- 1Nursing Department, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Orthopaedic Surgery, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3Zhejiang Key Laboratory of Mechanism Research and Precision Repair of Orthopaedic Trauma and Aging Diseases, Hangzhou, Zhejiang, China
Purpose: This review aims to systematically evaluate the incidence, risk factors, and international guideline discrepancies for venous thromboembolism (VTE) following hip arthroscopy (HA).
Methodology: A search of four databases from the inception to April 20, 2025, identified studies reporting VTE outcomes post-HA. Relevant practice guideline recommendations were concurrently analyzed.
Results: Twenty-one studies encompassing 135,377 patients and five clinical guidelines were included. Female patients constituted 91,013 cases (67.2%). The mean patient age was 37.08 years; however, the average follow-up duration was limited to 3.7 months, which may be a study limitation. Pooled incidence rates were: deep vein thrombosis (DVT) 0.441%, pulmonary embolism (PE) 0.216%, and overall VTE 0.656%. The majority of studies were Level IV evidence (57%), with Level III evidence comprising 33%. Identified risk factors for post-HA VTE included obesity, oral contraceptive use, ≥45 years, overweight status, coagulopathy, and arteriovenous anomalies. The reported VTE incidence ranged from 0% to 6.94%. International guidelines vary, but most advocate for risk-stratified thromboprophylaxis.
Conclusions: The incidence of VTE following hip arthroscopy is low. Routine pharmacological thromboprophylaxis may not be necessary for standard-risk patients. However, high-risk individuals warrant personalized prophylaxis regimens, with pharmacological prophylaxis when clinically indicated based on risk assessment.
Introduction
Since Dr. Michael Burman performed the first hip arthroscopy in 1931 (1), the annual volume of hip arthroscopic procedures has progressively increased, with a marked acceleration in recent years (2). HA helps doctors better diagnose and treat hip conditions such as femoroacetabular impingement, a leading cause of labral tears and early arthritis. With its widespread adoption, complications associated with HA have garnered increasing attention, including femoral neck fractures, avascular necrosis of the femoral head, iatrogenic chondral injury, temporary neuropraxia, DVT, and PE (3). Reported rates of postoperative thrombosis following HA range from 0.2% to 9.5% (4). Despite lower VTE rates compared to joint replacement (5), post-arthroscopic thrombosis has received limited attention. However, the annual performance of tens of thousands of hip arthroscopies ensures that the absolute number of thromboembolic complications is not trivial (6).
A UK study analyzed hip arthroscopy patients through England's National Health Service admissions database, reporting a 0.16% venous thromboembolism (VTE) rate (7). In contrast, a US study using the Mariner Ortho Pearl Database (2015–2021) found a higher incidence of 0.75% post-surgery (8). A recent study leveraging The TriNET Research Network (2003–2024)—which houses one of the largest clinical datasets across North America and Western Europe—documented the highest VTE rate at 1% following these procedures (9). Among untreated proximal DVT cases, approximately 10% to 30% progress to symptomatic PE. However, imaging studies suggest that the proportion of asymptomatic PE may reach 50% (10). The incidence of untreated PE is approximately 5% to 30%, which prolongs hospitalization, increases costs, and necessitates prolonged anticoagulation (>3 months) (10, 11), thereby compromising the minimally invasive advantage of arthroscopic surgery.
Multiple factors have been confirmed as risk factors for thrombosis, including obesity, oral contraceptive use, age ≥45 years, and overweight status (12). However, no detailed guidelines exist for thromboprophylaxis for hip arthroscopy because orthopedic surgeons are not fully aware of the risks. This review aims to examine and compile the existing literature reporting VTE after HA to evaluate the incidence and risk factors for VTE following HA. This review synthesizes incidence, risk factors, and prophylaxis strategies for VTE following HA.
Methods
Registration
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and prospectively registered with PROSPERO (registration number: CRD420251035449).
Eligibility criteria
Inclusion criteria comprised (1): studies reporting incidence of VTE, DVT, or PE following HA; (2) investigations evaluating physical or pharmacological VTE prophylaxis strategies post-HA; and (3) clinical guidelines addressing VTE prevention after arthroscopic procedures. Exclusion criteria encompassed animal studies, editorials, commentaries, and studies lacking explicit quantitative VTE outcome data.
Search strategy
Two independent reviewers systematically searched PubMed, Web of Science, China National Knowledge Infrastructure and Embase databases from inception to April 20, 2025. Search terms included: (‘venous thromboembolism’ OR ‘VTE’ OR ‘deep vein thrombosis’ OR ‘DVT’ OR ‘pulmonary embolism’ OR ‘PE’) AND (‘arthroscopic hip surgery’ OR ‘hip arthroscopy’). Only English and Chinese publications were included. We additionally searched for relevant clinical guidelines and international consensus statements on HA and VTE. The PRISMA framework guided all search and reporting processes.
Study screening
Two reviewers independently screened titles and abstracts against eligibility criteria. Articles deemed potentially relevant by either reviewer underwent full-text assessment. Discrepancies during title/abstract screening were resolved by inclusive retention to maximize sensitivity. Full-text screening disagreements were resolved through consensus or, if necessary, adjudication by a senior reviewer. Reference lists and citation tracking were also performed.
