- 1Nursing Department, Huashan Hospital of Fudan University, Shanghai, China
- 2School of Nursing, Fudan University, Shanghai, China
- 3Department of Vascular Surgery, Huashan Hospital of Fudan University, Shanghai, China
- 4Department of Vascular Surgery, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China
- 5Department of Vascular Surgery, The First Affiliated Hospital, Zhejiang University, School of Medicine, Hangzhou, China
- 6Department of Vascular Surgery, Liyuan Hospital Affiliated Tongji Medical Collage of Huazhong University of Science & Technology, Wuhan, China
- 7Department of Vascular Surgery, Xuanwu Hospital, Capital Medical University and Institute of Vascular Surgery, Capital Medical University, Beijing, China
- 8Department of Vascular Surgery, Zhongshan Hospital of Fudan University, Shanghai, China
- 9Department of Vascular Surgery, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 10Department of Vascular Surgery, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Background: Patients with femoropopliteal (FP) occlusive disease encounter considerable obstacles concerning health-related quality of life (HRQoL), which serve as the primary objectives of their interventions. While Drug-Coated Balloons (DCBs) present potential advantages, they are not constitute definitive cures. There is a paucity of research concerning postoperative HRQoL in these patients. This study evaluates HRQoL 12 months post-DCB treatment and examines influencing risk factors through a multicenter cross-sectional study.
Methods: This retrospective, multicenter study involved 1012 patients with FP occlusive disease who underwent DCB at 8 vascular centers from August 2021 to December 2023. Data on initial hospitalizations and 12-month follow-up were gathered, and logistic regression was utilized to examine the influencing factors.
Results: According to the median HRQoL at 12 months postoperatively, patients were categorized into low (N = 503) and high (N = 509) HRQoL groups. Significant differences were found in several variables such as renal insufficiency, calcification degree and TLR incidence (P < 0.05), while intervention approach (P = 0.781), DCB diameter (P = 0.301) and DCB length (P = 0.368) showed no significant differences. Logistic regression demonstrated that arterial calcification (OR = 0.33–0.44, P < 0.001), postoperative Rutherford classification (grade 1–6, OR = 0.0000 to 0.0367, P < 0.001), the Rutherford classification progression within 12 months (OR = 9.53, P < 0.001), and target lesion revascularization (TLR) occurrence (OR = 0.09, P = 0.011) were significantly linked to HRQoL at 12 months postoperatively, with no significant differences for other factors.
Conclusions: Overall, the Rutherford classification progression over 12 months was significantly positively linked to HRQoL 12 months postoperatively. Conversely, HRQoL was notably diminished in patients who exhibited arterial calcification, elevated postoperative Rutherford classification, and experienced TLR. Nevertheless, intervention approach, DCB length and diameter had no significant relationship to postoperative HRQoL.
1 Introduction
Peripheral Artery Disease (PAD) is a chronic, progressive disease primarily caused by atherosclerosis, predominantly impacting middle-aged and elderly individuals (1), with Femoropopliteal (FP) occlusive disease being the most prevalent (2). In 2019, approximately 113 million individuals globally were afflicted with PAD (3). The initial symptoms of the disease are often atypical or absent, but as the disease progresses, patients are likely to experience chronic limb ischemia symptoms, such as pain, intermittent claudication and ulcers (4, 5). A systematic review indicates that PAD, whether symptomatic or not, is linked to increased cardiovascular morbidity and mortality, in conjunction with a deterioration in health-related quality of life (HRQoL) (6). As a prevalent global health issue characterized by its chronic nature and propensity for recurrence, FP occlusive disease significantly impacts the physical and mental well-being of individuals who are afflicted.
Multiple guidelines recommend endovascular intervention as the preferred approach for femoropopliteal (FP) occlusive disease (7). Standard percutaneous transluminal angioplasty (PTA) and arterial stenting have been commonly employed for the treatment of FP occlusive disease (8). However, in-stent restenosis (ISR) may arise post-procedure due to factors such as elastic recoil of the vessel wall and the irritation from the stent (9). Drug-Coated Balloons (DCBs), which are balloons coated with pharmacological agents that inhibit endothelial cell proliferation, have seen a growing application in the treatment of FP occlusive disease in recent years (10). Numerous studies have indicated that DCBs enhance patency rates and decrease the frequency of target lesion revascularization (TLR) (11–16). Nevertheless, despite the positive outcomes associated with DCBs for patients with PAD, the challenge of restenosis following DCB persists as a significant concern (11, 17, 18).
In contrast to other cardiovascular diseases, the management of peripheral artery disease (PAD) primarily emphasizes the enhancement of overall health, rather than exclusively prioritizing survival or limb preservation (19). For patients with FP occlusive disease, enhancements in HRQoL, including pain alleviation and enhanced walking capacity, represent the primary objectives of their interventions. These improvements also serve as critical metrics for patients to evaluate the effectiveness of their medical intervention. According to the European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines (1), emphasis on HRQoL following endovascular therapy in patients with PAD has been advocated as one of the recommended directions for future research in lower extremity arterial disease. However, the majority of existing studies have not prioritized HRQoL as a primary outcome measure following DCB treatment for FP occlusive disease. Furthermore, there is a notable lack of studies that specifically examine the determinants influencing HRQoL following DCB treatment among patients with FP occlusive disease. Consequently, the objective of this study was to assess HRQoL in individuals with FP occlusive disease who received DCB therapy, evaluated at 12 months postoperatively, utilizing a multicenter cross-sectional design. Additionally, the study sought to conduct a preliminary investigation into the risk factors impacting postoperative HRQoL.
2 Methods and methods
2.1 Study design
This retrospective study, conducted across various centers at one time point, was designed to identify factors affecting HRQoL of patients suffer from FP occlusive disease who underwent DCB at 12 months postoperatively, and the dependent variable was patients’ HRQoL at 12 months postoperatively.
2.2 Setting and study population
Patients diagnosed with FP occlusive disease who received DCB treatment were chosen using a convenience sampling method and were admitted to the Department of Vascular Surgery at 8 vascular centers in China between August 2021 and December 2023. The study data were authorized by the respective hospital. The types of variables included in the study were systematically collected across all centers. The surgical techniques employed across all centers are standardized, which were vascular interventions, including DCB, Debulking procedures, and stent implantation when necessary.
