- 1Department of Thyroid & Hernia Surgery, Medical Department of General Surgery, Chinese People’s Liberation Army General Hospital, Beijing, China
- 2Department of Ultrasound, Seventh People’s Hospital of Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background: Robotic thyroidectomy has shown good acceptance results and improved cosmetic outcomes. This study aimed to evaluate the safety and efficacy of bilateral axillary-breast approach (BABA) robotic thyroidectomy compared with conventional open surgery for the treatment of thyroid cancer.
Methods: The clinicopathological features and surgical outcomes of 73 papillary thyroid cancer patients treated by robotic surgery using the BABA approach and 62 papillary thyroid cancer patients treated by open surgery in our department from January 2024 to January 2025 were analyzed and compared.
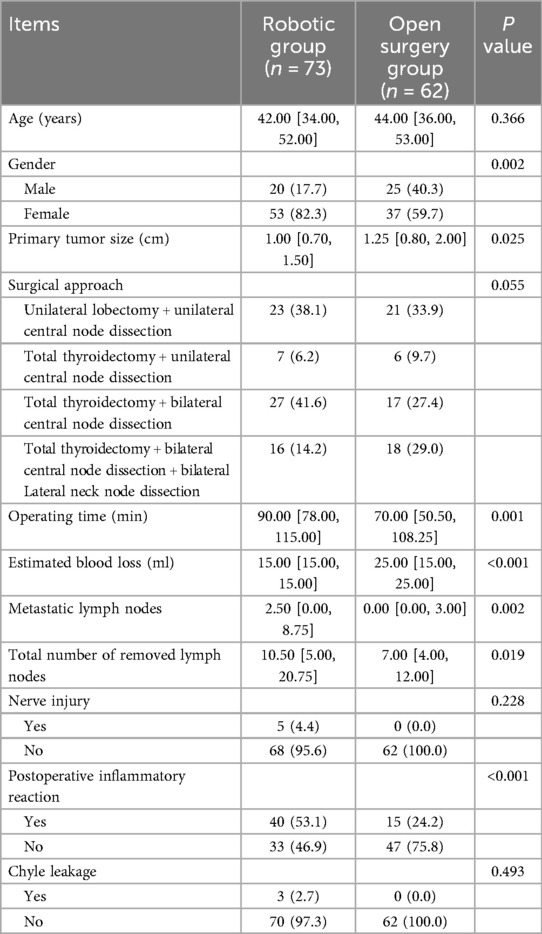
Results: The operation time was longer in the robotic group than in the open surgery group (P = 0.001). However, when thyroid cancer requires lymph node dissection in the lateral neck area, the open operation time is longer than that of robotic surgery, although it is no significant (P > 0.05). The estimated blood loss was lower in the robotic group than in the open surgery group (P < 0.001). Both total number of removed lymph nodes (P = 0.019) and metastatic lymph nodes (P = 0.002) were higher in the robotic group than in the open surgery group. Postoperative inflammatory reaction was higher in the robotic group than in the open surgery group (P < 0.001). No significant difference was observed in the nerve injury or Chyle leakage between the two groups. No recurrence or metastasis was found.
Conclusions: Compared with open surgery, BABA robot radical thyroid cancer surgery with lymph node dissection is safety and efficacy, and have advantages such as less intraoperative blood loss, no neck scar. Especially in lateral cervical lymph node dissection, robotic surgery has obvious advantages.
Introduction
Thyroid cancer is one of the most common malignant tumors, and its annual incidence rate is on the rise (1, 2). Compared with other malignant tumors, most thyroid cancer patients have a good prognosis, so they not only require complete removal of the lesion, but also hope to have a higher quality of life after surgery (3, 4). Endoscopic surgery is an ideal option for treating thyroid disease because it maintains the aesthetics of the neck. As one of the advanced endoscopic systems, the da Vinci robot has advantages such as 3D high-definition vision and remote control that can filter out operator shaking; it also has flexible internal joints with 7 degrees of freedom, which is more conducive to delicate operations than traditional open surgery (5, 6). Therefore, it has been widely used in thyroid surgery (7–10). Although recent studies have shown that robot-assisted radical thyroidectomy is as safety and efficacy as traditional open surgery (11, 12). However, it is unclear whether robots are superior to open surgery in radical thyroid cancer surgeries of different degrees, especially in lateral neck lymph node dissection.
From January 2024 to January 2025, we treated 73 thyroid cancer patients by robotic surgery via a bilateral axillo-breast approach (BABA) (robotic group). Another 62 thyroid cancer patients who underwent open surgery in the same period served as the control group (open surgery group). The data pertaining to the efficacy and safety of the two groups were analyzed.
