- 1Department of Neurosurgery, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China
- 2Department of Image Center, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China
- 3Department of Cardiology, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, China
Background: Cerebellar Mutism Syndrome (CMS) is a significant neurological complication following posterior fossa tumor surgery in children. The pathophysiological mechanisms of CMS remain elusive, and there is a growing interest in the potential influence of sex on its incidence. This study aims to evaluate sex as an independent risk factor for the development of CMS.
Methods: A retrospective cohort study of 385 pediatric patients who underwent posterior fossa tumor surgery at Beijing Children's Hospital (2013–2024) was conducted. Comprehensive demographic, clinical, and pathological data were collected. Statistical analysis involved Chi-square tests for categorical variables, Kruskal–Wallis tests for non-parametric comparisons among groups, and logistic regression to identify independent predictors of CMS.
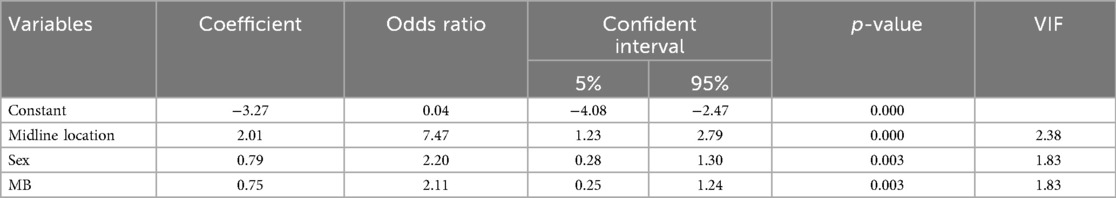
Results: CMS occurred in 29.9% of all cases, with annual incidence ranging from 14.3% to 37.9%. Medulloblastoma was the most common pathology (38.4%), with a median maximal tumor diameter of 47.2 mm. Tumors were predominantly located at the midline (68.1%), and gross total resection was achieved in 86.3% of patients. Male patients exhibited a significantly higher incidence of CMS compared to females (73.0% vs. 53.0%, p = 0.003). Independent risk factors for CMS included male sex [OR 2.25; 95% CI (1.30–3.70)], midline tumor location [OR 7.47; 95% CI (2.79–19.98)], and medulloblastoma diagnosis [OR 2.11; 95% CI (1.24–3.59)].
Conclusion: This study indicates a notable male predominance in CMS occurrence, suggesting the existence of sex-specific differences in cerebellar function and language development. These findings highlight the need for heightened monitoring and tailored interventions for male patients undergoing posterior fossa tumor surgery and suggest a potential biological basis for sex-specific differences in cerebellar function and vulnerability to surgical injury.
Importance of the study: This study provides critical insights into the significant role of sex as an independent risk factor for Cerebellar Mutism Syndrome (CMS) following posterior fossa tumor surgeries in pediatric patients. By identifying male sex, midline tumor location, and medulloblastoma pathology as independent predictors, this research addresses a gap in understanding sex-based disparities in CMS development. These findings suggest potential gender-specific differences in cerebellar and language development, offering a foundation for future translational research and targeted clinical strategies. The results emphasize the need for heightened monitoring and tailored interventions, especially for male patients, to mitigate CMS risk and improve surgical outcomes in pediatric neurosurgery.
Key points
1. Cerebellar mutism syndrome is significantly more common in male patients after posterior fossa tumor surgery.
2. Midline tumor location and medulloblastoma diagnosis are independent risk factors for cerebellar mutism syndrome.
Introduction
Cerebellar Mutism Syndrome (CMS) is a common complication following following resection of posterior fossa tumors, affecting approximately 25% of this patient population (1, 2). Characterized by the sudden onset of mutism and emotional lability within 1–6 days post-surgery, CMS often persists for several months (3). Additional symptoms include ataxia, hypotonia, and irritability (4).
Although the exact pathogenesis of CMS remains elusive, the prevailing hypothesis attributes its development to injury of the deep cerebellar nuclei and their efferent pathways (5). Evidence implicates bilateral damage to the dentato-thalamo-cortical tracts in CMS pathogenesis (6). While midline tumor location and medulloblastoma have been recognized as risk factors (2, 7), the influence of age, sex, tumor size, hydrocephalus, and ventriculoperitoneal (VP) shunts remains controversial (8–10).
The role of sex in CMS development is not well understood. The literature presents mixed findings on the role of sex in CMS development. While some studies have suggested a possible male predisposition, other multicenter or review studies have reported no significant association or have shown conflicting results (3, 9, 11, 12). This discrepancy highlights a critical gap in our understanding and underscores the necessity for validation in large, well-characterized patient cohorts. Sex-based differences in the developmental trajectories of cerebellar function and language networks represent a compelling, yet unproven, hypothesis to explain possible sex disparities in CMS susceptibility across populations. Research indicates that girls exhibit superior expressive language skills compared to boys during the early development while boys tend to experience a relative delay in language acquisition and increased vulnerability to linguistic interference (13, 14). Functional MRI studies have also revealed sex-related differences in language processing, suggesting a potential link between sex and CMS susceptibility (15).
In this study, we aim to investigate the sex disparities in CMS incidence among pediatric patients undergoing posterior fossa tumor resection. Through multivariable and subgroup analyses, our goal is to discern definitive risk factors associated with CMS.