The following criteria were used for inclusion: individuals aged 18 years and older with a projected survival time exceeding 24 months; a Rutherford Classification ranging from 1 to 6; evidence from computed tomography angiography (CTA) indicating the occurrence of an occlusive lesion in the FP artery; inclusion of DCB in the proposed endovascular treatment strategy; for patients presenting with concurrent aortoiliac artery disease, endovascular luminal reconstruction was conducted to achieve flow recanalization with residual stenosis not exceeding 50%; and the presence of at least one healthy infrapopliteal outflow tract measuring 10 cm or more, which is continuous with the infrapopliteal artery either directly or through lateral branches, with a healthy outflow tract defined as having no more than 50% stenosis upon visual inspection; individuals administered double anti platelet therapy (DAPT).
The following criteria were used for exclusion: individuals presenting with concurrent severe insufficiency of cardiac, cerebral, renal, or other critical organ systems; individuals with documented allergies or hypersensitivity to contrast agents, heparin, aspirin, and other anticoagulant or antiplatelet therapies; individuals experiencing intraoperative procedural changes, intermediate incisional thrombectomy, and/or hybridization; individuals’ important data has been lost, such as the basic data, demographics and follow-up information; and individuals enrolled in other research studies at that time. The study relied on secondary data without any personal identification details. Informed consent was waived due to the study's retrospective approach.
2.3 Study size
Ni at al.,(2020) suggest a sample size 5–10 times greater than the count of independent variables when calculating for influencing factors (20). The study incorporated 32 independent variables and account for a data loss rate of 15%, resulting in a required minimum sample size ranging from 184 to 368 cases.
2.4 Variables
The researchers systematically collected data by reviewing the hospital case system, which included patients’ baseline information, lesion characteristics, treatment information, follow-up information, and HRQoL assessmentsat at both admission and 12 months postoperatively. YZ and WS subsequently organized the data into standardized, pre-agreed data extraction sheets, and any discrepancies were addressed through collaborative discussion.
2.4.1 Basic information and demographics
Baseline data was gathered through a researcher-designed form, developed following a comprehensive review of pertinent literature. The form contained variables involving body mass index (BMI), gender, age, household income, household sizes, smoking, account of chronic ailments [including hyperlipidemia, diabetes mellitus, renal insufficiency, coronary artery disease, hypertension, chronic obstructive pulmonary disease (COPD), and cerebral infarction], previous treatments for PAD, and admission HRQoL.
2.4.2 Lesion characteristics
The lesion characteristics, including preoperative Rutherford classification, preoperative manifestations of acute ischemia, lesion site, the presence of occlusive disease at other sites, the Trans-Atlantic Inter-Society Consensus II (TASC II) classification, lesion length, Vascular calcification (VC), preoperative outflow tract status, stenosis or occlusion, were assessed. One to three days prior to treatment, Rutherford classification and the presence of acute ischemic manifestations of each patient were documented.
Computed tomography (CT) scans were employed to measure the calcification traits of the occluded FP artery occlusion. VC was categorized as follows: mild calcification was characterized by calcification appearing on a single side of the artery, measuring under 5 cm; moderate calcification was identified by calcification on both sides of the artery, measuring between 5 and 10 cm in length; severe calcification was defined by the presence of calcification on both sides of the artery, exceeding 10 cm in length.
Prior to treatment, angiography was utilized to examine lesion site, the existence of occlusive disease at other sites, lesion length, and the infrapopliteal outflow tract status. Lower extremity arterial lesions were classified according to TASC II based on imaging findings (21). To evaluate the below-knee outflow tracts, a runoff score was calculated using the angiographic observations from anterior tibial, posterior tibial, and peroneal arteries (22), Each infrapopliteal artery was independently evaluated on a scale from 0 to 3 (0 = normal artery with minimal lesion; 1 = artery open with stenosis ranging from 20% to 49% of its diameter; 2 = artery open with narrowing ranging from 50% to 99% of its diameter; 2.5 = artery blocked by a clot with less than 50% of its height; 3 = an artery with a thrombus exceeding 50% of its height). The tibial runoff score was calculated by adding 1 to the sum of the three values. A score below 7 was classified as good, while a score of 7 or higher was classified as poor.
2.4.3 Treatment and follow-up information
The treatment information encompassed variables such as endovascular intervention approach, the diameter and length of DCB, and the postoperative Rutherford classification. The approachs encompassed DCB monotherapy, DCB in conjunction with remedial stenting, DCB with stent coverage, directional atherectomy (DA) combined with DCB, and laser atherectomy (LA) combined with DCB.
Follow-up information assessments involved monitoring the consistency of antithrombotic medication administration, the progression of the Rutherford Classification within 12 month, Limb retention status (including minor or major amputation) and TLR status at 12 months following the treatment.
2.4.4 HRQoL
In this research, HRQoL in patients suffering from FP occlusive disease was evaluated at the time of admission and subsequently re-evaluated 12 months following surgical intervention. HRQoL at 12 months post-treatment with DCB was designated as the dependent variable. The assessment utilized the Chinese version visual Vascular Quality of Life Questionnaire (VascuQoL). VascuQoL, initially created by Morgan et al. in 2001, was a specifical tool for evaluating quality of life in PAD (23), and was recommended by clinical guidelines for evaluating HRQoL in patients suffering from PAD (1, 4). The questionnaire comprised 5 dimensions and 25 items: pain (4 items), activity (8 items), symptoms (4 items), socialization (2 items), and mood (7 items). Scores varied between 1 and 7 for each item, with higher scores indicating enhanced HRQoL. In 2023, Zhu Jingpu and colleagues adapted and visualized the VascuQoL for cross-cultural contexts (24). They modified the response options of the original VascuQoL to a graded underlined format and incorporated facial expression —frustrated, smiling, and laughing—as rating anchors corresponding to scores of 1, 4, and 7, respectively.
Exhibiting by a Cronbach's α of 0.969, a KMO value of 0.783, and a Bartlett's test of sphericity at 1040.15 (p < 0.01), the Chinese version visual VascuQoL proved to be highly reliable and valid. Consequently, this study utilized the Chinese version visual VascuQoL to evaluate HRQoL in patients.
2.4.5 Statistical methods
This study employed SPSS27.0 for statistical data analysis software to analyze the data. At present, there is no standardized grading system for VascuQoL. Therefore, this paper categorized patients into two groups—those with a low HRQoL and those with a high HRQoL—based on the median HRQoL score at 12 months postoperatively. The analysis was conducted on these two groups. For continuous variables following a normal distribution, data were expressed as mean ± standard deviation, with univariable analysis of variance (ANOVA) used for comparisons across multiple groups. For continuous variables not following a normal distribution, data were expressed as median (quartiles) [M(P25,P75)], and Intergroup comparisons were conducted using a non-parametric rank-sum test. Categorical data were reported as frequencies and percentages [cases (%)], with group comparisons conducted using either the chi-square test or Fisher's exact probability method, contingent upon the data characteristics. In the logistic regression analysis, potential risk factors were detected by initially performing a univariable logistic regression., using HRQoL at 12 months post-DCB treatment as the dependent variable. Based on statistically significant results in variance analysis (P < 0.05), independent variables were selected. To mitigate the risk of omitting important clinical variables, all items with P < 0.10 in the results of univariable logistic regression analysis were subsequently incorporated as independent variables in the multivariable logistic regression to conduct further analysis.