Materials and methods
Patients
Patients who met the following criteria were included in the study: (1) aged between 18 and 65 years; (2) diagnosed with thyroid cancer by preoperative fine needle aspiration (FNA) or suspected malignant tumors by preoperative FNA examination and confirmed by postoperative pathology; and (3) with a maximum tumor diameter of ≤5 cm as shown by ultrasonography; (4) preoperative nasofibrolaryngoscopy was performedand. The exclusion criteria are as follows: (1) a history of thyroid surgery or radiotherapy in the neck; (2) preoperative examination or intraoperative findings that the tumor has invaded the esophagus, trachea, recurrent laryngeal nerve, or large blood vessels in the neck; (3) preoperative examination showing suspected or clear metastasis to the lateral lymph nodes; (4) preoperative examination showing distant metastasis. The demographic and clinical data showed in Table 1. The sample size of this study was estimated based on the data from a previous pilot study. Choice of surgical approach: All patients were informed of the advantages and disadvantages of robotic surgery and traditional laparotomy and made their own decision before surgery without inducement behavior. All operations were performed by a single surgeon who completed over 1,000 cases of thyroid cancer surgeries using the robotic BABA approach and 5,000 cases of open thyroid cancer surgeries. We analyzed the patients’ medical records and evaluated the surgical outcomes, including the total operative time, estimated intraoperative blood loss, number of resected lymph nodes, number of metastatic lymph nodes, nerve injury, postoperative inflammatory response, and chylous leak, among other data for all patients.
Open surgery
After satisfactory anesthesia, the patient lies in a supine position, with the shoulder and neck in an overextended position. The surgical area of the neck and shoulder is disinfected and covered with a sterile towel. Two transverse fingers above the suprasternal fossa are made along the skin lines to make a transverse arc-shaped incision about 5–12 cm long. The skin, subcutaneous tissue, and latissimus neck platysma are incised. A free flap is made on the deep surface of the latissimus neck platysma, up to the hyoid level and down to the suprasternal fossa. Cut the anterior cervical fascia along the white line of the neck. Separate the bilateral thyroid tissues from the space between the infrahyoid muscles and the external layer of the thyroid gland. Carefully separate the thyroid gland with an electric knife within the surgical capsule, expose the thyroid gland, inject 0.2 mL of nano-carbon suspension into the thyroid lobe, and cut and ligate the middle thyroid vein. Separate the annular thyroid space, cut and ligate the superior thyroid artery and vein closely at the upper pole of the thyroid gland. Lift the lower pole of the thyroid gland, cut and ligate the blood vessels of the lower pole of the thyroid gland to expose and protect the parathyroid blood supply. Use scissors to free the recurrent laryngeal nerve from the tumor. The electromyographic signals of the recurrent laryngeal nerve and vagus monitored by the nerve monitor show normal responses. Cut the tight tissue between the isthmus of the thyroid gland and the trachea, cut and ligate the papillary ligament of the thyroid gland. Be careful not to damage the site where the recurrent laryngeal nerve enters the larynx. Remove the thyroid gland and isthmus simultaneously. Clear the Lymphoid adipose tissue from below the thyroid cartilage to the clavicle plane, the medial side of the common carotid artery, the area around the recurrent laryngeal nerve, and the pretracheal lymphoid adipose tissue above the sternal incision. The ultrasonic knife was used to free the space between the sternocleidomastoid muscle and the ribbon-like muscle. The right sternocleidomastoid muscle was pulled outward to expose the carotid sheath. The carotid sheath was cut vertically from the surface of the internal jugular vein. The tissues were separated from front to back respectively. The lymphoid adipose tissue in front of the carotid sheath, including the carotid triangle, was lifted from bottom to top, and then this tissue was turned back through the superficial surface of the internal jugular vein. It ascends to the posterior abdomen of the right biceps femoris muscle, exposing and protecting the accessory nerve, and clearing the adipose lymphoid tissue in area II around this segment of the accessory nerve. The ultrasonic scalpel opens the intermuscular system of the sternocleidomastoid muscle, downward to the supraclavicular level and backward to the prevertebral fascia, revealing and protecting the phrenic nerve. The right lymphatic duct was explored at the jugular Angle. The tissues in this area were cut in batches to protect the main trunk of the transverse jugular artery and vein. The entire dissection specimen was separated and dissected backward from the lymph nodes in areas III, IV, and V, and the dissected lymph node specimens were removed.