Method and materials
Study cohorts
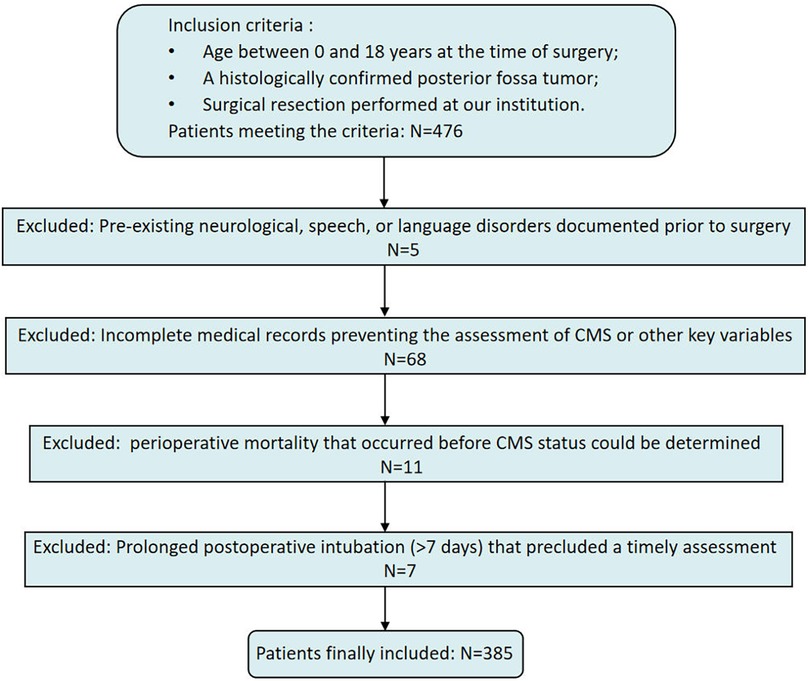
This single-center, retrospective cohort study was conducted at Beijing Children's Hospital, National Center for Children's Health, Beijing, China. From July 2013 to March 2024, a total of 476 pediatric patients diagnosed with a posterior fossa tumor underwent surgical resection. Inclusion criteria were as follows: (1) age 0–18 years at the time of surgery; (2) a histologically confirmed posterior fossa tumor; and (3) surgical resection performed at our institution.
Exclusion criteria were as follows: (1) documented pre-existing neurological, speech, or language disorders documented; (2) incomplete medical records hat precluded assessment of CMS or other key variables; (3) perioperative mortality that occurred before CMS status could be determined; and (4) prolonged postoperative intubation (>7 days) that precluded a timely assessment for CMS. After applying these criteria, 385 patients were included in the final analysis (Figure 1).
All participants underwent a preoperative brain MRI. Data collection included demographic information, CMS status, radiological and histological characteristics, operative records, and follow-up data. CMS status was determined from clinical records or telephone interviews. Follow-up assessments were conducted every 6–12 months, either during outpatient visits or via telephone. During follow-up, data regarding the resolution of mutism were collected to determine the duration of symptoms for patients in the CMS group. Informed consent was obtained from all patients or their guardians, and the study was approved by the local Ethics Committee (Approval No. [2019-k-344).
Diagnosis of CMS and follow-up
The diagnosis of CMS was determined according to the Iceland Delphi consensus diagnostic criteria, characterized by the delayed onset of impaired speech or complete mutism, accompanied by emotional lability, occurring after posterior fossa surgery (16).
The primary diagnosis was made by the neurosurgical team during the patient's hospitalization and documented in the clinical records. For cases where CMS status was ambiguous in the records, or for confirmation during follow-up, structured telephone interviews were conducted with the patients’ parents or legal guardians. The interview followed a standardized questionnaire designed to elicit parental recall of key symptoms in a non-leading and unbiased manner. The interview structure included the following components:
1. Timing and Onset: Questions to establish the timeline of any speech changes, specifically to identify a delay of 1–6 days post-surgery (e.g., “Thinking back to the week after the surgery, do you recall any changes in your child's ability to speak? If so, on which day did this change begin?”).
2. Nature of Speech Deficit: Questions to describe the severity of the speech change (e.g., “Could you describe the change? Did your child stop speaking completely, or were they just very quiet?”).
3. Emotional and Behavioral Changes: Probes for symptoms of emotional lability (e.g., “During that same period, did you notice any significant shifts in their mood, such as being unusually irritable, withdrawn, or crying more than usual?”).
4. Cross-Validation: A concluding question inquiring whether the term “Cerebellar Mutism Syndrome” or “Posterior Fossa Syndrome” was ever used by the clinical team, thereby validating the parent's descriptive recall against a formal diagnosis.
For pre-verbal patients (typically under 2 years of age), the diagnosis was based on a sudden and marked loss of previously acquired vocalizations (e.g., cessation of babbling or single-word use), combined with pronounced emotional lability (e.g., apathy, inconsolable crying) and other features of posterior fossa syndrome, as observed by both clinicians and parents.
Definitions of interested variables
The analyzed variables included: age at surgery, sex, year of diagnosis, tumor size, tumor consistency (solid or non-solid), tumor location (midline or off-midline), presence of a VP shunt prior to resection, extent of resection [gross total resection (GTR) or Non-GTR], tumor pathology [medulloblastoma (MB) or Non-MB], Evans’ index, and presence of paraventricular and peritumoral edema.
Tumor size was determined by measuring the maximum diameter across axial, sagittal, and coronal MRI planes. Tumors were classified as non-solid if a cystic component accounted for more than 50% of the tumor volume. The categorization of tumor location was based on MRI findings, and the Evans’ index was calculated as the ratio of the maximum width of the frontal horns to the maximum internal width of the cranial cavity (normal range ≤0.30). Paraventricular and peritumoral edema were identified using T2-weighted or fluid-attenuated inversion recovery (FLAIR) MRI sequences. Surgical approach was categorized into four types: (1) Telovelar, (2) Transvermian, (3) Others, and (4) Unknown. MRI scans were reviewed and measurements were taken by two experienced neurosurgeons to ensure accuracy and consistency.
