- 1Yiling Hospital of Yichang, Affiliated Yiling Hospital of China Three Gorges University, Yichang, Hubei, China
- 2Yunnan St. John’s Hospital, Kunming, Yunnan, China
- 3First Ward of Hepatobiliary Pancreatic Surgery Department, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
- 4Fourth Ward of Hepatobiliary Pancreatic Surgery Department, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
Background: Liposarcoma (LPS) is a rare mesenchymal soft tissue sarcoma, and dedifferentiated liposarcoma (DDLPS) represents a clinically significant and aggressive subtype. Retroperitoneal DDLPS poses significant clinical challenges due to its insidious onset, large tumor size at the time of diagnosis, complex anatomical relationships, and high rates of recurrence and multi-organ involvement.
Case presentation: We present the case of a 64-year-old male who presented with a progressively enlarging abdominal mass, fatigue, and reduced appetite persisting for over five years. Imaging studies and interdisciplinary evaluation revealed a massive retroperitoneal tumor with invasion into multiple adjacent organs. The patient underwent comprehensive surgical resection involving the tumor, left kidney, spleen, and distal pancreas, along with diaphragmatic repair. Histopathological analysis confirmed high-grade dedifferentiated liposarcoma. Postoperatively, the patient developed mild complications, including pneumothorax, which were effectively managed. Postoperative imaging and laboratory examinations demonstrated that the tumor was completely resected, with the majority of organ functions effectively preserved.
Conclusion: This case highlights the complexity of treating a large and multi-organ-involving retroperitoneal dedifferentiated liposarcoma. Radical surgical resection remains the most effective treatment approach, but while achieving complete resection, it is also necessary to take into account the maximum protection of the patient's vital organ functions.
1 Introduction
Liposarcoma (LPS) is a rare mesenchymal soft tissue sarcoma that originates from the adipocyte lineage and is generally believed to arise from adipocytes in soft tissues (1). LPS accounts for approximately 13%–20% of all soft tissue sarcomas and is one of the most common types of soft tissue sarcoma worldwide (2). According to the 2020 World Health Organization (WHO) classification criteria for LPS, its subtypes include atypical lipomatous tumor (ALT)/well-differentiated liposarcoma (WDLPS), dedifferentiated liposarcoma (DDLPS), myxoid liposarcoma (MLPS), pleomorphic liposarcoma (PLPS), and myxoid pleomorphic liposarcoma (MPLPS) (3). This type of tumor is frequently diagnosed at an advanced stage due to its indolent growth pattern and nonspecific early symptoms.
Among all subtypes of LPS, DDLPS holds significant clinical importance, accounting for approximately 20% of all LPS (4). Its clinical manifestations are characterized by significant local invasiveness and a high recurrence rate, and it is more frequently found in the retroperitoneum (5). Due to the strong extensibility of the retroperitoneal space, DDLPS lacks specific clinical manifestations in the early stage, and patients may only present with mild abdominal distension or discomfort at the initial stage (6). It is not until the tumor compresses or invades adjacent organs that obvious clinical symptoms occur (7). Therefore, many patients are diagnosed at an advanced stage with large tumor volumes, significantly increasing the difficulty of surgical treatment.
In clinical diagnosis, early LPS is often misdiagnosed due to its similar imaging manifestations to benign lipomas. Although imaging examinations such as CT and MRI are helpful in assessing the extent of the tumor and its relationship with adjacent structures, a retrospective analysis of 291 cases by Morosi indicates that, except for WDLPS, there are currently no clear radiological criteria to accurately distinguish all types of retroperitoneal sarcomas (8). Consequently, an accurate diagnosis typically necessitates a combination of imaging studies and histopathological evaluation (9).
DDLPS, due to its anatomical location and inherent biological features, frequently infiltrates neighboring vital organs, consequently elevating the complexity of surgical resection (10). During clinical treatment, multi-organ resection is often necessary to achieve complete tumor removal. The case described in this report features a large tumor volume, recurrence, multi-organ resection, and multidisciplinary decision-making, providing important reference value for the clinical management of complex liposarcoma and the multidisciplinary collaboration model. This case report is written in accordance with the SCARE 2025 (11) statements. For detailed content, please refer to Supplementary Materials.
2 Case presentation
2.1 Patient information and chief complaint
The patient is a 64-year-old male. He has no history of smoking or alcohol consumption and denies a family history of genetic disorders. The patient presented to the clinic primarily due to a progressively enlarging abdominal mass associated with fatigue and decreased appetite for more than five years. There was no notable weight loss, melena, or hematuria.
2.2 Past medical history and medication history
Five years ago, he was admitted to a local hospital due to gastrointestinal bleeding and incidentally discovered a renal mass. After discharge, he self-administered traditional Chinese medicine for treatment, but the specific formula is unknown. He has not undergone regular followup since then. The patient denies a history of diabetes, hypertension, or cardiovascular and cerebrovascular diseases. He also denies any previous surgical history.