Robotic surgery
After the patient was placed in a supine position with the recurrent laryngeal nerve monitoring tube inserted under general anesthesia, the shoulder was elevated and the neck was extended backward. The routine disinfection and sheet were made. A 1.2 cm long incision was made at two points on the right areola. A long pneumoperitoneum needle was inserted into the suprasternal fossa, and about 50 mL of expansion fluid and 20 mL of air were injected. A separation rod was inserted, and a 12 mm Trocar was placed in the superficial layer of the deep fascia in the chest as the main observation hole. Set the pressure at 8mmHg to establish the CO2 residual cavity. 0.8 cm incisions were made respectively in the left and right axilla and the left areola at 11 points, and an 8 mm Trocar was inserted. The Da Vinci robot is placed in the predetermined position. The 1st, 2nd and 3rd arms are respectively connected to the ultrasonic scalpel, the gripper and the Maryland forceps (Figure 1). A latent separated flap is made between the deep surface of the latissimus neck platysma and the deep cervical fascia, reaching up to the thyroid cartilage, down to the supraspinal fossa, and on both sides to the anterior edge of the sternocleidomastoid muscle. Cut the white line of the neck to reach the thyroid capsule, separate the anterior cervical muscle groups on both sides, and expose the thyroid gland. The bilateral surgical capsules were carefully separated with an ultrasonic scalpel to expose the bilateral lobules of the thyroid gland. The nano-carbon suspension was percutaneous injected at the neck with 0.2 mL under the capsule of the bilateral lobules of the thyroid gland. Subsequent operations were carried out after the nano-carbon black staining diffused uniformly. Expose the thyroid gland, cut and ligate the middle thyroid vein, separate the cricothyroid space, use a surgical clip to ligate and cut the superior thyroid artery and vein close to the thyroid at the upper pole of the thyroid gland, lift the lower pole of the thyroid gland, cut and ligate the blood vessels at the lower pole of the thyroid gland, expose and protect the parathyroid blood supply, release the recurrent laryngeal nerve, and the electromyographic signals of the recurrent laryngeal nerve and vagus nerve monitored by a nerve monitor showed normal responses. Cut the tight tissue between the isthmus of the thyroid gland and the trachea, cut and ligate the papillary ligament of the thyroid gland, and be careful not to damage the site where the recurrent laryngeal nerve enters the larynx. Remove both the thyroid gland and the isthmus at the same time, and clear the lymphoid adipose tissue below the thyroid cartilage to the clavicle plane, the medial side of the common carotid artery, the lymphoid adipose tissue around the recurrent laryngeal nerve, and the lymphoid adipose tissue in front of the trachea above the sternal incision (Figure 2). The ultrasonic knife is used to free the space between the sternocleidomastoid muscle and the band-shaped muscle. The sternocleidomastoid muscle is pulled outward to expose the carotid sheath. Make a longitudinal incision along the surface of the internal jugular vein to open the carotid sheath. The tissues are separated from the front to the back respectively. The lymphoid adipose tissue in front of the carotid sheath, including the carotid triangle, is lifted from bottom to top. Then, this tissue is flipped back through the superficial surface of the internal jugular vein and released upward to the posterior abdomen of the biceps. Expose and protect the accessory nerve, and clear the adipose lymphoid tissue in area II around this segment of the accessory nerve. The intermuscular system of the sternocleidomastoid muscle was opened with an ultrasonic knife, moving downward to the supraclavicular level and backward to the prevertebral fascia to expose and protect the phrenic nerve. Lymphatic ducts were detected at the jugular Angle. The tissues in this area were cut in batches to protect the main trunk of the transverse carotid artery and vein. The entire dissection specimen was separated and dissected backward from the lymph nodes in areas III, IV, and V, and the dissected lymph node specimens were removed (Figure 3).

Figure 1. Diagrams of robotic surgery and open surgery approaches for thyroid cancer. (A) Drawing instrument arm trajectory lines and working area. (B) Each of the four trocars was docked with a robotic arm. (C) Open the lateral incision in the neck for surgery to expose the thyroid gland.
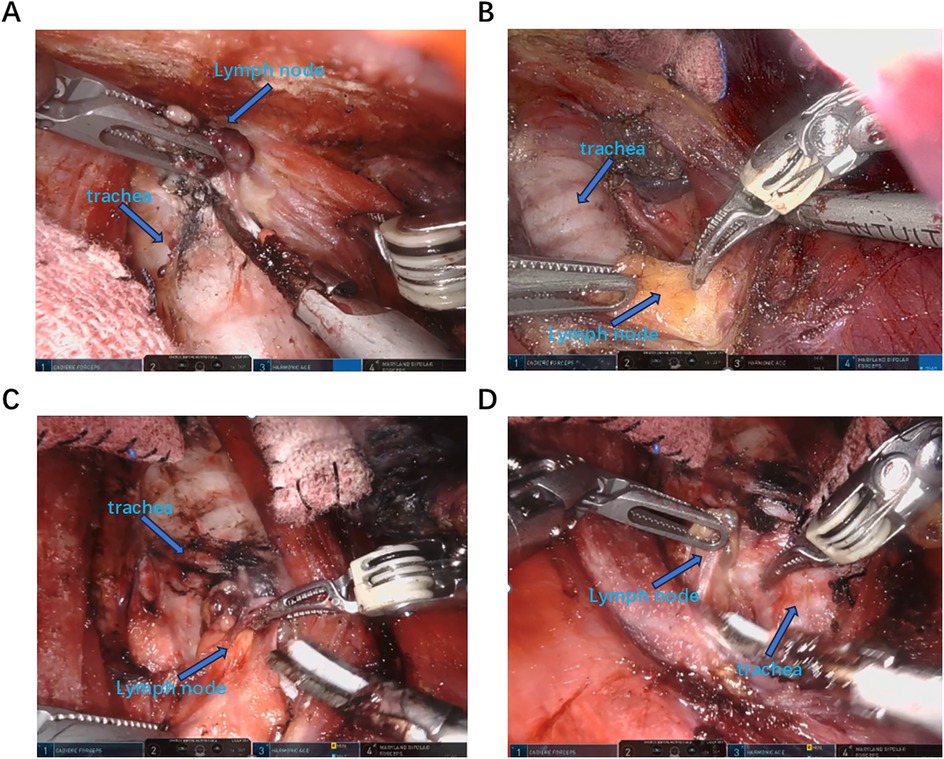
Figure 2. Identify and dissect the lymph nodes in the central area. (A,B) The strap muscle was drawn to the left and the trachea was drawn to the right using Maryland and cartier to expose the left median area for central lymph node dissection. (C,D) The strap muscle was drawn to the right and the trachea was drawn to the left using Maryland and cartier to expose the right median area for central lymph node dissection.