Statistical methods
Statistical analyses were performed using python 3.7. Categorical variables were compared using the Chi-square test or Fisher's exact test, as appropriate. For continuous variables with non-normal distributions, the Kruskal–Wallis test was employed to compare median values across groups.
Univariable logistic regression was performed to identify potential risk factors for CMS. Variables with a p-value ≤ 0.10 in univariable analysis were entered into the multivariable logistic regression model. A stepwise selection procedure was used, with entry and removal criteria set at p = 0.01 and p = 0.1, respectively. The final model retained variables that were independently associated with CMS.
Collinearity among predictor variables was assessed using variance inflation factors (VIFs), with VIF > 5 indicating potential multicollinearity. Statistical significance was set at a threshold of P < 0.05 for all tests performed.
Results
CMS occurrence
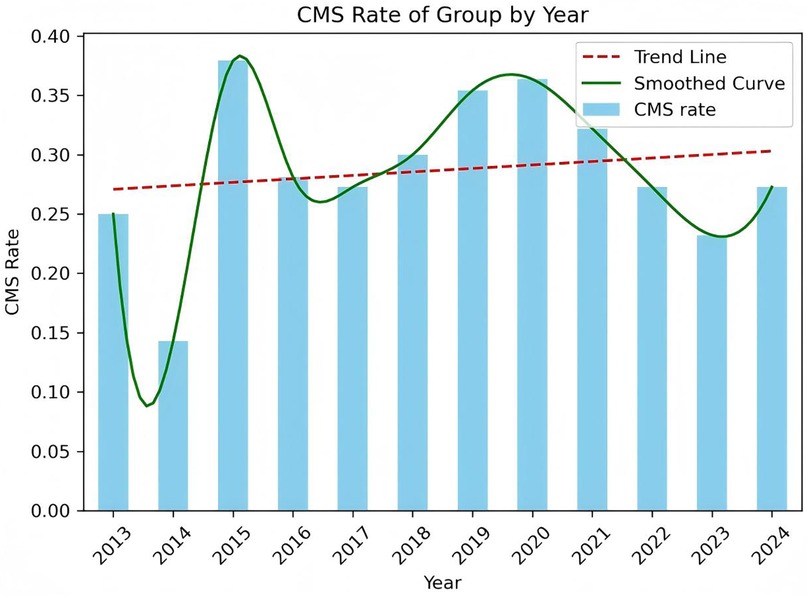
A total of 385 pediatric patients met the inclusion criteria and were analyzed. The cohort included 59.0% males, with a median age at surgery of 5.0 years (IQR: 3.0–7.9 years). The age distribution of the cohort, CMS and Non-CMS group were presented in Figures 2, 3. Tumors were predominantly located at the midline (68.1%), and gross total resection was achieved in 86.3% of cases. Medulloblastoma represented the most common pathology (38.4%), with tumors exhibiting a median maximal diameter of 47.2 mm (IQR: 39.1–55.2 mm). Preoperative VP shunt placement was performed in 9.1% of patients. CMS was observed in 29.9% of patients (115 out of 385). The incidence of CMS varied across study periods, ranging from 14.3% to 37.9% (Figure 4).
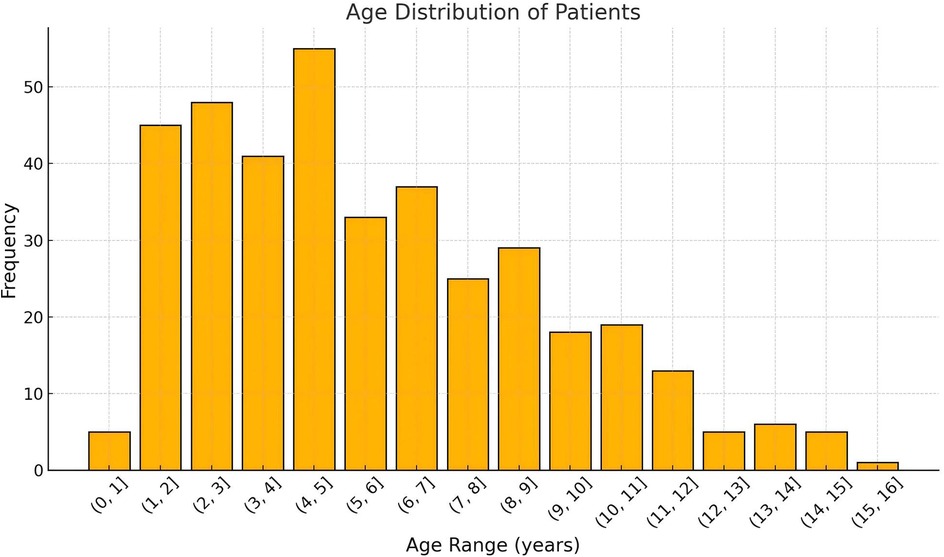
Figure 2. The overall age distribution of patients undergoing posterior fossa tumor surgery. The x-axis represents the age range in years, grouped in intervals of 1 year, while the y-axis indicates the number of patients in each age range. The histogram shows the frequency of patients in each age group, highlighting the predominant age ranges for surgical cases.
