- 1The First School of Clinical Medicine, Lanzhou University, Lanzhou, China
- 2School of Philosophy and Sociology, Lanzhou University, Lanzhou, China
- 3The Second School of Clinical Medicine, Lanzhou University, Lanzhou, China
- 4School of Mathematics and Computer Science, Shantou University, Shantou, China
- 5Faculty of Engineering, The Chinese University of Hong Kong, Hong Kong SAR, China
- 6Department of Anesthesia, First Hospital of Lanzhou University, Lanzhou, China
- 7Department of Trauma Surgery, University Hospital Regensburg, Regensburg, Germany
Background: Abdominal tumors, including those in the stomach, colon, pancreas, and gallbladder, significantly impact global morbidity and mortality. Surgical resection is the primary treatment, but postoperative outcomes and long-term survival are often affected by factors such as preoperative nutritional status. Malnutrition is common in these patients, making its management crucial for improving outcomes. This systematic review and meta-analysis aim to consolidate evidence on the role of preoperative nutritional status in postoperative survival for patients undergoing abdominal tumor surgery, offering insight into its prognostic value.
Methods: A systematic literature search was conducted using electronic databases to report the impact of the preoperative nutritional status on OS (overall survival) of patients with abdominal tumor surgery as of January 1st, 2025. The hazard ratio (HR) with a 95% confidence interval (CI) was used to evaluate the impact of the preoperative nutritional status on OS.
Results: A total of 32 studies involving 10352 patients were included in the meta-analysis. The results (pooled HR: 1.61, 95% CI: 1.49–1.73, I² = 43.0%, p < 0.001) indicated that preoperative malnutrition is significantly associated with poorer OS. Subgroup and meta-regression analyses based on methods of nutritional status assessment, country, sample size, study design, follow-up duration, analytical model, and tumor type all showed a consistent association between preoperative malnutrition and worse OS. The robustness of these pooled results was further verified through sensitivity analysis. Additionally, the heterogeneity of pooled HR of OS was attributed to differences in study designs, as indicated by meta-regression analysis (p = 0.005). Funnel plots did not show significant publication bias.
Conclusion: Based on existing evidence, the preoperative nutritional status is a valuable predictor of postoperative OS in patients with abdominal tumor surgery.
Systematic Review Registration: PROSPERO CRD420251008979.
1 Introduction
Abdominal tumors, primarily consisting of gastrointestinal malignancies such as gastric cancer, colorectal cancer, pancreatic cancer, and liver cancer, are often associated with varying degrees of malnutrition and muscle wasting (1–3). These conditions are typically characterized by high mortality rates, primarily due to the subtle onset of symptoms, with many patients being diagnosed at advanced stages (4–7). Given that the majority of these cancers are diagnosed at later stages, treatment options are limited, and surgical resection remains the primary therapeutic approach (8, 9). Therefore, improving postoperative outcomes for these patients is crucial to enhancing their overall health and well-being.
Cancer patients often present with complex conditions and multiple comorbidities, with numerous factors influencing their prognosis (10). Identifying more controllable, simple factors that can improve postoperative outcomes is essential. Since the 1990s, numerous studies have highlighted the widespread issue of poor nutritional status among cancer patients, which has been associated with unfavorable postoperative outcomes (11–13). The growth of abdominal tumors and symptoms such as anorexia can impair gastrointestinal function, leading to malnutrition (14). Surgical interventions further increase metabolic demands, exacerbating pre-existing nutritional deficiencies (15). Research indicates that malnutrition not only compromises immune function but may also result in slower postoperative recovery, increased complication rates, and prolonged hospitalization (16, 17). Therefore, early assessment and intervention of the preoperative nutritional status may have a positive impact on the postoperative prognosis of patients undergoing abdominal cancer surgery.
Previous studies have demonstrated that preoperative malnutrition is associated with adverse postoperative outcomes in cancer patients (18). However, many of these studies rely on subjective questionnaires to assess nutritional status (19–21), which may introduce biases such as communication difficulties, recall errors, social desirability biases, and comprehension issues, potentially affecting the accuracy of the results. This underscores the need for more objective tools to assess the risk of malnutrition.
In response to this need, several objective nutritional assessment tools have been proposed, offering a more accurate and reliable means of evaluating nutritional status. These tools include the Prognostic Nutritional Index (PNI) (22), the Controlling Nutritional Status (CONUT) score (23), and the Geriatric Nutritional Risk Index (GNRI) (24). PNI, which includes serum albumin levels and lymphocyte count, has been widely used to assess immune-nutritional status and predict postoperative outcomes in gastrointestinal cancers (22). The CONUT score, a system for evaluating nutritional status, incorporates serum albumin levels, total lymphocyte count, and total cholesterol levels, and has become an important prognostic tool for patients undergoing abdominal tumor resections, including pancreatic cancer, liver cancer, and other abdominal cancers (23). The GNRI, calculated using serum albumin levels and the ratio of ideal to actual body weight, has also been identified as a key predictor of overall survival in patients undergoing abdominal tumor resections (25).
In the context of modern oncologic surgery, the emergence of the Enhanced Recovery After Surgery (ERAS) program further supports the integration of nutritional assessment into preoperative evaluation. These multidisciplinary pathways, which emphasize early mobilization, pain control, and nutritional support, have been shown to improve postoperative outcomes and shorten hospital stays (26). Despite the substantial evidence linking poor nutritional status with increased postoperative complications and reduced survival rates, the current literature remains fragmented, with variations in assessment methods and outcome measures.
To address the gap in this field, our systematic review and meta-analysis aim to evaluate the prognostic role of preoperative nutritional status on overall survival (OS) in patients undergoing abdominal tumor surgery, based on objective nutritional assessment tools. This study seeks to support clinical risk stratification and intervention strategies, provide a theoretical foundation for future research, and ultimately optimize the management of patients undergoing abdominal tumor surgery, thereby improving their long-term outcomes.
2 Materials and methods
This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (27). The study is registered with PROSPERO under registration number CRD420251008979.
2.1 Search strategies
PubMed, Embase, Web of Science, and Cochrane Library databases were searched for eligible articles up to January 1st, 2025. The search was conducted using medical subject headings (MeSH) in combination with free text words. The search strategy in PubMed database was the following: (nutritional status[MeSH Terms] OR malnutrition[MeSH Terms) AND (preoperative malnutrition[Title/Abstract] OR preoperative dystrophy[Title/Abstract] OR preoperative GNRI[Title/Abstract] OR preoperative PNI[Title/Abstract] OR preoperative CONUT[Title/Abstract) AND prognosis[Title/Abstract]. The search strategies used in all databases are available in Supplementary File S1. In the study selection phase, blinding was implemented to reduce bias and ensure the objectivity and accuracy of the study selection process.
2.2 Inclusion criteria
The selection criteria for this research adhere to the PICOS framework (Population, Intervention/Exposure, Comparator, Outcomes, Study Designs). Studies meeting these criteria will be included, with no restrictions regarding language or publication date.
2.2.1 Population
Adult patients (18 years or older) who have undergone abdominal cancer surgery, with no exclusions based on nationality, race, ethnic background, gender, or professional status.
2.2.2 Exposure
Preoperative nutritional status or preoperative nutritional assessment, specifically based on the PNI, CONUT, or GNRI, to identify patients diagnosed with malnutrition.
2.2.3 Comparator
Adults with normal preoperative nutritional status, when applicable.
2.2.4 Primary outcome
Postoperative overall survival.
2.2.5 Study designs
Randomized controlled trials, cohort studies, case-control studies, and observational studies.
2.3 Exclusion criteria
This research will exclude studies involving non-adult patients (under 18 years of age) and those with incomplete data, including insufficient or missing preoperative nutritional assessment information, or those not reporting the specified outcomes, such as postoperative overall survival. Studies focusing on patients with non-abdominal cancers, including those undergoing surgery for thoracic, brain, or other non-abdominal malignancies, will also be excluded. Additionally, studies that concentrate on postoperative nutritional interventions or outcomes, rather than preoperative assessments, will not be considered. Research articles lacking comprehensive clinical data, even after repeated attempts to contact the authors, will be excluded. Correspondence, conference abstracts, editorial pieces, case studies, review articles, and any studies that fail to provide sufficient clinical data will also be excluded. Finally, full-text scholarly works that are inaccessible despite thorough search efforts will not be included.
2.4 Data extraction
Two investigators (ZYS and XHC) independently extracted the necessary data from the included studies, and any disagreements were resolved through discussion until a consensus was reached. The following data were extracted from each study: first author, publication year, country, study type, study design, sample size, male/female distribution, tumor type, surgical procedure, duration of follow-up, postoperative chemotherapy, overall survival (OS) with hazard ratio (HR) and its 95% confidence interval (CI), type of analysis and the cutoff values for nutritional status scores. In cases where both univariate and multivariate analyses were performed, multivariate analysis was preferred for obtaining HRs for OS, due to adjustments for confounding factors. If HR with 95% CI was not provided in the original studies, the data were extracted from the survival curve using Engauge Digitizer software (28). During the entire data extraction phase, repeated extraction procedures were implemented to ensure the objectivity and accuracy of the data.
2.5 Quality assessment
The Newcastle-Ottawa Quality Assessment Scale (NOS) was used to assess the methodological quality of the included studies (29). The NOS evaluates studies across three key domains: selection (with a maximum score of 4 points), comparability (with a maximum score of 2 points), and outcomes (with a maximum score of 3 points). Studies that achieved a score of six or higher were considered to be of high quality (30). This assessment was carried out independently by two investigators (YFL and XHC) to ensure the reliability and objectivity of the evaluation process. When there was a difference, the disagreement was resolved through discussion with a third investigator (YFZ) until consensus was reached. The detailed results of the quality assessment can be found in Supplementary Table S1.
2.6 Statistical analysis
Statistical analyses and graphical representations were conducted using R 4.3.3 and STATA 16.0. Pooled HRs with 95% CIs were calculated to evaluate the association between preoperative nutritional status and postoperative OS in patients undergoing abdominal cancer surgery. Heterogeneity among the studies was assessed using the chi-square test and I² statistic. If no significant heterogeneity was detected (P ≥ 0.10 or I² ≤ 50%), a fixed-effect model was applied for the meta-analysis. In the presence of significant heterogeneity (I² > 50% or P < 0.10), a random-effects model was employed.
To explore and account for heterogeneity across studies, subgroup analyses, meta-regression, and sensitivity analyses were performed. The subgroup factors included:
1. Preoperative nutritional status, categorized based on the PNI, CONUT, and GNRI scores.
2. Study country (China vs. Japan).
3. Sample size (<200 vs. ≥200).
4. Study design (Multicenter vs. Single-center).
5. Follow-up duration, comparing studies with clearly defined median or average follow-up times to those without.
6. Type of analysis (Univariate vs. Multivariate).
7. Tumor type, categorized as Cholangiocarcinoma, Gallbladder cancer, Renal cancer, Colorectal cancer, Liver cancer, Gastric cancer, or Pancreatic cancer.
If the original studies included in the research have excessive missing data for certain clinical variables (such as the duration of follow-up and postoperative chemotherapy), subgroup analysis will be conducted based on whether the study lacks data for those variables, rather than grouping based on the variable values. Furthermore, publication bias was visually assessed using a funnel plot and quantitatively examined using Begg's and Egger's tests. All statistical tests were two-sided, and P-values less than 0.05 were considered statistically significant.
