- 1College of Medicine, Alfaisal University, Riyadh, Saudi Arabia
- 2Department of Pediatric Surgery, Universitätsklinikum Jena, Jena, Germany
Congenital midline cervical cleft (CMCC) is a rare developmental anomaly of the anterior neck, often misdiagnosed due to its similarity to other cervical malformations. It results from impaired midline fusion of the branchial arches, leading to a linear skin defect with a fibrotic cord and, in some cases, a sinus tract. Left untreated, CMCC can cause progressive contracture, restricted neck mobility, and aesthetic deformities. This review examines the embryological basis, clinical presentation, histopathological characteristics, differential diagnosis, and surgical management of CMCC, with a focus on Z-plasty as the preferred reconstructive technique. Z-plasty effectively lengthens the scar, prevents recurrent contracture, and restores normal neck contour. In addition, we present a case of a 3-day-old female neonate with CMCC, successfully treated with Z-plasty reconstruction, reinforcing the importance of early intervention. Emerging genetic research suggests a potential hereditary component in CMCC, warranting further investigation into its molecular underpinnings. Advances in regenerative medicine and surgical innovation may improve treatment outcomes, offering new possibilities for personalized management of congenital cervical anomalies.
1 Introduction
Congenital midline cervical cleft (CMCC) is a rare congenital anomaly of the anterior neck, with sporadic occurrences reported in the literature (1, 2). It is characterized by a thin, slender, atrophic midline fissure in the skin, underlain by a subcutaneous fibrous cord that creates a soft tissue protuberance (1, 3, 4). Clinically, CMCC presents as a vertically oriented erythematous strip with fluid exudation and, in some cases, mucoid discharge from a blind-ended sinus (1, 3, 4). This sinus extends between the mandibular symphysis and the suprasternal notch (1, 3, 4). CMCC may also be associated with additional developmental anomalies, such as bifid mandible and microgenia (1).
The etiology of CMCC is hypothesized to originate from a failure of midline fusion of the branchial arches during embryonic development, disrupting the normal migration of mesodermal cells from the developing tongue to the ventral neck (3, 5). This aberrant migration results in the formation of a complex unique composite structure composed of skin, skeletal muscle, fibrous tissue, and exocrine elements (3, 5). Although CMCC is an uncommon condition, it can significantly impact a patient's quality of life if left untreated (6). Progressive complications may include neck extension impairment, microgenia, exostosis, torticollis, and recurrent infections, underscoring the importance of early diagnosis and surgical intervention (6).
The management of CMCC requires complete excision of anomalous tissue to prevent the formation of cicatricial contractures and associated morbidities over time (2, 6, 7). However, simple linear closure technique should be avoided, due to its high risk of hypertrophic scarring and recurrent neck contracture (2, 6, 7). Instead, Z-plasty closure is the preferred surgical technique, which involves the transposition of two triangular skin flaps to effectively elongate a linear scar contracture, reconstruct the cervicomental crease, and reduce contracture formation (6, 7). In this report, we describe a case of CMCC successfully managed by using Z-plasty closure. Furthermore, we provide a comprehensive review of the literature on CMCC and its surgical management.
2 Case presentation
We report the case of a full-term, healthy 3-day-old female newborn who was referred to the pediatric surgery clinic due to a congenital anomaly identified at birth. The malformation was localized at the midline of the anterior neck. On examination, a painless lesion measuring approximately 3 cm in length and 1 cm in width was observed. The lesion did not impair breathing or swallowing (Figure 1). It was characterized by an open sinus covered with a mucous membrane, raising suspicion of a potential fistulous tract at its caudal terminus. Notably, the newborn had no other congenital anomalies, and there was no relevant family history. This suggests a non-related genetic predisposition. The lesion remained stationary during tongue protrusion and swallowing, indicating no attachment to the hyoid bone or thyroid gland, both of which were assessed to be structurally normal on physical examination.
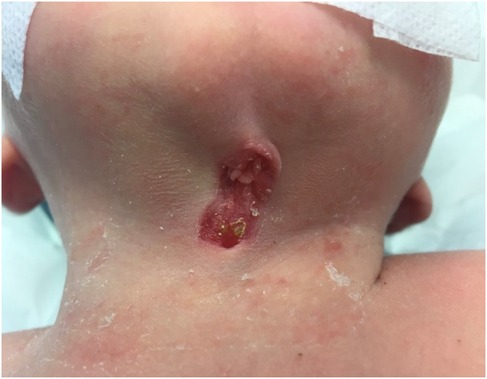
Figure 1. Clinical presentation of the CMCC. The lesion is characterized by erythematous skin, a midline cleft with an upper skin tag, a mucosal sulcus, and a caudal sinus. Neck extension accentuates skin webbing toward the mandible.
Given the unusual presentation and potential clinical implications, a comprehensive diagnostic evaluation was initiated. Preoperative clinical assessment revealed normal neck mobility, with no restriction of extension or rotation. Ultrasonography served as the primary imaging modality to explore the lesion's depth and its relationship to surrounding structures (Figure 2). The ultrasound revealed a superficial anomaly with no evidence of deep tissue involvement or communication with adjacent anatomical structures. These findings guided a multidisciplinary team—including specialists from pediatric surgery, neonatology, and pediatrics—to proceed with surgical intervention to fully delineate the extent of the malformation and prevent possible complications.
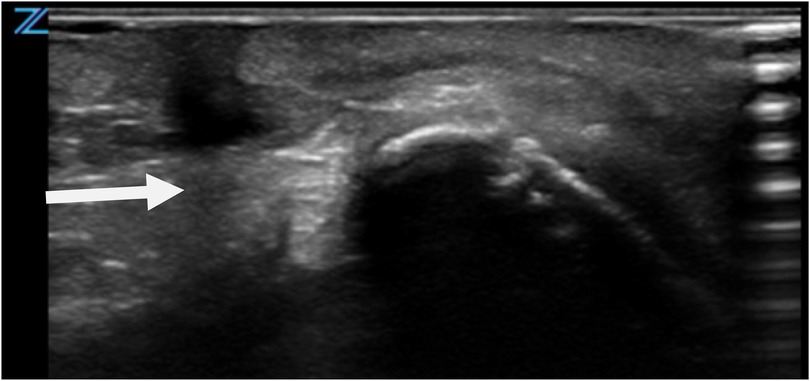
Figure 2. Ultrasonographic evaluation of CMCC. Imaging reveals a blind-ended tract extending caudally, with no evidence of deeper structural involvement.