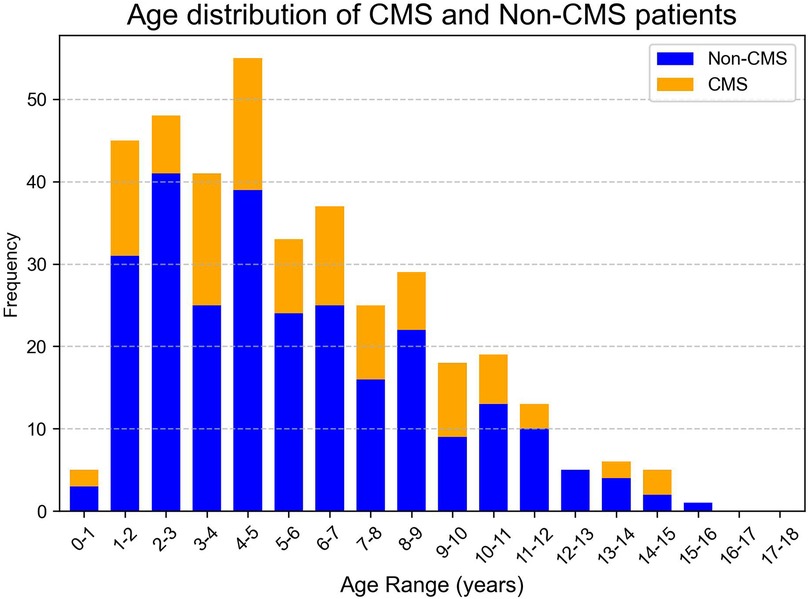
Figure 3. Age frequency distribution of CMS and Non-CMS groups. The x-axis represents the age range in years, grouped in intervals of 1 year, while the y-axis indicates the frequency as a proportion of the total number of patients in each group. The blue bars represent the CMS group, and the orange bars represent the Non-CMS group.
Comparison of CMS and non-CMS group
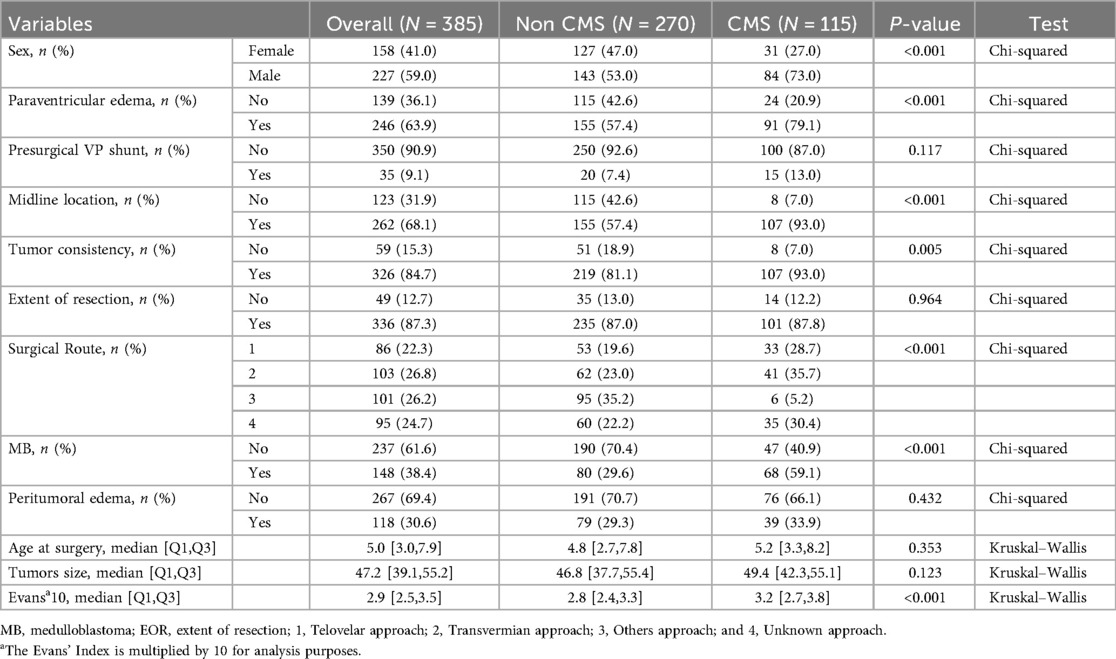
The comparative analysis between the CMS (n = 115) and non-CMS (n = 270) groups revealed several statistically significant differences (Table 1). The CMS group exhibited a markedly higher proportion of male patients (73.0% vs. 53.0%; p < 0.001), midline tumor location (93.0% vs. 57.4%; p < 0.001), solid tumor texture (93.0% vs. 81.1%; p = 0.004), paraventricular edema (79.1% vs. 57.4%; p < 0.001), and medulloblastoma pathology (59.1% vs. 29.6%; p < 0.001). The adjusted Evans’ index was also significantly higher in the CMS group (median 3.2 vs. 2.8; p < 0.001). Additionally, the selection of surgical routes presented a significant difference between the CMS and Non-CMS groups, as detailed in Table 1.
No significant disparities were observed between the two groups regarding VP shunt placement, age at surgery, extent of resection, maximal tumor diameter, and the presence of peritumoral edema.
Follow-up and symptom duration
Follow-up data on the duration of mutism were available for 98 of the 115 patients with CMS (85.2%). The median duration of mutism was 6.5 weeks [Interquartile Range (IQR): 3.0–12.0 weeks]. A comparison between sexes revealed no statistically significant difference in the duration of mutism (median 7.0 weeks for males vs. 6.0 weeks for females, p = 0.48, Kruskal–Wallis test).