3 Results
3.1 Study selection
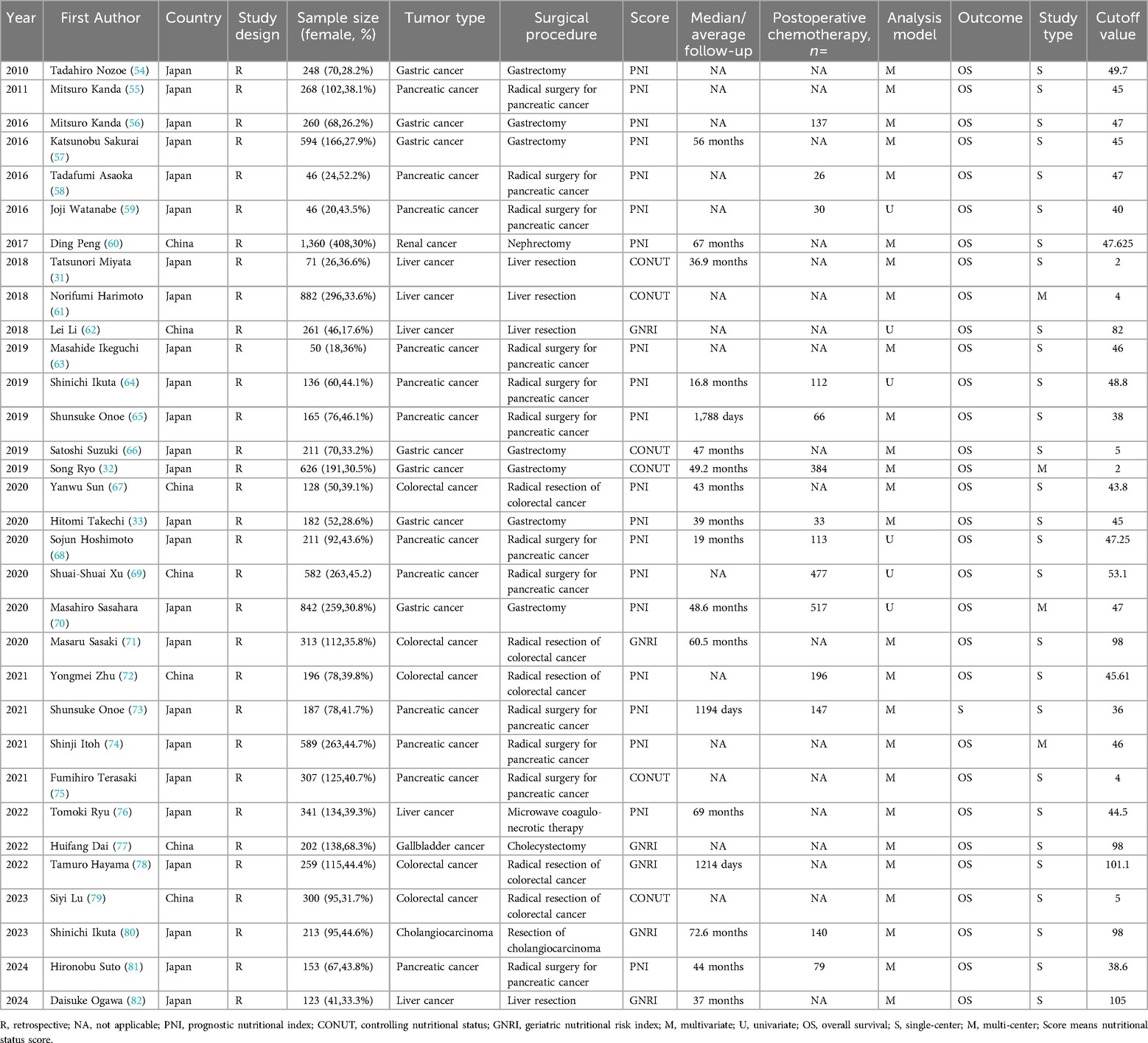
We conducted a systematic search in PubMed, Embase, Web of Science, and the Cochrane Library databases, initially identifying 473 articles. After removing 152 duplicate records, 321 articles remained. Following the screening of titles and abstracts, 258 studies were excluded due to irrelevant topics, being reviews or meta-analyses, conference abstracts, or conference proceedings. Of the remaining 63 articles, 31 were excluded due to missing data, quality issues, inability to access full text, or inconsistencies between the outcomes and the analysis objectives. Ultimately, 32 studies were included in the meta-analysis, encompassing a total of 10,352 patients. Detailed information on the included studies is provided in Table 1, and the selection process is outlined in Figure 1.
3.2 Clinical characteristic of enrolled studies
The main characteristics of the included studies are presented in Table 1. These studies were retrospective in design and primarily published within the past fifteen years. All included studies assessed the preoperative nutritional status of surgical patients using PNI, CONUT, or GNRI. Among them, 20 studies mainly used PNI to assess preoperative nutritional status, 6 studies primarily used the GNRI score to assess preoperative nutritional status, and the remaining 6 studies mainly used the CONUT score to evaluate patients’ preoperative nutritional status. Among the 32 studies, 12 were based on pancreatic cancer surgery populations, 7 on gastric cancer surgery populations, 5 on liver cancer surgery populations, 5 on colorectal cancer surgery populations, and 1 each on kidney cancer, gallbladder cancer, and cholangiocarcinoma surgery populations. Of the 32 studies, 25 were from Japan, and 7 were from China. Twenty-eight studies were single-center, while 4 were multi-center. The sample sizes of the included studies ranged from 46 to 1,360, with 20 studies having a sample size of 200 or more. All included studies investigated the correlation between preoperative nutritional status and OS. Multivariate analysis was performed in 26 out of the 32 studies. The NOS scores ranged from 6 to 8, as shown in Supplementary Table S1.
3.3 Relationship between preoperative nutritional status and OS
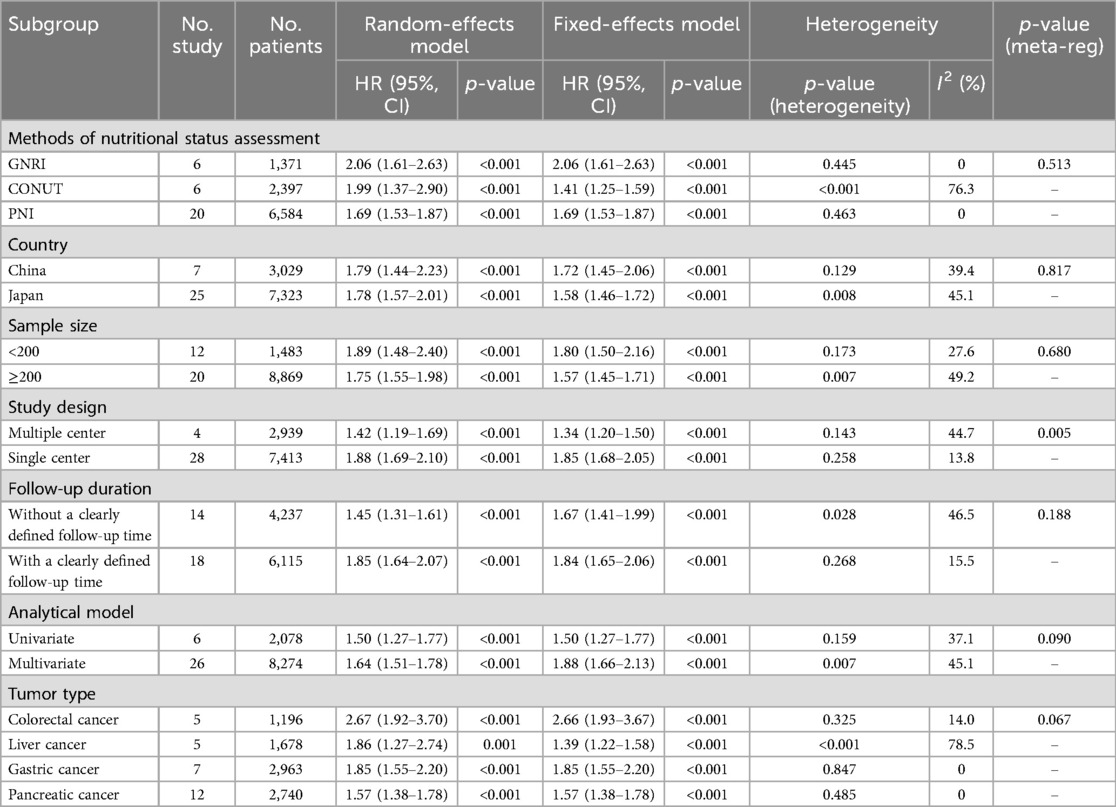
A total of 32 studies, including 10,352 patients, investigated the relationship between preoperative nutritional status and OS. The combined forest plot demonstrated that preoperative malnutrition was associated with poorer OS in patients with abdominal cancer surgery (HR = 1.61, 95% CI 1.49–1.73, p < 0.001) (Figure 2). Subgroup analyses based on methods of nutritional status assessment, country, sample size, study design, follow-up duration, analytical models, and cancer types revealed consistent results (Table 2). Specifically, whether assessed using the GNRI score (HR = 2.06, 95% CI 1.21–2.63, p < 0.001), CONUT score (HR = 1.99, 95% CI 1.37–2.90, p < 0.001), or PNI score (HR = 1.69, 95% CI 1.53–1.87, p < 0.001), preoperative malnutrition was consistently associated with worse OS in patients (Figures 3–5). Studies from China (HR = 2.13, 95% CI 1.50–3.02, p < 0.001) and Japan (HR = 1.58, 95% CI 1.46–1.72, p < 0.001) both supported this association between preoperative malnutrition and poorer OS (Figures 6 and 7). Furthermore, studies with a sample size of less than 200 (HR = 1.80, 95% CI 1.50–2.16, p < 0.001) and those with a sample size of 200 or more (HR = 1.84, 95% CI 1.60–2.12, p < 0.001) also demonstrated that preoperative malnutrition was linked to worse OS (Figures 8, 9). Both multicenter (HR = 1.34, 95% CI 1.20–1.50, p < 0.001) and single-center studies (HR = 1.85, 95% CI 1.68–2.05, p < 0.001) yielded similar findings (Figures 10, 11). Studies that clearly documented median or mean follow-up durations (HR = 1.84, 95% CI 1.65–2.06, p < 0.001) and those that did not (HR = 1.45, 95% CI 1.31–1.60, p < 0.001) also showed comparable results (Figures 12, 13). Both univariate analysis (HR = 1.50, 95% CI 1.27–1.77, p < 0.001) and multivariate analysis (HR = 1.64, 95% CI 1.51–1.78, p < 0.001) studies confirmed the same association (Figures 14, 15). After categorizing by tumor type, subgroup analyses for colorectal cancer (HR = 2.66, 95% CI 1.93–3.67, p < 0.001), liver cancer (HR = 1.86, 95% CI 1.27–2.74, p = 0.001), gastric cancer (HR = 1.85, 95% CI 1.55–2.20, p < 0.001), and pancreatic cancer (HR = 1.57, 95% CI 1.38–1.78, p < 0.001) also demonstrated that preoperative malnutrition was associated with poorer OS, as shown in Figures 16–19. In addition, studies focus on cholangiocarcinoma (HR = 1.73, 95% CI 1.11–1.2.70, p < 0.001), gallbladder cancer (HR = 2.21, 95% CI 1.13–4.31, p = 0.020), and renal cancer (HR = 1.65, 95% CI 1.15–2.35, p = 0.006) each demonstrated the correlation between preoperative malnutrition and poorer OS (Supplementary Figures S1–S3).