2.3 Disease course review and timeline
In 2020, he was first admitted to a local hospital where an abdominal mass was detected during a physical examination, but no further treatment was pursued. At the end of 2022, an imaging follow-up at the local hospital indicated an increase in tumor size, and surgery was recommended. However, the patient and his family refused and continued with conservative treatment using traditional Chinese medicine. In May 2024, due to the increasing size of the abdominal mass, he underwent a biopsy at a hospital in Guiyang, Guizhou Province, China.
The results of the puncture biopsy pathology are as follows (Supplementary Materials):
1. CD34 is diffusely positive, suggesting a mesenchymal tumor.
2. This histological examination rules out neurogenic and myogenic tumors, but does not rule out well-differentiated liposarcoma.
The immunohistochemical results are as follows: Vimentin (+), CD34 (+),Bcl2 (+), CD99 (−), SATB2 (−), β-catenin (−), SMA (−), MSA (−), Desmin (−)CD117(−), DOG-1(−), S-100(−), CK(−), Ki67 (approximately 3%+), STAT-6(−), CDK4(+), MDM2(−). The patient refused further treatment due to personal reasons. On March 15, 2025, the patient was transferred to our hospital due to a significant increase in the abdominal mass.
2.4 Physical examination
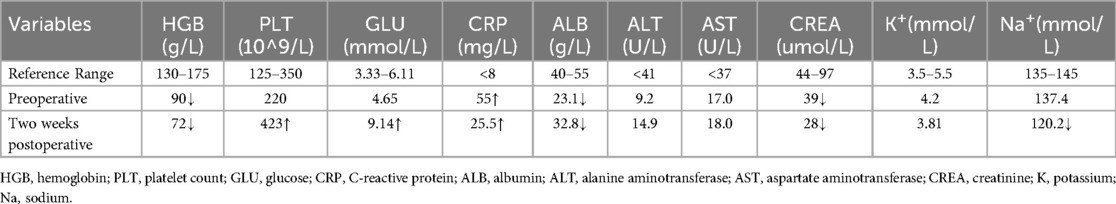
On admission, the patient's pulse was 112 beats per minute, respiration 30 times per minute, and blood pressure 106/75 mmHg. The abdomen was distended. A large abdominal mass could be palpated in both the supine and standing positions (Figures 1A,B), and it exhibited a medium consistency. Varicosities of the abdominal wall veins were evident. Abdominal respiration was the primary mode of breathing, and no signs of gastric or intestinal patterns or peristaltic waves were detected. Mild abdominal tenderness was noted, without rebound tenderness, while muscular tension of the abdominal wall was present. Enlargement of the liver and spleen was not palpable below the costal margin. The upper border of the hepatic dullness was located at the fifth intercostal space on the right side. The fluid thrill test was negative, as was the shifting dullness test. The bowel sound frequency was 4 times per minute. No percussion pain was elicited over the hepatic and renal regions. Murphy's sign was negative. There was no tenderness at the costal margin point, upper ureteral point, middle ureteral point, costovertebral angle point, or costolumbar point. Moreover, no vascular murmurs were auscultated.
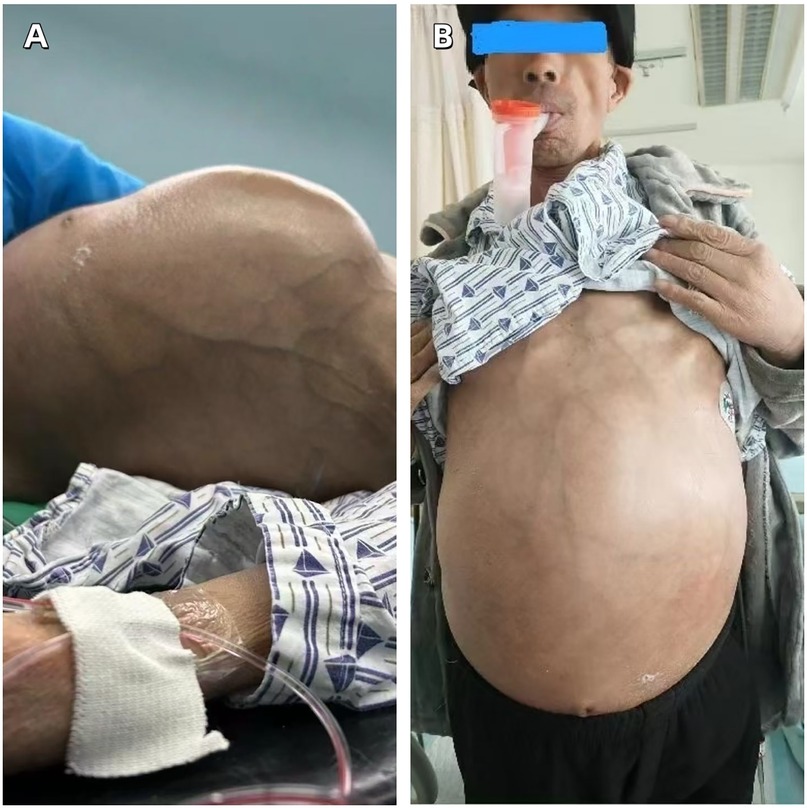
Figure 1. (A) A massive abdominal mass in the supine position. (B) A massive abdominal mass in the upright position.