Univariable and multivariable analysis
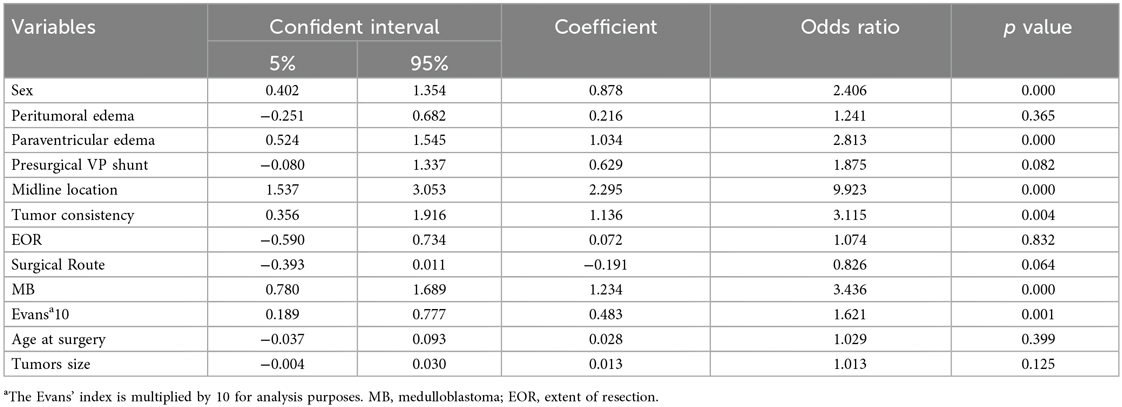
Univariable analysis identified male sex [OR 2.37; 95% CI (1.50–3.76); p < 0.001], midline tumor location [OR 9.02; 95% CI (4.31–18.87); p < 0.001], MB pathology [OR 3.37; 95% CI (2.13–5.34); p < 0.001], paraventricular edema [OR 2.73; 95% CI (1.70–4.39); p < 0.001], solid tumor consistency [OR 3.23; 95% CI (1.43–7.29); p = 0.004], and the adjusted Evans’ Index [OR 1.07 per 0.01 increase; 95% CI (1.03–1.11); p = 0.001] as factors significantly associated with CMS (Table 2).
A stepwise logistic regression analysis was performed to identify independent risk factors for CMS. Variables with a p-value less than 0.01 were included in the model, and those with a p-value greater than 0.1 were excluded. The analysis revealed that male sex (OR = 2.20, 95% CI: 1.30–3.70, p = 0.003), midline tumor location (OR = 7.47, 95% CI: 2.79–19.98, p < 0.001), and MB pathology type (OR = 2.11, 95% CI: 1.24–3.59, p = 0.003) were independently associated with an increased risk of CMS. These results are detailed in Table 3. A collinearity analysis confirmed the absence of multicollinearity among these variables (VIF < 5 for all variables).
Discussion
This study investigated the risk factors associated with CMS in pediatric patients undergoing posterior fossa tumor surgery, focusing on the role of sex, which has been a subject of debate with inconsistent findings across studies (17, 18). We found that male sex is an independent risk factor for CMS in a large Chinese cohort, along with midline tumor location and medulloblastoma pathology.
Our findings suggest that males are more susceptible to developing CMS. Importantly, this association persisted in our multivariable model after adjusting for both midline location and medulloblastoma pathology, indicating that the observed male predisposition is not solely explained by these established risk factors. Although the effect size for male sex is smaller than for midline location, this more than two-fold increased risk remains clinically relevant as a non-modifiable factor. Such findings can inform surgical planning, enhance parental counseling, and guide targeted postoperative monitoring strategies. The underlying mechanism may be related to sex-specific differences in language development and cerebellar function. The precise mechanism linking male gender to CMS is yet to be elucidated. We hypothesize that CMS is associated with language deficits stemming from brain development, with the cerebellum potentially playing a significant role in early language acquisition. The prevailing hypothesis attributes CMS to damage in the cerebral-cerebellar circuit; however, the specific functioning of this circuit in maintaining language and the cerebellum's role in speech processing remain obscure.
Several studies provide supporting evidence for our hypothesis: (1) A recent study indicates that children with CMS exhibit involvement in brain networks associated with Autism Spectrum Disorder (ASD) (19), highlighting similarities between the two language disorders. ASD is characterized by communication and social interaction deficits and shows a notable gender bias, with a male-to-female ratio of 4:1 (20). The enhanced plasticity theory posits that males have a lower threshold for developing an enhanced plastic response to environmental stimuli compared to females, which could affect specific brain regions involved in perception and language (21). (2) Girls tend to have an advantage over boys in the early stages of language acquisition (22). Girls outperform boys in word production tasks during early language development, with this disparity widening with age. However, by school age, the gender effect on language performance diminishes and may vanish (23). Yet, previous studies have not consistently identified males as an independent risk factor for CMS (2, 18, 24). The discrepancy may be attributed to differences between Chinese and Western language systems, as phonological processing in English and Chinese engages distinct brain regions (25). Chinese phonological processing recruits the middle frontal cortex, motor cortex, and supplementary motor area in the left hemisphere (26), whereas for native English speakers, it engages the left inferior prefrontal cortex and superior temporal gyrus (27). Furthermore, research has shown that language processing differences between children and adults differs developmentally between language, indicating developmental effects on the language network (28). This evidence may explain why male gender was not identified as an independent risk factor in some studies.