Surgery was performed under general anesthesia with complete excision of the epithelial cleft and underlying fibrous cord. Intraoperative exploration confirmed a CMCC with a caudally positioned duct terminating above the sternum, without extension into deeper structures, thereby excluding the possibility of a complex fistulous tract. A fusiform incision was fashioned, and the specimen was excised en bloc to the level of the investing fascia, measuring approximately 3.5–4.0 cm in length. Hemostasis was secured with bipolar cautery. Reconstruction was accomplished using a 60° Z-plasty. The central limb, corresponding to the excised tract, measured 3.5 cm. From each terminus of the central limb, lateral limbs of equal length (3.5 cm) were designed at 60° angles. Triangular flaps were carefully elevated in the subdermal plane, preserving vascularity, and transposed across the axis without undue tension. This maneuver provided effective scar lengthening and reoriented the closure along relaxed skin tension lines. The 60° angle was selected for its reliable balance between flap viability and length gain. Layered closure was performed with 5-0 Vicryl sutures to the dermis and 6-0 Vicryl sutures to the skin. Flaps demonstrated excellent viability with precise inset and alignment. A light sterile dressing was applied. Postoperative recovery was uneventful, with routine wound care and satisfactory healing (Figure 3).
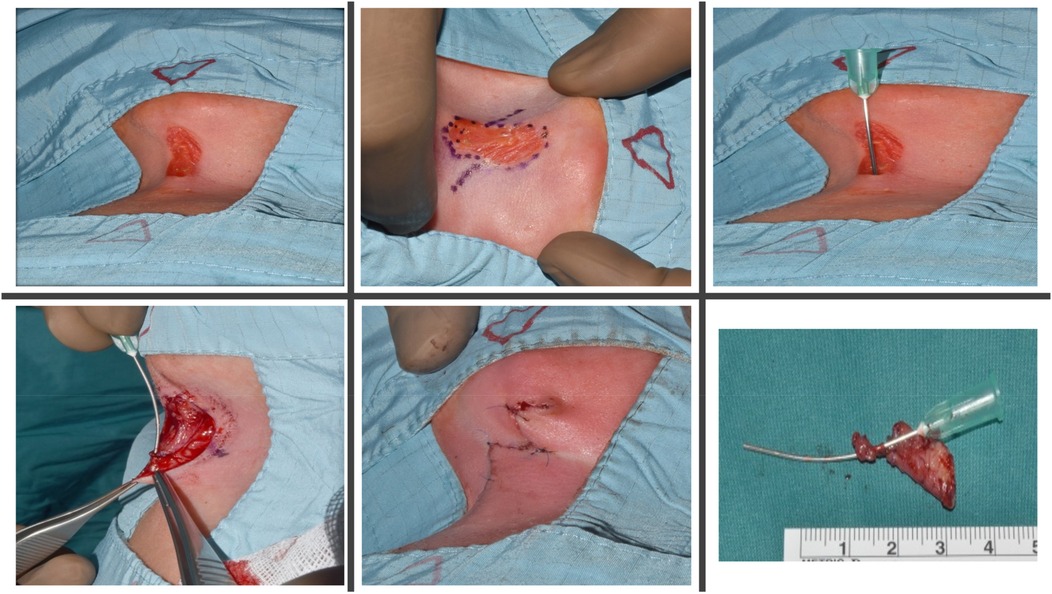
Figure 3. Intraoperative findings and surgical technique. A 60° Z-plasty was performed with an elliptical incision. The upper portion of the lesion was completely excised, while the lower portion, including the fibrotic cord extending to the manubrium, was removed. The resulting Z-plasty flaps were meticulously transposed and sutured in two layers to achieve optimal functional and cosmetic outcomes.
Postoperative histopathological analysis of the excised tissue confirmed the diagnosis of a CMCC, with no evidence of fistulous structures or ectopic salivary gland tissue. These findings were critical in excluding other differential diagnoses and solidifying the classification of the anomaly (Figure 4).
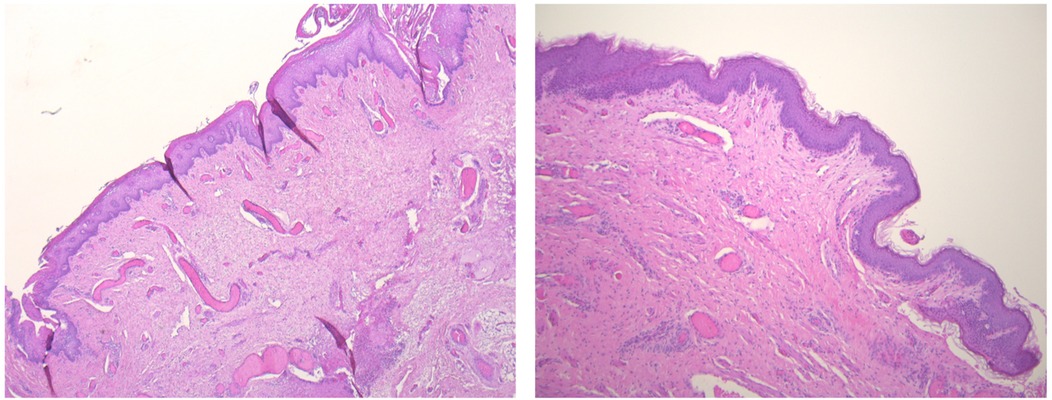
Figure 4. Histopathological features of the CMCC. Hematoxylin and eosin staining (40× magnification) reveals a cleft lined by stratified squamous epithelium with surface parakeratosis. The underlying dermis lacks adnexal structures but contains abundant striated muscle bundles at deeper levels. Similarly, the cephalic papule is composed of a stratified squamous epithelial lining overlying muscle bundles, which progressively transition into normal skin with adnexal structures, highlighting the lesion's interaction with surrounding tissue.
