- Department of Thoracic Surgery, Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
Background: Lung cancer and chronic obstructive pulmonary disease (COPD) commonly coexist simultaneously and individuals with COPD are at a higher risk of developing lung cancer. Nutritional intervention has a restorative effect on COPD combined with lung cancer, but there is almost no research on lung rehabilitation training, and there are even fewer studies on the combination of the two.
Objectives: Our study aimed to assess the role of preoperative lung rehabilitation training plus nutritional intervention on surgical tolerance and accelerated recovery indicators in patients with moderate to severe COPD complicated with lung cancer.
Methods: A total of 92 patients with COPD complicated by lung cancer who underwent surgery at our hospital between February 2023 and March 2024 were enrolled. Using a block randomization method, patients were divided into two groups: the control group (n = 47) receiving only nutritional intervention, and the observation group (n = 45) receiving a combination of lung rehabilitation training and nutritional intervention. The following indicators were compared between the two groups: pulmonary function parameters [forced expiratory volume in 1 s (FEV1), FEV1/forced vital capacity (FVC), maximum voluntary minute ventilation percentage (MVV%), and lung carbon monoxide diffusion capacity (DLCO)], Modified Medical Research Council (MMRC) dyspnea scale score, 6 min walking distance (6MWD), blood gas indicators [arterial partial pressure of oxygen (PaO2) and arterial partial pressure of carbon dioxide (PaCO2)], quality of life, and postoperative complication rate.
Results: After intervention, the observation group showed significantly higher levels of FEV1, FEV1/FVC, MVV%, DLCO, and 6MWD compared with the control group, while the MMRC score was significantly lower (all p < 0.05). Regarding blood gas indicators, the observation group had a significantly higher PaO2 level and a significantly lower PaCO2 level than the control group (p < 0.05). Additionally, the quality of life score in the observation group was significantly higher, and the postoperative complication rate was significantly lower than those in the control group (both p < 0.05).
Conclusion: Preoperative lung rehabilitation training combined with nutritional intervention can effectively improve pulmonary function and respiratory function in patients with moderate to severe COPD complicated by lung cancer, enhance their surgical tolerance, improve quality of life, and reduce the incidence of postoperative complications.
Introduction
As the most common malignant tumor, lung cancer continues to increase its incidence rate and mortality, and currently ranks first in cancer deaths (1–3). With the continuous aggravation of air pollution and the induction of risk factors such as long-term smoking, patients with lung cancer combined with COPD are becoming increasingly common in clinical practice, especially those with moderate to severe obstructive ventilation dysfunction, who lose their best surgical opportunities due to poor lung function reserve (4–6). Video assisted thoracoscopy surgery (VATS) has the characteristics of minimal trauma and short postoperative recovery time, making it an ideal surgical method for lung cancer complicated with COPD, which can promote rapid recovery of patients during the perioperative period (7, 8). Nevertheless, surgical treatment for patients can cause metabolic hyperactivity in the body, greatly reducing the synthesis of carbohydrates, fats, and proteins, resulting in poor nutrition and immune regulation disorders in patients (9). Hence, various intervention methods are often used in clinical to improve this situation (10). Nutritional intervention is simple and easy to implement, convenient to operate, but the focus is relatively single, resulting in poor overall efficacy and affecting the treatment process and prognosis (11, 12). Preoperative lung rehabilitation training plays an important role in the prevention of cancer and the treatment of pulmonary disease complications, but there are few reports on the impact on COPD complicated with lung cancer (13). A recent systematic review and meta-analysis of randomized controlled trials confirmed that preoperative pulmonary rehabilitation can significantly improve perioperative outcomes (e.g., pulmonary function, exercise tolerance) in lung cancer patients (14). Additionally, a scoping review on prehabilitation in the lung cancer pathway emphasized that combined interventions (e.g., pulmonary rehabilitation + nutritional support) may yield greater benefits than single strategies, though data on COPD-complicated lung cancer remain limited (15). Hence, this study aimed to discuss the role of preoperative lung rehabilitation training plus nutritional intervention on surgical tolerance and accelerated recovery indicators in patients with moderate to severe COPD complicated with lung cancer.