Risk of bias assessment
Methodological quality of included studies was appraised in duplicate using validated tools: the Methodological Index for Non-Randomized Studies (MINORS) for case series and the Newcastle-Ottawa Scale (NOS) for cohort studies (13, 14).
• MINORS: This 12-item instrument assigns scores of 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). Maximum scores are 16 for non-comparative and 24 for comparative studies.
• NOS: This 8-item scale evaluates studies across three domains: Selection (4 items), Comparability (1 item), and Outcome/Exposure (3 items). Studies scoring ≥6 points were classified as high quality; scores ≤5 indicated low quality.
Data extraction
Two reviewers independently extracted data using a standardized form in Microsoft Excel. Extracted variables included: first author, publication year, study design, evidence level (I-V), sample size, patient demographics (age, sex), follow-up duration, preoperative VTE prophylaxis use, traction duration, surgical time, VTE diagnostic methods, incidence rates (DVT, PE, overall VTE), and reported VTE risk factors (e.g., obesity, oral contraceptive use).
Statistical analysis
The occurrence rates of VTE, DVT, and PE in the included studies were all dichotomous data, which was extracted in the form as an absolute number and patient number. The Mantel-Haenszel (M-H) method was used Heterogeneity was quantitatively assessed using I2. Descriptive statistics were calculated. Continuous variables are expressed as weighted means ± standard deviations (SD). Categorical data are presented as frequencies and percentages. Statistical significance was defined as p < 0.05 for all analyses; reported p-values were either directly extracted or calculated from study data. An intraclass correlation coefficient (ICC) was used to evaluate the inter-reviewer agreement of the MINORS/NOS scores. The ICC value ranges from 0 to 1, with values closer to 1 indicating higher consistency.
Results
Literature screening
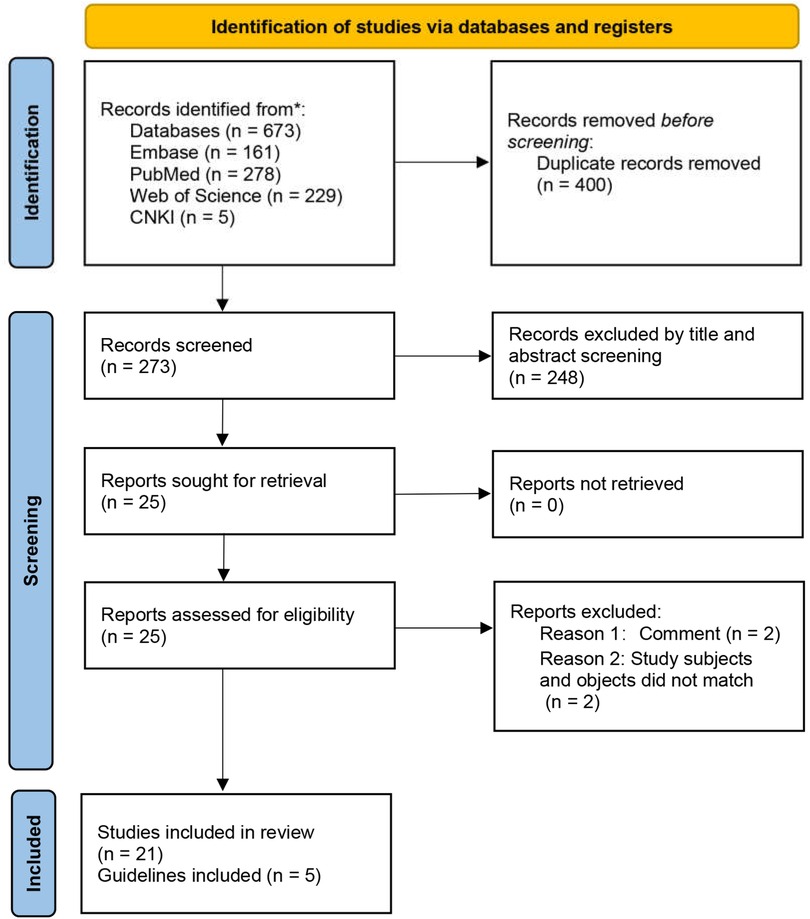
The initial database search yielded 673 records (Figure 1). After duplicate removal and independent title/abstract screening by two reviewers, 21 articles proceeded to full-text review and met inclusion criteria. The literature comprised: retrospective studies (n = 14), prospective investigations (n = 2), meta-analyses (n = 1), case reports (n = 1), and clinical guidelines (n = 3) (Tables 1, 2). This systematic process ensured the inclusion of relevant, high-quality evidence.