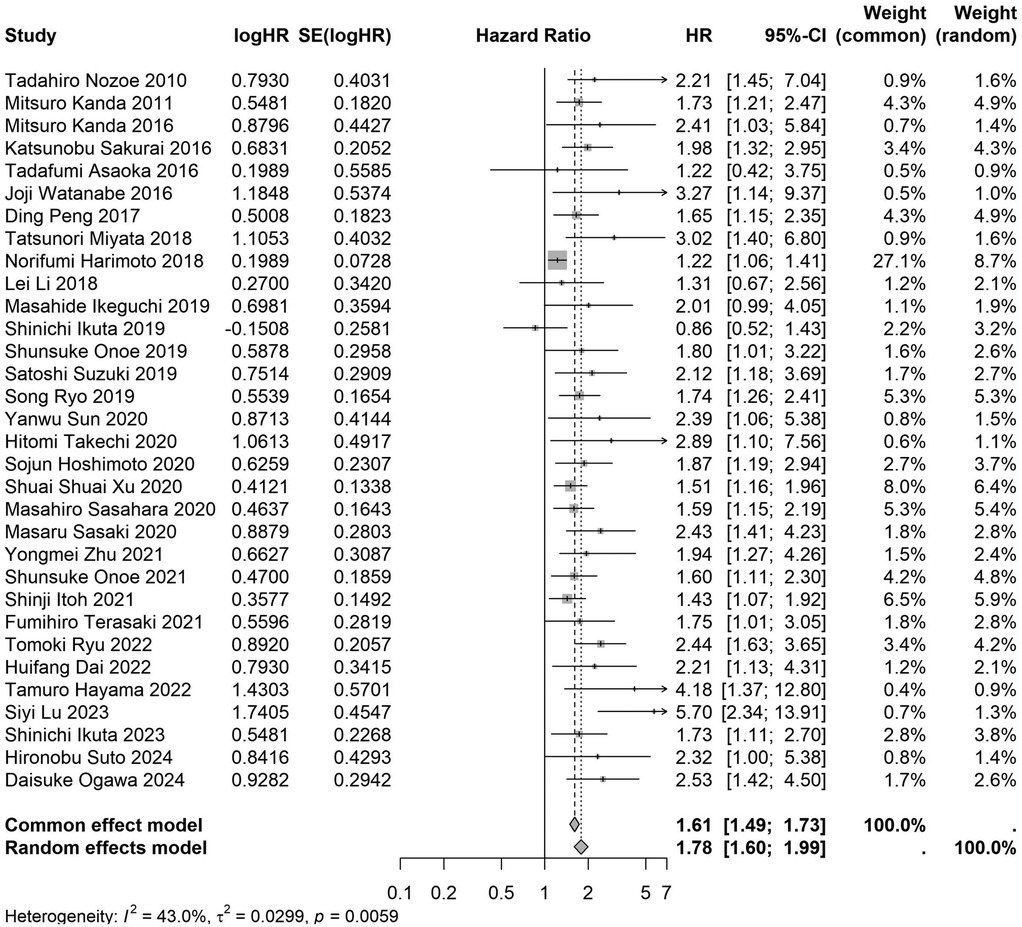
Figure 2. Forest plot of the association between preoperative nutritional status and OS. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
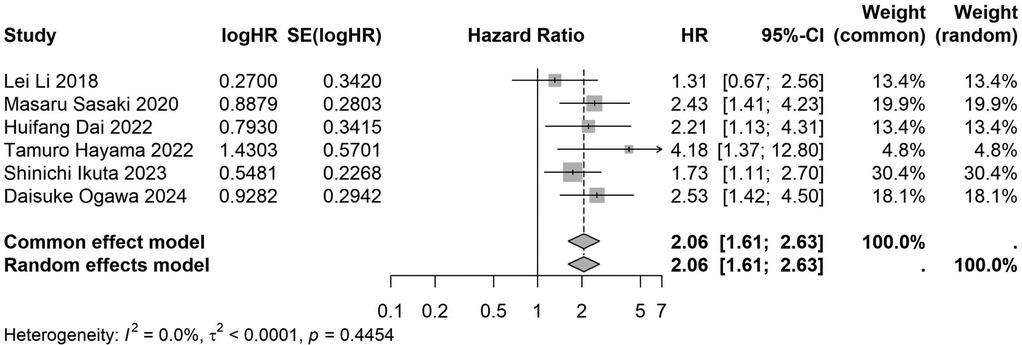
Figure 3. Forest plot of the association between preoperative GNRI and OS. (GNRI, geriatric nutritional risk index; OS, overall survival; HR, hazard ratio; Cl, confidence interval).
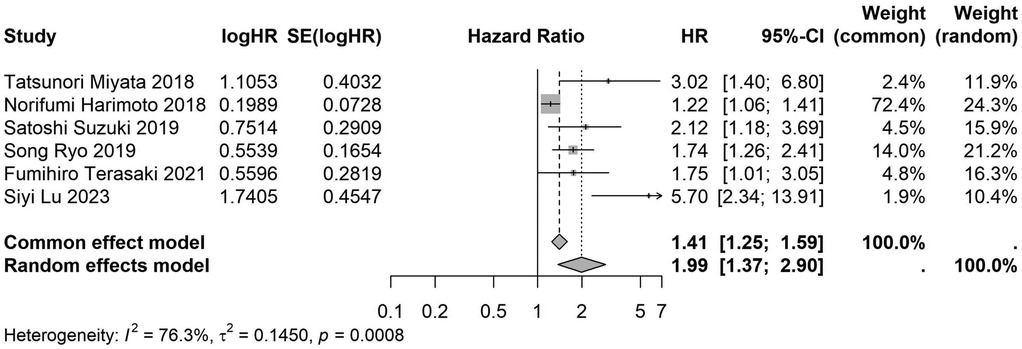
Figure 4. Forest plot of the association between preoperative CONUT and OS. (CONUT, controlling nutritional status; OS, overall survival; HR, hazard ratio; Cl, confidence interval).
![Forest plot showing hazard ratios (HR) for different studies, with each study listed alongside its logHR, standard error, HR with 95% confidence interval, and weights. The plot includes squares representing individual study estimates and a diamond for the overall effect. Heterogeneity statistics: I² = 0.0%, τ² = 0, p = 0.4634. Overall HR is 1.69 with 95% CI [1.53; 1.87].](https://www.frontiersin.org/files/Articles/1645392/fsurg-12-1645392-HTML/image_m/fsurg-12-1645392-g005.jpg)
Figure 5. Forest plot of the association between preoperative PNI and OS. (PNI, prognostic nutritional index; OS, overall survival; HR, hazard ratio; Cl, confidence interval).
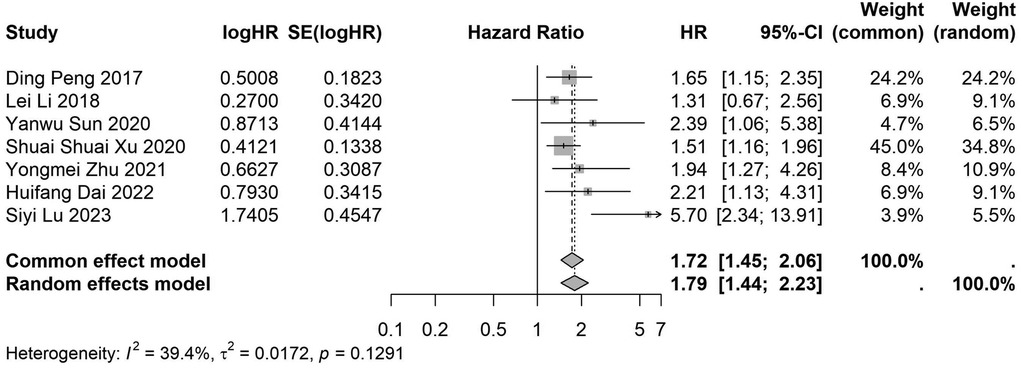
Figure 6. Forest plot of the association between preoperative nutritional status and OS based on the Chinese study. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
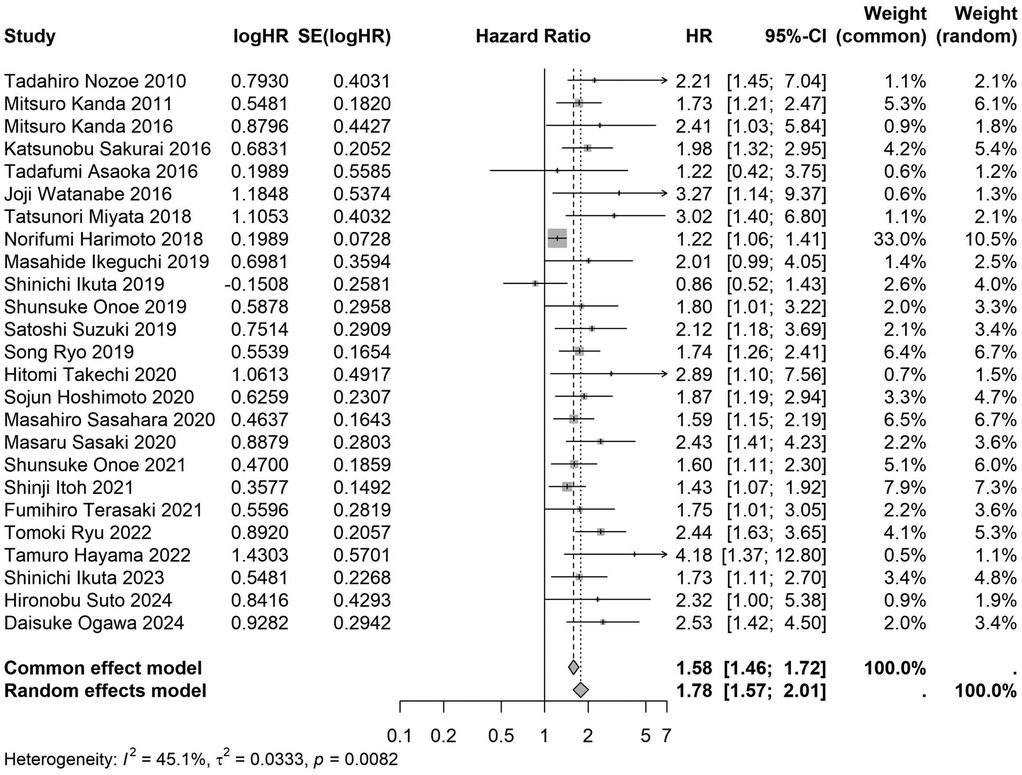
Figure 7. Forest plot of the association between preoperative nutritional status and OS based on the Japanese study. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
![A forest plot from a meta-analysis displays studies' hazard ratios (HR) with confidence intervals (CI). Twelve studies are listed with logHR values, standard errors, HR, 95% CI, and weights for common and random effects models. Studies plotted show effects on a scale from 0.1 to 7, with some data points extending beyond the confidence interval. Summary diamonds at the bottom indicate pooled estimates: HR of 1.80 with CI [1.50; 2.16] for common effects, and HR of 1.89 with CI [1.48; 2.40] for random effects. Heterogeneity is indicated by I² of 27.6%.](https://www.frontiersin.org/files/Articles/1645392/fsurg-12-1645392-HTML/image_m/fsurg-12-1645392-g008.jpg)