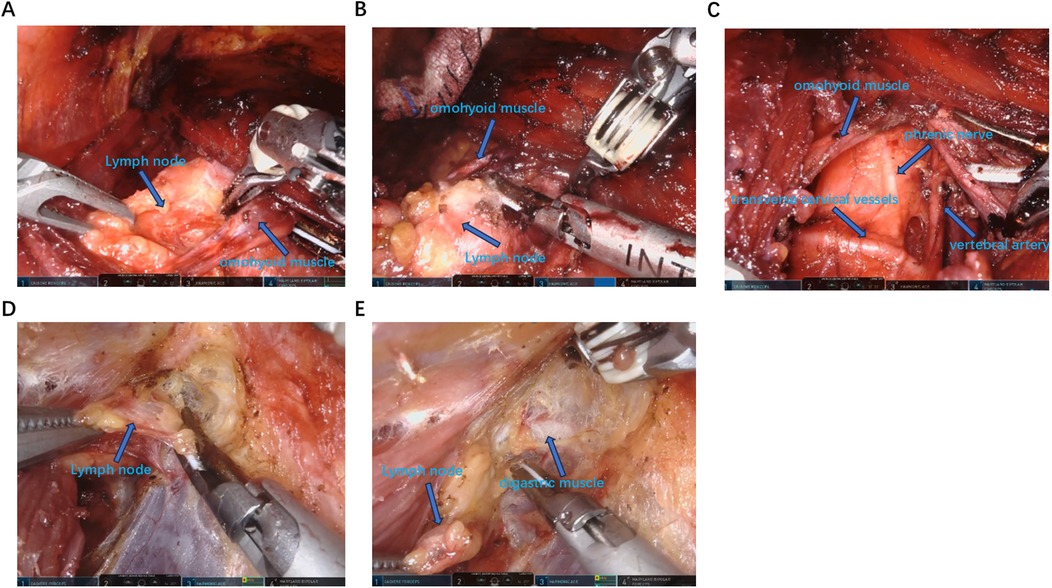
Figure 3. Identify and dissect the lymph nodes in the lateral cervical area. (A–C) From the intermuscular space of the sternocleidomastoid muscle to expose the omohyoid muscle and dissect the lymph nodes in area III, IV and V. (D,E) Expose the digastric muscle from the inner side of the sternocleidomastoid muscle and dissect the lymph nodes in area II.
Postoperative treatment and follow-up
The patient's voice was observed postoperatively. The color and volume of the drainage fluid were recorded daily, and the drainage tube was removed when the drainage fluid became light red and the daily volume was less than 15 mL. Patients were followed up in the clinic at 1, 3, and 6 months after surgery and every 6 months thereafter. Follow-up evaluations included thyroid function tests, thyroglobulin (TG) measurements, serum calcium and parathyroid hormone (PTH) levels, and neck ultrasound. Based on laboratory test results, a serum calcium level of less than 2.2 mmol/L was defined as hypocalcemia. A parathyroid hormone (PTH) level of less than 15 pg/mL was defined as hypoparathyroidism. If PTH returned to normal levels within 6 months, the patient was diagnosed with transient hypoparathyroidism, otherwise permanent hypoparathyroidism. Any bleeding requiring surgical intervention was considered postoperative bleeding.
Statistical analysis
For descriptive statistics of quantitative variables, mean ± standard deviation (SD) and range were used to describe central tendency and dispersion. For analysis of the differences in proportions, Chi-square test was used. Fisher's exact test was used if the assumptions of Chi-square test were violated. Independent samples t-test was used to compare the level of quantitative variables between the two groups. Data were analyzed using SPSS 15.0 on Windows (SPSS Inc., Chicago, IL, USA). A P < 0.05 was considered statistically significant.
Results
Overall comparison of the two groups
No significant differences were observed in patient's age, gender, size of tumor, Nerve injury (intraoperative nerve detection and postoperative voice changes), Chyle leakage (color of the postoperative drainage fluid) between the open surgery group and the robotic group. The operation time (initial incision to the suturing of the approach) was longer in the robotic group than in the open surgery group (P = 0.001). The estimated blood loss (reference anesthesia record) was lower in the robotic group than in the open surgery group (P < 0.001). Both total number of removed lymph nodes (P = 0.019) and metastatic lymph nodes (postoperative pathological results) (P = 0.002) were higher in the robotic group than in the open surgery group. Postoperative inflammatory reaction [postoperative check white blood cell (WBC) and C-reactive protein (CRP)] was higher in the robotic group than in the open surgery group (P < 0.001) (Table 2).