Study characteristics and patient demographics
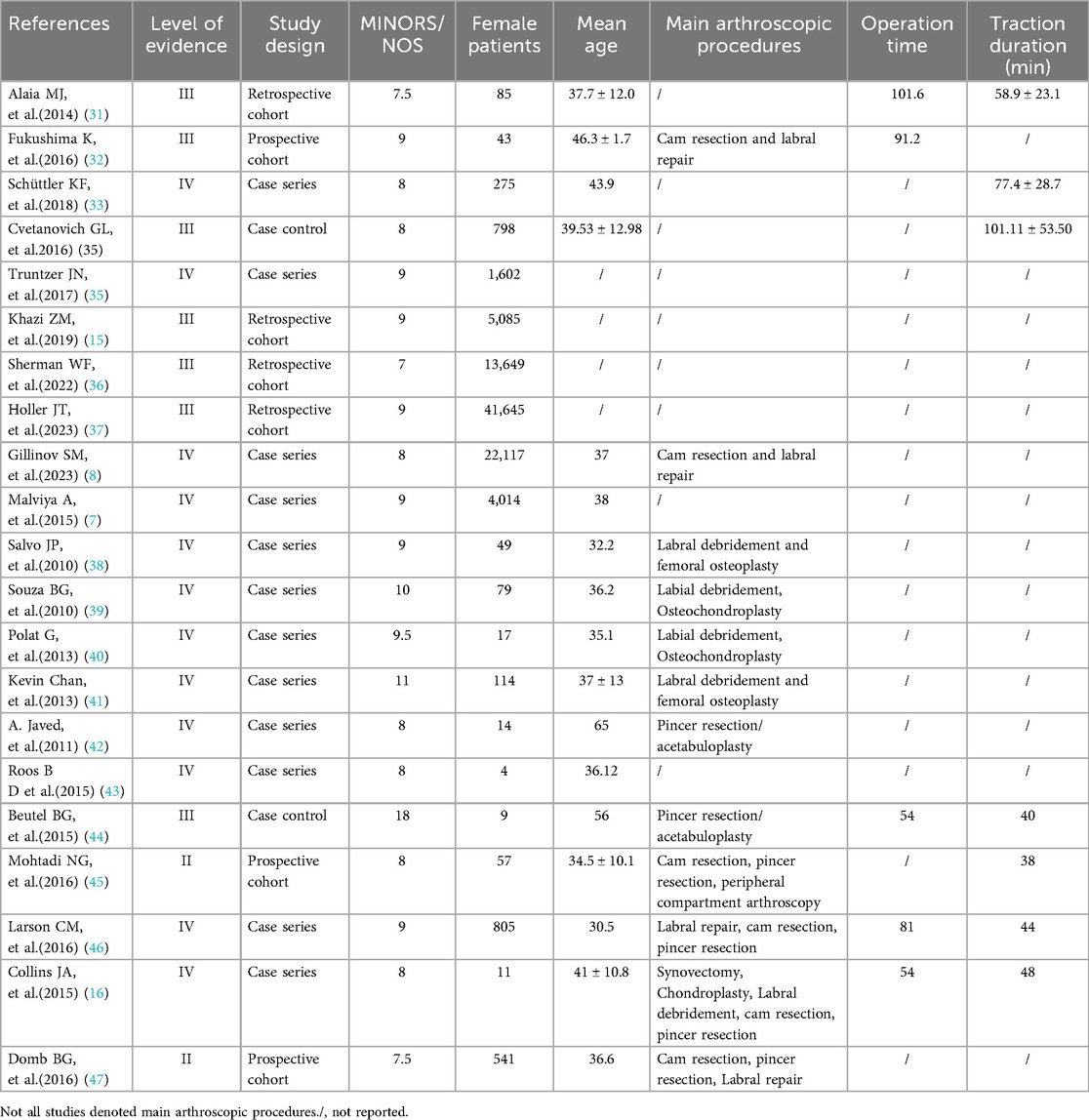
The 21 included studies, published between 2010 and 2025, reported outcomes for 135,377 patients undergoing HA. Evidence levels were: Level II (n = 2), Level III (n = 7), and Level IV (n = 12). Study designs included: cohort studies (n = 7) and case series/case-control studies (n = 14). Methodological quality assessment yielded a mean MINORS score of 9.5 for case series/control studies and a mean NOS score of 8.1 for cohort studies (Table 2).
The incidence of VTE after HA
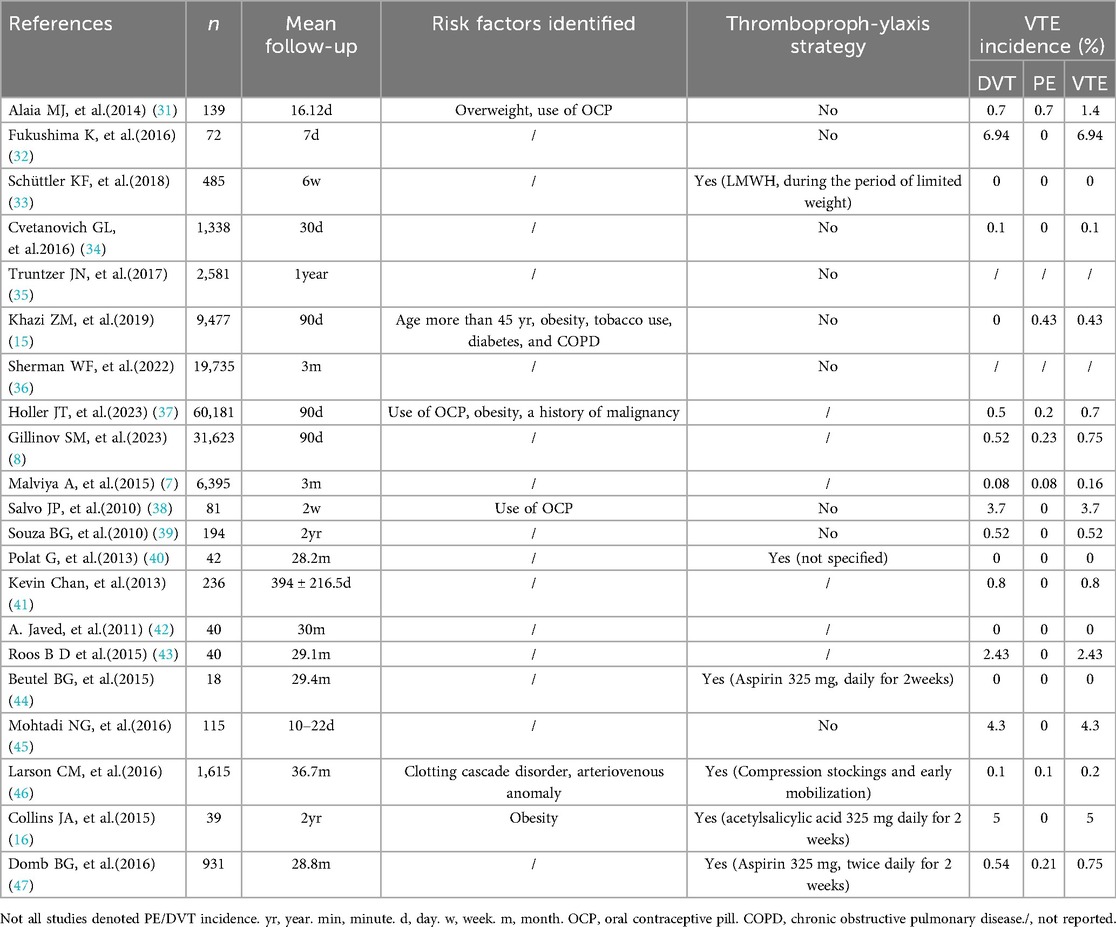
As shown in Supplementary Tables 1, 2; Supplementary Table S1, these studies demonstrated substantial heterogeneity in VTE incidence (range: 0%–6.94%). Meanwhile, it was observed that the incidence rate of VTE in prospective cohort studies was higher than in retrospective cohort studies (Tables 1, 2). When subgroup analysis was conducted according to the study design, the case-control group showed low heterogeneity, with a combined incidence of VTE of 0.07% (Supplementary Table S2; Supplementary Figure S1). Large-scale analyses (n > 30,000) reported lower rates (0.7%-0.75%), whereas smaller cohorts (n = 18–81) showed higher incidences, suggesting detection bias or underpowered outcomes. Pharmacological prophylaxis (e.g., LMWH, aspirin) reduced VTE risk to very low levels (0%-0.54%), contrasting sharply with no-prophylaxis cohorts (1.4%-5.0%). The incidence rate of pulmonary embolism following hip arthroscopy ranges from 0% to 0.7%.
Risk factors after HA
The risk factors for VTE following hip arthroscopy have been reported in various studies. As shown in Table 1, multiple studies suggest that obesity and oral contraceptive use are significant risk factors for VTE after HA. One study indicates age ≥45 is also a risk factor for VTE after HA (15). Additionally, smoking, chronic obstructive pulmonary disease (COPD), diabetes mellitus, history of malignancy, and vascular malformations have also been identified as risk factors for VTE after HA.
