- 1Department of Otorhinolaryngology, Head and Neck Surgery, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 2Université Paris Cité, Institut Pasteur, AP-HP, Inserm, CNRS, Fondation Pour l’Audition, Institut de l’Audition, IHU ReConnect, Technologies et Thérapie Génique Pour la Surdité, Paris, France
- 3Department of Otorhinolaryngology, Head and Neck Surgery, CHUV, University of Lausanne, Lausanne, Switzerland
- 4The Sense Innovation and Research Center, Lausanne and Sion, Lausanne, Switzerland
Purpose: To compare the outcomes of endoscopic and microscopic tympanoplasty (TPL) types I-III in pediatric patients.
Methods: A retrospective case-control study was conducted on 70 TPL cases in 58 pediatric patients at Inselspital, Bern University Hospital, Switzerland, from June 2017 to December 2023. Data on hearing function, graft intake, residual disease, operating time and complications were collected.
Results: We observed mean postoperative air-bone gap (ABG) of 16.83 dB using the endoscopic and 19.37 dB for the microscopic techniques, as well as a higher graft intake rate (91%) for the endoscopic compared to the microscopic group (80%), although these differences did not reach statistical significance. No residual cholesteatoma was found in the endoscopic group, while the microscopic group had a significantly higher incidence of residual disease (42%; p = .03). The mean operative time was shorter in the endoscopic group (87 min vs. 113 min; p < .01). Postoperative complications were lower in the endoscopic group compared to a 14% incidence in the microscopic group.
Conclusion: Endoscopic tympanoplasty in pediatric patients achieves similar audiological outcomes and graft intake rates compared to the microscopic approach while offering significant advantages, including reduced operation times and lower complication rates. This minimally invasive approach is highly effective especially regarding cholesteatoma resection and provides excellent functional and structural outcomes.
Introduction
Children with chronic otitis media (COM) suffer from tympanic membrane (TM) perforation, which may be associated with ossicular chain disruption, leading to conductive hearing loss and more or less frequent superinfections. At a young age impaired hearing negatively affects language acquisition and learning development (1). Tympanoplasty (TPL) represents the surgical gold standard treatment aiming to remove the disease, repair the TM and restore ossicular chain integrity. While the operation was historically commonly approached with a microscope, the use of an endoscope has been increasingly reported in the last decades. Studies on surgical hearing restoration favor less invasive transcanal methods, which reduce the need for extensive bone drilling and postauricular incisions (2). These surgical approaches result in shorter hospital stays and faster return to physical activities (3).
While at birth the dimensions of the TM and middle ear are comparable to those of adults, the external auditory canal (EAC) is subject to significant growth approximately until the age of six (4, 5). The question arises, whether transcanal surgery and in particular endoscopic surgery is suited for the pediatric population. It has been observed, that the relatively short EAC in children permits wide-ranging maneuverability of the endoscope and instruments, compensating for its narrow diameter. Moreover, the wide-angle view from the tip of the endoscope provides a panoramic view of the middle ear recesses (6). The extent to which a microscope or an endoscope is used in pediatric ear surgery depends largely on the need of bone drilling, the ear canal condition, available resources, and surgeon expertise. With appropriate circumstances, a full range of otologic procedures such as cholesteatoma removal, all types of TPL, stapes surgery and treatment of skull base pathology can be performed partially or entirely using the endoscopic technique (7–10).
Comparative studies between endoscopic and microscopic techniques have reported similar auditory outcomes and graft intake rates. However, the endoscopic approach has shown potential benefits, such as reduced postoperative pain, faster recovery, and lower rates of residual cholesteatoma (11–14). At our center, both transcanal endoscopic and microscopic approaches are routinely employed. The aim of this retrospective case-control study is to compare the outcome of the two techniques in TPL type I-III according to the Wullstein classification (15) in pediatric patients.
Materials and methods
Pediatric patients who underwent endoscopic or microscopic TPL type I-III with or without cholesteatoma at our tertiary referral center, in the period between June 2017 and December 2023 were retrospectively reviewed. A total of 70 consecutive ears involving 58 patients younger than 18 years old, who underwent Type I-III TPL were included. Exclusion criteria included lack of available pre- and postoperative audiometric data and less than 3 months of audiometric and clinical follow up (FU). Institutional review board approval was obtained at the institutional and regional review board (Kantonale Ethikkomission, KEK-BE 2019-00555), and a waiver of informed consent was granted for this retrospective study.