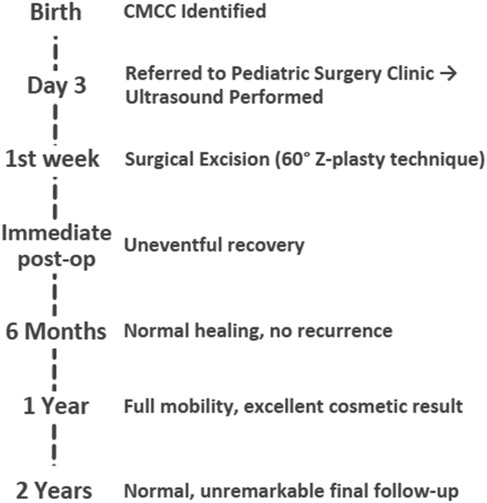
At the 6-week follow-up, clinical examination demonstrated satisfactory wound healing with localized erythema and crusting at the surgical site (Figure 5). The postoperative course was uneventful. Follow-up examinations demonstrated excellent wound healing with no signs of functional impairment or cosmetic disfigurement. The absence of complications or recurrence during the follow-up period underscores the success of the surgical approach and highlights the importance of early intervention in such cases. Chronological timeline of the presented case of CMCC, from birth through 2-year follow-up (Figure 6).
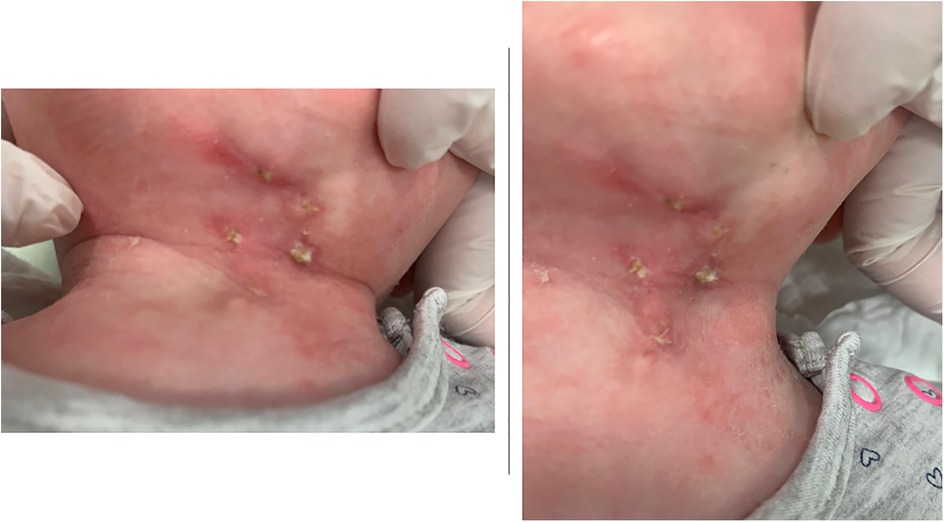
Figure 5. Postoperative wound appearance at 6 weeks follow-up. Clinical photographs of the cervical region showing the surgical site with evidence of partial healing and residual erythema, crusting, and localized inflammatory changes.
3 Discussion
CMCC is a rare congenital anomaly of the anterior neck, first described in the medical literature in the 1940s (2, 3, 6, 8, 9). It has been referred to by various terminologies, including congenital midline cervical cord/cleft, medial cleft, median fissure of the neck, mentosternal dysraphia, and pterygium colli medianum (2, 3, 6, 8, 9). CMCC exhibits a slight female predominance, with an estimated female-to-male incidence ratio of approximately 2:1 (6).
The etiology of CMCC remains poorly understood, necessitating consideration of both genetic and environmental factors (2). Researchers have proposed various hypotheses about their origin. A predominant theory suggests that these clefts represent a spectrum of developmental abnormalities in the branchial arches, originating from a disruption in the fusion between the first and second branchial arches at the midline during embryonic development (2, 6, 9–11). This theory explains the observed variations in the anomaly, which range from a simple cleft-less cord to the complete absence of the thyroid cartilage and hyoid bone (6, 9, 12). The hypothesis suggests that the underlying mechanism for the incomplete branchial fusion is associated with vascular anomalies that leads to ischemia, tissue necrosis, and subsequent scarring (6, 13–17). Other factors that contribute to CMCC development include the persistence of remnants of the thyroglossal duct and sinus cysts, pressure exerted on the cervical area by the pericardial roof, rupture of pathological epithelial adhesions in the first branchial arch between the cardiohepatic fold and the ventral part, and the absence of mesenchymal tissue in the cervical midline (6, 13–17). Additional proposed mechanisms involve aberrant interactions between ectoderm and mesoderm, impaired mesodermal fusion along the distal branchial arches, and defective differentiation of mesenchymal tissue (6, 17, 18). These hypotheses enhance our understanding on the formation of CMCC in the anterior neck.
3.1 Genetic contributions to CMCC
Genetic data suggest that CMCC arises from distinct mechanisms in sporadic and familial disease (4, 19). Sporadic cases often harbor heterogeneous variants, such as those in PARD3, MDM4, and EP300, typically inherited from unaffected parents (19). These changes converge on core cellular pathways, supporting a model of polygenic risk with incomplete penetrance rather than a single causal mutation (19). Familial CMCC, in contrast, shows a clearer hereditary signal: truncating variants in TYW1B and SSPO, together with frameshifts in OVGP1, ZAN, and FOLR3, implicate pathways in gamete interaction, embryonic development, and neural organization (4). This divergence underscores the genetic heterogeneity of CMCC, sporadic disease emerging from multifactorial or de novo events, and familial disease reflecting pathogenic inheritance (4, 19). Broader sequencing efforts are needed to define whether these represent convergent molecular disruptions or distinct genetic etiologies (4, 19). Table 1 summarizes the current genes and its molecular mechanism in CMCC.