Thromboprophylaxis recommendations after HA: different guidelines
Currently, no dedicated guidelines exist for thromboprophylaxis following HA. Recommendations for HA are addressed only in brief within select general orthopedic thromboprophylaxis guidelines. Table 3 summarizes the part of the guidelines for the prevention of venous thromboembolism that mentions hip arthroscopy. These recommendations collectively advocate for risk-adapted prophylaxis, though variations in implementation reflect differing clinical priorities. The American College of Chest Physicians (ACCP) emphasizes a risk-stratified approach, reserving pharmacological prophylaxis (e.g., anticoagulants) for high-risk patients with factors such as prior VTE, obesity, or immobility, while recommending physical prophylaxis (e.g., compression devices) for those without additional thrombotic risks. The UK National Institute for Health and Care Excellence (NICE) employs the Caprini score to guide prophylaxis: low-to-moderate risk patients receive mechanical prophylaxis combined with early mobilization, whereas high-risk cases are advised to incorporate low-molecular-weight heparin (LMWH).
The German Association of Scientific Medical Societies (AWMF) discourages routine anticoagulation unless patients exhibit a history of VTE or immobility, prioritizing mechanical methods as first-line interventions. The Chinese Expert Committee on Thrombotic Diseases opposes universal prophylaxis for HA patients but endorses pharmacological measures for high-risk subgroups.
A recent International Consensus Meeting (ICM), involving over 600 experts from 68 countries and 135 societies. The ICM recommends that routine administration of VTE prophylaxis to patients undergoing HA is not supported by the current data. However, patients at higher risk of VTE may benefit from the use of mechanical and/or pharmacological prophylaxis, which may include aspirin.