Figure 8. Forest plot of the association between preoperative nutritional status and OS based on the study with a sample size <200. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
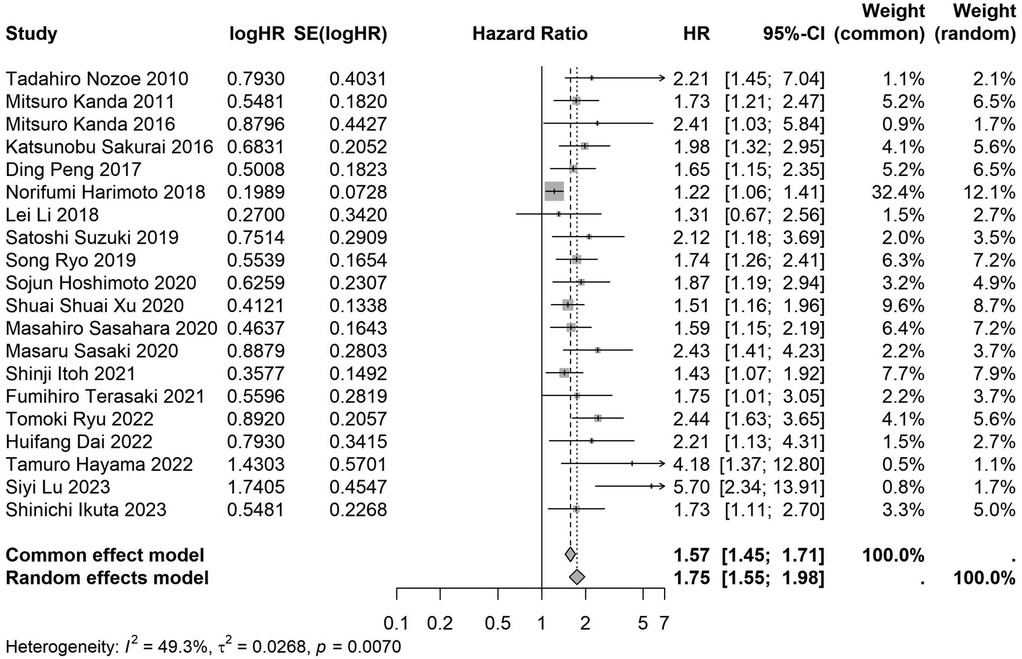
Figure 9. Forest plot of the association between preoperative nutritional status and OS based on the study with a sample size ≥200. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
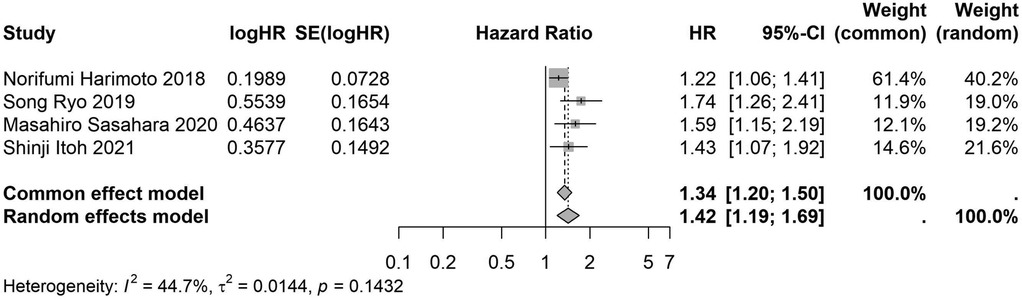
Figure 10. Forest plot of the association between preoperative nutritional status and OS based on the study multicenter design. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
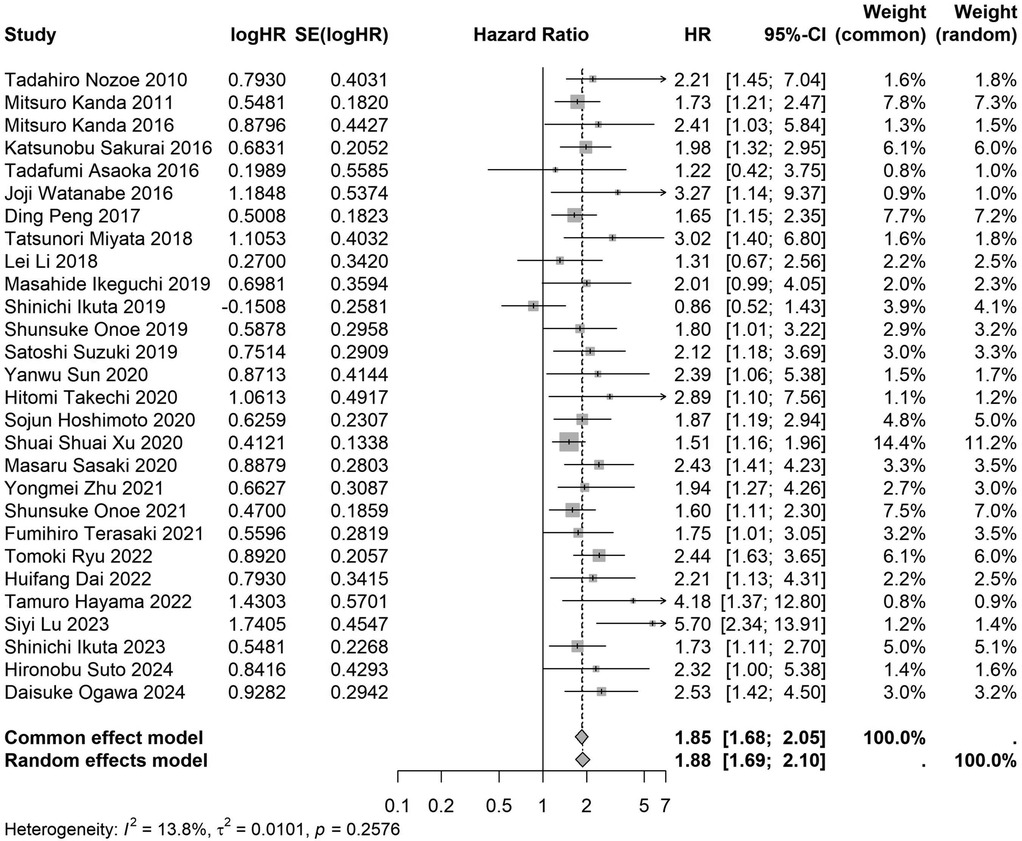
Figure 11. Forest plot of the association between preoperative nutritional status and OS based on the study single-center design. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
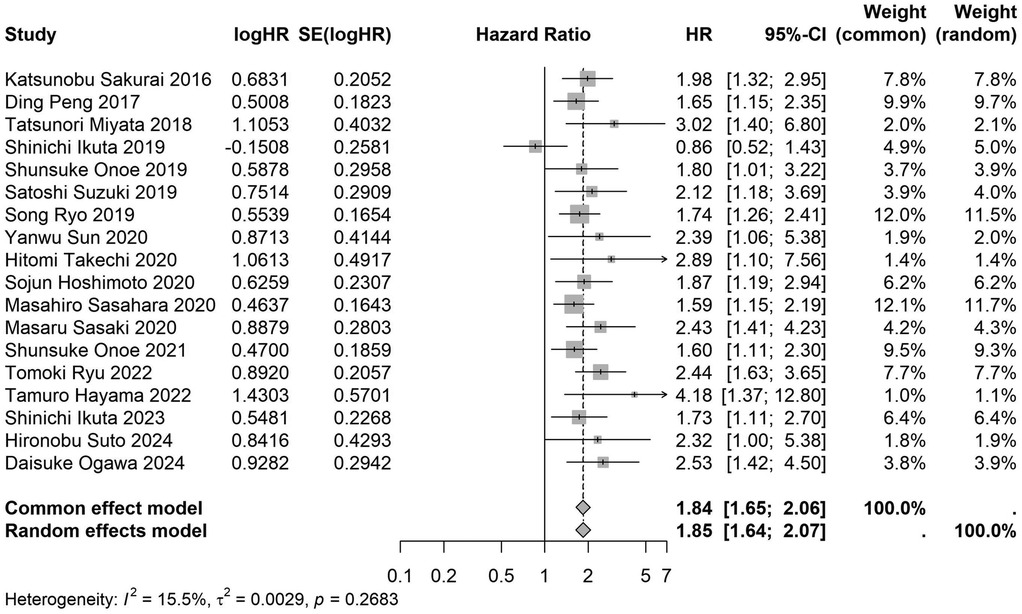
Figure 12. Forest plot of the association between preoperative nutritional status and OS based on the study that clearly documented median or mean follow-up durations. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
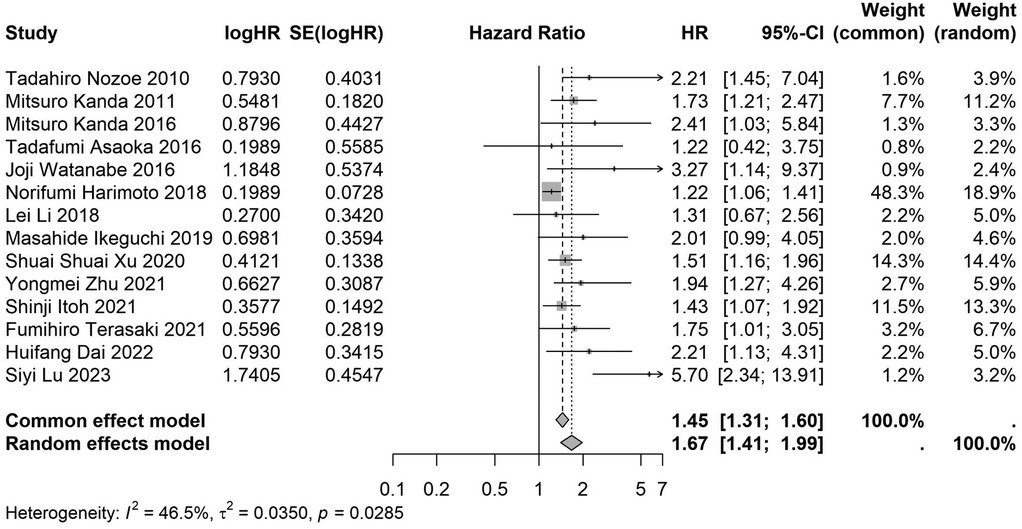
Figure 13. Forest plot of the association between preoperative nutritional status and OS based on the study that did not clearly documented median or mean follow-up durations. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
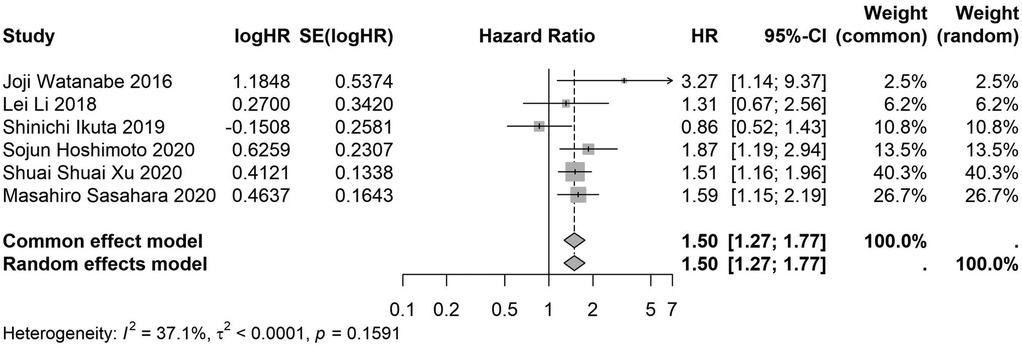
Figure 14. Forest plot of the association between preoperative nutritional status and OS based on the study using univariate analytical model. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
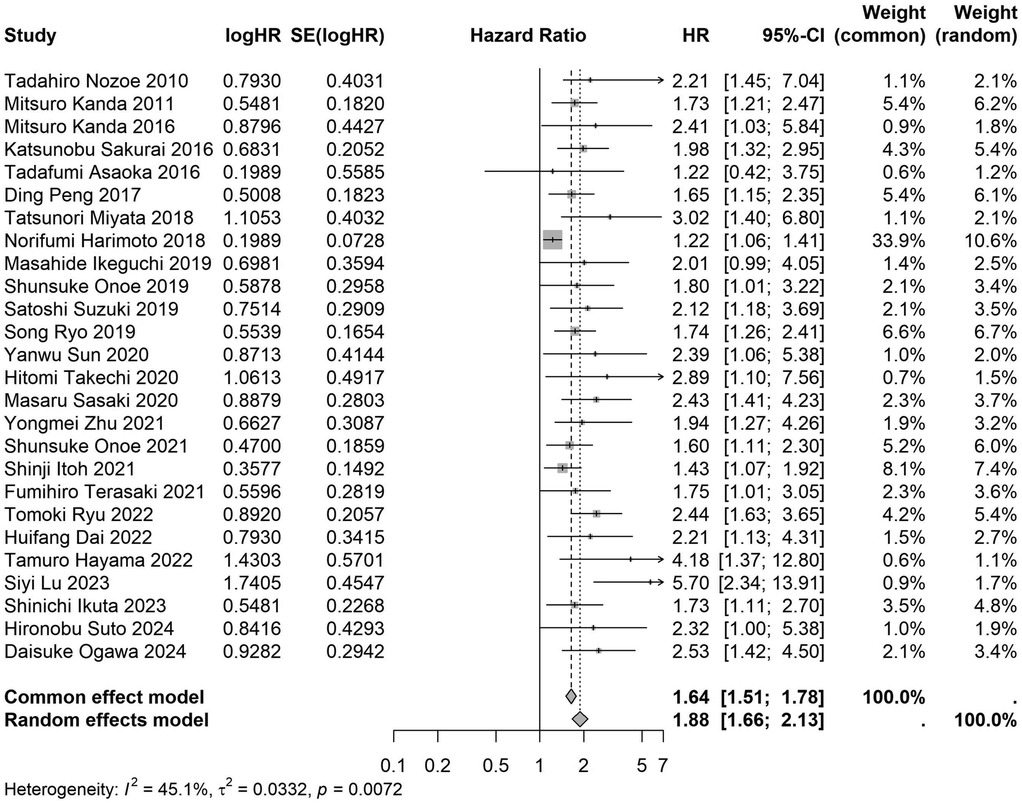
Figure 15. Forest plot of the association between preoperative nutritional status and OS based on the study using multivariate analytical model. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