2.5 Laboratory and imaging findings
The blood test results showed an elevated white blood cell count, accompanied by mild anemia and a decreased albumin level. The remaining routine laboratory test results are detailed in Table 1. The plain chest CT combined with abdominal enhanced CT indicated the presence of a large mixed-density mass in the thoracic, abdominal and pelvic cavities (Figures 2A,B), which exerted compression and invasion on the surrounding organs, and was accompanied by left pleural effusion (Figure 2C), left lung atelectasis (Figure 2D), and pelvic effusion. The echocardiogram showed a small amount of pericardial effusion; abdominal ultrasound also indicated effusion in both the abdominal and thoracic cavities. The patient did not undergo PET-CT examination.
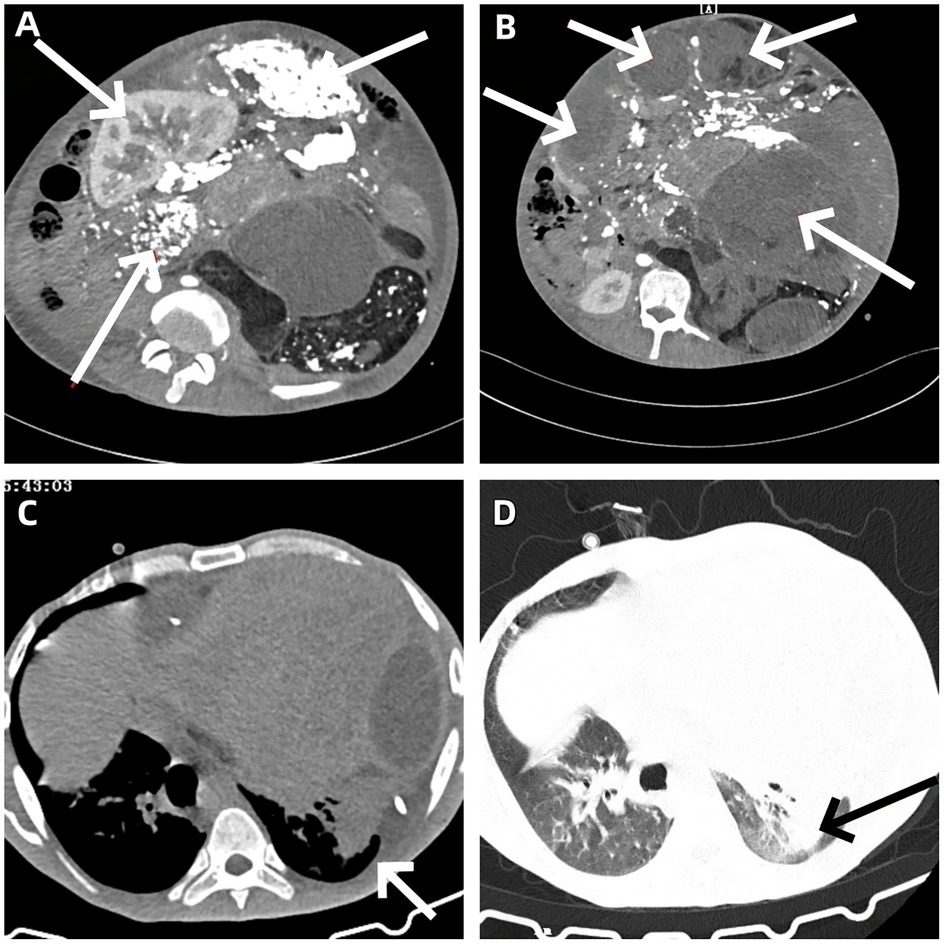
Figure 2. (A) A large heterogeneous-density mass is observed in the abdominal cavity, causing compression and displacement of the left kidney to the right abdominal region. (B) A large heterogeneous-density mass lesion in the pelvic cavity, with evidence of displacement and infiltration of adjacent organs. (C) Presence of left-sided pleural effusion. (D) Partial atelectasis of the left lung.