Discussion
VTE incidence after HA
Venous thromboembolism (VTE), comprising deep vein thrombosis and pulmonary embolism, carries a mortality risk when not managed. Thus, VTE constitutes a serious complication following hip arthroscopy (HA). This systematic review identifies a post-HA VTE incidence ranging from 0% to 6.94%, while pulmonary embolism specifically occurs in 0%–0.7% of cases. Collins et al. reported patients undergoing pharmacological prophylaxis exhibited a higher incidence of VTE. However, this finding was derived from a study with a limited sample size of 39 participants (16). Pooled data from these studies show marked variability in VTE incidence, potentially reflecting underreporting or inconsistent prophylaxis adherence. The observed heterogeneity in VTE incidence after hip arthroscopy arises from multiple interrelated factors. First, study design and detection bias are critical factors. Prospective cohorts using active screening methods (e.g., ultrasound) report significantly higher DVT rates, ranging from 4.3% to 6.94%. In contrast, retrospective studies relying on symptomatic diagnosis show lower rates of 0.08% to 3.7%. This disparity underscores how surveillance intensity affects outcome reporting. Prophylaxis practices also influenced variability. Pharmacological interventions (e.g., aspirin, LMWH) reduced VTE risk by over 90%, outperforming mechanical or no prophylaxis. This underscores the need for standardized preventive protocols. Additionally, surgical complexity increased risks-especially prolonged traction (>50 min) and bony resection procedures. However, inconsistent reporting of operative details hindered cross-study comparisons.
Risk factors
This review establishes obesity, oral contraceptive use, age ≥45 years, smoking, chronic obstructive pulmonary disease, diabetes mellitus, history of malignancy, and vascular malformations as significant VTE risk factors post-HA. Oral contraceptive use enhances thrombin production, evidenced by elevated D-dimer and prothrombin fragment (F1 + 2) levels. Concurrently, hormone therapy modulates endothelial activity. Emerging evidence indicates that estrogen dosage correlates with matrix metalloproteinase expression, triggering degradation of vascular collagen/elastin networks. This cascade promotes venous stasis, heightened vascular permeability, and ultimately predisposes to thrombus formation (12). However, a meta-analysis revealed that obesity, smoking, and age >45 years are significant risk factors for postoperative VTE following hip arthroscopy (17). This discrepancy might be due to variations in the included studies, as this meta-analysis only incorporated five studies. In contrast, factors like obesity, COPD, history of malignant tumor, and vascular abnormalities have been identified as VTE risk factors in multiple studies and are integrated into the Caprini risk assessment score (18, 19). Among 21 included studies, six implemented pharmacological thromboprophylaxis, with three reporting zero VTE events and two documenting low incidences (0.2% and 0.75%). Notably, Collins et al. observed 5% VTE incidence despite prophylaxis-a finding attributable to their exclusive focus on obese patients (BMI ≥ 30), a high-risk subgroup. Obesity is a recognized risk factor for venous thromboembolism. Obesity may cause thrombosis due to the activity of adipocytokines, such as leptin and adiponectin, increasing coagulation activity and inflammation and decreasing the fibrinolytic cascade (20). Prolonged surgical and traction durations appear associated with elevated VTE risk, as orthopedic traction generates tourniquet-like effects. Simultaneously, anesthesia-induced calf muscle paralysis promotes venous stasis. Although surgeons routinely monitor traction duration, the cumulative anesthesia duration (including postoperative recovery) remains clinically overlooked.
VTE risk assessment tools
Numerous risk assessment tools have been developed for thromboembolism evaluation, including the Caprini Risk Assessment Model (RAM), Rogers Score, and Padua Prediction Score (18, 19, 21, 22). The Padua Prediction Score is primarily employed for medical inpatients, while the Caprini RAM finds extensive application in surgical populations. Unlike the Caprini RAM, the Rogers Score omits consideration of certain critical VTE risk factors, notably personal or familial history of VTE and inherited thrombophilia. However, there remains a paucity of evidence regarding the optimal risk assessment tool for patients undergoing HA. The Caprini Risk Assessment Model was originally developed for general and vascular surgery patients. It provides a systematic approach to quantify thrombotic risk. Based on calculated risk scores, clinicians can formulate corresponding prophylactic strategies. Notably, the Caprini scoring system has demonstrated successful clinical adaptation and validation in plastic surgery (23–27), suggesting potential applicability for direct implementation in HA populations. Crucially, no existing risk assessment instrument has undergone rigorous clinical validation specifically for HA procedures. When using scoring systems for postoperative thromboprophylaxis decisions in this surgical setting, clinicians should practice careful interpretation. They must integrate both quantitative risk stratification and patient-specific considerations. A comprehensive patient-specific considerations is essential.
Guidelines and recommendations
Current global thromboprophylaxis guidelines advocate for personalized approaches, yet substantial regional disparities persist in anticoagulant utilization patterns (28). Notably, no consensus exists regarding optimal pharmacologic prophylaxis following orthopedic procedures. Emerging evidence suggests that in non-major orthopedic surgery, the risks of VTE and bleeding complications remain comparable between rivaroxaban and LMWH regimens (29). Regarding total knee arthroplasty (TKA), a growing body of research indicates that aspirin demonstrates inferior efficacy to LMWH in VTE prevention, with studies reporting significantly higher postoperative thrombosis rates with aspirin monotherapy (30). This finding may partially explain why European and Asian clinical frameworks exercise greater restraint in routine pharmacological prophylaxis, prioritizing bleeding risk mitigation over universal anticoagulation. Moving forward, the standardization of risk stratification protocols and the development of procedure-specific outcome data will be critical to establishing globally harmonized clinical practices.
Limitations
This study is constrained by methodological limitations inherent to the extant low-quality evidence base. Among the 21 included studies, case-control or case series accounted for 14 (predominantly Level IV evidence), which may compromise the reliability of the conclusions. Substantial discrepancies in reported surgical parameters (anesthesia duration, traction time, and postoperative follow-up intervals) across studies in both VTE prophylaxis regimens and detection protocols, significantly complicate the formulation of definitive clinical recommendations. Furthermore, while acknowledging the inherent difficulty in disaggregating hip-specific outcomes from general patient data within published literature, such confounding variables exerted negligible impact on pooled rates.