Materials and methods
General data
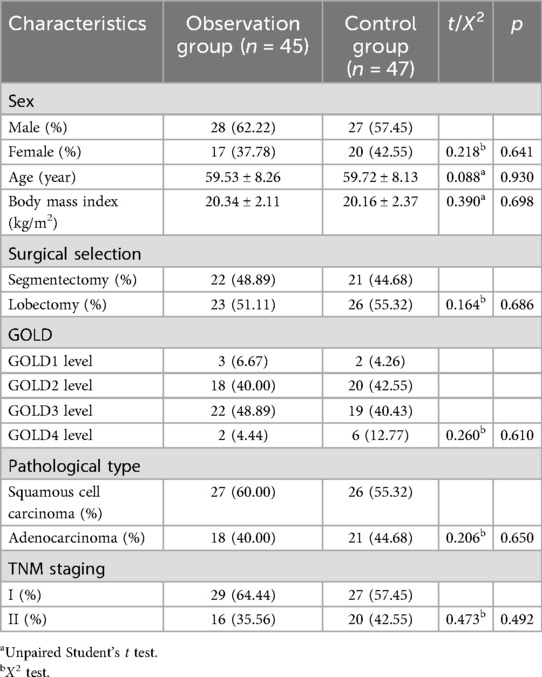
The clinical data of 92 patients who diagnosed with COPD complicated with lung cancer at our hospital between February 2023 and March 2024 were analyzed, adopting the method of block randomization. All patients were diagnosed with lung cancer through preoperative biopsy, and moderate to severe COPD was defined when the FEV1/FVC < 70%, −2.51 < Z-value <−4.00 is moderate, and Z-value < −4.10 is severe. there were no statistically significant differences between the two groups in terms of gender, age, body mass index, surgical selection, pathological type, and TNM staging (p > 0.05), as shown in Table 1. This study was approved via the Ethics Committee of our hospital.
Inclusion and exclusion criteria
Inclusion criteria: (1) Diagnosed with COPD complicated with lung cancer through pathological examination; (2) The patient's clinical data is complete; (3) The patient has no mental cognitive impairment; (4) All patients have signed informed consent.
Exclusion criteria: (1) The patient has malignant tumors in other parts of the body; (2) Patient's tumor has distant metastasis; (3) Severe impairment of liver, liver, and kidney function; (4) The patient has a mental illness.
Treatment method
The control group was treated with nutritional intervention: Dietitian provide nutritional guidance and dietary matching to each patient, and calculate the patient's daily total energy and intake of various nutrients based on the dietary survey results. The patient's daily energy and protein intake should reach 70% of the target value, with a total energy requirement of 25–30 kcal·kg−1·d−1 and a protein requirement of 1.5–2.0 g·kg−1·d−1. The weight is calculated based on the ideal weight: ideal weight (kg) = height (cm) −105. If the patient's independent diet is insufficient, oral enteral nutrition supplement (ONS) can be given. Provide ONS nutrition related health education 1d before surgery, and provide personalized guidance according to the patient's chosen surgical sequence. Start taking ONS orally 10 h before surgery, 200–300 ml each time, 2–4 times in total, and stop taking it 2 h before surgery. If the ONS is not well executed and the dietary survey shows that the dietary intake for 7 consecutive days does not reach 60% of the energy intake standard, the clinical physician and nutritionist will discuss the nutritional support plan, and combine the patient's wishes. Decide whether to provide enteral nutrition or parenteral nutrition support through tube feeding. The observation group was treated with lung rehabilitation training + nutritional intervention on the basis of the control group. The specific treatment is as follows: (1) Breathing training: Keep the patient naturally relaxed, take a slow deep breath and hold it for about 5 s. At the end of the deep breath, slowly exhale through the mouth and inwardly contract the abdomen. Train 2–3 times a day for about 15 min each time. (2) Coughing and expectoration training: While taking deep breaths, cross your hands in front of your chest and exhale continuously in large mouthfuls. When the phlegm accumulates in your throat, cough it up vigorously and gently tap the patient's back if necessary to help expel the phlegm. (3) Respiratory gymnastics training: Guide patients to perform appropriate limb training on the basis of deep breathing, including abduction and chest expansion of both arms, lifting during inhalation, etc. At the same time, select aerobic training with appropriate intensity for patients, such as walking, skipping rope, etc., adjust according to the patient's physical condition, about 10 min each time, and train 2–3 times/d. (4) Stair climbing training: Accompanied by medical staff, exercise by pursed lip breathing and exhaling with force. Depending on the individual's condition, if there is slight wheezing, continue. If there is obvious difficulty breathing, take a short break before continuing, 15–30 min/time, twice a day. (5) Weightlifting training: Patients lift objects weighing 0.5–3 kg above their head and shoulders for 10–15 min each time, twice a day.