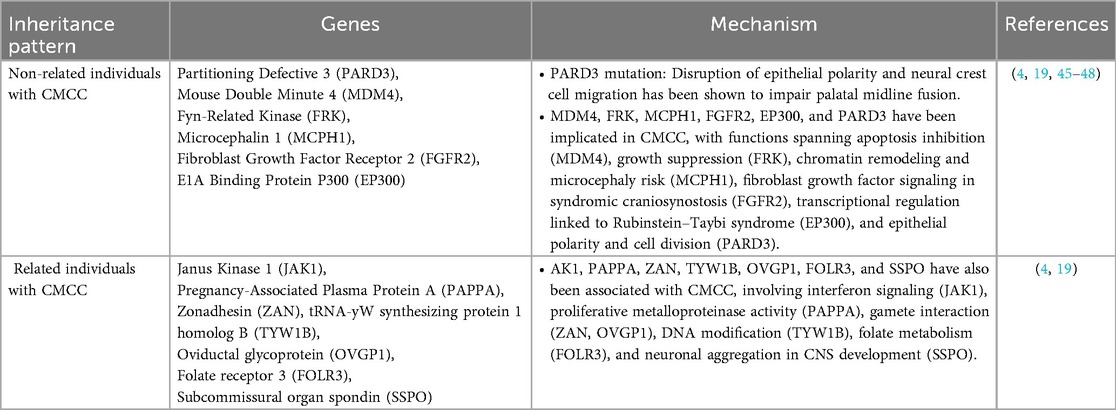
Table 1. Reported genes in CMCC, grouped by inheritance pattern with their proposed molecular mechanisms.
3.2 Clinical features and differential diagnosis
CMCC manifests as a variable-length vertical defect, extending from the mandible to the sternum, affecting skin and subcutaneous tissues in the midline of the anterior neck (2, 5, 14, 18, 20). CMCC is histologically characterized by an atrophied epidermal layer with a mucosal surface accompanied by either fibrous connective or glandular tissue (1, 2, 6, 13). Furthermore, CMCC can be associated with the cartilage, skeletal muscle, absence of epithelial adnexa in the dermis, ectopic salivary gland tissue, and pseudostratified ciliated columnar epithelium, particularly in the presence of the sinus tracts (6, 14, 15, 17). At the cephalic end of the anomaly, there is a cleft, nipple-like protrusion, confined to the skin that can extend to the tongue, lip, mandible, and sternum in severe cases (2, 16). The anomaly may also involve a fistula, sinus tract, or duct, which is shallow, blind-ended, and secretes mucous (7, 16, 21). This seromucous secretion is observed to extend towards the manubrium, sternum, or caudally towards the hyoid bone (16, 18, 22). The seromucous discharge tends to resolve spontaneously during infancy (18). A fibrous cord connects the cleft, which appears as a reddish or pinkish linear area with a moist surface and atrophic epidermis, which are the characteristics of CMCC (6, 16).
CMCC diagnosis happens at birth and can be done by physical examination (3, 10). However, correctly diagnosing CMCC can be challenging due to its potential to resemblance to other anomalies since it often appears as a spot-like scar, include branchial cleft anomalies, dermoid cyst, or a thyroglossal duct remnants (Table 2) (2, 3, 6, 17). CMCC may occur as an isolated anomaly or coexist with various conditions, including ectopic bronchogenic cyst, midline hemangiomas, thyroglossal duct cyst, cleft lip, and cleft mandible (3, 6, 17). Additionally, it can be associated with the absence of the hyoid bone or thyroid cartilage, and congenital heart disease (3, 6, 17). Early recognition of these potential concurrent conditions is crucial for accurate diagnosis and effective management.
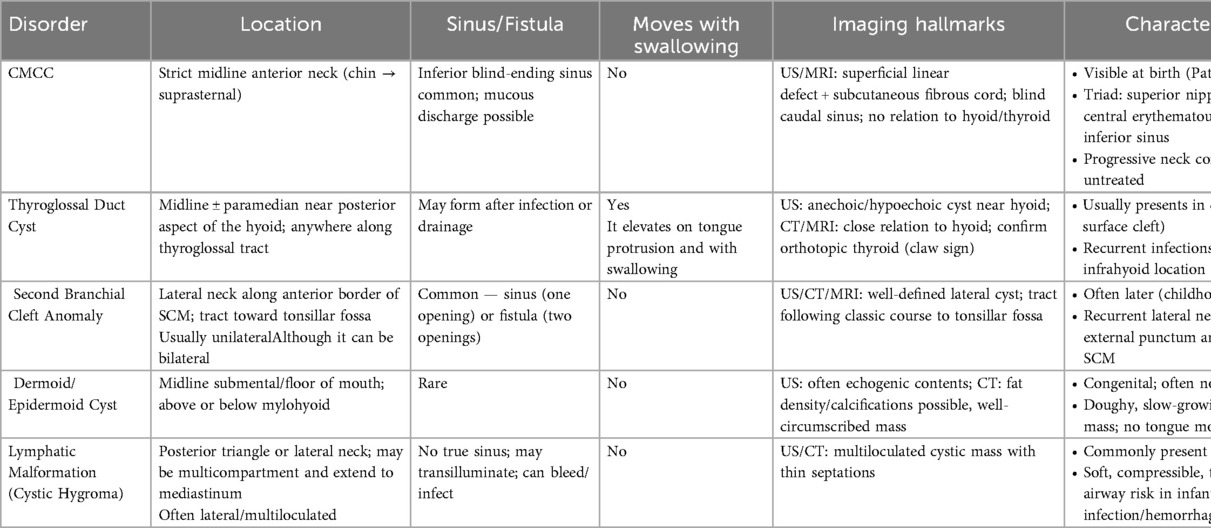
Table 2. Differential diagnosis of CMCC compared with other congenital neck anomalies, highlighting key clinical, imaging, and management features.