3 Results
3.1 Patient characteristics and difference analysis results
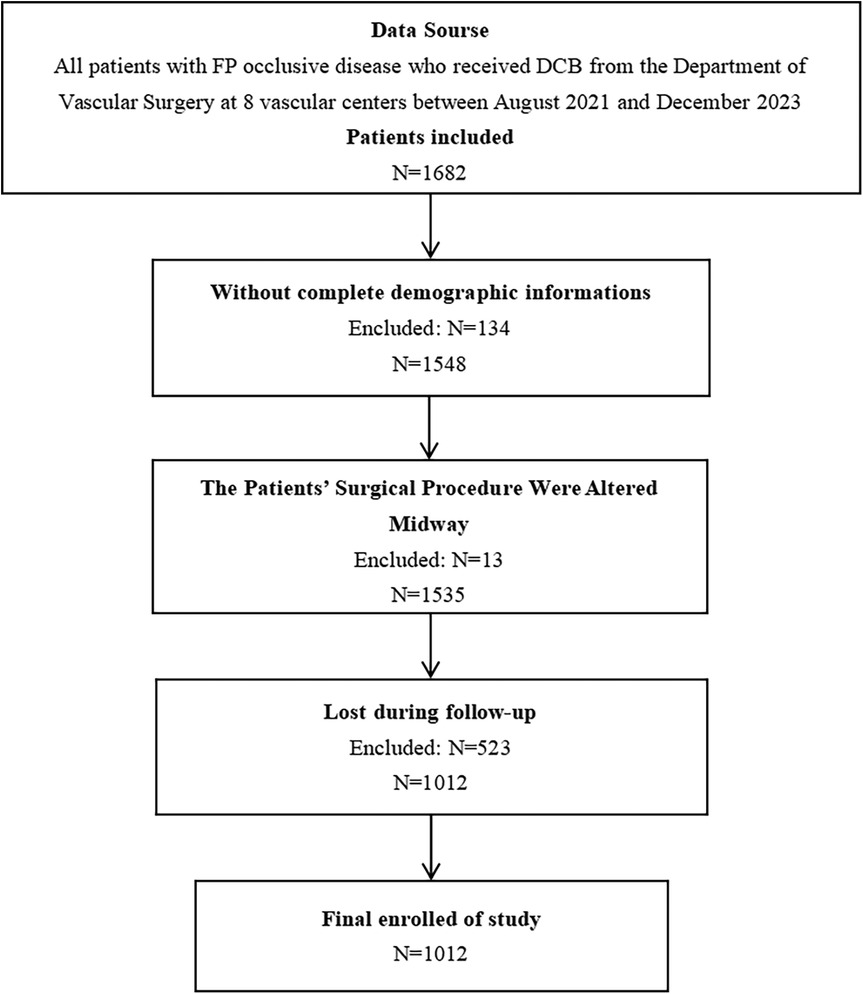
According to the established criteria for inclusion and exclusion, this study encompassed 1,012 patients diagnosed with FP occlusive disease, comprising 727 males (71.8%) and 285 females (28.2%), as shown in Figure 1. The median HRQoL score at 12 months following treatment with DCB was 141. Those with scores below 141 were classified as the low HRQoL group, comprising 503 patients (49.70%), while those with scores of 141 or higher were classified as having a high HRQoL group, comprising 509 (50.30%). The HRQoL scores at 12 months postoperatively served as a binary dependent variable. A detailed comparison of the patients’ characteristics was shown in Supplementary Table S1.
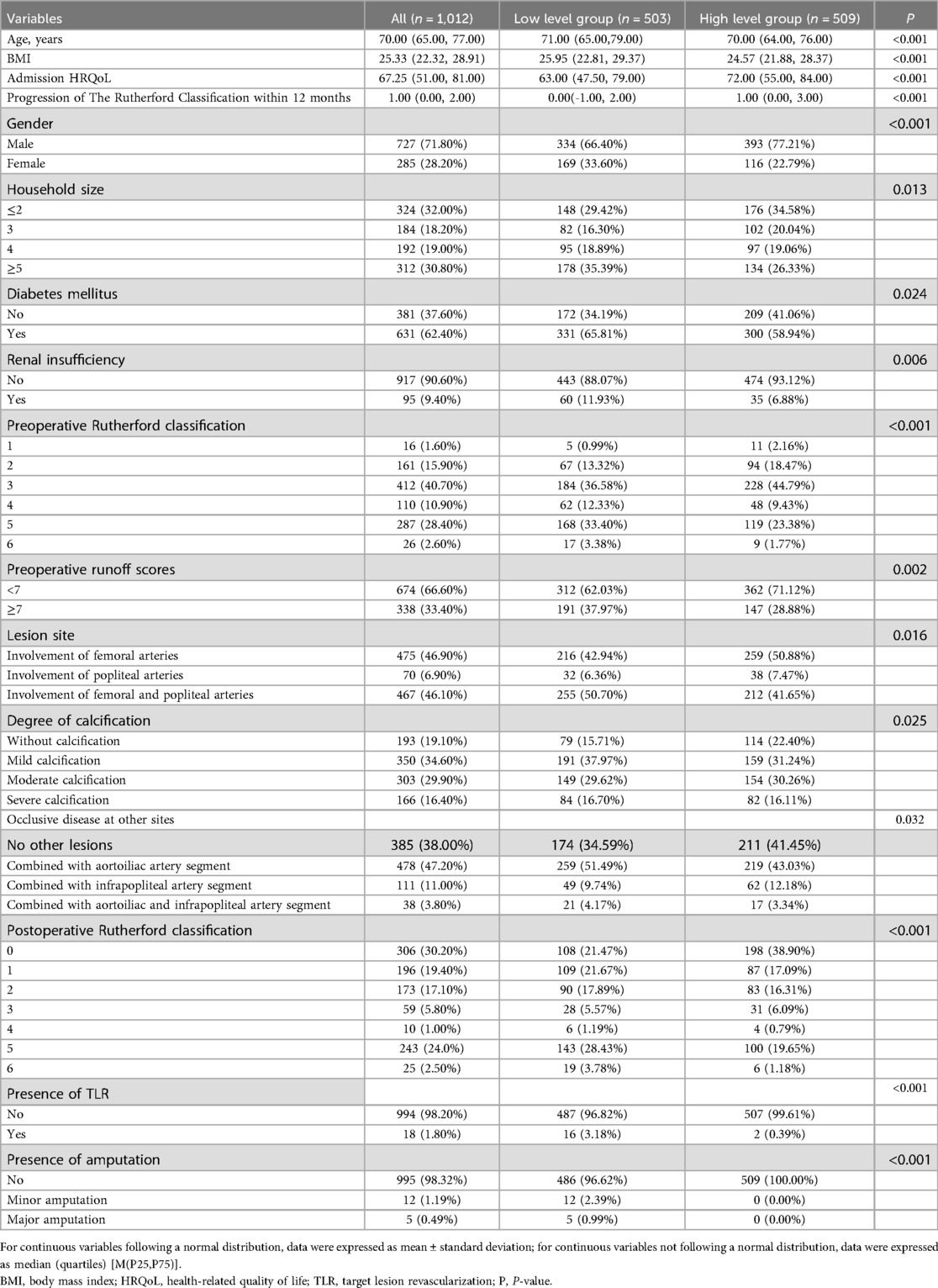
Statistically significant differences (P < 0.05), as detailed in Table 1, in BMI, HRQoL on admission, progression of the Rutherford classification within 12 months, gender, age, household size, diabetes mellitus, renal insufficiency, preoperative Rutherford classification, preoperative runoff scores, lesion site, degree of calcification, the presence of occlusive disease at other sites, postoperative Rutherford classification, the presence of TLR and amputation between the two groups. Conversely, DCB length, DCB diameter, TASC classification, monthly household income, smoking, hypertension, hyperlipidemia, coronary artery disease, cerebral infarction, COPD, history of PAD treatment, preoperative manifestations of acute ischemia, stenosis or occlusion, lesion length, endovascular intervention approach and the presence of regular antithrombotic medication were not statistically significant (P > 0.05).