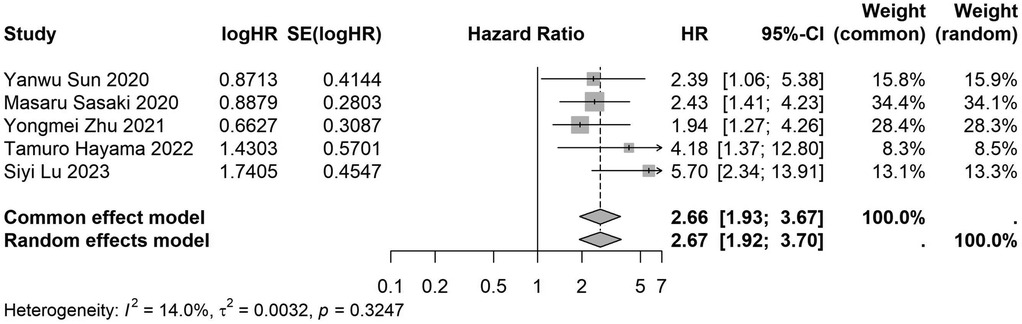
Figure 16. Forest plot of the association between preoperative nutritional status and OS based on the study focus on colorectal cancer. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
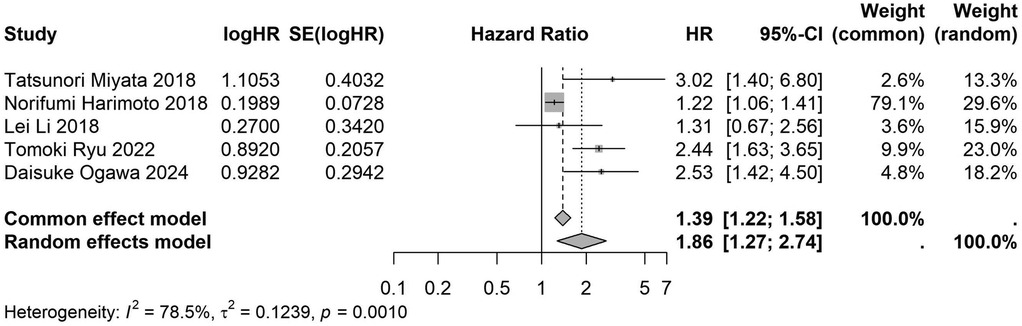
Figure 17. Forest plot of the association between preoperative nutritional status and OS based on the study focus on liver cancer. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
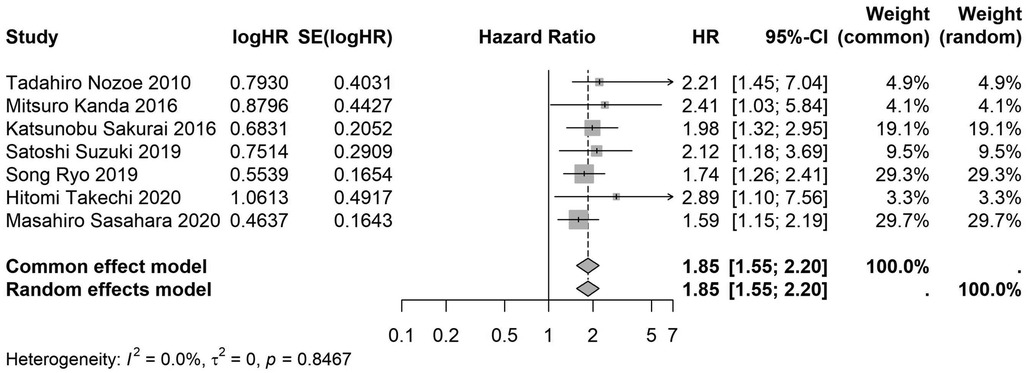
Figure 18. Forest plot of the association between preoperative nutritional status and OS based on the study focus on gastric cancer. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
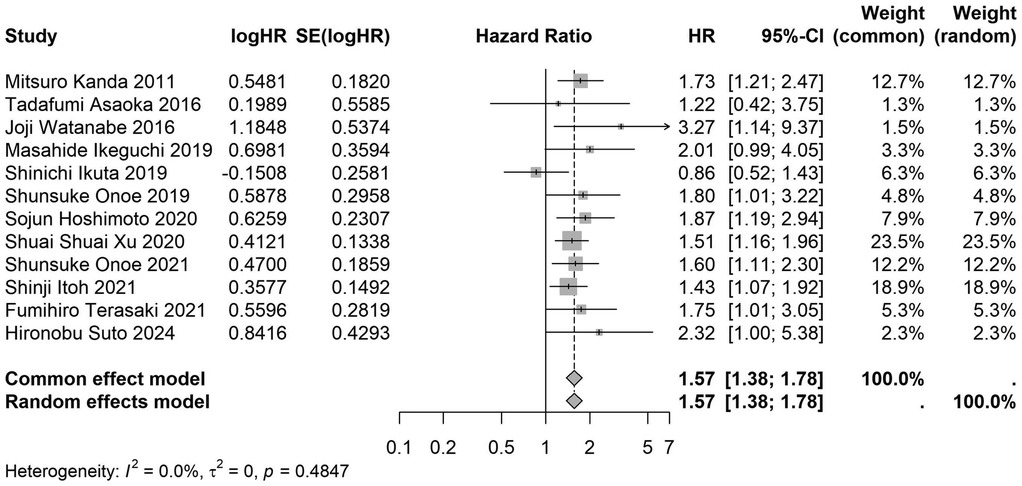
Figure 19. Forest plot of the association between preoperative nutritional status and OS based on the study focus on pancreatic cancer. (OS, overall survival; HR, hazard ratio; Cl, confidence interval).
3.4 Meta-regression
The meta-regression analysis revealed that, with the exception of the subgroup analysis based on study design (single-center vs. multi-center studies) (p = 0.005), no significant heterogeneity was observed in the other subgroup analyses, suggesting that our results are robust (Table 2). Furthermore, these findings imply that the type of study design (single-center or multi-center) may contribute to the observed heterogeneity.
3.5 Sensitivity analysis and publication bias
A sensitivity analysis was performed to evaluate the impact of individual studies on the pooled HR for OS. The results indicated that excluding any single study did not significantly affect the pooled HR (Figure 20). Additionally, publication bias was assessed, and visual inspection of the funnel plot revealed no apparent asymmetry (Figure 21).
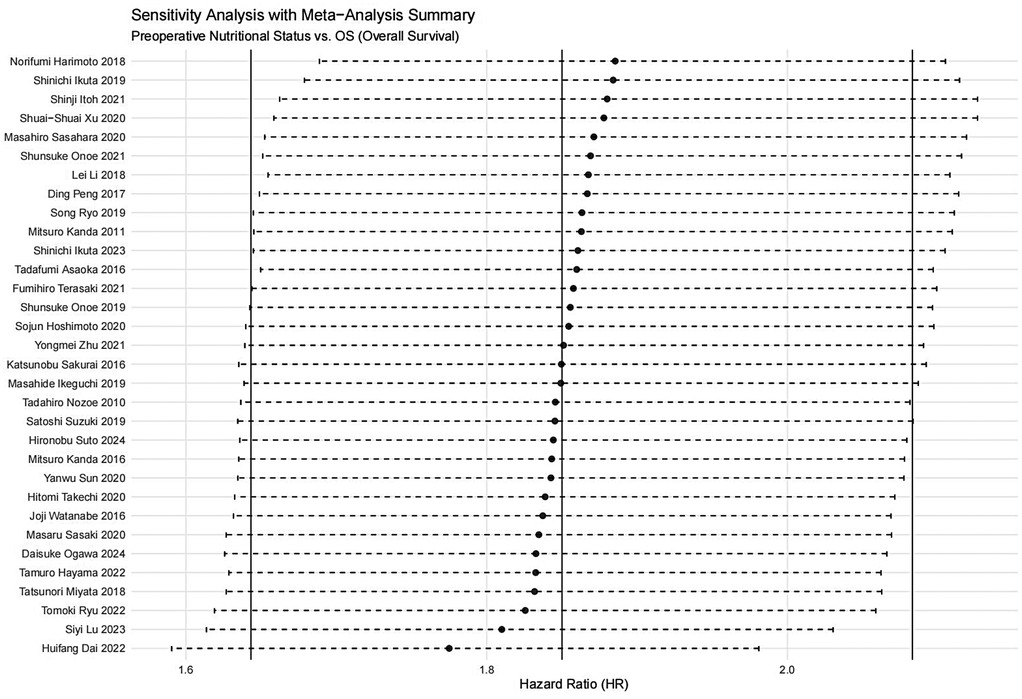
Figure 20. Sensitivity analysis for the association between preoperative nutritional status and OS (OS, overall survival).
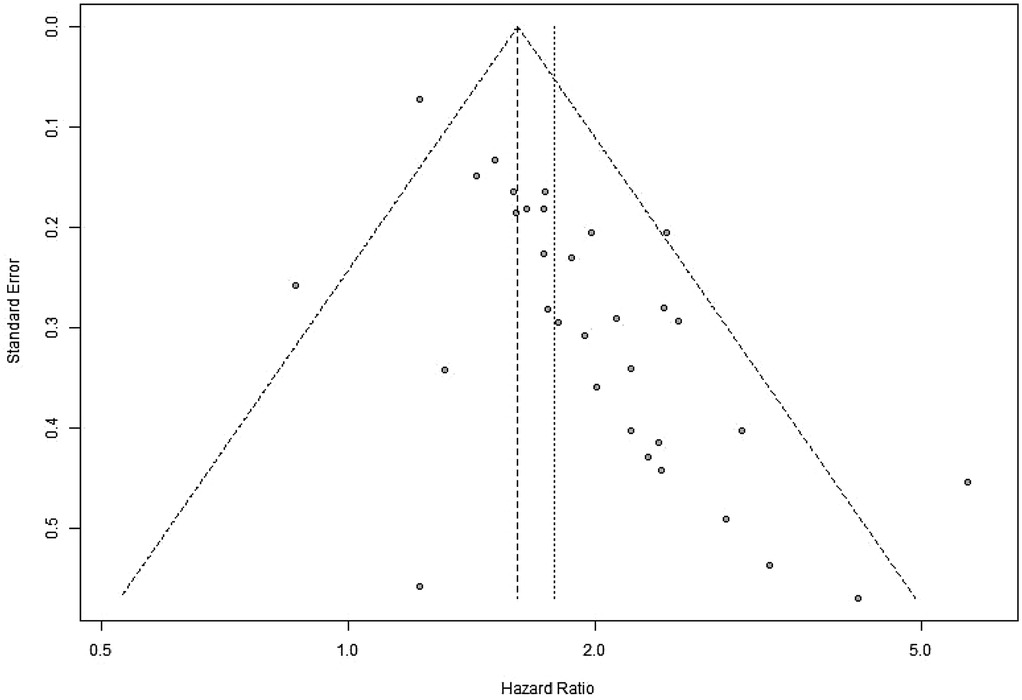
Figure 21. Funnel plot for evaluation of publication bias or small-study effects in OS (OS, overall survival).