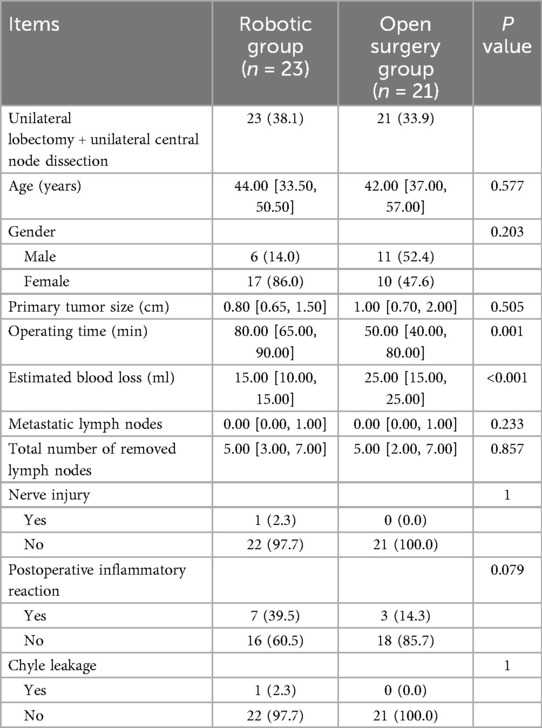
Comparison of unilateral lobectomy + unilateral central node dissection in the two groups
No significant differences were observed in patient's age, gender, size of tumor, Nerve injury, Chyle leakage, total number of removed lymph nodes, metastatic lymph nodes between the open surgery group and the robotic group. The operation time was longer in the robotic group than in the open surgery group (P = 0.001). The estimated blood loss was lower in the robotic group than in the open surgery group (P < 0.001) (Table 3).
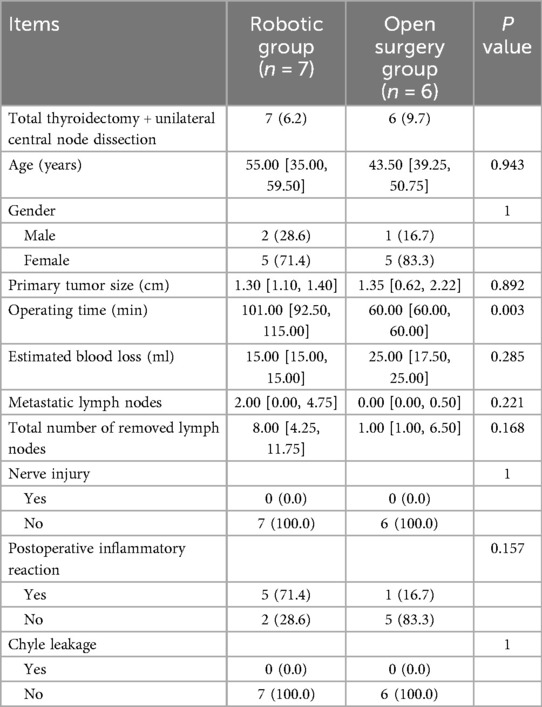
Comparison of total thyroidectomy + unilateral central node dissection in the two groups
No significant differences were observed in patient's age, gender, size of tumor, Nerve injury, Chyle leakage, total number of removed lymph nodes, metastatic lymph nodes, estimated blood loss between the open surgery group and the robotic group. The operation time was longer in the robotic group than in the open surgery group (P = 0.003) (Table 4).
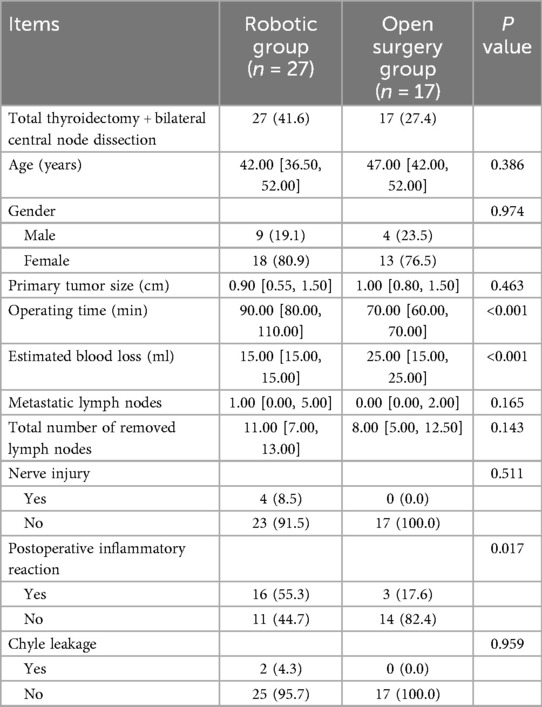
Comparison of total thyroidectomy + bilateral central node dissection in the two groups
No significant differences were observed in patient's age, gender, size of tumor, Nerve injury, Chyle leakage, total number of removed lymph nodes, metastatic lymph nodes between the open surgery group and the robotic group. The operation time was longer in the robotic group than in the open surgery group (P < 0.001). The estimated blood loss was lower in the robotic group than in the open surgery group (P < 0.001). Postoperative inflammatory reaction was higher in the robotic group than in the open surgery group (P = 0.017) (Table 5).
Comparison of total thyroidectomy + bilateral central node dissection + bilateral lateral neck node dissection in the two groups
No significant differences were observed in patient's age, gender, size of tumor, Nerve injury, Chyle leakage, total number of removed lymph nodes, metastatic lymph nodes between the open surgery group and the robotic group. The estimated blood loss was lower in the robotic group than in the open surgery group (P = 0.002). However, only when thyroid cancer requires lymph node dissection in the lateral neck area, the open operation time is longer than that of robotic surgery, although it is no significant (P > 0.05) (Table 6).