3.3 Surgical management and prognosis
The prognosis and management of CMCC significantly depend on their size and location (2). Early detection and surgical intervention are critical to prevent complications (2, 6, 17). If not addressed promptly, the cleft can heal into a longitudinal scar with a fibrous band, leading to cicatricial contracture of the neck, restricting neck movement, and potentially causing complications like micrognathia, mandibular exostosis, or hypoplasia (2, 6, 17). Simple excision followed by a simple straight-line closure may lead to scarring and contracture, making Z-plasty the preferred surgical technique for these clefts (2, 7).
Z-plasty is a widely recognized surgical technique in plastic and reconstructive surgery (2). This procedure involves creating a central limb incision with bilateral limb incisions to form two opposing triangular transposition flaps in a “Z” pattern (23, 24). This technique facilitates the release of scar contracture, alters the direction and length of a contracted scar or defect, allows for tissue mobilization and realignment, reduces skin tension, and enhances soft tissue contour, which makes Z-plasty uniquely valuable in the neck by both preventing recurrent contracture and camouflaging scars within natural skin creases (1, 2, 23, 25–29).
The extent of tissue lengthening in Z-plasty is correlates with the angle between the central and bilateral incisions. Table 3 shows the length of scar resulting from different angles between the limbs in a Z-Plasty procedure, highlighting how different geometries influence lengthening and clinical application (1, 2, 23, 28–30). Larger angles yield more lengthening but increase skin tension, possibly causing tissue distortion and dog-ear deformities (2, 23). Narrower angles ease closure but limit lengthening and increase the risk of flap necrosis due to reduced blood flow to the flap tips (2, 23). A 60° angle in Z-plasty is often ideal for maximizing tissue lengthening while ensuring ease of closure, achieving a 90° scar rotation and a 75% increase in length. However, variations in angles and limb lengths are feasible, leading to “skew Z-plasties” (2, 23, 24, 29). These are particularly useful in situations where anatomical constraints hinder the application of symmetric Z-plasties (2, 23, 24, 29). For angles over 60°, the usage of a “compound Z-plasty” is indicated by splitting the angle into smaller equal flaps to reduce skin deformities with an extra scar (29). For longer scars, “serial Z-plasty” distributes tension by adding multiple flaps along the scar, improving flexibility and outcome (29).
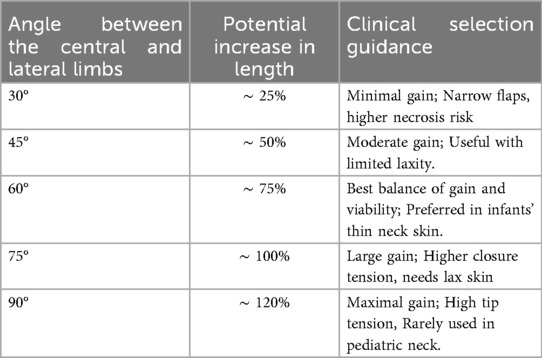
Table 3. Z-plasty limb angle with corresponding potential scar length gain and clinical selection guidance.
Compared with alternative scar-revision strategies, Z-plasty confers distinct advantages that are directly relevant to CMCC (31, 32). W-plasty effectively camouflages linear scars by irregularizing their contour along relaxed skin-tension lines, but it provides no true lengthening and is therefore inadequate when contracture release is required (33, 34). Local flap transfers, such as advancement, rotation, or transposition flaps, can supply well-vascularized adjacent tissue and are useful for larger or composite defects, though they necessitate broader dissection and carry risks of donor-site morbidity and contour irregularities (35–37). By contrast, Z-plasty simultaneously lengthens contracted skin, reorients the central limb into favorable vectors, and breaks the scar into a less conspicuous pattern (29, 31). A 60° design reliably achieves ∼75% lengthening with ∼90° scar rotation, and serial or multiple Z-plasties can emulate the irregularizing effect of W-plasty while still ensuring measurable lengthening (29, 31, 38). For CMCC, most reported series favor excision with Z-plasty closure, as it reliably restores neck extension and cervicomental contour while minimizing recurrent tethering and visible scarring (2, 22).
3.4 Regenerative adjuncts to Z-plasty
Emerging regenerative strategies, particularly stem cell–assisted flap transplantation, represent a promising frontier for CMCC repair. Preclinical studies demonstrate that mesenchymal stem cells (MSCs) can enhance skin flap survival and neovascularization thereby improving long-term viability (39–42). More recently, a 2023 large-animal model of cervical skin injury showed that MSCs, particularly when combined with platelet-rich plasma, accelerate re-epithelialization, organize collagen fibers, and limit contraction—hallmarks of regenerative rather than reparative healing (40). Meta-analyses further affirm that MSC–scaffold treatments markedly boost wound closure, angiogenesis, collagen deposition, and growth-factor expression in preclinical burn wound models, and exosome-based MSC derivatives show emerging promise for restoring skin structure and function in reconstructive contexts (39, 43, 44). These mechanisms are directly relevant to CMCC, where atrophic epidermis, subdermal fibrosis, and absent adnexal structures predispose to postoperative scarring and contracture despite meticulous Z-plasty. While CMCC-specific trials are lacking, such data support hypothesis-generating studies exploring biologically augmented flap repair as a complement to surgical technique.
4 Conclusion & future directions
In conclusion, CMCC is a rare anomaly that demands early surgical intervention to prevent progressive contracture, functional limitation, and cosmetic deformity. Z-plasty remains the gold standard of repair, offering reliable lengthening and favorable outcomes. The present report, however, is inherently limited by its single-case design, which cannot establish the universality of surgical success or permit comparison across different Z-plasty techniques. Longer follow-up in larger cohorts, ideally through multicenter case-control studies, will be necessary to determine the durability of functional and aesthetic results and to clarify the relative advantages of different flap angles.