In our investigation, we have identified midline tumor location and MB as independent risk factors for CMS. Extensive literature supports the idea that midline location is associated with an increased risk of CMS, whereas tumors located in the cerebellar hemispheres are linked to a protective effect and seldom progress to CMS. While midline location was a powerful predictor in our model, we did not specifically analyze brainstem invasion as a variable. We acknowledge this as a limitation. However, it is a clinical reality that the large midline tumors typically seen in this pediatric population (median diameter 47.2 mm in our cohort) almost invariably lead to brainstem compression. Thus, the “midline location” variable likely reflects the significant risk associated with brainstem involvement. MB is recognized as a significant and established risk factor for CMS, with recent findings highlighting associations a correlation between the molecular subtypes of MB and the development of CMS. Specifically, the Wingless, Group 3, and Group 4 subtypes are associated with a notably higher incidence of CMS than the sonic hedgehog subtype independent of other risk factors. Additionally, a compelling body of evidence has emerged linking CMS with the malignancy grade of tumors, with MB exhibiting a higher grade in comparison to astrocytoma and ependymoma. Despite the majority of MBs being midline in location, the interplay between MB and midline positioning has not been extensively explored. Utilizing stepwise logistic regression analysis in our study, we have determined that both midline location and MB independently contribute to the risk of CMS. Furthermore, our collinearity analysis has confirmed the absence of multicollinearity among the variables examined.
Beyond the clinical risk factors identified in this large cohort, the specific neural structures damaged during surgery are paramount in the development of CMS. The reviewer rightly points to the importance of features like superior cerebellar peduncle (SCP) and dentate nucleus (DN) involvement. Indeed, our group has previously published a detailed analysis of postoperative MRI features in a cohort from our institution (29). That study demonstrated that postoperative injury to the bilateral SCPs and DNs, quantified as a cerebro-cerebellar circuit injury score on T2-weighted MRI, was a powerful independent predictor of both the occurrence and duration of CMS. The current study complements these detailed radiological findings by validating, in a larger and more heterogeneous population, that male sex stands as an independent clinical risk factor alongside broad predictors like midline location and medulloblastoma pathology.
In our study, we investigated the association of various factors, including age at the time of surgery, maximum tumor diameter, presence of paraventricular edema, and Evan's index, with CMS. However, none of these variables showed an independent association with CMS. The link between age and CMS remains enigmatic. Our results align with several previous studies (2, 9, 30), which also reporting no significant association between age and CMS. In contrast, other studies have identified a younger age as an independent risk factor for CMS (17, 31–33). This discrepancy in findings might be due to sample variability or potential biases in the diagnosis of CMS. Further research is warranted to elucidate the relationship between age at the time of surgery and the development of CMS.
The association between Evans’ index and CMS remains controversial. Although Tian et al. proposed that a rapid decrease in intracranial pressure may contribute to CMS development (34), a meta-analysis did not uncover any significant association between CMS and hydrocephalus (18). This finding is further supported by Keating et al., who observed no significant difference in cerebrospinal fluid (CSF) diversion between the CMS and non-CMS groups (35), which corroborates our results. Despite these findings, the role of certain risk factors remains ambiguous and necessitates future validation. Establishing a rigorous, explicit, practical, and universally applicable definition of CMS, potentially through multicenter collaboration, could address these ambiguities. As Wickenhauser proposed, defining specific diagnostic criteria for CMS would enhance both research and clinical practice (36).
Our data presented a significant year-to-year variation in CMS incidence, which ranged from 14.3% to 37.9%. During 2013–2014, the patient cohort size was particularly small (n < 10), which lead to the statistical instability and larger fluctuations in the CMS rates. In the subsequent period, when the annual patient numbers were larger and more stable, the CMS incidence rate also stabilized, generally fluctuating between 25% and 35%.
Our study, however, has limitations. Firstly, it is a single-center, retrospective study, which may limit the generalizability of our findings. Secondly, data on factors such as handedness, medulloblastoma molecular subtypes, and surgical approaches were incomplete and not included in the analysis. Third, while our group has previously published on the importance of detailed postoperative radiological features like superior cerebellar peduncle and dentate nucleus injury in predicting CMS (29), a detailed re-analysis of these specific features for the entire 385-patient cohort was beyond the scope of the current clinical validation study. Furthermore, data on brainstem invasion, medulloblastoma molecular subtypes, intraoperative technologies such as intraoperative imaging and neuromonitoring were not consistently available across the study period and were therefore not included in our analysis. These factors represent important areas for future prospective, multicenter studies. Finally, the lack of a universally adopted, stringent definition for CMS remains a challenge for the entire field.
Conclusion
This study demonstrates that male sex is an independent risk factor for developing CMS in pediatric patients undergoing posterior fossa tumor surgery. Along with midline tumor location and medulloblastoma pathology, male sex contributes to increased CMS susceptibility. The identification of male sex as an independent risk factor, despite a moderate effect size, underscores its value for risk stratification and future mechanistic studies. The intrinsic mechanisms driving this sex-based disparity in CMS susceptibility are yet to be fully elucidated. One plausible explanation may lie in the divergent developmental milestones of language acquisition between the sexes, potentially predisposing males to a greater risk of CMS. Further research is needed to elucidate the mechanisms behind this sex disparity and to develop targeted interventions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Beijing Children's Hospital Ethic Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective study. Informed consent was obtained from all patients or their guardians.