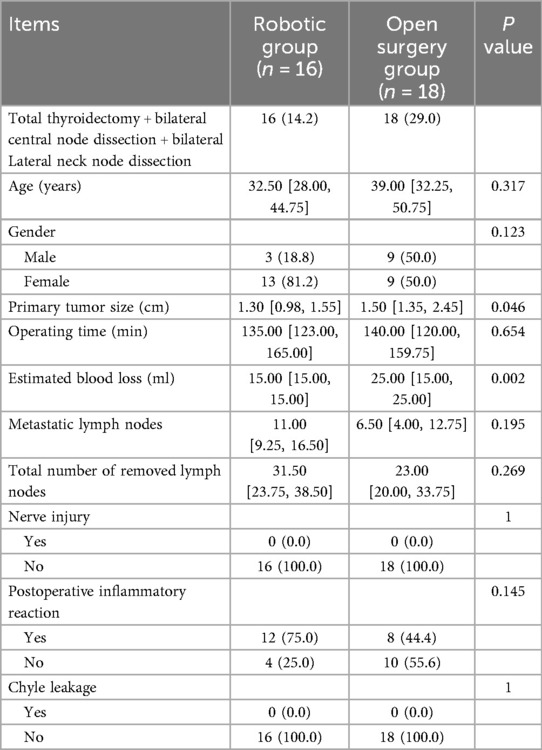
Table 6. Comparison of total thyroidectomy + bilateral central node dissection + bilateral lateral neck node dissection in the two groups.
Discussion
The da Vinci robotic system has been used for surgical treatment of thyroid cancer (13) because of its high-definition 3D field of view, remote-controlled operation to eliminate hand tremors, and internally articulated instruments (14, 15). However, whether robotic surgery is superior to traditional open surgery remains controversial due to its high cost and concerns about clinical outcomes (16–18). It is worth recognizing that this is the first study to compare BABA robotic surgery with conventional open surgery, and our results show that BABA robotic thyroidectomy can maintain the aesthetic appearance of the neck and has significant results.
Traditionally, the incision has been enlarged into a larger transverse incision to complete the required neck resection. Since the front of the neck is prominent and a frequently exposed part of the body, this may leave an unsightly neck scar, which can be a nuisance to patients. In addition, most thyroid cancer patients are young women who prefer to avoid scarring on their necks. Therefore, surgeons have gone to great lengths to address this scarring issue. In 1997, the first endoscopic thyroidectomy was performed (19). In 2009, the feasibility of robotic thyroidectomy via the axillary approach was demonstrated, which further improved the aesthetic outcome of the surgery (20). To our knowledge, several improved approaches have been reported in the literature, which can be roughly divided into the following categories: smaller neck incisions, including incisions in natural body cavities or body surfaces; more distal incisions, such as chest wall, periauricular, axillary, retroauricular, and transoral incisions; and combined incisions. All surgical approaches have their own advantages and disadvantages. For example, the axillary approach has limitations in reaching the contralateral central lymph nodes (21, 22). The BABA procedure provides a complete and symmetrical surgical field of view of anatomical structures (such as the superior and inferior thyroid vessels, recurrent laryngeal nerve, parathyroid glands, and trachea), enables exploration of both thyroid glands, provides more space for instrument manipulation, and enables the removal of larger nodules. No brachial plexus injury or axillary skin flap perforation occurred during the BABA procedure (13, 22, 23). Robotic total thyroidectomy with neck dissection via BABA had similar complication rates as open procedure. In terms of clinical safety and efficacy, robotic total thyroidectomy with neck lymph node dissection is equivalent to open surgery. The small scars in the bilateral axilla and nipple areola were almost invisible. However, some young women may refuse to undergo this procedure because the BABA procedure involves their breasts. Further research is needed to explore other better methods to minimize this disadvantage (24, 25).
As with any emerging treatment technology, careful patient selection is critical. While it is important to tailor the surgical approach to the patient's concerns and expectations, it is always imperative to adhere to basic surgical oncology principles. The oncological safety is more important than the cosmetic demand. We analyzed the clinical data of 73 thyroid cancer patients who underwent robotic surgery using the BABA approach and 62 thyroid cancer patients who underwent conventional open surgery. The results showed that there are no significant differences were observed in patient's age, gender, size of tumor, Nerve injury, Chyle leakage between the open surgery group and the robotic group. The operation time was longer in the robotic group than in the open surgery group (P = 0.001). The estimated blood loss was lower in the robotic group than in the open surgery group (P < 0.001). Both total number of removed lymph nodes (P = 0.019) and metastatic lymph nodes (P = 0.002) were higher in the robotic group than in the open surgery group. Postoperative inflammatory reaction was higher in the robotic group than in the open surgery group (P < 0.001), because the BABA robotic approach for thyroid cancer radical surgery requires the establishment of an operating space under the chest skin, this might be the main cause of postoperative inflammatory reaction. The long operation time of thyroid cancer surgery using the BABA robotic approach might also be a factor contributing to the postoperative inflammatory response. We checked the inflammatory response through postoperative white blood cell (WBC) and C-reactive protein (CRP) levels, the surgical stress response can also lead to an increase in postoperative WBC and CRP levels. Although recent studies have shown that robot-assisted radical thyroidectomy is no different from traditional open surgery in terms of safety and effectiveness (11, 12). However, it is unclear whether robots are superior to open surgery in radical thyroid cancer surgeries of different degrees, especially in lateral neck lymph node dissection. We grouped the surgeries for thyroid cancer of different degrees, The robot has obvious advantages in Total thyroidectomy + bilateral central node dissection + bilateral Lateral neck node dissection, compared with other degrees of thyroid cancer, the robotic surgery time for this thyroid cancer is shorter than that for open surgery, although it is no significant (P > 0.05). This fully reveals the safety and efficacy of robot-based radical thyroidectomy, especially in the lymph node dissection of the lateral neck region, where the advantages of robot-based surgery are obvious.