4 Discussion
Our systematic review and meta-analysis indicate that preoperative nutritional status is a critical prognostic factor for OS following abdominal tumor surgery. Several studies involving colorectal cancer, gastric cancer, pancreatic cancer, and hepatocellular carcinoma have shown that poor nutritional status, characterized by low serum albumin levels, decreased PNI, reduced GNRI, elevated CONUT, reduced skeletal muscle mass, and adverse inflammation-nutrition composite scores, is associated with lower long-term survival rates and increased postoperative complications (30–35). These findings underscore the necessity of incorporating routine and standardized nutritional assessments into the preoperative evaluation of patients with abdominal tumors. Over the past few decades, advances in surgical techniques and perioperative care have further emphasized the need to reassess the relationship between nutrition and clinical outcomes. In the era of ERAS, optimizing patients’ preoperative physiological status has gained increasing importance. ERAS protocols advocate for early mobilization, minimally invasive surgical techniques, and targeted perioperative nutritional support as key strategies to reduce postoperative complications and improve recovery times (36–38). Recent studies have demonstrated that adequate nutritional intervention can mitigate the harmful impact of preoperative malnutrition on OS (39), highlighting the potential positive effects of improving preoperative nutritional status on patient prognosis.
Additionally, with the promotion of precision medicine and the implementation of individualized treatment models, there is a growing demand for comprehensive assessments of patients’ overall condition, including their nutritional and functional status. Clarifying the prognostic role of preoperative nutritional status in abdominal tumor surgery not only provides risk stratification and decision-making support for surgeons but also plays a pivotal role in multidisciplinary clinical pathways. This is particularly relevant for elderly patients and high-risk groups, where optimizing preoperative nutritional status can greatly enhance postoperative recovery, improve overall survival, and quality of life. Such improvements can profoundly impact the development of modern surgical treatment models, transitioning toward an integrated approach of “prevention-intervention-recovery” (40).
Malnutrition often leads to increased postoperative complication rates, prolonged hospital stays, and heightened mortality risk (40). In addition to these adverse outcomes, malnourished patients are more prone to infections, exhibit poorer responses to cancer treatments, and experience impaired overall prognosis (30, 41). Previous studies have indicated that nutrition plays a critical role in immune response, with malnutrition leading to immune deficiencies (42). Furthermore, malnutrition is a major cause of zinc deficiency, which impairs cell-mediated immune function and is associated with host cell-mediated immune damage (43). Beyond impairing host immunity, poor nutritional status can alter drug metabolism, reduce tolerance to adjuvant therapies, and diminish patients’ overall functional reserves (44). In summary, maintaining optimal preoperative nutritional status is crucial for the recovery of patients undergoing tumor surgery.
While there are existing studies investigating the prognostic impact of individual preoperative nutritional assessments such as PNI, GNRI, or CONUT scores on tumor surgery outcomes, there is still a lack of integrative evaluations on the combined application of these scoring systems, particularly in terms of their applicability and effectiveness across different tumor types. Therefore, we conducted this meta-analysis by integrating studies that included these three nutritional scoring systems and abdominal tumors. This approach enhances the evidential value of the conclusions. To the best of our knowledge, our study is the first meta-analysis to combine multiple simple, objective nutritional scores with various types of abdominal tumors.
Ultimately, this study included a total of 32 studies and 10,352 patients who underwent abdominal cancer surgery. Our findings indicate that preoperative malnutrition negatively impacts the OS of patients. To assess the heterogeneity between the included studies and the influence of different study characteristics on the prognostic value of preoperative nutritional status scoring, we performed subgroup and meta-regression analyses based on the preoperative nutritional status assessment methods, the country of origin of the study, sample size, study design, follow-up duration, data analysis methods, and tumor type. We found that, regardless of these factors, preoperative malnutrition was consistently associated with significantly poorer OS. Sensitivity analyses further confirmed the robustness of our results. This suggests that preoperative nutritional status may serve as an important prognostic indicator for patients undergoing abdominal tumor surgery.
The PNI is calculated using serum albumin levels and peripheral blood lymphocyte count to assess the immune-nutritional status and a low PNI indicates poor prognosis (22); The CONUT score is calculated based on serum albumin levels, total lymphocyte count, and total cholesterol levels, with higher scores indicating worse nutritional statu (23). The GNRI is calculated using serum albumin concentration and the ratio of actual body weight to ideal body weight, and it is also used to evaluate nutritional risk, with lower values indicating higher risk of adverse events (24). All three indices are key tools for evaluating patients’ nutritional status and prognosis. These measures, based on specific laboratory markers, offer higher reliability compared to many subjective scales or imaging markers. These measures, based on specific laboratory markers, offer higher reliability compared to many subjective scales or imaging markers. They are also simpler and more practical for clinical use. The objective nature of these indices, when applied widely in clinical research and practice, can assist physicians in developing more targeted treatment strategies, thus improving overall health outcomes and quality of life for patients. Effective utilization of these scoring systems allows medical teams to better identify high-risk patients, enabling timely intervention to reduce complications and improve survival rates.
Of course, there are certain limitations to our study. For instance, subgroup analyses based on study design (single-center vs. multi-center studies) revealed some heterogeneity, highlighting the need for further investigation to address this potential source of variability in future studies. Additionally, careful and rigorous consideration of research designs related to preoperative nutritional status and prognosis is required. Another limitation is the variability in the definition of “abdominal tumors.” Although our systematic review attempted to encompass a broad range of abdominal tumors, the majority of studies focused on gastric, liver, pancreatic, and colorectal cancers, with relatively few studies on gallbladder and bile duct cancers, as well as renal cancer. This limitation emphasizes the need for future research to include a broader spectrum of abdominal tumors and to analyze outcomes based on tumor type, which may reveal more subtle prognostic differences.
One important factor to consider is the potential cultural or geographical bias inherent in the studies included in our analysis. The majority of the included studies originated from Japan and China, and these regions may have distinct healthcare systems, nutritional practices, and patient demographics compared to Western populations. These geographical differences could influence the generalizability of our findings, as nutritional status assessments, treatment protocols, and patient care approaches may differ across regions. In particular, the prevalence of certain abdominal cancers, as well as the approach to preoperative nutritional interventions, may vary significantly between Eastern and Western countries (45).
While the current meta-analysis provides valuable insights into the impact of preoperative nutritional status on postoperative outcomes in abdominal cancer surgery, it is essential to consider how these findings can be applied to Western populations or global practices. In Western countries, the prevalence of abdominal cancers such as colorectal and liver cancer may differ, and the typical patient demographic may also exhibit different nutritional challenges, such as varying rates of obesity or malnutrition (46, 47). Moreover, the healthcare infrastructure and access to preoperative nutritional interventions may differ, potentially influencing the effectiveness of these interventions.
Future studies in Western or other diverse populations are needed to confirm whether the results from this analysis are consistent across different geographic and cultural settings. It will be important to examine how nutritional assessment tools like PNI, CONUT, and GNRI are applied in Western healthcare systems, as well as to explore the role of different treatment regimens, tumor types, and preoperative interventions in these settings. Such studies could provide a more global perspective on the role of nutrition in cancer surgery and help standardize clinical practices worldwide.
By addressing these cultural and geographical variations, future research will be better equipped to tailor preoperative nutritional strategies for diverse patient populations and enhance the global applicability of these findings.
In addition, heterogeneity in defining malnutrition using various nutritional assessment tools such as PNI, CONUT, and GNRI cutoffs may have influenced the pooled estimates. Future studies should aim to standardize these thresholds to minimize this variability. Although the overall heterogeneity in our study was moderate to low, the CONUT subgroup exhibited substantial heterogeneity (I² = 76.3%). This finding may be attributed to the differences in the standardization of the CONUT score across studies (48). The CONUT score, as a tool for assessing nutritional status, may have been applied differently in various studies, influenced by patient characteristics, tumor types, and treatment protocols. For example, different studies may have used varying CONUT score thresholds or assessment methods, which could be affected by factors such as tumor staging and treatment regimens (49). Certain tumor types, such as gastric, liver, and pancreatic cancers, may show a higher sensitivity to the CONUT score, while other cancers may present different results (50). These factors likely explain the observed heterogeneity in the CONUT subgroup. Future studies should aim to standardize the use of the CONUT score, particularly in multi-center studies involving diverse tumor types and treatment approaches, to reduce such heterogeneity.
Moreover, while study design differences account for some of the heterogeneity, other potential confounders remain unexplained, including tumor stage, neoadjuvant therapies, the complexity of the surgical procedures, and the missing data related to these factors. These factors are likely to contribute to residual heterogeneity and should be carefully considered in future research.
Despite these limitations, our study holds significant clinical value. Integrating nutritional status assessment into preoperative evaluations can help identify patients at risk of reduced survival. This understanding provides a window for preoperative nutritional interventions, which can optimize patient outcomes through tailored dietary counseling, oral nutritional supplementation, and even prehabilitation programs such as resistance training to improve muscle mass. These interventions may be especially important for elderly or frail populations, who typically have limited physiological reserves.
Looking ahead, future research should focus on several key areas. First, large-scale, prospective trials are needed to examine the impact of targeted nutritional interventions on short- and long-term outcomes in abdominal cancer surgery. Standardized nutritional assessment methods (such as PNI, CONUT, and GNRI scores) should be used in studies to facilitate data aggregation and improve the reliability of meta-analytic estimates. Second, future studies should explore the combined effects of nutritional and inflammatory biomarkers, as their interaction appears to play a critical role in tumor progression and postoperative recovery (51).
Moreover, research should explore the integration of nutritional optimization into multimodal prehabilitation programs, which may include physical training, psychosocial support, and medical optimization. Such comprehensive programs can not only improve nutritional status but also enhance patients’ overall psychological resilience, leading to better surgical outcomes (52). Finally, integrating genomic and metabolomic research with nutritional assessment could identify new biomarkers, further refining our understanding of the relationship between host nutritional status and cancer outcomes (53). These interdisciplinary approaches may pave the way for personalized nutritional therapies in oncologic surgery.
In conclusion, our meta-analysis confirms that preoperative nutritional status is one of the key determinants of OS in patients undergoing abdominal cancer surgery. The significant association between poor nutritional indicators and adverse postoperative outcomes underscores the necessity for comprehensive preoperative assessments and the potential for nutritional interventions to improve long-term survival. Despite some heterogeneity and research limitations, a large body of evidence from various cancer types and clinical settings supports the inclusion of nutritional assessments in routine surgical planning and the development of targeted preoperative optimization strategies. Future research, particularly prospective multi-center studies, should standardize assessment tools and develop evidence-based nutritional intervention protocols that can be seamlessly integrated into clinical practice. Ultimately, addressing preoperative nutritional deficiencies may significantly improve surgical outcomes and increase the OS rate of patients with abdominal malignancies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
ZS: Writing – original draft, Writing – review & editing. YiL: Writing – review & editing, Writing – original draft. ML: Writing – review & editing, Writing – original draft. YY: Writing – review & editing, Writing – original draft. XC: Writing – review & editing, Writing – original draft. YZ: Writing – original draft, Writing – review & editing. YM: Writing – review & editing, Writing – original draft. ZH: Writing – review & editing, Writing – original draft. YaL: Writing – original draft, Writing – review & editing. NM: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research reported in this publication was supported by Ministry of Science and Technology Senior Foreign Expert Program (G20200028011, G2021175002l), Joint Research Fund Project of Gansu Province (23JRRA1496), National College Students Innovation and Entrepreneurship Training Program (202210730172), Medical Innovation and Development Project of Lanzhou University (lzuyxcx-2022-99), Innovation and Entrepreneurship Action Plan of Lanzhou University (20240060040), 2022 Gansu Provincial Key Talent Program (202277) and 2022 Chengguan District Science and Technology Program (2022HFSZ0015). The funders had no contribution to this article.