2.6 Diagnosis and multidisciplinary evaluation
After a comprehensive analysis of the patient's imaging data, previous pathological results and clinical manifestations by the multidisciplinary team (MDT), a preliminary diagnosis of a huge DDLPS in the retroperitoneum was made. The tumor has invaded the left kidney, spleen, body and tail of the pancreas, and the diaphragm, which is a highly aggressive and recurrent soft tissue sarcoma. Due to the large tumor volume, it has continuously compressed and infiltrated the organs, blood vessels and lymphatic system in the abdominal, thoracic and pericardial cavities, resulting in obstruction of venous and lymphatic return, and subsequently causing ascites, pleural effusion and pericardial effusion. The increased volume load has also led to an increase in the patient's heart rate. These pathological and physiological changes have further exacerbated the patient's abdominal pain and distension symptoms after admission, and the condition has been progressively worsening. Given the locally aggressive growth characteristics of the tumor and the patient's aggravated clinical symptoms and significantly decreased quality of life, the multidisciplinary team unanimously believes that surgical intervention is a necessary treatment measure to improve the patient's quality of life.
3 Surgical procedure
3.1 Surgical findings and procedures
Following comprehensive communication with the patient and his family members, surgical intervention was implemented at our hospital on April 1, 2025. A midline incision of approximately 45 cm was made in the abdomen, and the skin, subcutaneous tissue, and peritoneum were successively incised to enter the abdominal cavity. During the operation, it was found that there was a small amount of light yellow fluid in the abdominal cavity, about 200 ml in total, which was removed with an aspirator. A huge tumor was visible in the abdominal cavity, enveloping and pushing the left kidney and spleen to the right side, invading the left kidney, spleen, and diaphragm, making it difficult to separate. The intestinal tubes were gathered on the right side, with no obvious adhesions and no invasion by the tumor.
The patient underwent a comprehensive surgical procedure involving abdominal tumor resection, total splenectomy, left nephrectomy, distal pancreatectomy, and diaphragmatic repair. The tumor was completely resected and measured 42.0 cm × 36.0 cm × 18.0 cm, with an intact fibrous capsule observed macroscopically (Figures 3A,B). Postoperative imaging confirmed the absence of the previously noted large abdominal mass (Figure 3C).
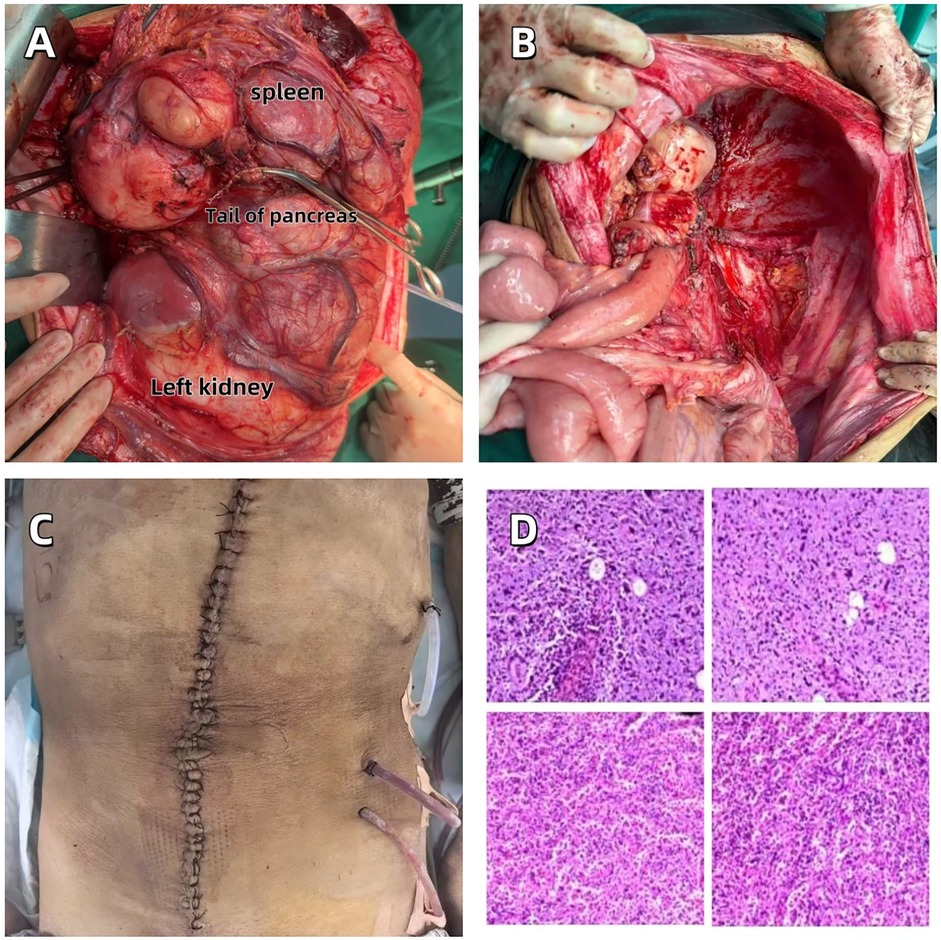
Figure 3. (A) Completely resected huge abdominal tumor. (B) The abdominal surgical field after tumor resection. (C) Abdominal incision in the postoperative recovery period. (D) Pathological section of well-differentiated dedifferentiated liposarcoma.