The absence of genetic testing in this patient represents another important limitation, as it prevents exclusion of a contributory hereditary factor and underscores the need for systematic molecular investigations. Comprehensive genomic profiling could help define the role of genetic predisposition in CMCC, refine risk stratification, and improve counseling for affected families. Future advances in regenerative medicine—particularly stem cell–assisted flap transplantation and other biologically augmented strategies—may complement conventional Z-plasty by enhancing flap survival, reducing contracture recurrence, and improving long-term scar quality. Rigorous, collaborative studies will be essential to translate these approaches into evidence-based, personalized care for patients with CMCC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving human samples in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
GA: Writing – review & editing, Conceptualization, Writing – original draft. FE: Supervision, Writing – review & editing, Writing – original draft. IA: Supervision, Conceptualization, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI (ChatGPT, OpenAI) was used to assist with language refinement and phrasing of select sentences in the abstract, introduction, and conclusion. The authors reviewed, edited, and approved all content to ensure accuracy, originality, and compliance with ethical standards. No AI tools were used to generate data, perform analysis, or draw conclusions. All clinical content, case description, data interpretation, and conclusions were developed entirely by the authors.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Erçöçen AR, Yilmaz S, Aker H. Congenital midline cervical cleft: case report and review. J Oral Maxillofac Surg. (2002) 60(5):580–5. doi: 10.1053/joms.2002.31859
2. Cochran CS, DeFatta RJ, Brenski AC. Congenital midline cervical cleft: a practical approach to Z-plasty closure. Int J Pediatr Otorhinolaryngol. (2006) 70(3):553–9. doi: 10.1016/j.ijporl.2005.07.024
3. Crippa BL, Bedeschi MF, Cantarella G, Colombo L, Agosti V, Amodeo I, et al. Congenital midline cervical cleft: clinical approach to a congenital anterior neck defect. Congenit Anom (Kyoto). (2015) 55(2):112–5. doi: 10.1111/cga.12086
4. Masood MM, Mieczkowski P, Malc EP, Foreman AKM, Evans JP, Clark JM, et al. Congenital midline cervical cleft: first report and genetic analysis of two related patients. Ann Otol Rhinol Laryngol. (2020) 129(7):653–6. doi: 10.1177/0003489420906180
5. Goldfisher R, Bawa P, Ibrahim Z, Amodio J. Clinical and imaging features of a congenital midline cervical cleft in a neonate: a rare anomaly. Case Rep Pediatr. (2015) 2015:1–3. doi: 10.1155/2015/439596
6. Sinopidis X, Kourea HP, Panagidis A, Alexopoulos V, Tzifas S, Dimitriou G, et al. Congenital midline cervical cleft: diagnosis, pathologic findings, and early stage treatment. Case Rep Pediatr. (2012) 2012:1–5. doi: 10.1155/2012/463628
7. Maschka DA, Clemons JE, Janis JF. Congenital midline cervical cleft. Case report and review. Ann Otol Rhinol Laryngol. (1995) 104(10 Pt 1):808–11. doi: 10.1177/000348949510401011
8. Kang B, Kim B. Congenital midline cervical cleft: an easily misdiagnosed disease. Arch Craniofac Surg. (2020) 21(6):372. doi: 10.7181/acfs.2020.00388
9. Salawu AI, Aremu SK, Olakunle BF, Olajide TG, Okunlola AI, Samuel OA, et al. A delayed diagnosis of congenital midline cervical cleft. Clin Case Rep. (2022) 10(3). doi: 10.1002/ccr3.5540
10. Derbez R, Nicollas R, Roman S, Estève A, Triglia JM. Congenital midline cervical cleft of the neck: a series of five cases. Int J Pediatr Otorhinolaryngol. (2004) 68(9):1215–9. doi: 10.1016/j.ijporl.2004.03.018
11. Foley DS, Fallat ME. Thyroglossal duct and other congenital midline cervical anomalies. Semin Pediatr Surg. (2006) 15(2):70–5. doi: 10.1053/j.sempedsurg.2006.02.003
12. Minami RT, Pletcher J, Dakin RL. Midline cervical cleft. A case report. J Maxillofac Surg. (1980) 8(1):65–8. doi: 10.1016/S0301-0503(80)80074-9
13. Gardner ROE, Moss ALH. The congenital cervical midline cleft. Case report and review of literature. Br J Plast Surg. (2005) 58(3):399–403. doi: 10.1016/j.bjps.2004.10.015
14. Eastlack JP, Howard RM, Frieden IJ. Congenital midline cervical cleft: case report and review of the English language literature. Pediatr Dermatol. (2000) 17(2):118–22. doi: 10.1046/j.1525-1470.2000.01727.x
15. McInnes CW, Benson AD, Verchere CG, Ludemann JP, Arneja JS. Management of congenital midline cervical cleft. J Craniofac Surg. (2012) 23(1):e36–8. doi: 10.1097/SCS.0b013e318241db99
16. Bajaj Y, Dunaway D, Hartley BEJ. Surgical approach for congenital midline cervical cleft. J Laryngol Otol. (2004) 118(7):566–9. doi: 10.1258/0022215041615074
17. Mlynarek A, Hagr A, Tewfik TL, Nguyen VH. Congenital mid-line cervical cleft: case report and review of literature. Int J Pediatr Otorhinolaryngol. (2003) 67(11):1243–9. doi: 10.1016/S0165-5876(03)00201-5
18. van der Staak FHJ, Pruszczynski M, Severijnen RSVM, van de Kaa CA, Festen C. The midline cervical cleft. J Pediatr Surg. (1991) 26(12):1391–3. doi: 10.1016/0022-3468(91)91042-W
19. Jakobsen LP, Pfeiffer P, Andersen M, Eiberg H, Hansen L, Mang Y, et al. Genetic studies in congenital anterior midline cervical cleft. Am J Med Genet A. (2012) 158A(8):2021–6. doi: 10.1002/ajmg.a.35466
20. Farhadi R, Sahebpour AA, Ghasemi M. Congenital midline cervical cleft: can it be treated in newborn? Iran J Pediatr. (2012) 22(4):547. Available online at: /pmc/articles/PMC3533160/.23431110
21. Helal AA, Mahmoud BA. Congenital midline cervical cleft. J Pediatr Surg Case Rep. (2018) 36:3–6. doi: 10.1016/j.epsc.2018.06.007
22. Çelikoyar M, Aktan E, Doǧusoy G. Congenital midline cervical cleft: a case report. J Med Case Rep. (2019) 13(1). doi: 10.1186/s13256-019-2116-6
23. Hove CR, Williams EF, Rodgers BJ. Z-plasty: a concise review. Facial Plast Surg. (2001) 17(4):289–93. doi: 10.1055/s-2001-18828
24. Rohrich RJ, Zbar RIS. A simplified algorithm for the use of Z-plasty. Plast Reconstr Surg. (1999) 103(5):1513–7. doi: 10.1097/00006534-199904050-00024
25. Seyhan T, Kýlýnç H. Median cleft of the lower lip: report of two new cases and review of the literature. Ann Otol Rhinol Laryngol. (2002) 111(3 Pt 1):217–21. doi: 10.1177/000348940211100305
26. Freiling D, Galla M, Lobenhoffer P. Arthrolysis for chronic flexion deficits of the knee. An overview of indications and techniques of vastus intermedius muscle resection, transposition of the tibial tuberosity and z-plasty of the patellar tendon. Unfallchirurg. (2006) 109(4):285–96. doi: 10.1007/s00113-005-1039-4
27. Robson MC, Barnett RA, Leitch IOW, Hayward PG. Prevention and treatment of postburn scars and contracture. World J Surg. (1992) 16(1):87–96. doi: 10.1007/BF02067119
28. Hudson DA. Some thoughts on choosing a Z-plasty: the Z made simple. Plast Reconstr Surg. (2000) 106(3):665–71. doi: 10.1097/00006534-200009010-00024
29. Zito PM, Jawad BA, Hohman MH, Mazzoni T. Z-Plasty. In: StatPearls. Treasure Island, FL: StatPearls Publishing (2023). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK507775/ (Accessed March 29, 2024).