Studies have shown that the learning curve for performing robotic thyroid surgery requires more than 50 surgical experiences (26–29). However, this surgeon should be more experienced and skilled in handling complex situations in thyroid surgery. The limitations of this study are as follows: (I) To avoid the influence of the surgeon's proficiency on the surgical outcomes, we selected patients who were operated on by the most experienced surgeons in both the robotic surgery group and the open surgery group. Even so, the selection bias is inevitable. (II) Our study was a single-center clinical trial with a small sample size, so more multicenter randomized controlled studies are needed for further verification. (III) Although the prognosis of thyroid cancer is generally good after surgical treatment, the effectiveness of tumor radicalization is evaluated based on the postoperative pathological lymph node metastasis situation. However, the assessment of tumor safety mainly relies on long-term postoperative follow-up. This study did not adequately evaluate the safety of the tumor due to the short follow-up period for the patients after surgery. In the future, longer follow-up periods are needed. In the future, we will conduct a large-sample retrospective study in multiple centers to further determine the superiority and safety of robotic thyroid cancer surgery.
Conclusion
The priority of any surgical procedure is to ensure patient safety with the best patient outcomes. This study demonstrates that BABA robot radical thyroid cancer surgery with lymph node dissection is safe and effective, and have advantages such as less intraoperative blood loss, no neck scar. Especially in lateral cervical lymph node dissection, robotic surgery has obvious advantages.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Chinese PLA General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FK: Conceptualization, Project administration, Software, Writing – original draft. MZ: Data curation, Formal analysis, Methodology, Writing – original draft. WW: Data curation, Writing – original draft. WT: Writing – review & editing. LL: Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82172573).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
3. Roman BR, Morris LG, Davies L. The thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol Diabetes Obes. (2017) 24:332–6. doi: 10.1097/MED.0000000000000359
4. Carling T, Udelsman R. Thyroid cancer. Annu Rev Med. (2014) 65:125–37. doi: 10.1146/annurev-med-061512-105739
5. Fan LJ, Jiang J. Present and future of robot-assisted endoscopic thyroid surgery. Chin Med J. (2012) 125:926–31. doi: 10.3760/cma.j.issn.0366-6999.2012.05.034
6. Rabinovics N, Aidan P. Robotic transaxillary thyroid surgery. Gland Surg. (2015) 4:397–402. doi: 10.3978/j.issn.2227-684X.2015.04.08
7. Kim EB, Cho JW, Lee YM, Sung TY, Yoon JH, Chung KW, et al. Postsurgical outcomes and surgical completeness of robotic thyroid surgery: a single surgeon’s experience on 700 cases. J Laparoendosc Adv Surg Tech A. (2018) 28:540–5. doi: 10.1089/Lap.2017.0597
8. Bae DS, Koo DH. A propensity score-matched comparison study of surgical outcomes in patients with differentiated thyroid cancer after robotic versus open total thyroidectomy. World J Surg. (2019) 43:540–51. doi: 10.1007/s00268-018-4802-8
9. Chai YJ, Suh H, Woo JW, Yu HW, Song RY, Kwon H, et al. Surgical safety and oncological completeness of robotic thyroidectomy for thyroid carcinoma larger than 2 cm. Surg Endosc. (2017) 31:1235–40. doi: 10.1007/s00464-016-5097-1
10. Pan JH, Zhou H, Zhao XX, Ding H, Wei L, Qin L, et al. Robotic thyroidectomy versus conventional open thyroidectomy for thyroid cancer: a systematic review and meta-analysis. Surg Endosc. (2017) 31:3985–4001. doi: 10.1007/s00464-017-5433-0
11. Zhang Y, Du J, Ma J, Liu J, Cui X, Yuan J, et al. Unilateral axilla-bilateral areola approach for thyroidectomy by da vinci robot vs. Open surgery in thyroid cancer: a retrospective observational study. Gland Surg. (2021) 10:1291–9. doi: 10.21037/gs-20-831
12. He QQ, Zhu J, Zhuang DY, Fan ZY, Zheng LM, Zhou P, et al. Comparative study between robotic total thyroidectomy with central lymph node dissection via bilateral axillo-breast approach and conventional open procedure for papillary thyroid microcarcinoma. Chin Med J. (2016) 129:2160–6. doi: 10.4103/0366-6999.189911