3.2 Univariable logistic regression analysis
3.2.1 Basic information and demographics
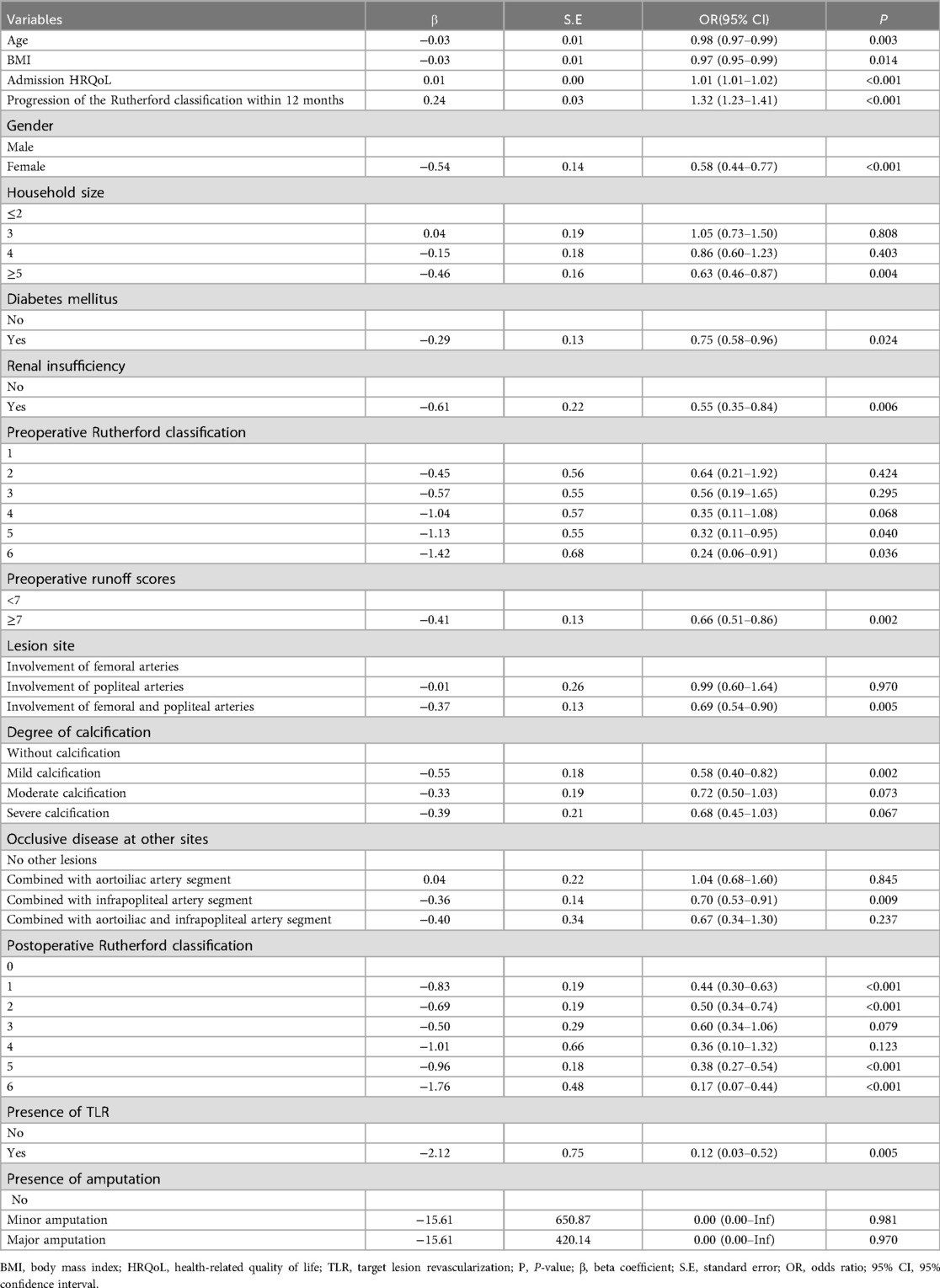
The results of univariable logistic regression analysis indicated that multiple independent variables exerted a statistically significant impact on patients’ HRQoL at 12 months following treatment with DCB (P < 0.05), as depicted in Table 2. In the analysis of variables in Base information and demographics, female patients had a lower likelihood of higher HRQoL scores, with an Odds Ratio (OR) of 0.58 compared to males [95% confidence interval (CI): 0.44–0.77, P < 0.001]. The OR for HRQoL scores decreased by 2% for each additional year of age (95% CI: 0.97–0.99, P = 0.003) and by 3% for every unit rise in BMI (95% CI: 0.95–0.99, P = 0.014), suggesting that both advancing age and elevated BMI may be correlated with diminished HRQoL. Larger household size was linked to better HRQoL, with an OR of 0.37 for those with five or more household members compared to those with two or fewer (95% CI: 0.46–0.87, P = 0.004). The OR for HRQoL scores in diabetic patients was 25% lower compared to non-diabetic patients (95% CI: 0.58–0.96, P = 0.024). Similarly, the OR for HRQoL scores in patients with renal insufficiency was 0.45% lower than in those without renal insufficiency (95% CI: 0.35–0.84, P = 0.006). These observations imply that diabetes and renal insufficiency may serve as risk factors adversely affecting HRQoL. Additionally, each 1-point increase in admission HRQoL correlated with a 1% rise in OR for post-surgery HRQoL (95% CI: 1.01–1.02, P < 0.001), revealing that patients exhibiting a higher HRQoL upon admission may experience an elevated HRQoL following surgical intervention.