The postoperative pathological diagnosis results are as follows (Supplementary Materials):
1. Considered to be liposarcoma;
2. A small amount of tumor infiltration was observed in the perirenal adipose tissue;
3. The tumor was found to involve the capsule of the spleen.
After consultation with experts from Jiangsu Provincial People's Hospital, China, the final diagnosis was high-grade dedifferentiated liposarcoma (Figure 3D).
The immunohistochemical test results are as follows: Tumor cells (−3): CD34 (+), SMA (−), S-100 (−), CD68 (a few +), Ki-67 (+, approximately 10%), CDK4 (−), MDM2 (scattered +), ALK (−); (−7): TFE-3 (−), CD68 (scattered +), CD163 (partially +), S-100 (−), PAX-8 (−), CAIX (−), CK7 (−), CgA (−), Syn (−); (−21): CgA (−), Syn (−).
Two weeks after the operation, the patient underwent a blood routine test and related biochemical index tests (see Table 1). The results showed mild hyperglycemia and a decrease in hemoglobin levels, while no significant abnormalities were found in liver and kidney functions. The increase in blood sugar might be related to the systemic stress response after the operation, and the decrease in hemoglobin levels was consistent with the amount of blood loss during the operation. In the early postoperative follow-up of chest CT, the patient was found to have pneumothorax (Figure 4A). After closed thoracic drainage treatment, subsequent CT examinations indicated that the gas was gradually absorbed (Figure 4B). Abdominal enhanced CT confirmed that the tumor had been completely resected (Figure 4C), and the chest CT also showed that the space-occupying lesion under the diaphragm had disappeared (Figure 4D). The patient was discharged smoothly on April 20, 2025, without any other postoperative complications.
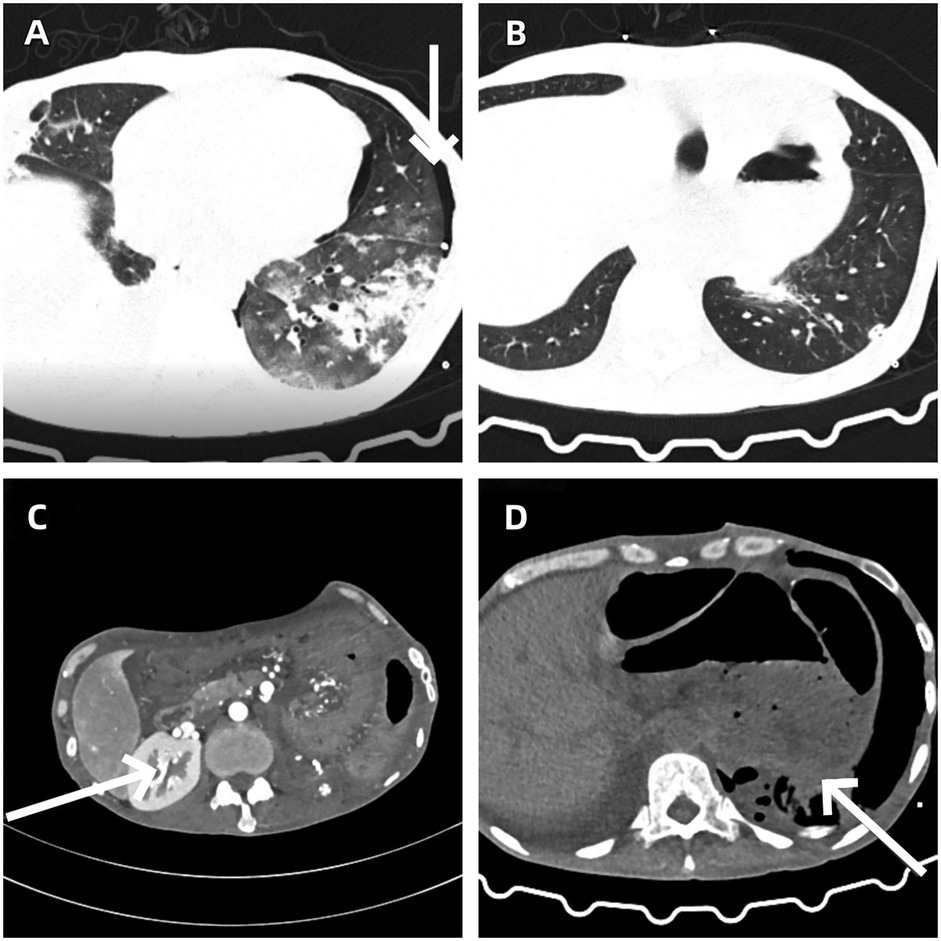
Figure 4. (A) Left-sided pneumothorax as a postoperative complication. (B) Gradual resolution of pneumothorax following closed thoracic drainage. (C) Abdominal contrast-enhanced CT confirms complete resection of the tumor. (D) The resolution of the subphrenic space-occupying lesion.