Conclusions
Current evidence indicates a clinically significant discrepancy between symptomatic (low incidence) and imaging-detected (up to 6.9%) VTE rates after hip arthroscopy, underscoring the necessity of protocolized screening for accurate risk stratification. Thus, the distinction between symptomatic and imaging-detected VTE is critical for risk stratification. While routine prophylaxis may not be necessary for all patients, omitting preventive measures should be based on thorough risk assessment-especially given the high rate of imaging-detected VTEs that often go unnoticed. High-risk patients (e.g., those with obesity, oral contraceptive use, age ≥45 years) should receive tailored thromboprophylaxis, including pharmacological prophylaxis when appropriate. To clarify current uncertainties, future studies should focus on standardized screening methods and risk models specific to hip arthroscopy. Until stronger evidence is available, doctors must weigh each patient's unique risks, hospital guidelines, and the shortcomings of symptom-based monitoring to prevent VTE effectively while avoiding unnecessary anticoagulation side effects.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TG: Writing – original draft. KL: Writing – original draft, Methodology. HZ: Writing – original draft. JC: Writing – review & editing. XZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1658428/full#supplementary-material
References
1. Sonnenfeld JJ, Trofa DP, Mehta MP, Steinl G, Lynch TS. Hip arthroscopy for femoroacetabular impingement. JBJS Essent Surg Tech. (2018) 8:e23. doi: 10.2106/JBJS.ST.18.00043
2. Adelstein JM, Sanghvi PA, Smith K, Burkhart RJ, Moyal AJ, Fortier LM, et al. Increased use of hip arthroscopy in the United States from 2015 to 2023 and projected growth through 2030. Arthroscopy. (2025). doi: 10.1016/j.arthro.2025.03.034
3. Mohammed C, Kong R, Kuruba V, Rai V, Munazzam SW. Outcomes and complications of hip arthroscopy for femoroacetabular impingement syndrome: a narrative review. J Clin Orthop Trauma. (2024) 58:102797. doi: 10.1016/j.jcot.2024.102797
4. Parsa A, Bedi A, Domb BG. Current trends for venous thromboembolic prophylaxis for hip arthroscopy: a modified delphi and nominal group technique consensus study. J Hip Preserv Surg. (2024) 11:192–7. doi: 10.1093/jhps/hnae014
5. Ma G, Zhang R, Wu X, Wang D, Ying K. Direct factor xa inhibitors (rivaroxaban and apixaban) versus enoxaparin for the prevention of venous thromboembolism after total knee replacement: a meta-analysis of 6 randomized clinical trials. Thromb Res. (2015) 135:816–22. doi: 10.1016/j.thromres.2015.02.008
6. Zusmanovich M, Haselman W, Serrano B, Banffy M. The incidence of hip arthroscopy in patients with femoroacetabular impingement syndrome and labral pathology increased by 85% between 2011 and 2018 in the United States. Arthroscopy. (2022) 38:82–7. doi: 10.1016/j.arthro.2021.04.049
7. Malviya A, Raza A, Jameson S, James P, Reed MR, Partington PF. Complications and survival analyses of hip arthroscopies performed in the national health service in England: a review of 6,395 cases. Arthroscopy. (2015) 31:836–42. doi: 10.1016/j.arthro.2014.12.013
8. Gillinov SM, Kim DN, Moran J, Lee MS, Fong S, Mahatme RJ, et al. Low rates of 5-year secondary surgery and postoperative complications after primary hip arthroscopy in more than 30,000 patients. Arthroscopy. (2023) 39:1639–48. doi: 10.1016/j.arthro.2023.01.100
9. Mittal MM, Acevedo KV, Lee TM, Singh A, Hosseinzadeh P. Assessing venous thromboembolism risk in hip arthroscopy: a propensity-matched comparison of adolescents and adults. J Pediatr Orthop. (2025) 45:e706–10. doi: 10.1097/BPO.0000000000002987
10. Giordano NJ, Jansson PS, Young MN, Hagan KA, Kabrhel C. Epidemiology, pathophysiology, stratification, and natural history of pulmonary embolism. Tech Vasc Interv Radiol. (2017) 20:135–40. doi: 10.1053/j.tvir.2017.07.002
11. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. (2016) 149:315–52. doi: 10.1016/j.chest.2015.11.026
12. Pastori D, Cormaci VM, Marucci S, Franchino G, Del Sole F, Capozza A, et al. A comprehensive review of risk factors for venous thromboembolism: from epidemiology to pathophysiology. Int J Mol Sci. (2023) 24:3169. doi: 10.3390/ijms24043169
13. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
15. Khazi ZM, An Q, Duchman KR, Westermann RW. Incidence and risk factors for venous thromboembolism following hip arthroscopy: a population-based study. Arthroscopy. (2019) 35:2380–4. doi: 10.1016/j.arthro.2019.03.054