Surgical techniques
A total of 35 consecutive endoscopic approaches were matched with consecutive microscopic cases. At our institution, the endoscopic technique was newly introduced during the study period. Surgeons who adopted the endoscopic approach consistently applied it to all cases, whereas those not yet familiar with the technique continued to utilize the microscopic approach. Thus, the choice of surgical method was not made on a per-case basis, but rather reflected a transition in institutional practice and individual surgeon expertise. This allowed for a direct comparison of outcomes between the two approaches within a comparable pediatric population. The surgical approach involved either the endoscopic method or the microscopic “inside-out” technique, eradicating the disease from the middle ear toward the mastoid (16). The use of the endoscope in the respective cases was classified by Cohen et al. (17) Cases rated as class 2b or higher were categorized within the endoscopic group, including cases requiring mastoidectomy due to disease extension beyond the lateral semicircular canal into the mastoid. In these cases, after mastoidectomy, the reconstruction of the middle ear was conducted endoscopically (Cohen Class 2b). In all cases, the defect of the posterior canal wall end epitympanum was reconstructed with cartilage. Only one case requiring canal wall down mastoidectomy underwent obliteration of the cavity using a pedicled random flap. Matching was based on surgical indication, type of ossicular chain reconstruction, and age. All type I TPL were performed in underlay technique. If a cartilaginous graft was used, the perichondrium was removed from the concave surface while preserved on the convex side, and the graft was positioned with the perichondral surface facing laterally. Incus interposition, Titanium partial ossicular replacement prostheses (PORP) and total ossicular replacement prosthesis (TORP), cartilage interposition, and bone cement ossiculoplasty (OPL) were included as methods of OPL.
Data collection
Data regarding pre- and postoperative hearing function, status of the TM and the ossicular chain, presence and extent of the cholesteatoma according to EAONO-JOS classification (18) or the underlying disease, surgical procedure, type of graft or prosthesis used, and perioperative complications were retrospectively collected from patients' charts. Where necessary, the surgical videos were reviewed. Postoperative assessment was carried out 3 months after surgery. Bone conduction (BC) and air conduction (AC) pure-tone average (PTA) were calculated, from pre- and postoperative pure-tone audiometry, as the mean value among thresholds at 0.25, 0.5, 1, 2, and 4 kHz frequencies. Mean air–bone gap (ABG) was calculated as the difference between AC-PTA and BC-PTA, while ABG improvement was calculated as the difference between mean pre- and mean postoperative ABG, with a positive value indicating an improvement and a negative value indicating a worse postoperative gap. All cases of cholesteatoma underwent primary OPL and received radiological FU with diffusion-weighted MRI one year after the surgery. Residual cholesteatoma was defined as cholesteatoma behind an intact neotympanum. In contrast, recurrent cholesteatoma is the appearance of a recurrent TM retraction and cholesteatoma.
Statistical analysis
Continuous variables such as audiometric outcomes and operative times were compared between the endoscopic and microscopic techniques using unpaired t-tests, whereas paired t-tests were applied within each group to compare pre- and postoperative audiometric outcomes. Categorical variables including graft intake and complications were compared using Fisher's exact test.
Statistical significance was set at p < .05. Results are presented as mean ± standard deviation for continuous variables and as proportions for categorical variables. Statistical analyses were performed with GraphPad Prism 10.0 (GraphPad, La Jolla, CA).