4 Discussion
This case fully demonstrates that through the close collaboration of a multidisciplinary team, complete resection of a large and anatomically complex retroperitoneal DDLPS is still achievable. During the operation, the key and challenge lies in ensuring complete tumor resection while maximizing the protection of the functions of surrounding organs. It is worth emphasizing that after tumor resection, the original compression on the internal organs was relieved, and the patient's clinical symptoms were significantly alleviated. Throughout the operation, the surgical team constantly balanced and adjusted between “thorough tumor resection” and “minimizing damage to important organs and maintaining the patient's physiological stability”, which is the core challenge in the surgical treatment of complex retroperitoneal tumors. Overall, this case provides valuable clinical experience and practical reference for surgical decision-making and implementation in similar complex situations.
Given the rarity of DDLPS and the intricacies of its biological behavior, accurate diagnosis poses a substantial challenge. This often leads to misdiagnosis or treatment delays, thereby forfeiting the optimal intervention window (1). In this case, the patient presented with progressive abdominal distension. Abdominal contrast-enhanced CT revealed multiple large masses, initially suggesting multiple malignant tumors. Although imaging studies can delineate the tumor's extent, the definitive confirmation of the subtype still hinges on multiple-site biopsies, histopathological examination, and immunohistochemical analysis. Eventually, the patient was diagnosed with DDLPS. However, due to the patient's financial constraints, PET-CT was not performed, potentially impeding the assessment of distant metastases.
Currently, surgical resection remains the most effective treatment modality for retroperitoneal DDLPS. Nevertheless, as the tumor frequently invades multiple organs, surgery often necessitates the combined resection of multiple organs. This not only escalates the risk of postoperative complications but also imposes more stringent demands on postoperative management (12). Surgeons should ensure tumor-free resection margins while endeavoring to preserve normal organ function to maintain the patient's physiological equilibrium and quality of life (13). In this case, via a midline abdominal incision, the abdominal tumor, spleen, left kidney, pancreatic body and tail, and a portion of the diaphragm were completely excised, achieving R0 resection. Postoperative pathology confirmed tumor involvement of the perirenal fat and the splenic capsule, further attesting to the thoroughness of the surgical resection. Systemic review studies have indicated that R0 resection is closely associated with improved patient prognosis (14). Although the patient experienced complications such as pneumothorax after surgery, these were within a manageable range. However, the literature also indicates that even with R0 resection margins, recurrence remains difficult to entirely prevent (15–17). This may be intricately linked to the degree of tumor dedifferentiation; the lower the degree of differentiation, the higher the recurrence risk and the poorer the prognosis (18). Consequently, surgery alone is insufficient to fully address the high recurrence rate of DDLPS, and a comprehensive treatment approach is required to optimize patient outcomes.
Regarding the role of chemotherapy in DDLPS, existing research has demonstrated its limited efficacy (19). However, individual case reports suggest that in some patients, neoadjuvant chemotherapy has led to a significant reduction in tumor volume, ultimately enabling radical resection. This implies that under specific circumstances, the combination of chemotherapy and surgery may confer clinical benefits (20). Nevertheless, such cases are infrequent and do not warrant routine recommendation. Emerging evidence suggests that trabectedin, as a second-line chemotherapeutic agent, demonstrates promising efficacy in the treatment of liposarcoma. By modulating the tumor microenvironment and extracellular matrix, it offers a viable therapeutic option for patients with refractory or recurrent disease (21). The combination of radiotherapy and surgery in the treatment of DDLPS has been extensively reported, encompassing preoperative, intraoperative, and postoperative modalities. Preoperative radiotherapy is the primary approach for most patients. For those with a low preoperative recurrence risk assessment but unfavorable pathological findings during surgery, postoperative radiotherapy serves a complementary purpose (3). Thus, the combination of surgery and radiotherapy may represent an ideal treatment strategy for DDLPS.