3.2.2 Lesion characteristics
In the analysis of variables in lesion characteristics, the OR for HRQoL scores in patients with a preoperative Rutherford classification of grade 6 was 0.24 compared to those with grade 1 (95% CI: 0.06–0.91, P = 0.036), which indicates that patients with a higher severity of illness preoperatively experienced significantly lower HRQoL scores. Moreover, an OR of 0.66 was found for individuals with a preoperative runoff score of ≥7 (95% CI: 0.51–0.86, P = 0.002), revealing that poorer status of below-knee outflow tracts may be associated with reduced HRQoL. The OR for patients exhibiting mild calcification was 0.58 (95% CI: 0.40–0.82, P = 0.002), indicating that the extent of calcification may have an impact on HRQoL. Furthermore, the OR for HRQoL scores was 31% lower in individuals with lesions affecting both the femoral and popliteal arteries (95% CI: 0.54–0.90, P = 0.005) and 30% lower in those with combined infrapopliteal arterial occlusive lesions (95% CI: 0.53–0.91, P = 0.009), demonstrating that HRQoL may be diminished in patients with a greater number of lesion sites.
3.2.3 Treatment and follow-up information
In the analysis of variables in treatment information and postoperative follow-up, patients with several postoperative Rutherford classification grades (grades 1, 2, 5 and 6) demonstrated a significantly lower OR for HRQoL scores, ranging from 0.17 to 0.44 (P < 0.001). Moreover, the OR for HRQoL scores increased by 27% for each additional level of improvement in the Rutherford classification, indicating that patients exhibiting a greater degree of improvement in the Rutherford classification tend to experience an enhanced HRQoL (95% CI: 1.19–1.36, P < 0.001). Furthermore, the OR for patients who underwent TLR was only 0.12 (95% CI: 0.03–0.52, P = 0.005), signifying that TLR events were associated with a markedly diminished HRQoL scores. In contrast, the multivariable model showed that statistical significance was not achieved by the presence of amputation (p > 0.1).
3.3 Multivariable logistic regression analysis
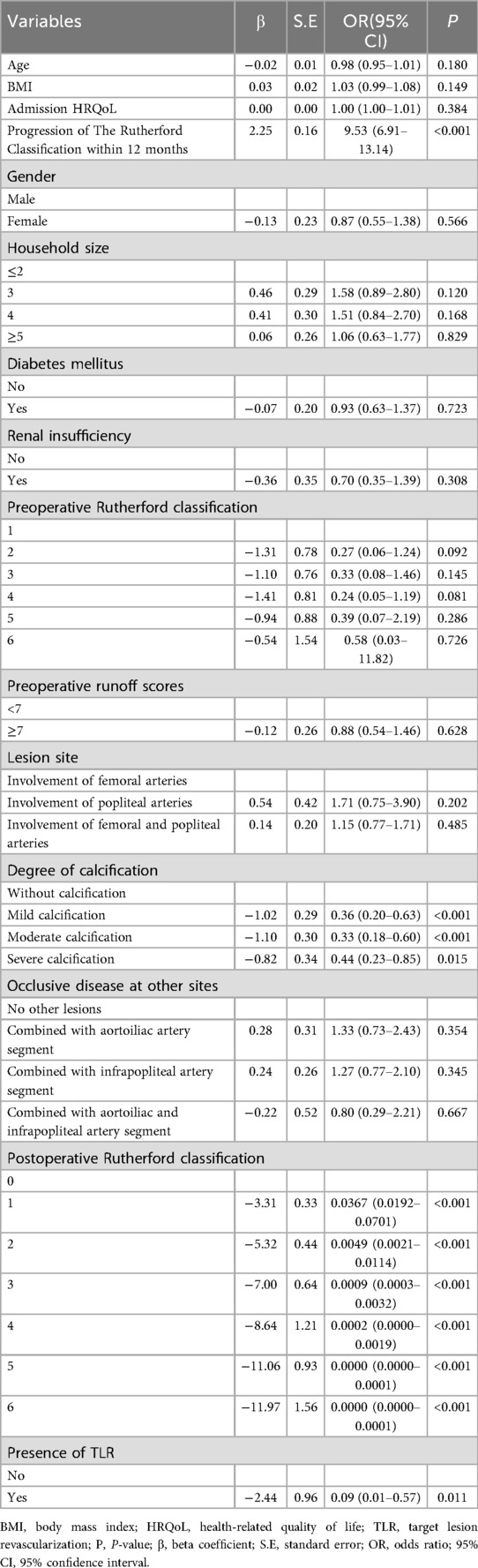
After adjusting for confounders in the multivariable logistic regression analysis, as detailed in Table 3, it was found that degree of calcification, postoperative Rutherford classification, progression of the Rutherford classification within a 12-month period and TLR status, independently influenced patients’ HRQoL scores at 12 months postoperatively (P < 0.05). Notably, the OR for patients with mild calcification, moderate calcification and severe calcification were 0.36 (95% CI 0.20–0.63, P < 0.001), 0.33 (95% CI 0.18–0.60, P < 0.001) and 0.44 (95% CI 0.23–0.85, P = 0.015), respectively. These findings suggested that vascular calcification is correlated with a reduced benefit in HRQoL scores. Furthermore, the OR for HRQoL scores among patients classified as postoperative Rutherford class 1, 2, 3 and 4 were found to be 3.67% (95% CI 0.0192–0.0701, P < 0.001), 0.49% (95% CI 0.0021–0.0114, P < 0.001), 0.09% (95% CI 0.0003–0.0032, P < 0.001) and 0.02% (95% CI 0.0000–0.0019, P < 0.001) lower than those of class 0, respectively, while the OR for Rutherford class 5 and 6 was so minimal that it was represented as 0.0000 (95% CI 0.0000–0.0001, P < 0.001).These findings indicated that a higher postoperative classification was significantly associated with a diminished advantage in HRQoL scores. Additionally, for each 1-point rise in the Rutherford classification over 12 months, the OR of HRQoL scores increased to 9.53 times (95% CI 6.91–13.14, P < 0.001), which suggested that a greater degree of improvement in the Rutherford classification was more likely to lead to higher HRQoL postoperatively. The OR for patients who experienced TLR was 0.09 (95% CI 0.01–0.57, P = 0.011), further corroborating the association between TLR events and significantly lower HRQoL scores. In contrast, the multivariable model showed that statistical significance was not achieved by other variables, such as gender, renal insufficiency and admission HRQoL (P > 0.05), indicating that their independent effects on the HRQoL scores were no longer significant after adjustment for confounders.