Results
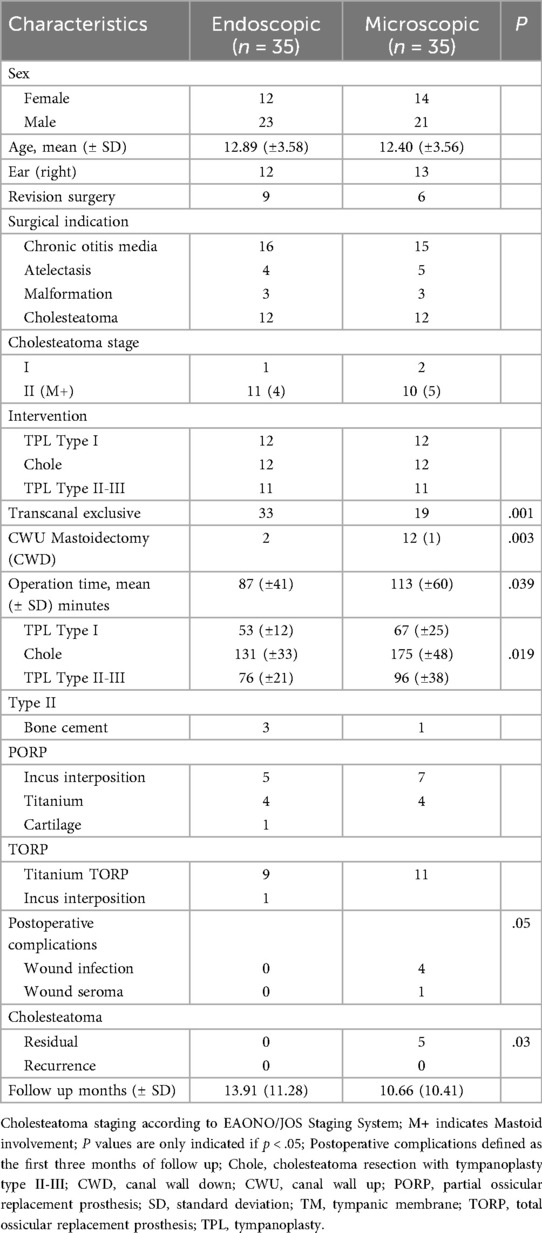
In this retrospective case-control study we matched a total of 70 consecutive endoscopic and microscopic TPL type I–III. The patients' age ranged from 4 to 17 with a mean age of 12.89 years (SD ± 3.58) in the endoscopic and 12.40 years (±3.56) in the microscopic group. The patient's characteristics are summarized in Table 1. COM was the primary surgical indication in both groups, with 16 cases in the endoscopic and 15 in the microscopic group. Cholesteatoma was found in an additional 12 cases per group. Other indications included conductive hearing loss due to atelectasis or malformation of the ossicular chain with intact footplate mobility. Nine endoscopic and six microscopic cases were revision surgeries due to persistent or recurrent disease.
In type I TPL the reconstruction material in the endoscopic group included cartilage (n = 8), temporalis fascia (n = 3), and artificial membrane (n = 1), achieving a graft intake rate of 91.66%. In contrast, the microscopic group used fascia (n = 8) and cartilage (n = 4), with a lower graft intake rate of 58.33% (p = .16). A total of 46 ossicular chain reconstructions were conducted, including 13 PORP and 10 TORP in the endoscopic group, and 12 PORP and 11 TORP in the microscopic group, utilizing various reconstruction materials. Both techniques showed comparable graft intake rates, as indicated in Figure 1, with one postoperative prosthesis dislocation in the microscopic group.
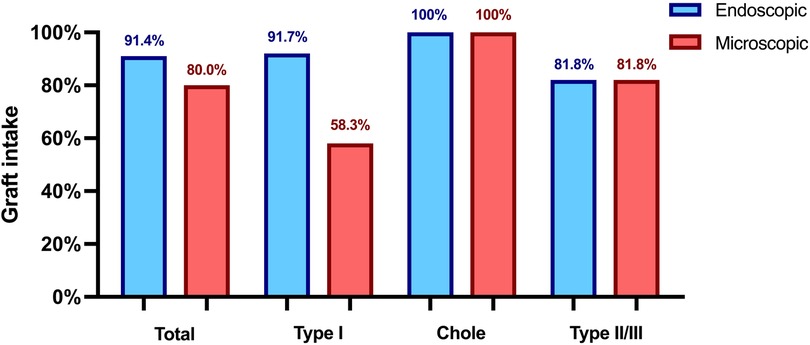
Figure 1. Graft intake rate 3 months postoperatively. Chole, cholesteatoma resection with tympanoplasty type II or III; Type I, tympanoplasty type I; Type II/III, tympanoplasty type II or III.
Audiological assessment revealed a statistically significant higher ABG improvement in the endoscopic group, with an average improvement of 14.7 dB compared to 7.9 dB in the microscopic group (p = .02). Preoperative ABG was higher in the endoscopic cohort with 31.5 dB (±12.13), compared to 26.4 dB (±12.10) not reaching statistical significance (p = .09). Tables 2a, 2b illustrate pre- and postoperative ABG according to the two techniques. As depicted in Figure 2, the postoperative ABG values were comparable between the endoscopic 16.83 dB (±7.74) and microscopic 19.37 dB (±11.01) approaches (p = .27).