Acknowledgments
We would like to thank the researchers and study participants for their contributions. And we would like to thank the Stork software (https://www.storkapp.me) for it's grammar correction and language polishing function.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1645392/full#supplementary-material
Abbreviations
PNI, prognostic nutritional index; CONUT, the controlling nutritional status; GNRI, geriatric nutritional risk index; ERAS, enhanced recovery after surgery; PRISMA, preferred reporting items for systematic reviews and meta-analyses; MeSH, medical subject headings; 95% Cl, 95% confidence interval; NOS, Newcastle-Ottawa scale; OS, overall survival.
References
1. Wang B, Hu S, Teng Y, Chen J, Wang H, Xu Y, et al. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct Target Ther. (2024) 9:200. doi: 10.1038/s41392-024-01889-y
2. Shen Y, Chen JX, Li M, Xiang Z, Wu J, Wang YJ. Role of tumor-associated macrophages in common digestive system malignant tumors. World J Gastrointest Oncol. (2023) 15:596–616. doi: 10.4251/wjgo.v15.i4.596
3. Yin L, Yan H, Chen K, Ji Z, Zhang X, Ji G, et al. Diet-derived circulating antioxidants and risk of digestive system tumors: a Mendelian randomization study. Nutrients. (2022) 14(16):3274. doi: 10.3390/nu14163274
4. Huang F, Dai C, Zhang Y, Zhao Y, Wang Y, Ru G. Development of molecular mechanisms and their application on oncolytic Newcastle disease virus in cancer therapy. Front Mol Biosci. (2022) 9:889403. doi: 10.3389/fmolb.2022.889403
5. Liu N, Liu M, Fu S, Wang J, Tang H, Isah AD, et al. Ang2-targeted combination therapy for cancer treatment. Front Immunol. (2022) 13:949553. doi: 10.3389/fimmu.2022.949553
6. Zhang G, Peng Z, Yan C, Wang J, Luo J, Luo H. A novel liver cancer diagnosis method based on patient similarity network and DenseGCN. Sci Rep. (2022) 12:6797. doi: 10.1038/s41598-022-10441-3
7. He J, Wu F, Han Z, Hu M, Lin W, Li Y, et al. Biomarkers (mRNAs and non-coding RNAs) for the diagnosis and prognosis of colorectal cancer—from the body fluid to tissue level. Front Oncol. (2021) 11:632834. doi: 10.3389/fonc.2021.632834
8. Mollinedo F, Gajate C. Direct endoplasmic Reticulum targeting by the selective alkylphospholipid analog and antitumor ether lipid edelfosine as a therapeutic approach in pancreatic cancer. Cancers (Basel). (2021) 13(16):4173. doi: 10.3390/cancers13164173
9. Liu P, Tan J, Tan Q, Xu L, He T, Lv Q. Application of carbon nanoparticles in tracing lymph nodes and locating tumors in colorectal cancer: a concise review. Int J Nanomedicine. (2020) 15:9671–81. doi: 10.2147/IJN.S281914
10. Li X, Zheng J, Lu Y, Pan X. Risk assessment of death of tumor-related PTE by CAR combined with DD detection. Vasc Health Risk Manag. (2022) 18:445–51. doi: 10.2147/VHRM.S365323
11. Rong A, Franco-Garcia E, Zhou C, Heng M, Akeju O, Azocar RJ, et al. Association of nutrition status and hospital-acquired infections in older adult orthopedic trauma patients. JPEN J Parenter Enteral Nutr. (2022) 46:69–74. doi: 10.1002/jpen.2096
12. Deepjyoti K, Bannoth S, Purkayastha J, Borthakur BB, Talukdar A, Pegu N, et al. Nasojejunal feeding is safe and effective alternative to feeding jejunostomy for postoperative enteral nutrition in gastric cancer patients. South Asian J Cancer. (2020) 9:70–3. doi: 10.1055/s-0040-1721218
13. August DA, Huhmann MB. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. (2009) 33:472–500. doi: 10.1177/0148607109341804
14. Yang ZY, Yang F. Nutritional status of patients with gastrointestinal cancers and analysis of factors for postoperative infections. BMC Cancer. (2024) 24:1389. doi: 10.1186/s12885-024-13093-w
15. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch. (2014) 68:263–7. doi: 10.5455/medarh.2014.68.263-267
16. Chen Y, Guo Y, Tong G, He Y, Zhang R, Liu Q. Combined nutritional status and activities of daily living disability is associated with one-year mortality after hip fracture surgery for geriatric patients: a retrospective cohort study. Aging Clin Exp Res. (2024) 36:127. doi: 10.1007/s40520-024-02786-8
17. Yu QS, Zheng Z, Peng H, Shen Y, Liu JD, Zhou FH. Effect of qihuang decoction combined with enteral nutrition on postoperative gastric cancer of nutrition and immune function. Evid Based Complement Alternat Med. (2020) 2020:1795107. doi: 10.1155/2020/1795107
18. Gupta A, Gupta E, Hilsden R, Hawel JD, Elnahas AI, Schlachta CM, et al. Preoperative malnutrition in patients with colorectal cancer. Can J Surg. (2021) 64:E621–e629. doi: 10.1503/cjs.016820
19. Kang J, Li H, Shi X, E MA, Chen W. Validation of the efficacy of the NUTRISCORE for the nutritional screening of cancer patients in China. BMC Cancer. (2022) 22:43. doi: 10.1186/s12885-021-09135-2
20. Akula B, Doctor N. A prospective review of preoperative nutritional status and its influence on the outcome of abdominal surgery. Cureus. (2021) 13(11):e19948. doi: 10.7759/cureus.19948
21. Yang D, Zheng Z, Zhao Y, Zhang T, Liu Y, Xu X. Patient-generated subjective global assessment versus nutritional risk screening 2002 for gastric cancer in Chinese patients. Future Oncol. (2020) 16:4475–83. doi: 10.2217/fon-2019-0539
22. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. (1980) 139:160–7. doi: 10.1016/0002-9610(80)90246-9
23. Ignacio De Ulíbarri J, González-Madroño A, De Villar NG, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. (2005) 20:38–45.
24. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
25. Shibutani M, Kashiwagi S, Fukuoka T, Iseki Y, Kasashima H, Maeda K. Impact of preoperative nutritional Status on long-term survival in patients with stage I–III colorectal cancer. In Vivo. (2023) 37:1765–74. doi: 10.21873/invivo.13265
26. Latka K, Kołodziej W, Rajski R, Pawuś D, Chowaniec J, Latka D. Outpatient spine surgery in Poland: a survey on popularity, challenges, and future perspectives. Risk Manag Healthc Policy. (2023) 16:1839–48. doi: 10.2147/RMHP.S425465
27. Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
28. Huang J, Zhao K, Zhao Z, Qu Y. Neuroprotection by transcranial direct current stimulation in rodent models of focal ischemic stroke: a meta-analysis. Front Neurosci. (2021) 15:761971. doi: 10.3389/fnins.2021.761971
29. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
30. Xie H, Tang S, Wei L, Gan J. Geriatric nutritional risk index as a predictor of complications and long-term outcomes in patients with gastrointestinal malignancy: a systematic review and meta-analysis. Cancer Cell Int. (2020) 20:530. doi: 10.1186/s12935-020-01628-7
31. Miyata T, Yamashita YI, Higashi T, Taki K, Izumi D, Kosumi K, et al. The prognostic impact of controlling nutritional Status (CONUT) in intrahepatic cholangiocarcinoma following curative hepatectomy: a retrospective single institution study. World J Surg. (2018) 42:1085–91. doi: 10.1007/s00268-017-4214-1
32. Ryo S, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al. The controlling nutritional status score serves as a predictor of short- and long-term outcomes for patients with stage 2 or 3 gastric cancer: analysis of a multi-institutional data set. Ann Surg Oncol. (2019) 26:456–64. doi: 10.1245/s10434-018-07121-w
33. Takechi H, Fujikuni N, Tanabe K, Hattori M, Amano H, Noriyuki T, et al. Using the preoperative prognostic nutritional index as a predictive factor for non-cancer-related death in post-curative resection gastric cancer patients: a retrospective cohort study. BMC Gastroenterol. (2020) 20:256. doi: 10.1186/s12876-020-01402-z
34. Dang C, Wang M, Zhu F, Qin T, Qin R. Controlling nutritional status (CONUT) score-based nomogram to predict overall survival of patients with pancreatic cancer undergoing radical surgery. Asian J Surg. (2022) 45:1237–45. doi: 10.1016/j.asjsur.2021.08.011
35. Lin ZX, Ruan DY, Jia CC, Wang TT, Cheng JT, Huang HQ, et al. Controlling nutritional status (CONUT) score-based nomogram to predict overall survival of patients with HBV-associated hepatocellular carcinoma after curative hepatectomy. Clin Transl Oncol. (2020) 22:370–80. doi: 10.1007/s12094-019-02137-4
36. Cavallaro P, Bordeianou L. Implementation of an ERAS pathway in colorectal surgery. Clin Colon Rectal Surg. (2019) 32:102–8. doi: 10.1055/s-0038-1676474
37. Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: enhanced recovery after surgery (ERAS®) society recommendations–part I. Gynecol Oncol. (2016) 140:313–22. doi: 10.1016/j.ygyno.2015.11.015
38. Ban KA, Berian JR, Ko CY. Does implementation of enhanced recovery after surgery (ERAS) protocols in colorectal surgery improve patient outcomes? Clin Colon Rectal Surg. (2019) 32:109–13. doi: 10.1055/s-0038-1676475
39. Wang C, Yang D. Effect of different preoperative nutritional treatments on postoperative recovery and clinical outcomes in patients with gastric cancer and early gastric outlet obstruction. Oncol Lett. (2024) 27:214. doi: 10.3892/ol.2024.14348
40. Okabayashi T, Nishimori I, Sugimoto T, Maeda H, Dabanaka K, Onishi S, et al. Effects of branched-chain amino acids-enriched nutrient support for patients undergoing liver resection for hepatocellular carcinoma. J Gastroenterol Hepatol. (2008) 23:1869–73. doi: 10.1111/j.1440-1746.2008.05504.x
41. Zhao P, Wu Z, Wang Z, Wu C, Huang X, Tian B. Prognostic role of the prognostic nutritional index in patients with pancreatic cancer who underwent curative resection without preoperative neoadjuvant treatment: a systematic review and meta-analysis. Front Surg. (2022) 9:992641. doi: 10.3389/fsurg.2022.992641
42. Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. (2016) 37:386–98. doi: 10.1016/j.it.2016.04.003
43. Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. (2017) 9(6):624. doi: 10.3390/nu9060624
44. Purcell SA, Kok DE, Ketterl T, Garcia MB, Joffe L, Brown JC, et al. Pharmacokinetics of cancer therapeutics and energy balance: the role of diet intake, energy expenditure, and body composition. J Natl Cancer Inst Monogr. (2023) 2023:3–11. doi: 10.1093/jncimonographs/lgad010
45. Akgül Ö, Ocak S, Gündoğdu SB, Yalaza M, Güldoğan CE, Tez M. Comparison of east and west survival nomograms in turkish gastric cancer patients who underwent radical surgery. Scand J Surg. (2018) 107:308–14. doi: 10.1177/1457496918766724