Author contributions
XP: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. ZZ: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. XC: Data curation, Investigation, Methodology, Writing – review & editing. HZ: Conceptualization, Formal analysis, Resources, Writing – review & editing. YC: Data curation, Formal analysis, Investigation, Resources, Writing – review & editing. KZ: Data curation, Formal analysis, Resources, Writing – review & editing. NZ: Data curation, Formal analysis, Methodology, Resources, Writing – review & editing. JW: Investigation, Methodology, Resources, Writing – review & editing. HS: Data curation, Resources, Writing – review & editing. GY: Data curation, Formal analysis, Resources, Writing – review & editing. WM: Conceptualization, Investigation, Project administration, Resources, Writing – review & editing. WY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. MG: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Beijing Municipal Science & Technology Commission Proof of Concept Center (No. 2024030119 to Xin Ni). Beijing Municipal Science & Technology Commission, Administrative Commission of Zhongguancun Science Park (No. Z241100007724008 to Shuli Liang).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issue please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fabozzi F, Margoni S, Andreozzi B, Musci MS, Del Baldo G, Boccuto L, et al. Cerebellar mutism syndrome: from pathophysiology to rehabilitation. Front Cell Dev Biol. (2022) 10:1082947. doi: 10.3389/fcell.2022.1082947
2. Robertson PL, Muraszko KM, Holmes EJ, Sposto R, Packer RJ, Gajjar A, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the children’s oncology group. J Neurosurg. (2006) 105(6 Suppl):444–51. doi: 10.3171/ped.2006.105.6.444
3. Catsman-Berrevoets CE. Cerebellar mutism syndrome: cause and rehabilitation. Curr Opin Neurol. (2017) 30(2):133–9. doi: 10.1097/WCO.0000000000000426
4. Schmahmann JD. Emotional disorders and the cerebellum: neurobiological substrates, neuropsychiatry, and therapeutic implications. Handb Clin Neurol. (2021) 183:109–54. doi: 10.1016/B978-0-12-822290-4.00016-5
5. Thacker N, Bouffet E. Posterior fossa syndrome-time to unmute the silence on cerebellar mutism. Neuro Oncol. (2021) 23(9):1427–8. doi: 10.1093/neuonc/noab147
6. Toescu SM, Hettige S, Phipps K, Smith RJP, Haffenden V, Clark C, et al. Post-operative paediatric cerebellar mutism syndrome: time to move beyond structural MRI. Childs Nerv Syst. (2018) 34(11):2249–57. doi: 10.1007/s00381-018-3867-x
7. Kupeli S, Yalcin B, Bilginer B, Akalan N, Haksal P, Buyukpamukcu M. Posterior fossa syndrome after posterior fossa surgery in children with brain tumors. Pediatr Blood Cancer. (2011) 56(2):206–10. doi: 10.1002/pbc.22730
8. Bakhshi SK, Mitha R, Mushtaq N, Shamim MS. Cerebellar mutism syndrome after surgical resection of posterior fossa neoplastic lesions. J Pak Med Assoc. (2020) 70(9):1667–8.33040137
9. Ashida R, Nazar N, Edwards R, Teo M. Cerebellar mutism syndrome: an overview of the pathophysiology in relation to the cerebrocerebellar anatomy, risk factors, potential treatments, and outcomes. World Neurosurg. (2021) 153:63–74. doi: 10.1016/j.wneu.2021.06.065
10. Catsman-Berrevoets CE, Aarsen FK. The spectrum of neurobehavioural deficits in the posterior Fossa syndrome in children after cerebellar tumour surgery. Cortex. (2010) 46(7):933–46. doi: 10.1016/j.cortex.2009.10.007
11. Gora NK, Gupta A, Sinha VD. Cerebellar mutism syndrome following midline posterior Fossa tumor resection in children: an institutional experience. J Pediatr Neurosci. (2017) 12(4):313–9. doi: 10.4103/JPN.JPN_23_17
12. Yang W, Ge M, Zhu K, Chen J, Yang P, Cai Y, et al. Male predisposition in cerebellar mutism syndrome: a cohort study. Cerebellum. (2023) 22(4):730–8. doi: 10.1007/s12311-022-01449-6
13. Adani S, Cepanec M. Sex differences in early communication development: behavioral and neurobiological indicators of more vulnerable communication system development in boys. Croat Med J. (2019) 60(2):141–9. doi: 10.3325/cmj.2019.60.141
14. Etchell A, Adhikari A, Weinberg LS, Choo AL, Garnett EO, Chow HM, et al. A systematic literature review of sex differences in childhood language and brain development. Neuropsychologia. (2018) 114:19–31. doi: 10.1016/j.neuropsychologia.2018.04.011
15. Yu VY, MacDonald MJ, Oh A, Hua GN, De Nil LF, Pang EW. Age-related sex differences in language lateralization: a magnetoencephalography study in children. Dev Psychol. (2014) 50(9):2276–84. doi: 10.1037/a0037470
16. Gudrunardottir T, Morgan AT, Lux AL, Walker DA, Walsh KS, Wells EM, et al. Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv Syst. (2016) 32(7):1195–203. doi: 10.1007/s00381-016-3093-3
17. Gronbaek JK, Wibroe M, Toescu S, Fric R, Thomsen BL, Moller LN, et al. Postoperative speech impairment and surgical approach to posterior fossa tumours in children: a prospective European multicentre cohort study. Lancet Child Adolesc Health. (2021) 5(11):814–24. doi: 10.1016/S2352-4642(21)00274-1
18. Pettersson SD, Kitlinski M, Miekisiak G, Ali S, Krakowiak M, Szmuda T. Risk factors for postoperative cerebellar mutism syndrome in pediatric patients: a systematic review and meta-analysis. J Neurosurg Pediatr. (2022) 29(4):467–75. doi: 10.3171/2021.11.PEDS21445