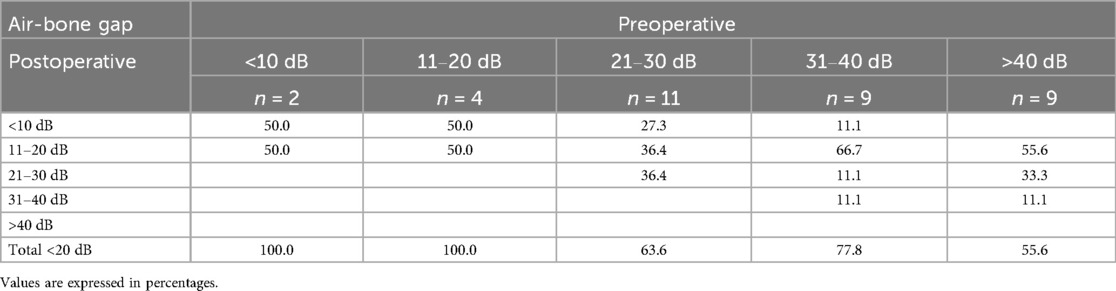
Table 2a. Endoscopic technique: breakdown of postoperative air-bone gap of 20 dB or lower according to the preoperative hearing loss.
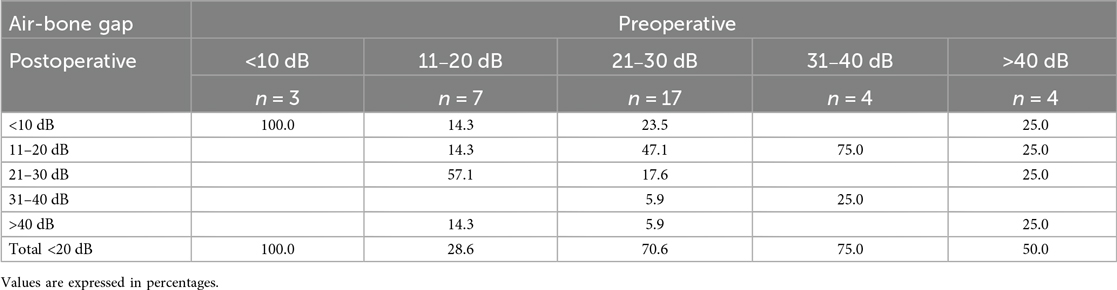
Table 2b. Microscopic technique: breakdown of postoperative air-bone gap of 20 dB or lower according to the preoperative hearing loss.
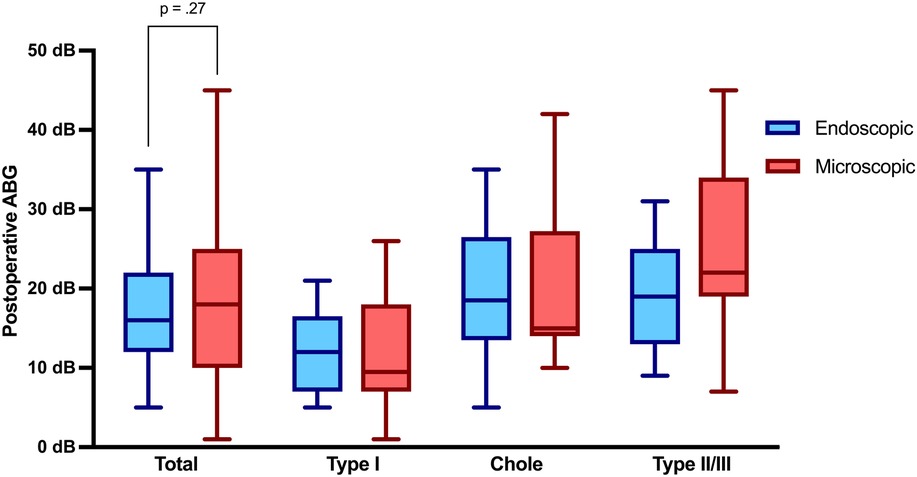
Figure 2. Air-bone gap 3 months postoperatively. ABG, air-bone gap; Chole, cholesteatoma resection with tympanoplasty Type II or III; Type I, tympanoplasty type I; Type II/III, tympanoplasty type II or III.