Among other treatment modalities, Brigimadlin (an MDM2 inhibitor) has exhibited potential antitumor activity in DDLPS/WDLPS (22). In DDLPS, CDK4 amplification occurs at a rate as high as 90%, suggesting its potential as a viable therapeutic target; however, the clinical efficacy of targeting CDK4 remains to be further investigated (23). Retrospective studies have also suggested that anlotinib, a multi-target vascular endothelial growth factor receptor inhibitor, may possess certain therapeutic efficacy for metastatic or recurrent DDLPS (20). Additionally, immunotherapy, radiofrequency ablation, CAR-T cell therapy, and TCR-T cell therapy offer alternative treatment options for inoperable patients (3).
The limitations of this study include the omission of FDG PET-CT, which may impact the assessment of distant metastases and the determination of long-term recurrence risk. Moreover, the dearth of long-term follow-up data precludes a comprehensive evaluation of the patient's long-term prognosis. In the future, the MDT collaborative mechanism should be further promoted, and the comprehensive treatment strategy centered on surgery should be fortified to enhance the clinical outcomes of patients with complex DDLPS.
4.1 Key clinical message
Successful management of giant dedifferentiated retroperitoneal liposarcoma necessitates a multidisciplinary approach and radical surgical intervention to achieve complete tumor resection while maximizing preservation of adjacent organs. Early diagnosis and individualized surgical planning play a critical role in improving clinical outcomes for patients with complex soft tissue sarcomas.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Yunnan St. John's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SZ: Writing – review & editing, Writing – original draft. WY: Data curation, Writing – review & editing. ZL: Conceptualization, Writing – review & editing. RL: Conceptualization, Writing – original draft. BF: Resources, Writing – review & editing, Methodology, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the doctoral research project (2023BS17) of the Second Affiliated Hospital of Kunming Medical University and the Yiling Hospital of Yichang internal research project (YLRMYY-YNKY -202510).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1650969/full#supplementary-material
References
1. Jonczak E, Grossman J, Alessandrino F, Seldon Taswell C, Velez-Torres JM, Trent J. Liposarcoma: a journey into a rare tumor’s epidemiology, diagnosis, pathophysiology, and limitations of current therapies. Cancers (Basel). (2024) 16(22):3858. doi: 10.3390/cancers16223858
2. Amer KM, Congiusta DV, Thomson JE, Elsamna S, Chaudhry I, Bozzo A, et al. Epidemiology and survival of liposarcoma and its subtypes: a dual database analysis. J Clin Orthop Trauma. (2020) 11:S479–S84. doi: 10.1016/j.jcot.2020.04.013
3. Liu H, Wang X, Wang X, Qiu F, Zhou B. Challenges and hope: latest research trends in the clinical treatment and prognosis of liposarcoma. Front Pharmacol. (2025) 16:1529755. doi: 10.3389/fphar.2025.1529755
4. Bock S, Hoffmann DG, Jiang Y, Chen H, Il’yasova D. Increasing incidence of liposarcoma: a population-based study of national surveillance databases, 2001–2016. Int J Environ Res Public Health. (2020) 17(8):2710. doi: 10.3390/ijerph17082710
5. Thway K. Well-differentiated liposarcoma and dedifferentiated liposarcoma: an updated review. Semin Diagn Pathol. (2019) 36:112–21. doi: 10.1053/j.semdp.2019.02.006
6. Yamazaki D, Ogihara N, Horiuchi T. Primary orbital dedifferentiated liposarcoma. World Neurosurg. (2020) 139:604–7. doi: 10.1016/j.wneu.2020.04.069
7. Rives-Lange C, Poghosyan T, Dariane C, Douard R, Mongeois E, Boudaoud AA, et al. Delayed retroperitoneal liposarcoma diagnosis and management in a patient with massive obesity. Eur J Clin Nutr. (2021) 75(10):1520–2. doi: 10.1038/s41430-020-00855-5
8. Morosi C, Stacchiotti S, Marchianò A, Bianchi A, Radaelli S, Sanfilippo R, et al. Correlation between radiological assessment and histopathological diagnosis in retroperitoneal tumors: analysis of 291 consecutive patients at a tertiary reference sarcoma center. Eur J Surg Oncol. (2014) 40(12):1662–70. doi: 10.1016/j.ejso.2014.10.005
9. Ma Y-D, Wu Z-Q, Liang X-R, Pi LJ, Gong M-Z, Tang Y. A case of fat-forming solitary fibrous tumor that is prone to be confused with liposarcoma. Diagn Pathol. (2024) 19(1):40. doi: 10.1186/s13000-024-01463-8
10. Agaimy A, Michal M, Hadravsky L, Michal M. Dedifferentiated liposarcoma composed predominantly of rhabdoid/epithelioid cells: a frequently misdiagnosed highly aggressive variant. Hum Pathol. (2018) 77:20–7. doi: 10.1016/j.humpath.2017.12.025
11. Kerwan A, Al-Jabir A, Mathew G, Sohrabi C, Rashid R, Franchi T, et al. Revised surgical case report (scare) guideline: an update for the age of artificial intelligence. Premier J Sci. (2025) 10(100079):2025. doi: 10.70389/PJS.100079
12. Gonzalez MR, Mendez-Guerra C, Goh MH, Pretell-Mazzini J. Principles of surgical treatment of soft tissue sarcomas. Cancers (Basel). (2025) 17(3):401. doi: 10.3390/cancers17030401
13. Santangelo A, Fernicola A, Santangelo D, Peluso G, Calogero A, Crocetto F, et al. Dark topics on giant retroperitoneal liposarcoma: a systematic review of 157 cases. Cancers (Basel). (2025) 17(5):740. doi: 10.3390/cancers17050740
14. Paik B, Seo CJ, Tan JW-S, Juan WKD, Soo KC, Ong C-AJ, et al. A systematic review of margin status in retroperitoneal liposarcomas: does the R0 margin matter? Front Oncol. (2022) 12:891710. doi: 10.3389/fonc.2022.891710
15. Fodor M, Maglione M, Kogler P, Kafka-Ritsch R, Ofner D, Perathoner A. Challenges in the treatment of a giant retroperitoneal liposarcoma. Ann Ital Chir. (2020) 9:S2239253X20033162-S2239253X.