4 Discussion
HRQoL is frequently defined as a concept that relates to the health aspect of quality of life, emphasizing how illness and treatment affect disability, daily activities, and the role of health status in one's capacity to experience a contented life. HRQoL evaluates quality of life by accounting for the effect of illness, injury, medical intervention, and policy on impairment, functional status, perception, and opportunities (25, 26). HRQoL generally encompasses physical, psychological, and social support dimensions, and comprehending HRQoL is critical for advancing symptom relief, patient treatment, and recovery. Patient self-reported HRQoL can offers valuable insights into the suitability and potential improvements of specific treatment modalities. As highlighted in the introduction, for many patients with FP occlusive disease, alterations in HRQoL are the primary motivation for seeking medical intervention, and serve as the principal metric for assessing procedural efficacy. Consequently, this study aims to propose a comprehensive large-scale study to preliminarily investigate the determinants affecting postoperative HRQoL in patients with FP occlusive disease undergoing DCB.
4.1 Vascular calcification
The findings of logistic regression analysis revealed that indicated that variables such as lesion site, DCB length, DCB diameter, and preoperative outflow tract condition did not exhibit a statistically significant association with HRQoL at 12 months postoperatively (P > 0.05). Conversely, degree of calcification and postoperative Rutherford classification demonstrated a significant association with HRQoL at 12 months following surgery. Vascular calcification, particularly medial arterial calcification, emerges as a critical risk factor for PAD and may compromise the effectiveness of Endovascular intervention (27, 28). Furthermore, VC adversely impacts the efficacy of stenting in standard PTA and elevates the risk of TLR (29, 30). The study carried out by Soga and colleagues demonstrated that DCB treatment for FP occlusive disease with mild calcification was associated with improved vascular patency; however, its effectiveness was reduced in patients with severe calcification (31). In contrast, the multivariate logistic regression analysis presented in the current study revealed that the OR for HRQoL scores in patients with mild calcification (OR = 0.36, P < 0.001) and moderate calcification (OR = 0.33, P < 0.001) was lower than that those with severe calcification (OR = 0.44, P < 0.001), which indicated that patients with mild or moderate calcification tend to experience a lower HRQoL at 12 months postoperatively, in comparison to severe calcification. This disparity could be explained by the fact that patients with severely calcified lesions in this research were potentially addressed using DA or LA in conjunction with DCB. Numerous studies have substantiated the effectiveness of these interventions for managing patients with severe calcification over the intermediate and long term (11, 12, 32), thus leading to a higher HRQoL at 12 months postoperatively among individuals with moderate and severe calcifications.
4.2 Postoperative rutherford classification and its progression
The results of this study suggested that individuals with a elevated postoperative Rutherford classification exhibited a comparatively lower HRQoL at 12 months. As the classification level increases, the HRQoL correspondingly decreased. The odds ratio for Rutherford classes 5 and 6 was negligible, being reported as 0.0000 (95% CI 0.0000–0.0001, P < 0.001). Meanwhile, as the Rutherford classification increases within 12 months, there was a corresponding significant improvement in the patient's HRQoL (OR = 9.53, 95% CI 6.91–13.14, P < 0.001). In brief, the aforementioned data collectively suggest a strong correlation between the R grade and patients’ HRQoL. The Rutherford classification is a widely utilized system for staging and grading patients with PAD based on clinical symptoms. An higher grading level within this system is associated with more severe ischemic symptoms in the affected limbs, including intermittent claudication and pain (33). The extent to which postoperative ischemic conditions are effectively ameliorated significantly influences the long-term HRQoL following surgery. Ambulatory capability constitutes a critical aspect of HRQoL, because adequate walking ability and the absence of pain are essential prerequisites for patients to fully engage in life. Alterations in the Rutherford classification during follow-up assessments can serve as direct indicators of enhancements in patients’ walking ability and pain levels.
4.3 The occurrence of TLR
Within one year, 18 patients (1.77%) underwent TLR, including 16 patients whose HRQoL scores were below the median of 141 at the 12-month mark. Multivariable logistic regression analysis revealed a significant negative correlation between the occurrence of TLR and HRQoL at 12 months postoperatively (OR = 0.11, P = 0.004). Interviews with these patients indicated that the primary reason for hospital readmission for TLR was the recurrence of lower extremity ischemic symptoms, such as coldness, pain, and intermittent claudication, which adversely affected their mobility and daily activities. Furthermore, TLR may induce concerns regarding disease prognosis among patients and impose economic burdens, which can adversely affect patients’ mental health and overall HRQoL.
4.4 Limitations
The present research is a retrospective, multicenter cross-sectional investigation, serving as a preliminary exploratory analysis. Convenience sampling was employed to select participants, prioritizing the study's feasibility. Consequently, it is essential to recognize that the findings may may not be representative, thereby limiting the generalizability of the conclusions compared to studies using probability sampling methods. To enhance the validity of these findings, future research should incorporate sampling methods such as simple random sampling or stratified sampling, and employ designs like cohort studies or randomized controlled trials.
The variables examined in this study were derived from patients’ baseline characteristics and treatment-related information, excluding laboratory indices. However, numerous studies have demonstrated that certain laboratory markers significantly influence postoperative restenosis and HRQoL in patients. A prospective observational study by Wachsmann-Maga et al. on patients with PAD identified a link between quality of life changes and markers of vascular inflammation including leukotrienes (LTE4) and thromboxane B2 (TXB2) (34). Additionally, TAO and colleagues performed a retrospective study to investigate the link between postoperative restenosis and anemia in 91 patients with FP occlusive disease who underwent DCB angioplasty (35). Their findings identified anemia (P = 0.030) as a risk factor for postoperative restenosis in this patient cohort. Consequently, future research should incorporate laboratory indicators to further explore and validate the relationships between additional variables and HRQoL outcomes following DCB treatment in individuals suffering from FP occlusive disease.