The overall mean operation time was statistically significantly shorter in the endoscopic group with 87 min (± 41) compared to 113 min (±60) in the microscopic group (p = .04), particularly during cholesteatoma resection. Exclusive transcanal surgery was performed in 33 of the 35 endoscopic cases, while two cases needed mastoidectomy due to disease extension (Cohen 2b). In contrast, 16 microscopic cases required a retroauricular approach, with a statistically significant increased number of mastoidectomies performed (p = .03). Postoperative FU revealed higher complication rates in cases requiring a retroauricular approach, with four incidents of wound infection and one seroma, necessitating surgical revision (p = .05). In our cohort, the mean FU period was 13.91 months (±11.28) for the endoscopic group and 10.66 months (±10.41) for the microscopic group. Specifically, among cholesteatoma cases, the mean FU period was longer with 18.58 months (±12.70) for the endoscopic group and 15.17 months (±10.45) for the microscopic group. Residual cholesteatoma was significantly more frequent in the microscopic group (p = .03), while no recurrences were reported in either group.
Discussion
This retrospective case-control study evaluates the outcomes of endoscopic vs. microscopic techniques in pediatric TPL types I-III. The two approaches showed comparable postoperative ABG values, while the endoscopic approach showed significantly higher ABG closure and shorter operating times. Additionally, it allowed for more frequent total transcanal surgeries, minimizing the need for retroauricular approaches, while reducing the incidence of residual cholesteatoma and postoperative complications.
Audiological outcome and graft intake
Previous studies have reported similar audiological outcomes between endoscopic and microscopic techniques in pediatric ear surgeries (12, 14, 19). Consistent with these findings, postoperative ABG values in our cohort were comparable between the two techniques. However, ABG closure was significantly increased (p = .02) in the endoscopic group, particularly in type II and III TPL without cholesteatoma. The enhanced visualization provided by endoscopy, which offers better illumination of anatomical details of the middle ear compared to an operating microscope, may contribute to more accurate prosthesis placement and, consequently, improved hearing outcomes. Another factor in the described cohort was the higher preoperative ABG in the endoscopic group facilitating an increased ABG closure. Among the type I TPL and cholesteatoma cases, ABG closure was comparable between the endoscopic and microscopic techniques. Enhanced graft placement in the endoscopic technique might also contribute to the higher graft intake rates observed in our type I TPL cohort (92% vs. 58%), although this difference did not reach statistical significance due to the cohort size. Moreover, the choice of graft material constitutes a relevant confounding factor, limiting the extent to which the observed differences in graft success can be attributed to the surgical approach alone. Previous studies have reported comparable hearing outcomes and graft intake rates for both endoscopic and microscopic type I TPL (20, 21). Therefore, the discrepancy in graft intake observed in our study may be related to differences in graft selection, with cartilage predominantly used in the endoscopic group and fascia in the microscopic group. This aligns with findings from other studies, which have reported differences in graft intake rates between tragal cartilage and fascia (22, 23). The superior visualization provided by the endoscopic technique, combined with the prevalent use of cartilage, may have contributed to the higher graft intake rates observed, while maintaining excellent audiological outcomes comparable to those achieved with fascia grafts.
Transcanal surgery
The limiting factors for transcanal surgery in children are the curvature and the narrow width of the bony meatus, which complicates especially microscopic inspection of the TM and middle ear. The endoscopic technique, with its varying diameters of rigid endoscopes, is less hindered by the small width. Moreover, the use of angled endoscopes allows complete examination of the hidden recesses of the middle ear, except in the rare occurrence of a type C retrotympanic recess (posterior and medial to the facial nerve) (24). Caloway et al. reported a significantly lower need for a postauricular approach with the endoscopic technique (19). These findings are supported by our study, showing a significantly higher frequency of postauricular approaches in the microscopic technique (p < .01). In our cohort, the smaller pediatric ear canal did not impede endoscopic transcanal access from the age of 4 years and for any type of TPL. Additionally, the thorough inspection of the middle ear allowed transcanal endoscopic cholesteatoma removal in most cases (83%). The two endoscopic cases that required a postauricular incision necessitated mastoidectomy due to disease spread into the mastoid. Our experience shows that depending on the specific anatomy of the middle ear and mastoid, cholesteatoma extending up to the dome of the lateral semicircular canal can be efficiently treated through an exclusive endoscopic approach. In cases of cholesteatoma extension beyond the lateral semicircular canal, managing the middle ear by the endoscope and supplement the resection by canal wall up mastoidectomy is reasonable as also proposed by James et al. (5) Given that radiological assessments frequently overestimate the extent of cholesteatoma in various middle ear subspaces compared to intraoperative findings, initiating surgery with a transcanal endoscopic approach appears to be a feasible strategy in order to minimize the needs for retroauricular incisions (25).