16. Mahjoubi Z, Zakhama W, Sakly A, Njima M, Mnasser A, Binous Y. Giant recurrent liposarcoma of the retroperitoneum–a surgical challenge: a case report. Int J Surg Case Rep. (2020) 77:486–9. doi: 10.1016/j.ijscr.2020.11.051
17. Bachmann R, Eckert F, Gelfert D, Strohäker J, Beltzer C, Ladurner R. Perioperative strategy and outcome in giant retroperitoneal dedifferentiated liposarcoma—results of a retrospective cohort study. World J Surg Oncol. (2020) 18(1):296. doi: 10.1186/s12957-020-02069-2
18. Yamada Y, Wakamatsu T, Imura Y, Tamiya H, Yagi T, Suzuki R, et al. Efficacy of surgery in the management of multiple recurrences of retroperitoneal dedifferentiated liposarcoma. World J Surg Oncol. (2024) 22(1):265. doi: 10.1186/s12957-024-03552-w
19. Gahvari Z, Parkes A. Dedifferentiated liposarcoma: systemic therapy options. Curr Treat Options Oncol. (2020) 21(2):15. doi: 10.1007/s11864-020-0705-7
20. Li Z-K, Liu J, Deng Y-T, Jiang Y. Efficacy and safety of anlotinib in patients with unresectable or metastatic well-differentiated/dedifferentiated liposarcoma: a single-center retrospective study. Anti-Cancer Drugs. (2021) 32(2):210–4. doi: 10.1097/CAD.0000000000001023
21. De Vita A, Recine F, Miserocchi G, Pieri F, Spadazzi C, Cocchi C, et al. The potential role of the extracellular matrix in the activity of trabectedin in ups and L-sarcoma: evidences from a patient-derived primary culture case series in tridimensional and zebrafish models. J Exp Clin Cancer Res. (2021) 40(1):165. doi: 10.1186/s13046-021-01963-1
22. LoRusso P, Yamamoto N, Patel MR, Laurie SA, Bauer TM, Geng J, et al. The Mdm2–P53 antagonist brigimadlin (Bi 907828) in patients with advanced or metastatic solid tumors: results of a phase Ia, first-in-human, dose-escalation study. Cancer Discov. (2023) 13(8):1802–13. doi: 10.1158/2159-8290.CD-23-0153
Keywords: case report, retroperitoneal liposarcoma, dedifferentiated liposarcoma, multidisciplinary treatment, surgical resection
Citation: Zhong S, Yang W, Li Z, Li R and Fu B (2025) Surgical resection of a giant retroperitoneal dedifferentiated liposarcoma: a case report. Front. Surg. 12:1650969. doi: 10.3389/fsurg.2025.1650969
Received: 20 June 2025; Accepted: 28 July 2025;
Published: 4 September 2025.
Edited by:
Alessandro De Vita, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyCopyright: © 2025 Zhong, Yang, Li, Li and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruixue Li, MjAyMDE2NTVAa21tdS5lZHUuY24=; Bimang Fu, MTMzMTI1NjE1NjZAMTYzLmNvbQ==
†ORCID:
Shijie Zhong
orcid.org/0000-0001-5853-1689
Ruixue Li
orcid.org/0000-0001-8305-7456
Bimang Fu
orcid.org/0009-0007-7128-0520
 Shijie Zhong
Shijie Zhong Wei Yang2
Wei Yang2