30. Garg S, Dahiya N, Gupta S. Surgical scar revision: an overview. J Cutan Aesthet Surg. (2014) 7(1):3. doi: 10.4103/0974-2077.129959
31. Aasi SZ. Z-Plasty made simple. Dermatol Res Pract. (2011) 2010(1):982623. doi: 10.1155/2010/982623
32. Salam GA, Amin JP, Zuber TJ. The basic Z-plasty. Am Fam Physician. (2003) 67(11). Available online at: http://www.aafp.org/afpAMERICANFAMILYPHYSICIAN2329 (Accessed August 30, 2025).
33. Papadakis M, Manios G, Zacharopoulos G, Koumaki D, Manios A. Biomechanical explanation of W-plasty effectiveness using a finite element method approach. Sci Rep. (2023) 13(1):1–12. doi: 10.1038/s41598-023-45400-z
34. Goutos I, Yousif AH, Ogawa R. W-plasty in scar revision: geometrical considerations and suggestions for site-specific design modifications. Plast Reconstr Surg Glob Open. (2019) 7(4):e2179. doi: 10.1097/GOX.0000000000002179
35. Masanovic MG, Téot L. Scar contractures. In: Téot L, Mustoe TA, Middelkoop E, Gauglitz GG, editors. Textbook on Scar Management: State of the Art Management and Emerging Technologies. Cham: Springer (2020). p. 117–22. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK586056/ (Accessed August 30, 2025).
36. Ogawa R. Usefulness of local flaps for scar contracture release. In: Téot L, Mustoe TA, Middelkoop E, Gauglitz GG, editors. Textbook on Scar Management: State of the Art Management and Emerging Technologies. Cham: Springer (2020). p. 301–9. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK586111/ (Accessed August 30, 2025).
37. Bednarek RS, Campos MBS, Hohman MH, Ramsey ML. Transposition flaps. In: StatPearls. Treasure Island, FL: StatPearls Publishing (2025). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK500028/ (Accessed August 30, 2025).
38. Căiță GA, Lascu CF, Bodog FD, Buhaș CL, Voiță-Mekeres F, Voiță GF. Surgical scar management - an evaluation of surgical techniques. Med Pharm Rep. (2024) 97(2):149. doi: 10.15386/mpr-2701
39. Aghayan AH, Mohammadi D, Atashi A, Jamalpoor Z. Synergistic effects of mesenchymal stem cells and their secretomes with scaffolds in burn wound healing: a systematic review and meta-analysis of preclinical studies. J Transl Med. (2025) 23(1):1–29. doi: 10.1186/s12967-025-06712-y
40. Iacopetti I, Perazzi A, Patruno M, Contiero B, Carolo A, Martinello T, et al. Assessment of the quality of the healing process in experimentally induced skin lesions treated with autologous platelet concentrate associated or unassociated with allogeneic mesenchymal stem cells: preliminary results in a large animal model. Front Vet Sci. (2023) 10:1219833. doi: 10.3389/fvets.2023.1219833
41. Berry CE, Le T, An N, Griffin M, Januszyk M, Kendig CB, et al. Pharmacological and cell-based treatments to increase local skin flap viability in animal models. J Transl Med. (2024) 22(1):1–19. doi: 10.1186/s12967-024-04882-9
42. Bazgir F, Karimi Rouzbahani A, Birjandi M, Chehelcheraghi F. Protective effect of bone marrow mesenchymal stem cells on the survival zone of the perforator flaps in rats. SAGE Open Med. (2024) 12:20503121241276280. doi: 10.1177/20503121241276278
43. Mcgraw IT, Wilson EE, Behfar A, Paradise CR, Rohrich RJ, Wyles SP. Evolving role of exosomes in plastic and reconstructive surgery and dermatology. Plast Reconstr Surg Glob Open. (2024) 12(8):e6061. doi: 10.1097/GOX.0000000000006061
44. Dutra Alves NS, Reigado GR, Santos M, Caldeira IDS, Hernandes HS, Freitas-Marchi BL, et al. Advances in regenerative medicine-based approaches for skin regeneration and rejuvenation. Front Bioeng Biotechnol. (2025) 13:1527854. doi: 10.3389/fbioe.2025.1527854
45. Thompson BJ. Par-3 family proteins in cell polarity & adhesion. FEBS J. (2021) 289(3):596. doi: 10.1111/febs.15754
46. Cui R, Chen D, Li N, Cai M, Wan T, Zhang X, et al. PARD3 gene variation as candidate cause of nonsyndromic cleft palate only. J Cell Mol Med. (2022) 26(15):4292. doi: 10.1111/jcmm.17452
47. Moore R, Theveneau E, Pozzi S, Alexandre P, Richardson J, Merks A, et al. Par3 controls neural crest migration by promoting microtubule catastrophe during contact inhibition of locomotion. Development (Cambridge). (2013) 140(23):4763–75. doi: 10.1242/dev.098509
48. Richardson RJ, Hammond NL, Coulombe PA, Saloranta C, Nousiainen HO, Salonen R, et al. Periderm prevents pathological epithelial adhesions during embryogenesis. J Clin Invest. (2014) 124(9):3891. doi: 10.1172/JCI71946
49. Villanueva-Meyer J, Glastonbury C, Marcovici P. Congenital midline cervical cleft. J Radiol Case Rep. (2015) 9(3):7. doi: 10.3941/jrcr.v9i3.2202
50. Amos J, Sutton AE, Shermetaro C. Thyroglossal duct cyst. In: StatPearls. Treasure Island, FL: StatPearls Publishing (2025). Available online at: https://www.ncbi.nlm.nih.gov/sites/books/NBK519057/ (Accessed August 29, 2025).