46. Liu S, Zhang L, Ge S, Shi J, Qiu J, Ge X, et al. Quality of plant-based diets in relation to all-cause and cardiovascular disease mortality in US adults with sarcopenia: a population-based study. Aging Clin Exp Res. (2025) 37:176. doi: 10.1007/s40520-025-03080-x
47. Cho YJ, Kawk JS, Yoon HJ, Park M. Body weight variability and cancer incidence in men aged 40 years and older-Korean national insurance service cohort. Sci Rep. (2021) 11:12122. doi: 10.1038/s41598-021-91601-9
48. Ma X, Zou W, Sun Y. Prognostic value of pretreatment controlling nutritional Status score for patients with pancreatic cancer: a meta-analysis. Front Oncol. (2021) 11:770894. doi: 10.3389/fonc.2021.770894
49. Qian Y, Liu H, Pan J, Yu W, Lv J, Yan J, et al. Preoperative controlling nutritional Status (CONUT) score predicts short-term outcomes of patients with gastric cancer after laparoscopy-assisted radical gastrectomy. World J Surg Oncol. (2021) 19:25. doi: 10.1186/s12957-021-02132-6
50. Xie T, Dong Z, Wu C, Ding Q, Zhan W, Fu S, et al. Association between CONUT scores and survival outcomes in patients with non-small cell lung cancer: meta-analysis from 4973 Asian cases. Front Oncol. (2025) 15:1522368. doi: 10.3389/fonc.2025.1522368
51. Gao X, Pan Y, Zhou L, Li Y, Lin B, Zheng Y. The fib-PNI-MLR score, an integrative model of coagulation cascades, nutrition Status, and systemic inflammatory response, predicts urological outcomes after surgery in patients with non-metastatic renal cell carcinoma. Front Oncol. (2020) 10:555152. doi: 10.3389/fonc.2020.555152
52. Carli F, Bousquet-Dion G, Awasthi R, Elsherbini N, Liberman S, Boutros M, et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg. (2020) 155:233–42. doi: 10.1001/jamasurg.2019.5474
53. Tuttolomondo A, Simonetta I, Daidone M, Mogavero A, Ortello A, Pinto A. Metabolic and vascular effect of the Mediterranean diet. Int J Mol Sci. (2019) 20(19):4716. doi: 10.3390/ijms20194716
54. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. (2010) 40:440–3. doi: 10.1007/s00595-009-4065-y
55. Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. (2011) 98:268–74. doi: 10.1002/bjs.7305
56. Kanda M, Mizuno A, Tanaka C, Kobayashi D, Fujiwara M, Iwata N, et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Medicine (Baltimore). (2016) 95:e3781. doi: 10.1097/MD.0000000000003781
57. Sakurai K, Ohira M, Tamura T, Toyokawa T, Amano R, Kubo N, et al. Predictive potential of preoperative nutritional status in long-term outcome projections for patients with gastric cancer. Ann Surg Oncol. (2016) 23:525–33. doi: 10.1245/s10434-015-4814-7
58. Asaoka T, Miyamoto A, Maeda S, Tsujie M, Hama N, Yamamoto K, et al. Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology. (2016) 16:434–40. doi: 10.1016/j.pan.2015.10.006
59. Watanabe J, Otani S, Sakamoto T, Arai Y, Hanaki T, Amisaki M, et al. Prognostic indicators based on inflammatory and nutritional factors after pancreaticoduodenectomy for pancreatic cancer. Surg Today. (2016) 46:1258–67. doi: 10.1007/s00595-016-1308-6
60. Peng D, He ZS, Li XS, Tang Q, Zhang L, Yang KW, et al. Prognostic value of inflammatory and nutritional scores in renal cell carcinoma after nephrectomy. Clin Genitourin Cancer. (2017) 15:582–90. doi: 10.1016/j.clgc.2017.04.001
61. Harimoto N, Yoshizumi T, Inokuchi S, Itoh S, Adachi E, Ikeda Y, et al. Prognostic significance of preoperative controlling nutritional Status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma: a multi-institutional study. Ann Surg Oncol. (2018) 25:3316–23. doi: 10.1245/s10434-018-6672-6
62. Li L, Wang H, Yang J, Jiang L, Yang J, Wu H, et al. Geriatric nutritional risk index predicts prognosis after hepatectomy in elderly patients with hepatitis B virus-related hepatocellular carcinoma. Sci Rep. (2018) 8:12561. doi: 10.1038/s41598-018-30906-8
63. Ikeguchi M, Goto K, Watanabe J, Urushibara S, Osaki T, Endo K, et al. Clinical importance of preoperative and postoperative prognostic nutritional index in patients with pancreatic ductal adenocarcinoma. Ann Hepatobiliary Pancreat Surg. (2019) 23:372–6. doi: 10.14701/ahbps.2019.23.4.372
64. Ikuta S, Aihara T, Yamanaka N. Preoperative C-reactive protein to albumin ratio is a predictor of survival after pancreatic resection for pancreatic ductal adenocarcinoma. Asia Pac J Clin Oncol. (2019) 15:e109–14. doi: 10.1111/ajco.13123
65. Onoe S, Maeda A, Takayama Y, Fukami Y, Takahashi T, Uji M, et al. The prognostic impact of the lymphocyte-to-monocyte ratio in resected pancreatic head adenocarcinoma. Med Princ Pract. (2019) 28:517–25. doi: 10.1159/000501017
66. Suzuki S, Kanaji S, Yamamoto M, Oshikiri T, Nakamura T, Kakeji Y. Controlling nutritional status (CONUT) score predicts outcomes of curative resection for gastric cancer in the elderly. World J Surg. (2019) 43:1076–84. doi: 10.1007/s00268-018-04889-6
67. Sun Y, Huang Z, Lin H, P CHI. Prognostic impact of preoperative immunonutritional status in rectal mucinous adenocarcinoma. Future Oncol. (2020) 16:339–51. doi: 10.2217/fon-2019-0793
68. Hoshimoto S, Hishinuma S, Shirakawa H, Tomikawa M, Ozawa I, Ogata Y. Validation and clinical usefulness of pre- and postoperative systemic inflammatory parameters as prognostic markers in patients with potentially resectable pancreatic cancer. Pancreatology. (2020) 20:239–46. doi: 10.1016/j.pan.2019.12.004
69. Xu SS, Li S, Xu HX, Li H, Wu CT, Wang WQ, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol. (2020) 26:828–38. doi: 10.3748/wjg.v26.i8.828
70. Sasahara M, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al. The preoperative prognostic nutritional index predicts short-term and long-term outcomes of patients with stage II/III gastric cancer: analysis of a multi-institution dataset. Dig Surg. (2020) 37:135–44. doi: 10.1159/000497454
71. Liao CK, Chern YJ, Hsu YJ, Lin YC, Yu YL, Chiang JM, et al. The clinical utility of the geriatric nutritional risk Index in predicting postoperative complications and long-term survival in elderly patients with colorectal cancer after curative surgery. Cancers (Basel). (2021) 13(22):5852. doi: 10.3390/cancers13225852
72. Zhu Y, Fan L, Geng X, Li J. The predictive value of the prognostic nutritional index to postoperative prognosis and nursing intervention measures for colorectal cancer. Am J Transl Res. (2021) 13(12):14096–101.35035753
73. Onoe S, Yokoyama Y, Kokuryo T, Igami T, Mizuno T, Yamaguchi J, et al. A presurgical prognostic stratification based on nutritional assessment and carbohydrate antigen 19-9 in pancreatic carcinoma: an approach with nonanatomic biomarkers. Surgery. (2021) 169:1463–70. doi: 10.1016/j.surg.2020.11.035
74. Itoh S, Tsujita E, Fukuzawa K, Sugimachi K, Iguchi T, Ninomiya M, et al. Prognostic significance of preoperative PNI and CA19-9 for pancreatic ductal adenocarcinoma: a multi-institutional retrospective study. Pancreatology. (2021) 21:1356–63. doi: 10.1016/j.pan.2021.08.003
75. Terasaki F, Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, et al. The preoperative controlling nutritional status (CONUT) score is an independent prognostic marker for pancreatic ductal adenocarcinoma. Updates Surg. (2021) 73:251–9. doi: 10.1007/s13304-020-00792-9
76. Ryu T, Takami Y, Wada Y, Sasaki S, Saitsu H. Predictive impact of the prognostic nutritional index in early-staged hepatocellular carcinoma after operative microwave ablation. Asian J Surg. (2022) 45:202–7. doi: 10.1016/j.asjsur.2021.04.043
77. Dai H, Xu J. Preoperative geriatric nutritional risk index is an independent prognostic factor for postoperative survival after gallbladder cancer radical surgery. BMC Surg. (2022) 22:133. doi: 10.1186/s12893-022-01575-2
78. Hayama T, Hashiguchi Y, Ozawa T, Watanabe M, Fukushima Y, Shimada R, et al. The preoperative geriatric nutritional risk index (GNRI) is an independent prognostic factor in elderly patients underwent curative resection for colorectal cancer. Sci Rep. (2022) 12:3682. doi: 10.1038/s41598-022-07540-6
79. Lu S, Chen Z, Peng R, Zhang Q, Wang Y, Li X, et al. Prognostic effect of preoperative controlling nutritional status score in patients with locally advanced rectal cancer: a two-center, retrospective study. Nutrition. (2023) 112:112078. doi: 10.1016/j.nut.2023.112078
80. Ikuta S, Nakajima T, Fujikawa M, Aihara T, Yamanaka N. Prognostic value of geriatric nutritional risk index for patients with biliary tract cancer undergoing surgical resection—a single-institution retrospective cohort study. Contemp Oncol (Pozn). (2023) 27(2):65–70. doi: 10.5114/wo.2023.127436
81. Suto H, Matsukawa H, Ando Y, Oshima M, Fuke T, Nagao M, et al. Predictive role of the prognostic nutritional index in patients with pancreatic ductal adenocarcinoma who underwent neoadjuvant chemoradiotherapy followed by curative pancreatic resection: a retrospective study using prospectively collected data. J Hepatobiliary Pancreat Sci. (2024). doi: 10.1002/jhbp.1424
82. Ogawa D, Miyata T, Yumoto S, Shiraishi Y, Matsumoto T, Takematsu T, et al. Prognostic value of preoperative geriatric nutritional risk index in intrahepatic cholangiocarcinoma after hepatectomy: a single-center retrospective cohort study. Langenbecks Arch Surg. (2024) 409:47. doi: 10.1007/s00423-023-03221-8
Keywords: preoperative nutritional status, abdominal tumor surgery, postoperative overall survival, enhanced recovery after surgery, surgery, meta-analyses
Citation: Su Z, Lin Y, Li M, Yang Y, Chen X, Zhu Y, Mo Y, Huang Z, Liu Y and Michael N (2025) Preoperative nutritional status as a predictor of postoperative overall survival in abdominal tumor surgery: a systematic review and meta-analysis. Front. Surg. 12:1645392. doi: 10.3389/fsurg.2025.1645392
Received: 11 June 2025; Accepted: 30 July 2025;
Published: 18 August 2025.
Edited by:
Luigi Marano, Academy of Applied Medical and Social Sciences, PolandReviewed by:
XiaoDong Chen, First Affiliated Hospital of Wenzhou Medical University, ChinaEva Karanikki, Hippokration General Hospital, Greece
Copyright: © 2025 Su, Lin, Li, Yang, Chen, Zhu, Mo, Huang, Liu and Michael. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yatao Liu, ZG9jdG9ybGl1eXRAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Zhaoyin Su
Zhaoyin Su Yifeng Lin
Yifeng Lin Molan Li1,†
Molan Li1,† Xiaohan Chen
Xiaohan Chen Yifan Mo
Yifan Mo Yatao Liu
Yatao Liu