Residual cholesteatoma
Surgical preservation of the mastoid air cell systems is thought to play an important role in maintaining pressure equilibrium within the middle ear by acting as a pressure buffer. Transcanal access with mastoid preservation may help prevent recurrences by maintaining middle ear gas homeostasis. Presutti et al. reported a significant reduction in recurrence rates for primary acquired attic cholesteatoma using transcanal endoscopic approach with mastoid preservation (26). According to James et al. young age, cholesteatoma extension and mastoid involvement increase the risk of recurrence in children. Surgical approach did not have a significant effect on this outcome (27). During the clinical and radiological FU in our cohort, no cases of recurrent disease were observed in both the endoscopic and the microscopic group, which is mainly due to the limited FU time. Our findings therefore refer exclusively to residual cholesteatoma. The microscopic group demonstrated a significantly higher incidence of residual cholesteatoma (p = .03), as depicted on FU MRI scans, and subsequently confirmed intraoperatively during revision surgery. The endoscopic technique showed no residual disease, whereas the microscopic technique showed residual disease in 42%. Simon et al. reported a 39% incidence of residual disease in children three years following a microscopic approach that did not routinely include endoscopic control (28). The enhanced visualization of the middle ear and the availability of digital enhancement technologies in endoscopic ear surgery facilitate complete disease elimination (29). A meta-analysis by Han et al. reported a significantly lower rate of residual disease with the endoscopic technique (p < .01) among children (14). Additionally, two authors reported a reduction in the risk of residual disease when the endoscope was used for dissection rather than just inspection (30, 31).
Postoperative complications
Sparing retroauricular incisions and mastoidectomy in children not only reduces operating time and postoperative pain but also appears to lower the incidence of postoperative complications (13, 32). In their systematic review including both pediatric and adult population, Gkrinia et al. reported a significantly higher incidence of wound infections in the microscopic group, at 5%, compared to the endoscopic group, which had an incidence of 1% (33). Our results showed a 0% incidence of wound complications in the endoscopic cohort, whereas the microscopic cohort had an incidence of 14%. Notably, all wound infections including one seroma occurred following a retroauricular approach. Among the 18 retroauricular approaches, the incidence of wound complications reached 28%. Given the considerable complication rate associated with retroauricular approaches, we recommend whenever possible total transcanal surgery using the endoscope, as it significantly reduces the need for extending to a retroauricular approach.
Limitations
This study has several limitations. The retrospective design and lack of randomization introduce a potential selection bias, as the choice of surgical technique was based on surgeon experience during a period of institutional transition. While matching was performed to improve comparability, residual confounding cannot be excluded. Additionally, the limited sample size may reduce the statistical power to detect more subtle differences between groups.
The relatively short FU period represents a major limitation, particularly in assessing long-term outcomes such as cholesteatoma recurrence, graft stability, and sustained hearing improvement. Although this reflects common challenges in pediatric otology FU, especially in asymptomatic children, it limits the ability to draw definitive conclusions on late outcomes. Prospective studies with extended follow-up are warranted to confirm these findings.
Conclusion
Endoscopic ear surgery is highly effective for pediatric otology. This analysis showed reduced operating times and complication rates with comparable graft intake and audiological outcome in the endoscopic group. This minimally invasive technique offering unprecedented visualization, leads to excellent functional and structural outcomes, making it an optimal choice for pediatric patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Kantonale Ethikkomission, KEK-BE 2019-00555. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because waived for retrospective study.