This study is a single time-point cross-sectional analysis, but FP occlusive disease is chronic and recurrent. Patients’ walking ability and HRQoL change over time after DCB treatment. For instance, a patient with FP occlusive disease who underwent DCB may exhibit significantly different HRQoL outcomes at 6 months and 12 months postoperatively. Therefore, Collecting data at multiple postoperative points would allow for a longitudinal analysis of HRQoL alterations, which would facilitate a comprehensive understanding of the HRQoL trajectory among patients with varying characteristics, thereby helping to identify factors influencing HRQoL in FP occlusive disease patients after DCB.
5 Conclusion
In the study, we performed a multicenter cross-sectional analysis of HRQoL at 12 months postoperatively among individuals suffer from FP occlusive disease who underwent DCB angioplasty. We examined the factors influencing HRQoL and found that a higher degree of calcification, a higher postoperative Rutherford classification, and those who experienced TLR exhibited lower postoperative HRQoL. Additionally, the progression of the Rutherford classification within 12 months was significantly positively correlated with HRQoL at 12 months postoperatively. This study represents a preliminary investigation into the risk factors affecting long-term HRQoL following DCB treatment. Future research should seek to validate this study's findings through the implementation of randomized sampling, the expansion and refinement of the variable set, and the assessment of patients at multiple time intervals. This method will enable a more accurate identification of factors affecting long-term HRQoL in patients with FP occlusive disease undergoing DCB treatment. Additionally, such studies will contribute to the enhancement of therapeutic interventions, ultimately aiming to improve patients’ ambulatory capabilities and overall HRQoL.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Huashan Hospital of Fudan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YZ: Conceptualization, Methodology, Formal analysis, Data curation, Writing – review & editing, Writing – original draft. SL: Writing – original draft, Writing – review & editing, Conceptualization. WF: Resources, Investigation, Writing – review & editing. MY: Resources, Supervision, Writing – review & editing. ZW: Writing – review & editing, Resources. ZF: Writing – review & editing, Resources. LG: Writing – review & editing, Resources. ZS: Resources, Writing – review & editing. XF: Writing – review & editing, Resources. CH: Resources, Writing – review & editing. WS: Supervision, Resources, Writing – review & editing, Formal analysis. YC: Writing – review & editing, Methodology, Conceptualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project received funding from the Shanghai Shenkang Hospital Development Center, specifically for the special training program focused on medical-enterprise integration and innovation support skills (SHDC2023CRS025).
Acknowledgments
We acknowledge the valuable contributions and insights provided by our colleagues on this article.
Conflict of interest
The authors affirm that there were no commercial or financial relationships involved in the research that could be perceived as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1657478/full#supplementary-material
References
1. Nordanstig J, Behrendt C, Baumgartner I, Belch J, Bäck M, Fitridge R, et al. Editor’s choice—European society for vascular surgery (ESVS) 2024 clinical practice guidelines on the management of asymptomatic lower limb peripheral arterial disease and intermittent claudication. Eur J Vasc Endovasc Surg. (2024) 67(1):9–96. doi: 10.1016/j.ejvs.2023.08.067
2. Gallagher K, Meltzerm A, Ravin R, Graham A, Shrikhande G, Connolly PH, et al. Endovascular management as first therapy for chronic total occlusion of the lower extremity arteries: comparison of balloon angioplasty, stenting, and directional atherectomy. J Endovasc Ther. (2011) 18(5):624–37. doi: 10.1583/11-3539.1
3. Eid MA, Mehta K, Barnes JA, Wanken Z, Columbo JA, Stone DH, et al. The global burden of peripheral artery disease. J Vasc Surg. (2023) 77(4):1119–26. doi: 10.1016/j.jvs.2022.12.015
4. Gornik HL, Aronow HD, Goodney PP, Arya S, Brewster LP, Byrd L, et al. 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS guideline for the management of lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2024) 149(24):e1313. doi: 10.1161/CIR.0000000000001329
5. Aboyans V, Ricco J, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS). Eur Heart J. (2018) 39(9):763–816. doi: 10.1093/eurheartj/ehx095
6. Giannopoulos S, Volteas P, Koudounas G, Virvilis D, Labropoulos N. Contemporary femoropopliteal stents: reporting gaps of randomized trials. Ann Vasc Surg. (2025) 118:68–82. doi: 10.1016/j.avsg.2025.04.110
7. Branch of Lower Extremity Artery Disease In Professional Committee of Vascular Surgery In National Committee of Cardiovascular Experts, Vascular Surgery Committee of China Medical Education Association. Chinese expert consensus on the diagnosis and treatment of femoral-popliteal artery occlusive disease. Chin Circ J. (2022) 37(7):669–76. doi: 10.3969/j.issn.1000-3614.2022.07.003
8. Aru RG, Tyagi SC. Endovascular treatment of femoropopliteal arterial occlusive disease: current techniques and limitations. Semin Vasc Surg. (2022) 35(2):180–9. doi: 10.1053/j.semvascsurg.2022.04.010
9. Abdullah K, Bou Dargham B, Steinbrecher M, Sun B, Huiqiang Z, Khalili H, et al. Drug-eluting stents for treatment of peripheral artery disease. Am J Cardiovasc Drugs. (2018) 18(3):175–80. doi: 10.1007/s40256-018-0265-4
10. Sallustro M, Peluso A, Turchino D, Maione I, Vita F, Martelli E, et al. Results of new dual-drug coated balloon angioplasty versus POBA for femoropopliteal lesions. Ann Vasc Surg. (2023) 89:52–9. doi: 10.1016/j.avsg.2022.09.047
11. Hu L, Wang H, Pan D, Wu S, Zhang H, Gu Y. Three-year results of combining debulking devices with drug-coated balloons for the treatment of de novo femoropopliteal arteriosclerosis obliterans. Ann Vasc Surg. (2025) 114:63–73. doi: 10.1016/j.avsg.2025.01.019
12. Li Y, Tong Z, Guo J, Guo L, Gu Y. Two-year outcomes of excimer laser ablation combined with drug-coated balloon for treating de novo lesions and in-stent restenosis in femoropopliteal artery of chronic limb-threatening ischemia patients. Ann Vasc Surg. (2025) 114:90–100. doi: 10.1016/j.avsg.2025.01.016
13. Tang X, Ma H, Zhou G, Shu X, Li Y, Guo M. Efficacy of supera stent in the treatment of complex femoropopliteal artery lesions: a short-term result analysis of real-world evidence. Ann Vasc Surg. (2024) 109:101–10. doi: 10.1016/j.avsg.2024.06.022
14. Wang H, Wu S, D'Oria M, Pan D, Hu L, Zhang H, et al. Comparison of different endovascular treatments of femoropopliteal artery in-stent restenosis: a systematic review and Bayesian network meta-analysis. Ann Vasc Surg. (2024) 104:205–16.