Observation indicators
The observation indicators were as follows: (1) Pulmonary function indicators: Before and after 4 weeks of intervention, compare the lung function indicators of the two groups of patients, including forced expiratory volume in one second (FEV1), forced vital capacity (FVC), MVV (Maximum autonomous minute ventilation volume), DLCO (diffusion capacity of the lung for carbon monoxide). (2) 6 min walking distance (6MWD): The six minute walking test was used to evaluate the exercise tolerance of two groups of patients. The test was conducted in a quiet, safe, and well ventilated corridor of 30 m. Before the test, the testing method was demonstrated to the patients, and records were kept. If breathing difficulties, chest pain, or other conditions occurred during the process, the test should be terminated and the cause identified, and symptomatic treatment should be given. (3) The modified dyspnea scale(mMRC): The mMRC scale was used to evaluate respiratory distress in both groups. It classifies respiratory distress into 5 levels (0–4), with higher scores indicating more severe dyspnea. (4) Blood gas indicators: Before and after 4 weeks of intervention, compare the blood gas indicators of two groups of patients, including arterial partial pressure of oxygen (PaO2) and arterial partial pressure of carbon dioxide (PaCO2), the above indicators were detected using ABL800FLEX blood gas analyzer. (5) Quality of life: The European Organization for the Treatment of Cancer Quality of Life Scale (EORTC QLQ-C30) was used to evaluate the quality of life of patients, including functional areas, general health, and symptom areas. The higher the score, the better the quality of life. (6) Incidence of complications: Compared the incidence of postoperative complications between two groups of patients, including pulmonary infection, dyspnea, atelectasis, and pulmonary leakage.
Statistical processing
Data were analyzed using SPSS 25.0 software. Measurement data were expressed as mean ± standard deviation (x ± s), and inter-group comparisons were conducted using the t-test. Counting data were presented as rate, and inter-group comparisons were performed using the χ2 test. P < 0.05 was considered statistically significant.
Results
Multidimensional outcome indicators of the comprehensive efficacy of intervention measures
Prior to intervention, there were no significant differences between the two groups in 6 min walk distance (6MWD), mMRC dyspnea scores, blood gas parameters (PaO2, PaCO2), or quality of life scores (all p > 0.05), indicating comparable baseline characteristics. Post-intervention, both groups showed significant improvements in the aforementioned indicators compared to pre-intervention (all p < 0.01). However, intergroup comparisons revealed that the observation group demonstrated significantly greater improvement than the control group across all measures. Specifically: the observation group demonstrated significantly longer 6MWD, significantly lower mMRC scores, higher PaO2 levels, lower PaCO2 levels, and significantly higher scores across all four quality-of-life dimensions (physical, emotional, social, and cognitive) compared to the control group (all p < 0.01). Detailed data are presented in Table 2.