Author contributions
RF: Writing – original draft, Writing – review & editing, Data curation. SB: Investigation, Writing – review & editing. SS: Writing – review & editing, Investigation. MC: Supervision, Writing – review & editing. LA: Writing – review & editing, Writing – original draft, Data curation, Supervision, Investigation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lieu JEC. Unilateral hearing loss in children: speech-language and school performance. B-ENT. (2013) Suppl 21:107–15.24383229
2. Pollak N. Endoscopic and minimally-invasive ear surgery: a path to better outcomes. World J Otorhinolaryngol Head Neck Surg. (2017) 3:129–35. doi: 10.1016/j.wjorl.2017.08.001
3. Nair S, Aishwarya JG, Vasu PK, Karthikeyan A, Shalini M. Outcomes of totally endoscopic versus microscopic techniques in middle ear cholesteatoma: a systematic review and meta-analysis. Indian J Otolaryngol Head Neck Surg. (2022) 74:4200–11. doi: 10.1007/s12070-021-02869-2
4. Dahm MC, Shepherd RK, Clark GM. The postnatal growth of the temporal bone and its implications for cochlear implantation in children. Acta Otolaryngol Suppl. (1993) 505:1–39.8379315
5. James AL. Endoscopic middle ear surgery in children. Otolaryngol Clin N Am. (2013) 46:233–44. doi: 10.1016/j.otc.2012.10.007
6. Bonali M, Anschuetz L, Fermi M, Villari D, Mariani GA, Manzoli L, et al. The variants of the retro- and hypotympanum: an endoscopic anatomical study. Eur Arch Otorhinolaryngol. (2017) 274:2141–8. doi: 10.1007/s00405-017-4492-0
7. Beckmann S, Mantokoudis G, Weder S, Borner U, Caversaccio M, Anschuetz L. Endoscopic cholesteatoma surgery. J Vis Exp. (2022) 179:e63315. doi: 10.3791/63315
8. Fink R, Molinari G, Beckmann S, Fernandez IJ, Burato A, Caversaccio M, et al. Techniques of endoscopic ossiculoplasty. J Vis Exp. (2024) 203:e66155. doi: 10.3791/66155
9. Isaacson B, Hunter JB, Rivas A. Endoscopic stapes surgery. Otolaryngol Clin North Am. (2018) 51:415–28. doi: 10.1016/j.otc.2017.11.011
10. Marchioni D, Alicandri-Ciufelli M, Rubini A, Masotto B, Pavesi G, Presutti L. Exclusive endoscopic transcanal transpromontorial approach: a new perspective for internal auditory canal vestibular schwannoma treatment. J Neurosurg. (2017) 126:98–105. doi: 10.3171/2015.11.JNS15952
11. Thomassin JM, Korchia D, Doris JM. Endoscopic-guided otosurgery in the prevention of residual cholesteatomas. Laryngoscope. (1993) 103:939–43. doi: 10.1288/00005537-199308000-00021
12. Hunter JB, Zuniga MG, Sweeney AD, Bertrand NM, Wanna GB, Haynes DS, et al. Pediatric endoscopic cholesteatoma surgery. Otolaryngol Head Neck Surg. (2016) 154:1121–7. doi: 10.1177/0194599816631941
13. Kwinter A, Purcell PL, Leonard CG, James AL. Comparing transcanal endoscopic ear surgery to post-auricular microscope-guided surgery in pediatric ossiculoplasty: hearing outcomes and post-operative pain. Otol Neurotol. (2021) 42:e1648–51. doi: 10.1097/MAO.0000000000003235
14. Han S-Y, Lee DY, Chung J, Kim YH. Comparison of endoscopic and microscopic ear surgery in pediatric patients: a meta-analysis. Laryngoscope. (2019) 129:1444–52. doi: 10.1002/lary.27556
15. Wullstein H. The restoration of the function of the middle ear, in chronic otitis media. Ann Otol Rhinol Laryngol. (1956) 65:1021–41. doi: 10.1177/000348945606500416
16. Roth TN, Haeusler R. Inside-out technique cholesteatoma surgery: a retrospective long-term analysis of 604 operated ears between 1992 and 2006. Otol Neurotol. (2009) 30:59–63. doi: 10.1097/mao.0b013e31818ee0a7
17. Cohen MS, Basonbul RA, Barber SR, Kozin ED, Rivas AC, Lee DJ. Development and validation of an endoscopic ear surgery classification system. Laryngoscope. (2018) 128:967–70. doi: 10.1002/lary.26802
18. Yung M, Tono T, Olszewska E, Yamamoto Y, Sudhoff H, Sakagami M, et al. EAONO/JOS joint consensus statements on the definitions, classification and staging of middle ear cholesteatoma. Int Adv Otol. (2017) 13:1–8. doi: 10.5152/iao.2017.3363
19. Caloway CL, Basonbul RA, Ronner EA, Tolisano AM, Zhu AW, Suresh H, et al. Pediatric endoscopic ossiculoplasty following surgery for chronic ear disease. Laryngoscope. (2020) 130:2896–9. doi: 10.1002/lary.28526