15. Khan MS, Zou F, Khan AR, Moustafa A, Schmid CH, Baig M, et al. Meta-analysis comparing endovascular treatment modalities for femoropopliteal peripheral artery disease. Am J Cardiol. (2020) 128:181–8. doi: 10.1016/j.amjcard.2020.05.015
16. Jaff MR, Nelson T, Ferko N, Martinson M, Anderson LH, Hollmann S. Endovascular interventions for femoropopliteal peripheral artery disease: a network meta-analysis of current technologies. J Vasc Interv Radiol. (2017) 28(12):1617–27. doi: 10.1016/j.jvir.2017.08.003
17. Wang Z, Sheng L, Gu H, Yang F, Xie H, Li M. Rivaroxaban and aspirin in drug-coated balloon angioplasty for femoropopliteal in-stent restenosis: a retrospective cohort study. Ann Vasc Surg. (2024) 108:338–45. 39013487
18. Wang J, Chen X, Zhao J, Zhang WW. Systematic review and meta-analysis of the outcomes of drug-eluting stent versus drug-coated balloon angioplasty for lower extremity peripheral artery diseases. Ann Vasc Surg. (2022) 85:1–8. doi: 10.1016/j.avsg.2022.04.039
19. Spertus J, Jones P, Poler S, Rocha-Singh K. The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J. (2004) 147(2):301–8. doi: 10.1016/j.ahj.2003.08.001
20. Pin N, Jingli C, Na L. Sample size estimation in quantitative nursing research. Chin J Nurs. (2010) 45(4):378–9. doi: 10.3761/j.issn.0254-1769.2010.04.037
21. Jaff MR WCHW. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the inter-society consensus for the management of peripheral arterial disease (TASC II): the TASC steering committee. Catheter Cardiovasc Interv. (2015) 86(4):611–25. doi: 10.1002/ccd.26122
22. Park UJ, Kim HT, Roh Y. Factors affecting outcomes after endovascular treatment for femoropopliteal atherosclerotic lesions. Asian J Surg. (2019) 42(1):209–16. doi: 10.1016/j.asjsur.2018.04.008
23. Morgan MB, Crayford T, Murrin B, Fraser SC. Developing the Vascular Quality of Life Questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg. (2001) 33(4):679–87. doi: 10.1067/mva.2001.112326
24. Jingpu Z, Ting Z, Qiong N, Yuli W. Chinese version visual vascular quality of life questionnaire for evaluating life quality of patients with peripheral artery disease in China. Chin J Interv Imaging Ther. (2023) 20(5):274–7. doi: 10.13929/j.issn.1672-8475.2023.05.005
25. Haraldstad K, Wahl A, Andenæs R, Andersen JR, Andersen MH, Beisland E, et al. A systematic review of quality of life research in medicine and health sciences. Qual Life Res. (2019) 28(10):2641–50. doi: 10.1007/s11136-019-02214-9
26. Mayo N. Dictionary of Quality of Life and Health Outcomes Measurement. Milwaukee: WI: International Society for Quality of Life Research (2015).
27. St. Hilaire C. Medial arterial calcification: a significant and independent contributor of peripheral artery disease. Arterioscler Thromb Vasc Biol. (2022) 42(3):253–60. doi: 10.1161/ATVBAHA.121.316252
28. Singh KJ R, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv. (2014) 83(6):212–20. doi: 10.1002/ccd.25387
29. Tepe G, Brodmann M, Bachinsky W, Holden A, Zeller T, Mangalmurti S, et al. Intravascular lithotripsy for peripheral artery calcification: mid-term outcomes from the randomized disrupt PAD III trial. J Soc Cardiovasc Angiogr Interv. (2022) 1(4):100341.
30. Kawaguchi R, Tsurugaya H, Hoshizaki H, Toyama T, Oshima S, Taniguchi K. Impact of lesion calcification on clinical and angiographic outcome after sirolimus-eluting stent implantation in real-world patients. Cardiovasc Revasc Med. (2008) 9(1):2–8. doi: 10.1016/j.carrev.2007.07.004
31. Soga Y, Takahara M, Iida O, Tomoi Y, Kawasaki D, Tanaka A, et al. Vessel patency and associated factors of drug-coated balloon for femoropopliteal lesion. J Am Heart Assoc. (2023) 12(1):e25677. doi: 10.1161/JAHA.122.025677
32. Rocha Singh KJ, Sachar R, DeRubertis BG, Nolte Ernsting CCA, Winscott JG, Krishnan P, et al. Directional atherectomy before paclitaxel coated balloon angioplasty in complex femoropopliteal disease: theVIVA REALITY study. Catheter Cardiovasc Interv. (2021) 98(3):549–58. doi: 10.1002/ccd.29777
33. Rutherford RB, Baker JD, Ernst C. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. (1997) 26(3):517–38. doi: 10.1016/S0741-5214(97)70045-4
34. Wachsmann-Maga A, Maga M, Polczyk R, Włodarczyk A, Pasieka P, Terlecki K, et al. Vascular inflammatory markers as predictors of peripheral arterial disease patients’ quality-of-life changes after endovascular treatment. J Clin Med. (2023) 12(10):3412. doi: 10.3390/jcm12103412
Keywords: femoropopliteal occlusive disease, drug-coated balloons, health-related quality of life, multicenter cross-sectional study, rutherford classification, vascuQoL
Citation: Zhang Y, Lu S, Fan W, Ye M, Wu Z, Feng Z, Guo L, Shi Z, Fang X, He C, Shi W and Cao Y (2025) Analysis of factors influencing health-related quality of life in patients with femoropopliteal atherosclerotic occlusive disease treated with drug-coated balloons 12 months after surgery. Front. Surg. 12:1657478. doi: 10.3389/fsurg.2025.1657478
Received: 18 August 2025; Accepted: 22 October 2025;
Published: 12 November 2025.
Edited by:
Apostolos Tassiopoulos, Stony Brook University, United StatesReviewed by:
George Galyfos, National and Kapodistrian University of Athens, GreeceEugenio Martelli, University of Rome Tor Vergata, Italy
Copyright: © 2025 Zhang, Lu, Fan, Ye, Wu, Feng, Guo, Shi, Fang, He, Shi and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weihao Shi, dmFzY3VsYXI3NDEwMjFAMTYzLmNvbQ==; Yanpei Cao, eWFucGVpY2FvQGZ1ZGFuLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Yan Zhang
Yan Zhang Shuangshuang Lu1,†
Shuangshuang Lu1,† Weijian Fan
Weijian Fan Ziheng Wu
Ziheng Wu Zibo Feng
Zibo Feng Zhenyu Shi
Zhenyu Shi Chunshui He
Chunshui He Weihao Shi
Weihao Shi Yanpei Cao
Yanpei Cao