51. Li X, Yang H, Deng X, Qiao DH. Thyroglossal duct cyst located in the thyroid gland misdiagnosed with thyroid carcinoma: a case report and literature review. Ear Nose Throat J. (2024) 103(2):NP124–7. doi: 10.1177/01455613211040583
52. Taha A, Enodien B, Frey DM, Taha-Mehlitz S. Thyroglossal duct cyst, a case report and literature review. Diseases. (2022) 10(1):7. doi: 10.3390/diseases10010007
53. Ndegbu CU, Olasehinde O, Adeyemo A, Alatise OI, Amusa YB. Management of thyroglossal cyst in adults: a single-institution experience. Niger J Surg. (2021) 27(1):38. doi: 10.4103/njs.NJS_25_20
54. Choi HI, Choi YH, Cheon JE, Kim WS, Kim IO. Ultrasonographic features differentiating thyroglossal duct cysts from dermoid cysts. Ultrasonography. (2017) 37(1):71. doi: 10.14366/usg.17027
55. Chen W, Zhou Y, Xu M, Xu R, Wang Q, Xu H, et al. Congenital second branchial cleft anomalies in children: a report of 52 surgical cases, with emphasis on characteristic CT findings. Front Pediatr. (2023) 11:1088234. doi: 10.3389/fped.2023.1088234
56. Prasad SC, Azeez A, Thada ND, Rao P, Bacciu A, Prasad KC. Branchial anomalies: diagnosis and management. Int J Otolaryngol. (2014) 2014:237015. doi: 10.1155/2014/237015
57. Holt AC, Lofgren DH, Shermetaro C. Branchial cleft anomalies. Indian J Dermatol. (2025) 61(6):701. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK499914/ (Accessed August 29, 2025).
58. Sutton AE, Goldman J. Branchial cleft cysts. In: StatPearls. Treasure Island, FL: StatPearls Publishing (2025). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK482467/ (Accessed August 29, 2025).
59. Shen LF, Zhou SH, Chen Q, Yu Q. Second branchial cleft anomalies in children: a literature review. Pediatr Surg Int. (2018) 34(12):1251–6. doi: 10.1007/s00383-018-4348-8
60. Zito PM, Scharf R. Epidermoid cyst. In: StatPearls. Treasure Island, FL: StatPearls Publishing (2023). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK499974/ (Accessed August 29, 2025).
61. Shareef S, Ettefagh L. Dermoid cyst. Brain Imaging with MRI and CT: An Image Pattern Approach. (2023) 9780521119443. p. 153–4. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK560573/ (Accessed August 29, 2025).
62. Celenk P, Celenk C, Kocasarac HD. Imaging features of sublingual dermoid cysts: a report of four cases. Radiol Case Rep. (2022) 17(8):2888. doi: 10.1016/j.radcr.2022.05.025
63. Dwivedi G, Saxena P, Patnaik U, Kumari A, Sood A. Dermoid cyst floor of mouth: a diagnostic conundrum. Indian J Otolaryngol Head Neck Surg. (2020) 74(Suppl 2):1961. doi: 10.1007/s12070-020-01939-1
64. Mittal MK, Malik A, Sureka B, Thukral BB. Cystic masses of neck: a pictorial review. Indian J Radiol Imaging. (2012) 22(4):334. doi: 10.4103/0971-3026.111488
65. Auerbach N, Gupta G, Mahajan K. Cystic hygroma. Clinical Anesthesia for the Newborn and the Neonate. (2023). p. 837–51. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK560672/ (Accessed August 29, 2025).
Keywords: congenital midline cervical cleft, Z-plasty reconstruction, neonatal neck anomalies, pediatric plastic surgery, branchial arch malformations, genetic associations in CMCC
Citation: Adi G, Eckoldt F and Alhussami I (2025) Z-Plasty technique in congenital midline cervical cleft; a rare case report & literature review. Front. Surg. 12:1660354. doi: 10.3389/fsurg.2025.1660354
Received: 5 July 2025; Accepted: 12 September 2025;
Published: 6 October 2025.
Edited by:
Joseph M. Escandón, Wyckoff Heights Medical Center, United StatesReviewed by:
Joo Ming Cheong, International Islamic University Malaysia, MalaysiaKailun Hu, Fujian Medical University Union Hospital, China
Copyright: © 2025 Adi, Eckoldt and Alhussami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ghaith Adi, Z2FkaUBhbGZhaXNhbC5lZHU=; Z2hhaXRoYWRpQGljbG91ZC5jb20=
†ORCID:
Ghaith Adi
orcid.org/0009-0005-0699-0708
 Ghaith Adi
Ghaith Adi Felicitas Eckoldt2
Felicitas Eckoldt2