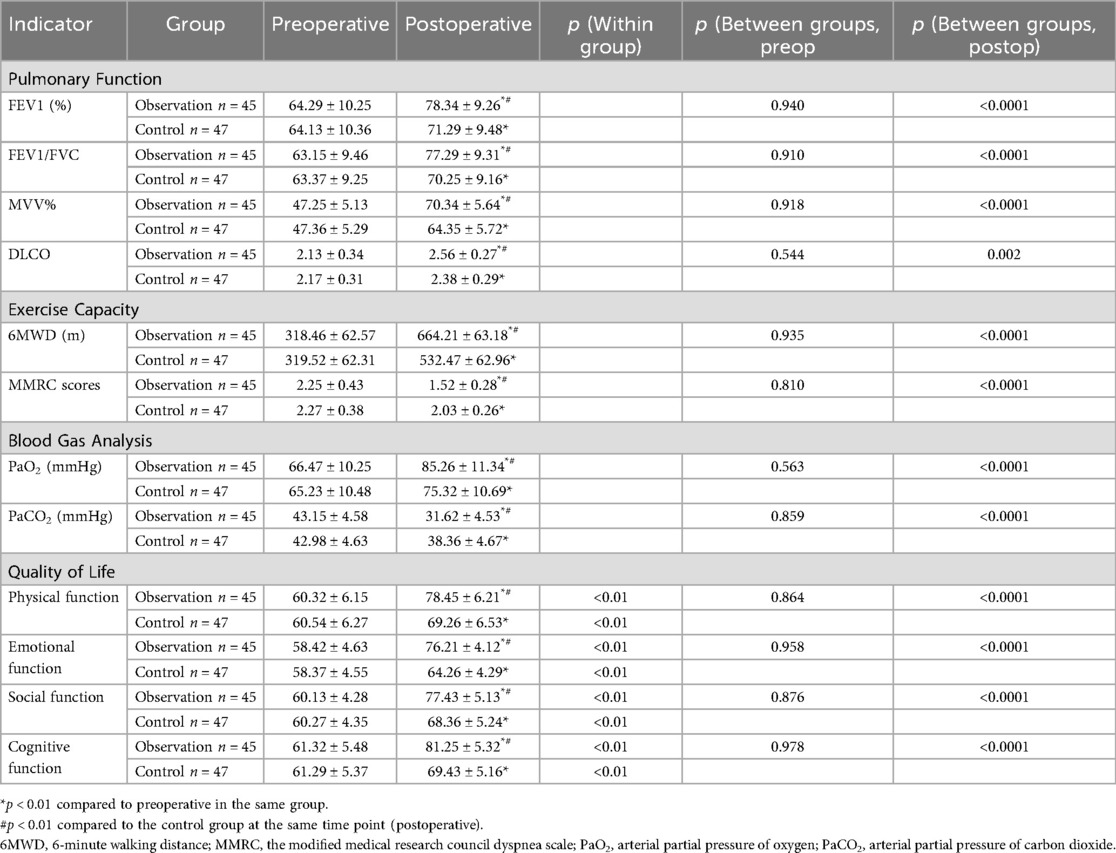
Table 2. Comparison of preoperative and postoperative pulmonary function, exercise capacity, blood gas indicators and quality of life between the two groups of patients .
Incidence of complications
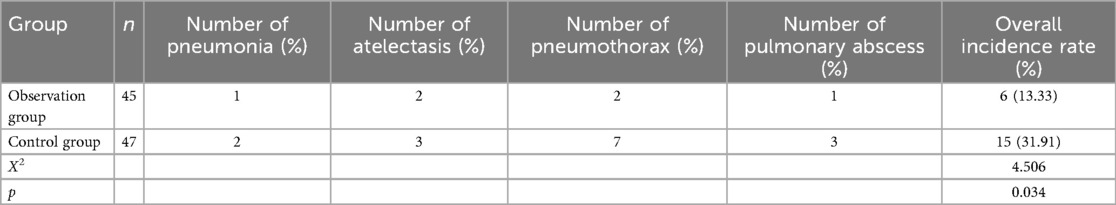
After intervention, The incidence of complications in the observation group (13.33%) was lower than that in the control group (31.91%, p < 0.05), as shown in Table 3.
Discussion
Lung cancer is one of the cancers with a high mortality rate worldwide, and its causes are mainly related to smoking, occupational exposure, environmental pollution, genetics, and other factors (16, 17). Research has shown that the incidence of lung cancer complicated with COPD is 10.8%, and lung cancer is the main cause of death for COPD patients, with approximately 4%–33% of COPD patients dying from lung cancer (18, 19). Thus, with the trend of population aging and the impact of environmental pollution, the number of lung cancer patients with COPD is increasing year by year (20). Video assisted thoracoscopy (VATS) is a minimally invasive surgery that can effectively remove tumor lesions (21). Compared with traditional surgery, it has the characteristics of less trauma, faster postoperative recovery, and lower complications (22). It is an ideal surgical method for COPD combined with lung cancer (23). However, due to the decrease in lung capacity and ventilation, the oxygen utilization rate decreases, and the ability of the lungs to clear secretions decreases, resulting in an increase in the viscosity of respiratory secretions, which seriously affects the patient's recovery and reduces their quality of life (24, 25). Therefore, it is particularly important to take effective intervention measures for COPD combined with lung cancer patients to improve their lung function and enhance their quality of life.
Early nutritional intervention can provide patients with the necessary nutrients for survival, maintain a state of metabolic balance, and improve their nutritional indicators (26). However, nutritional interventions lack personalization and specificity, making it difficult to achieve ideal improvement effects on patients (27). Preoperative pulmonary rehabilitation training is an intervention measure aimed at patients with respiratory system diseases (28). Through training of limb skeletal muscles, lung capacity, and respiratory muscles, it alleviates patients' respiratory distress symptoms and improves the recovery of limb function (29). It is a comprehensive and scientific non pharmacological intervention method (30). A study has found that preoperative lung rehabilitation training could improve lung function and prolong survival time in lung cancer patients (31). Howbeit, there is limited research on the effects of preoperative lung rehabilitation training combined with nutritional intervention on COPD complicated with lung cancer.