13. Kim WW, Jung JH, Park HY. A single surgeon’s experience and surgical outcomes of 300 robotic thyroid surgeries using a bilateral axillo-breast approach. J Surg Oncol. (2015) 111:135–40. doi: 10.1002/jso.23793
14. Shan L, Liu J. Meta-analysis comparison of bilateral axillo-breast approach robotic thyroidectomy and conventional thyroidectomy. Surg Innov. (2019) 26:112–23. doi: 10.1177/1553350618817145
15. Wang YC, Liu K, Xiong JJ, Zhu JQ. Robotic thyroidectomy versus conventional open thyroidectomy for differentiated thyroid cancer: meta-analysis. J Laryngol Otol. (2015) 129:558–67. doi: 10.1017/S002221511500122X
16. Paek SH, Kang KH, Kang H, Park SJ. Comparison of postoperative surgical stress following robotic thyroidectomy and open thyroidectomy: a prospective pilot study. Surg Endosc. (2016) 30:3861–6. doi: 10.1007/s00464-015-4689-5
17. Berber E, Bernet V, Fahey TJ, Kebebew E 3rd, Shaha A, Stack BC, et al. American Thyroid association statement on remote-access thyroid surgery. Thyroid. (2016) 26:331–7. doi: 10.1089/thy.2015.0407
18. Aidan P, Arora A, Lorincz B, Tolley N, Garas G. Robotic thyroid surgery: current perspectives and future considerations. ORL J Otorhinolaryngol Relat Spec. (2018) 80:186–94. doi: 10.1159/000488354
19. Lirici MM, Hüscher CS, Chiodini S, Napolitano C, Recher A. Endoscopic right thyroid lobectomy. Surg Endosc. (1997) 11:877. doi: 10.1007/s004649900476
20. Kang SW, Jeong JJ, Yun JS, Sung TY, Lee SC, Lee YS, et al. Robot-assisted endoscopic surgery for thyroid cancer: experience with the first 100 patients. Surg Endosc. (2009) 23:2399–406. doi: 10.1007/s00464-009-0366-x
21. Caruso G, Spinosi MC, Cambi J, Passali FM, Bellussi L, Passali D. Open versus robotic thyroidectomy: is it really a controversial choice? Kulak Burun Bogaz Ihtis Derg. (2015) 25:375–6. doi: 10.5606/kbbihtisas.2015.49035
22. Perrier ND. Why I have abandoned robot-assisted transaxillary thyroid surgery. Surgery. (2012) 152:1025–6. doi: 10.1016/j.surg.2012.08.060
23. Bae DS, Suh BJ, Park JK, Koo DH. Technical, oncological, and functional safety of bilateral axillo-breast approach (BABA) robotic total thyroidectomy. Surg Laparosc Endosc Percutan Tech. (2016) 26:253–8. doi: 10.1097/SLE.0000000000000262
24. Hinson AM, Kandil E, O'Brien S, Spencer HJ, Bodenner DL, Hohmann SF, et al. Trends in robotic thyroid surgery in the United States from 2009 through 2013. Thyroid. (2015) 25:919–26. doi: 10.1089/thy.2015.0066
25. Lee HY, Yang IS, Hwang SB, Lee JB, Bae JW, Kim HY. Robotic thyroid surgery for papillary thyroid carcinoma: lessons learned from 100 consecutive surgeries. Surg Laparosc Endosc Percutan Tech. (2015) 25:27–32. doi: 10.1097/SLE.0b013e3182a2b0ae
26. Kim WW, Jung JH, Park HY. The learning curve for robotic thyroidectomy using a bilateral axillo-breast approach from the 100 cases. Surg Laparosc Endosc Percutan Tech. (2015) 25:412–6. doi: 10.1097/SLE.0000000000000121
27. Park JH, Lee J, Hakim NA, Kim HY, Kang SW, Jeong JJ, et al. Robotic thyroidectomy learning curve for beginning surgeons with little or no experience of endoscopic surgery. Head Neck. (2015) 37:1705–11. doi: 10.1002/hed.23824
28. Lörincz BB, Busch CJ, Möckelmann N, Knecht R. Initial learning curve of single-incision transaxillary robotic hemi- and total thyroidectomy–a single team experience from Europe. Int J Surg. (2015) 18:118–22. doi: 10.1016/j.ijsu.2015.04.053
Keywords: thyroid cancer, bilateral axillo-breast approach (BABA), da vinci robot, open surgery, lateral neck lymph node dissection
Citation: Kuang F, Zhou M, Wang W, Tian W and Liu L (2025) Retrospective observational study between robotic thyroidectomy via bilateral axillo-breast approach and conventional open surgery for thyroid cancer. Front. Surg. 12:1663126. doi: 10.3389/fsurg.2025.1663126
Received: 10 July 2025; Accepted: 5 November 2025;
Published: 20 November 2025.
Edited by:
Pietro Princi, Ospedale Cristo Re, ItalyReviewed by:
Luis Alberto Gaitan, National Autonomous University of Mexico, MexicoSe Hyun Paek, Ewha Womans University, Republic of Korea
Copyright: © 2025 Kuang, Zhou, Wang, Tian and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Liu, Z2VvZmZyZXkzMDFAMTI2LmNvbQ==; Wen Tian, dGlhbndlbjMwMV9jdGEwMUAxNjMuY29t
†These authors have contributed equally to this work
 Fei Kuang
Fei Kuang Mengjia Zhou2,†
Mengjia Zhou2,†