Swallow CJ, Strauss D, Meeus P, et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: sTRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. (2020) 21:1366–77. doi: 10.1016/S1470-2045(20)30446-0
15. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. (2008) 113(3):573–81. doi: 10.1002/cncr.23592
16. US National Library of Medicine. ClinicalTrials.gov. Available online at: https://www.clinicaltrials.gov/ (Accessed April 13, 2022). identifier: NCT04031677.
17. von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, et al. Soft tissue sarcoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20(7):815–33. doi: 10.6004/jnccn.2022.0035
18. Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. (2016) 34:786–93. doi: 10.1200/JCO.2015.62.4734
19. Le Cesne A, Blay J-Y, Cupissol D, Italiano A. Results of a prospective randomized phase III T-SAR trial comparing trabectedin vs best supportive care (BSC) in patients with pretreated advanced soft tissue sarcoma (ASTS) [abstract]. Presented at the 2016 ESMO Congress; October 7–11. (2016). Copenhagen, Denmark.
20. Kollár A, Jones RL, Stacchiotti S, Gelderblom H, Guida M, Grignani G, et al. Pazopanib in advanced vascular sarcomas: an EORTC soft tissue and bone sarcoma group (STBSG) retrospective analysis. Acta Oncol. (2017) 56:88–92. doi: 10.1080/0284186X.2016.1234068
21. Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schöffski P, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol. (2009) 27:3126–32. doi: 10.1200/JCO.2008.21.3223
22. Toulmonde M, Pulido M, Ray-Coquard I, Andre T, Isambert N, Chevreau C, et al. Pazopanib or methotrexate-vinblastine combination chemotherapy in adult patients with progressive desmoid tumours (DESMOPAZ): a non-comparative, randomised, open-label, multicentre, phase 2 study. Lancet Oncol. (2019) 20:1263–72. doi: 10.1016/S1470-2045(19)30276-1
23. Iida T, Maeda T, Amari Y, Yurugi T, Tsukamoto Y, Nakajima F. Primary hepatic leiomyosarcoma in a patient with autosomal dominant polycystic kidney disease. CEN Case Rep. (2017) 6:74–8. doi: 10.1007/s13730-017-0247-4
24. Silveira SM, Villacis RAR, Marchi FA, Barros Filho M, Drigo SA, Neto CS, et al. Genomic signatures predict poor outcome in undifferentiated pleomorphic sarcomas and leiomyosarcomas. PLoS One. (2013) 8(6):e67643. doi: 10.1371/journal.pone.0067643
25. Zhao L-R, Tian W, Wang G-W, Chen K-X, Yang J-L. The prognostic role of PRUNE2 in leiomyosarcoma. Chin J Cancer. (2013) 32(12):648–52. doi: 10.5732/cjc.013.10069
26. Zhou Q, You Y, Zhao Y, Xiao S, Song Z, Huang C, et al. TRPV4 Drives the progression of leiomyosarcoma by promoting ECM1 generation and co-activating the FAK/PI3K/AKT/GSK3β pathway. Cell Oncol. (2024) 48(2):455–70. doi: 10.1007/s13402-024-01008-7
27. Ozturk H. High-grade primary renal leiomyosarcoma. Int Braz J Urol. (2015) 41(2):304–11. doi: 10.1590/S1677-5538.IBJU.2015.02.17
28. Karaosmanoğlu AD, Onur MR, Shirkhoda A, Ozmen M, Hahn PF. Unusual malignant solid neoplasms of the kidney: cross-sectional imaging findings. Korean J Radiol. (2015) 16(4):853–9. doi: 10.3348/kjr.2015.16.4.853
29. Wang MX, Menias CO, Elsherif SB, Segaran N, Ganeshan D. Current update on IVC leiomyosarcoma. Abdom Radiol. (2021) 46(11):5284–96. doi: 10.1007/s00261-021-03256-9
Keywords: renal leiomyosarcoma, case report, inferior vena cava tumor thrombus, review, comprehensive treatment
Citation: Wang P, Li J, Liu Q, Lv D and Liu L (2025) Incidentally detected renal leiomyosarcoma with inferior vena cava tumor thrombus: a case report with review of the literature. Front. Surg. 12:1640444. doi: 10.3389/fsurg.2025.1640444
Received: 3 June 2025; Accepted: 6 August 2025;
Published: 21 August 2025.
Edited by:
Mottaran Angelo, University of Bologna, ItalyReviewed by:
Zhuang Aobo, Xiamen University, ChinaÖzge Ertener, izmir University of Economics, Türkiye
Copyright: © 2025 Wang, Li, Liu, Lv and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Lv, bHYtMDkxOUAxNjMuY29t; Liangren Liu, bGl1bGlhbmdyZW5Ac2N1LmVkdS5jbg==
 Puze Wang
Puze Wang Jinze Li
Jinze Li Qian Liu2
Qian Liu2 Liangren Liu
Liangren Liu