19. Suresh H, Morgan BR, Mithani K, Warsi NM, Yan H, Germann J, et al. Postoperative cerebellar mutism syndrome is an acquired autism-like network disturbance. Neuro Oncol. (2024) 26(5):950–64. doi: 10.1093/neuonc/noad230
20. Dougherty JD, Marrus N, Maloney SE, Yip B, Sandin S, Turner TN, et al. Can the “female protective effect” liability threshold model explain sex differences in autism spectrum disorder? Neuron. (2022) 110(20):3243–62. doi: 10.1016/j.neuron.2022.06.020
21. Calderoni S. Sex/gender differences in children with autism spectrum disorder: a brief overview on epidemiology, symptom profile, and neuroanatomy. J Neurosci Res. (2023) 101(5):739–50. doi: 10.1002/jnr.25000
22. Chilosi AM, Brovedani P, Cipriani P, Casalini C. Sex differences in early language delay and in developmental language disorder. J Neurosci Res. (2023) 101(5):654–67. doi: 10.1002/jnr.24976
23. Rinaldi P, Pasqualetti P, Volterra V, Caselli MC. Gender differences in early stages of language development. Some evidence and possible explanations. J Neurosci Res. (2023) 101(5):643–53. doi: 10.1002/jnr.24914
24. de Laurentis C, Cristaldi PMF, Rebora P, Valsecchi MG, Biassoni V, Schiavello E, et al. Posterior fossa syndrome in a population of children and young adults with medulloblastoma: a retrospective, multicenter Italian study on incidence and pathophysiology in a histologically homogeneous and consecutive series of 136 patients. J Neurooncol. (2022) 159(2):377–87. doi: 10.1007/s11060-022-04072-x
25. Tan LH, Laird AR, Li K, Fox PT. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: a meta-analysis. Hum Brain Mapp. (2005) 25(1):83–91. doi: 10.1002/hbm.20134
26. Tan LH, Spinks JA, Eden GF, Perfetti CA, Siok WT. Reading depends on writing, in Chinese. Proc Natl Acad Sci U S A. (2005) 102(24):8781–5. doi: 10.1073/pnas.0503523102
27. Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong J, et al. Neural systems of second language reading are shaped by native language. Hum Brain Mapp. (2003) 18(3):158–66. doi: 10.1002/hbm.10089
28. Cao F, Fan Y, Yan X, Chen W, Dodson-Garrett M, Spray GJ, et al. Greater similarity between L1 and L2’s brain network in adults than in children. Front Neurosci. (2022) 16:816729. doi: 10.3389/fnins.2022.816729
29. Yang W, Zhang H, Cai Y, Peng X, Sun H, Chen J, et al. Postoperative MRI features of cerebellar mutism syndrome: a retrospective cohort study. J Neurosurg Pediatr. (2022) 30(6):567–77. doi: 10.3171/2022.8.PEDS22294
30. Pols S, van Veelen MLC, Aarsen FK, Gonzalez Candel A, Catsman-Berrevoets CE. Risk factors for development of postoperative cerebellar mutism syndrome in children after medulloblastoma surgery. J Neurosurg Pediatr. (2017) 20(1):35–41. doi: 10.3171/2017.2.PEDS16605
31. Boerger TF, Pahapill P, Butts AM, Arocho-Quinones E, Raghavan M, Krucoff MO. Large-scale brain networks and intra-axial tumor surgery: a narrative review of functional mapping techniques, critical needs, and scientific opportunities. Front Hum Neurosci. (2023) 17:1170419. doi: 10.3389/fnhum.2023.1170419
32. Ricci FS, D'Alessandro R, Soma A, Salvalaggio A, Rossi F, Rampone S, et al. Development and application of a diagnostic and severity scale to grade post-operative pediatric cerebellar mutism syndrome. Eur J Pediatr. (2022) 181(3):941–50. doi: 10.1007/s00431-021-04290-x
33. Khan RB, Patay Z, Klimo P, Huang J, Kumar R, Boop FA, et al. Clinical features, neurologic recovery, and risk factors of postoperative posterior fossa syndrome and delayed recovery: a prospective study. Neuro Oncol. (2021) 23(9):1586–96. doi: 10.1093/neuonc/noab030
34. Zhang H, Liao Z, Hao X, Han Z, Li C, Gong J, et al. Establishing reproducible predictors of cerebellar mutism syndrome based on pre-operative imaging. Childs Nerv Syst. (2019) 35(5):795–800. doi: 10.1007/s00381-019-04075-6
35. Cobourn K, Marayati F, Tsering D, Ayers O, Myseros JS, Magge SN, et al. Cerebellar mutism syndrome: current approaches to minimize risk for CMS. Childs Nerv Syst. (2020) 36(6):1171–9. doi: 10.1007/s00381-019-04240-x
Keywords: cerebellar mutism syndrome, sex differences, pediatric neurosurgery, posterior fossa tumor, risk factors
Citation: Peng X, Zhi Z, Chai X, Zhang H, Cai Y, Zhu K, Zhang N, Wang J, Sun H, Yin G, Ma W, Yang W and Ge M (2025) Sex as an independent risk factor for cerebellar mutism syndrome: a validation study. Front. Surg. 12:1645832. doi: 10.3389/fsurg.2025.1645832
Received: 17 June 2025; Accepted: 28 August 2025;
Published: 15 September 2025.
Edited by:
Junjie Jing, Fujian Medical University, ChinaReviewed by:
Iroda Mammadinova, National Center for Neurosurgery, KazakhstanEmily Hanzlik, St. Jude Children's Research Hospital, United States
Copyright: © 2025 Peng, Zhi, Chai, Zhang, Cai, Zhu, Zhang, Wang, Sun, Yin, Ma, Yang and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Ge, bWluZ19nZUAxMjYuY29t; Wei Yang, ZHIueWFuZ3dlaUBmb3htYWlsLmNvbQ==; Wenping Ma, bWF3ZW5waW5nQGJqbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Xiaojiao Peng1,†
Xiaojiao Peng1,† Xinyi Chai
Xinyi Chai Hong Zhang
Hong Zhang Yingjie Cai
Yingjie Cai Kaiyi Zhu
Kaiyi Zhu Nijia Zhang
Nijia Zhang Wenping Ma
Wenping Ma Wei Yang
Wei Yang Ming Ge
Ming Ge