20. Crotty TJ, Cleere EF, Keogh IJ. Endoscopic versus microscopic type-1 tympanoplasty: a meta-analysis of randomized trials. Laryngoscope. (2023) 133:1550–7. doi: 10.1002/lary.30479
21. Mitton TJ, Killeen DE, Momin ZK, Hunter JB, Isaacson B, Lee K, et al. Endoscopic versus microscopic pediatric tympanoplasty: is there a difference between closure rates and hearing outcomes? Otol Neurotol. (2022) 43:1205–11. doi: 10.1097/MAO.0000000000003694
22. Yegin Y, Çelik M, Koç AK, Küfeciler L, Elbistanlı MS, Kayhan FT. Comparison of temporalis fascia muscle and full-thickness cartilage grafts in type 1 pediatric tympanoplasties. Braz J Otorhinolaryngol. (2016) 82:695–701. doi: 10.1016/j.bjorl.2015.12.009
23. Dhoke PR, Dhote KS, Khadakkar S, Harkare V, Deosthale N, Singh A. Paediatric type 1 tympanoplasty: comparison of full thickness tragal cartilage versus temporalis fascia graft-a randomised controlled trial. Indian J Otolaryngol Head Neck Surg. (2023) 75:470–5. doi: 10.1007/s12070-022-03262-3
24. Bonali M, Fermi M, Alicandri-Ciufelli M, Mattioli F, Villari D, Presutti L, et al. Correlation of radiologic versus endoscopic visualization of the middle ear: implications for endoscopic ear surgery. Otol Neurotol. (2020) 41:e1122–7. doi: 10.1097/MAO.0000000000002787
25. Beckmann S, Hool S, Yacoub A, Hakim A, Caversaccio M, Wagner F, et al. Accuracy of high-resolution computed tomography compared to high-definition ear endoscopy to assess cholesteatoma extension. Otolaryngol–Head Neck Surg. (2023) 169:1276–81. doi: 10.1002/ohn.413
26. Presutti L, Anschuetz L, Rubini A, Ruberto M, Alicandri-Ciufelli M, Dematte M, et al. The impact of the transcanal endoscopic approach and mastoid preservation on recurrence of primary acquired attic cholesteatoma. Otol Neurotol. (2018) 39:445–50. doi: 10.1097/MAO.0000000000001712
27. James AL. Cholesteatoma severity determines the risk of recurrent paediatric cholesteatoma more than the surgical approach. J Clin Med. (2024) 13:836. doi: 10.3390/jcm13030836
28. Simon F, Remangeon F, Loundon N, Leboulanger N, Couloigner V, Garabédian N, et al. Pediatric cholesteatoma follow-up: residual and recurrence in 239 cases with over 5-year hindsight. Laryngoscope. (2024) 134(11):4789–98. doi: 10.1002/lary.31567
29. Ragonesi T, Niederhauser L, Fernandez IJ, Molinari G, Caversaccio M, Presutti L, et al. Digital image enhancement may improve sensitivity of cholesteatoma detection during endoscopic ear surgery. Clin Otolaryngol. (2023) 48:595–603. doi: 10.1111/coa.14049
30. James AL, Cushing S, Papsin BC. Residual cholesteatoma after endoscope-guided surgery in children. Otol Neurotol. (2016) 37:196–201. doi: 10.1097/MAO.0000000000000948
31. Ghadersohi S, Carter JM, Hoff SR. Endoscopic transcanal approach to the middle ear for management of pediatric cholesteatoma. Laryngoscope. (2017) 127:2653–8. doi: 10.1002/lary.26654
32. Kakehata S, Furukawa T, Ito T, Kubota T, Futai K, Watanabe T. Comparison of postoperative pain in patients following transcanal endoscopic versus microscopic ear surgery. Otol Neurotol. (2018) 39:847–53. doi: 10.1097/MAO.0000000000001864
Keywords: endoscopic ear surgery, microscopic ear surgery, pediatric, cholesteatoma, tympanoplasty, hearing loss
Citation: Fink R, Beckmann S, Sheppard SC, Caversaccio M and Anschuetz L (2025) Endoscopic vs. microscopic tympanoplasty in children: a retrospective case-control study. Front. Surg. 12:1649552. doi: 10.3389/fsurg.2025.1649552
Received: 26 June 2025; Accepted: 25 August 2025;
Published: 16 September 2025.
Edited by:
Hiroo Uchida, Nagoya University Graduate School of Medicine, JapanReviewed by:
Deniz Baklaci, Zonguldak Bulent Ecevit University, TürkiyeViorel Zainea, Carol Davila University of Medicine and Pharmacy, Romania
Copyright: © 2025 Fink, Beckmann, Sheppard, Caversaccio and Anschuetz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raffael Fink, cmFmZmFlbGRmaW5rQGdtYWlsLmNvbQ==
 Raffael Fink
Raffael Fink Sven Beckmann
Sven Beckmann Sean C. Sheppard
Sean C. Sheppard Marco Caversaccio
Marco Caversaccio Lukas Anschuetz
Lukas Anschuetz