16. Collins JA, Beutel BG, Garofolo G, Youm T. Correlation of obesity with patient-reported outcomes and complications after hip arthroscopy. Arthroscopy. (2015) 31:57–62. doi: 10.1016/j.arthro.2014.07.013
17. Li H, Zhang H, Zhou S, Hu C, Guan Y, Jin R. Risk factors for venous thromboembolism after hip arthroscopy: a systematic review and meta-analysis. J Orthop Surg Res. (2025) 20:134. doi: 10.1186/s13018-025-05536-2
18. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. (2005) 51:70–8. doi: 10.1016/j.disamonth.2005.02.003
19. Cronin M, Dengler N, Krauss ES, Segal A, Wei N, Daly M, et al. Completion of the updated caprini risk assessment model (2013 version). Clin Appl Thromb Hemost. (2019) 25:1076029619838052. doi: 10.1177/1076029619838052
20. Darvall KAL, Sam RC, Silverman SH, Bradbury AW, Adam DJ. Obesity and thrombosis. Eur J Vasc Endovasc Surg. (2007) 33:223–33. doi: 10.1016/j.ejvs.2006.10.006
21. Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua prediction score. J Thromb Haemost. (2010) 8:2450–7. doi: 10.1111/j.1538-7836.2010.04044.x
22. Golemi I, Salazar AJ, Tafur A, Caprini J. Venous thromboembolism prophylaxis using the caprini score. Dis Mon. (2019) 65:249–98. doi: 10.1016/j.disamonth.2018.12.005
23. Qiao L, Yao Y, You X, Wu D, Tsai H, Zhou G, et al. Identifying high-risk groups for deep vein thrombosis after primary total knee arthroplasty using preoperative caprini scores and d-dimer levels. J Orthop Surg Res. (2024) 19:616. doi: 10.1186/s13018-024-05074-3
24. Qiao L, Yao Y, Wu D, Xu R, Cai H, Shen Y, et al. The validation and modification of the caprini risk assessment model for evaluating venous thromboembolism after joint arthroplasty. Thromb Haemost. (2024) 124:223–35. doi: 10.1055/a-2122-7780
25. Pannucci CJ, Dreszer G, Wachtman CF, Bailey SH, Portschy PR, Hamill JB, et al. Postoperative enoxaparin prevents symptomatic venous thromboembolism in high-risk plastic surgery patients. Plast Reconstr Surg. (2011) 128:1093–103. doi: 10.1097/PRS.0b013e31822b6817
26. Krauss ES, Segal A, Dengler N, Cronin M, Pettigrew J, Simonson BG. Utilization of the caprini score for risk stratification of the arthroplasty patient in the prevention of postoperative venous thrombosis. Semin Thromb Hemost. (2022) 48:407–12. doi: 10.1055/s-0042-1742739
27. Krauss ES, Segal A, Cronin M, Dengler N, Lesser ML, Ahn S, et al. Implementation and validation of the 2013 caprini score for risk stratification of arthroplasty patients in the prevention of venous thrombosis. Clin Appl Thromb Hemost. (2019) 25:1076029619838066. doi: 10.1177/1076029619838066
28. Meng J, Tang H, Xiao Y, Liu W, Wu Y, Xiong Y, et al. Appropriate thromboprophylaxis strategy for COVID-19 patients on dosage, antiplatelet therapy, outpatient, and postdischarge prophylaxis: a meta-analysis of randomized controlled trials. Int J Surg. (2024) 110:3910–22. doi: 10.1097/JS9.0000000000001307
29. Zhu L, Zhu B, Bing P, Qi M, He B. Effectiveness and safety of rivaroxaban or low-molecular-weight heparin in non-major orthopedic surgery: a meta-analysis of randomized controlled trials. J Orthop Surg Res. (2024) 19:609. doi: 10.1186/s13018-024-05087-y
30. Meng J, Liu W, Xiao Y, Tang H, Wu Y, Gao S. The role of aspirin versus low-molecular-weight heparin for venous thromboembolism prophylaxis after total knee arthroplasty: a meta-analysis of randomized controlled trials. Int J Surg. (2023) 109:3648–55. doi: 10.1097/JS9.0000000000000656
31. Alaia MJ, Patel D, Levy A, Youm T, Bharam S, Meislin R, et al. The incidence of venous thromboembolism (VTE)–after hip arthroscopy. Bull Hosp Jt Dis (2013). (2014) 72:154–8.25150343
32. Fukushima K, Takahira N, Uchiyama K, Moriya M, Minato T, Takaso M. The incidence of deep vein thrombosis (DVT) during hip arthroscopic surgery. Arch Orthop Trauma Surg. (2016) 136:1431–5. doi: 10.1007/s00402-016-2508-7
33. Schüttler KF, Schramm R, El-Zayat BF, Schofer MD, Efe T, Heyse TJ. The effect of surgeon’s learning curve: complications and outcome after hip arthroscopy. Arch Orthop Trauma Surg. (2018) 138:1415–21. doi: 10.1007/s00402-018-2960-7
34. Cvetanovich GL, Chalmers PN, Levy DM, Mather RR, Harris JD, Bush-Joseph CA, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. (2016) 32:1286–92. doi: 10.1016/j.arthro.2016.01.042
35. Truntzer JN, Hoppe DJ, Shapiro LM, Abrams GD, Safran M. Complication rates for hip arthroscopy are underestimated: a population-based study. Arthroscopy. (2017) 33:1194–201. doi: 10.1016/j.arthro.2017.01.021
36. Sherman WF, Verzeaux NP, Freiberger C, Lee OC, Wilder JH, Flick TR, et al. Local and systemic complications of knee and hip arthroscopy: a matched-cohort study. Orthop J Sports Med. (2022) 10:23259671221131059. doi: 10.1177/23259671221131059
37. Holler JT, Halvorson RT, Salesky M, Ma CB, Feeley BT, Leavitt AD, et al. Incidence of venous thromboembolism after hip arthroscopy is low with or without prophylaxis but risk factors include oral contraceptive use, obesity, and malignancy. Arthroscopy. (2023) 39:981–7. doi: 10.1016/j.arthro.2022.10.029
38. Salvo JP, Troxell CR, Duggan DP. Incidence of venous thromboembolic disease following hip arthroscopy. Orthopedics. (2010) 33:664. doi: 10.3928/01477447-20100722-10
39. Souza BG, Dani WS, Honda EK, Ricioli WJ, Guimarães RP, Ono NK, et al. Do complications in hip arthroscopy change with experience? Arthroscopy. (2010) 26:1053–7. doi: 10.1016/j.arthro.2009.12.021
40. Polat G, Dikmen G, Erdil M, Aşık M. Arthroscopic treatment of femoroacetabular impingement: early outcomes. Acta Orthop Traumatol Turc. (2013) 47:311–7. doi: 10.3944/aott.2013.3041
41. Chan K, Farrokhyar F, Burrow S, Kowalczuk M, Bhandari M, Ayeni OR. Complications following hip arthroscopy: a retrospective review of the McMaster experience (2009–2012). Can J Surg. (2013) 56:422–6. doi: 10.1503/cjs.021712
42. Javed A, O'Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. (2011) 93:326–31. doi: 10.1302/0301-620X.93B3.25262
43. Roos BD, Roos MV, Júnior AC, Lima EM, Gyboski DP, Martins LS. Extracapsular approach for arthroscopic treatment of femoroacetabular impingement: clinical and radiographic results and complications. Rev Bras Ortop. (2015) 50:430–7. doi: 10.1016/j.rboe.2015.06.011
44. Beutel BG, Collins JA, Garofolo G, Youm T. Hip arthroscopy outcomes, complications, and traction safety in patients with prior lower-extremity arthroplasty. Int Orthop. (2015) 39:13–8. doi: 10.1007/s00264-014-2479-7
45. Mohtadi NG, Johnston K, Gaudelli C, Chan DS, Barber RS, Walker R, et al. The incidence of proximal deep vein thrombosis after elective hip arthroscopy: a prospective cohort study in low risk patients. J Hip Preserv Surg. (2016) 3:295–303. doi: 10.1093/jhps/hnw027
46. Larson CM, Clohisy JC, Beaulé PE, Kelly BT, Giveans MR, Stone RM, et al. Intraoperative and early postoperative complications after hip arthroscopic surgery: a prospective multicenter trial utilizing a validated grading scheme. Am J Sports Med. (2016) 44:2292–8. doi: 10.1177/0363546516650885
47. Domb BG, Gui C, Hutchinson MR, Nho SJ, Terry MA, Lodhia P. Clinical outcomes of hip arthroscopic surgery: a prospective survival analysis of primary and revision surgeries in a large mixed cohort. Am J Sports Med. (2016) 44:2505–17. doi: 10.1177/0363546516663463
48. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: american college of chest physicians evidence-based clinical practice guidelines. Chest. (2012) 141:e419S–96. doi: 10.1378/chest.11-2301
49. NICE. Venous Thromboembolism in Over 16s: Reducing the Risk of Hospital-acquired deep Vein Thrombosis or Pulmonary Embolism. Manchester: The UK National Institute for Health and Care Excellence (2018). Available online at: https://Www.Nice.Org.Uk/Guidance/Ng89
50. AWMF. S2k-leitlinie Diagnostik und Therapie der Venenthrombose und Lungenembolie. Katharina Lenz: The German Association of Scientific Medical Societies (2023). Available online at: https://Register.Awmf.Org/De/Leitlinien/Detail/065-002
51. China ECOG. Guidelines for prevention and treatment of thrombotic diseases in China. Nat Med J Chin. (2018) 98:2861–88. doi: 10.3760/cma.j.issn.0376-2491.2018.36.002
Keywords: arthroscopy, hip, practice guidelines, risk assessment, thromboprophylaxis, venous thromboembolism
Citation: Gao T, Lv K, Zhang H, Chen J and Zhao X (2025) Venous thromboembolism after hip arthroscopy: a systematic review of incidence, risk factors, and international guidelines. Front. Surg. 12:1658428. doi: 10.3389/fsurg.2025.1658428
Received: 2 July 2025; Accepted: 3 September 2025;
Published: 14 October 2025.
Edited by:
Alexandr Ceasovschih, Grigore T. Popa University of Medicine and Pharmacy, RomaniaReviewed by:
Binsheng He, Changsha Medical University, ChinaTamrat Assefa Tadesse, Addis Ababa University, Ethiopia
Richard H. Parrish II, Alice L Walton School of Medicine, United States
Copyright: © 2025 Gao, Lv, Zhang, Chen and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangde Zhao, emhhb3hpYW5nZGVAemp1LmVkdS5jbg==
 Ting Gao1
Ting Gao1 Jian Chen
Jian Chen Xiangde Zhao
Xiangde Zhao