Our study discovered that after 4 weeks of intervention, the FEV1/FVC, MVV%, and DLCO in observation group were significantly higher than those in the control group. Additionally, the level of PaO2 in observation group was higher than control group, while the level of PaCO2 in observation group was lower than control group, which indicated that preoperative lung rehabilitation training combined with nutritional intervention could help improve active respiratory function after COPD combined with lung cancer surgery. By increasing respiratory muscle exercise, alveolar ventilation function could be improved, thereby increasing lung capacity and improving lung function, which was consistent with the results of Lai et al. (32).
6MWD and MMRC as important indicators for lung cancer rehabilitation assessment (33). The longer the 6MWD, the higher the degree of lung rehabilitation. The higher the MMRC score, the more severe the breathing difficulties (34). Divisi et al. (35) found preoperative respiratory training could improve 6MWD in lung cancer patients and reduce postoperative complications, which was consistent with our results. After 4 weeks of intervention, the 6MWD was higher in observation group than in control group, while the MMRC scores in observation group was lower than control group. There were two main reasons. Firstly, Preoperative pulmonary rehabilitation training combined with nutritional intervention could effectively improve ventilation volume, increase patient surgical tolerance, eliminate residual gas, and reduce dead space ventilation (36). Secondly, this intervention method was able to improve respiratory muscle strength and exercise tolerance, thereby reducing postoperative respiratory distress and alleviating (37). What's more, the EORTC QLQ-C30 scores in all dimensions of the observation group were higher than control group, while the incidence of adverse events was lower than control group, which implied that preoperative lung rehabilitation training plus nutritional intervention could promote sufficient lung expansion, further clear lung cancer airway secretions and sputum, accelerate patient recovery, and lessen the occurrence of complications. Laurent et al. (38) also discovered the same result.
Yet, this study has several limitations that need to be acknowledged. First, as a single-center study, the sample may lack representativeness, and results may not be generalized to other populations. Second, the follow-up duration was short (4 weeks post-intervention), and we did not assess long-term outcomes such as progression-free survival (PFS) and overall survival (OS)—key indicators for evaluating the intervention's impact on patient prognosis. Third, although block randomization balanced baseline characteristics (e.g., gender, age, BMI), potential confounding factors were not fully adjusted for: (1) Baseline exercise tolerance (a key predictor of pulmonary rehabilitation response) was not measured; (2) Tumor staging was simplified to I/II (no sub-staging, e.g., T1a/T1b), which may correlate with surgical difficulty; (3) Surgical details (e.g., extent of resection) were not recorded. Fourth, the small sample size (92 patients) reduces statistical power to detect subtle between-group differences. In the future, multi-center studies with larger samples, extended follow-up (to assess PFS/OS), and adjustment for confounding factors (e.g., baseline exercise tolerance) are needed to validate our findings.
All in all, preoperative pulmonary rehabilitation training plus nutritional intervention was able to ameliorate lung function, enhance surgical tolerance, improve patients' quality of life, and reduce the occurrence of complications in COPD combined with lung cancer.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YH: Writing – original draft, Data curation, Conceptualization, Methodology. QC: Conceptualization, Methodology, Writing – original draft, Data curation. QG: Supervision, Writing – review & editing, Project administration.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bade BC, Cruz D, S C. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. (2020) 41(1):1–24. doi: 10.1016/j.ccm.2019.10.001
2. Li Y, Wu X, Yang P, Jiang G, Luo Y. Machine learning for lung cancer diagnosis, treatment, and prognosis. Genom Proteom Bioinform. (2022) 20(5):850–66. doi: 10.1016/j.gpb.2022.11.003
3. Abu Rous F, Singhi EK, Sridhar A, Faisal MS, Desai A. Lung cancer treatment advances in 2022. Cancer Invest. (2023) 41(1):12–24. doi: 10.1080/07357907.2022.2119479
4. Zhang D, Liu H, Zhao F, Guo P, Li J, Lu T, et al. Exploring the relationship between Treg-mediated risk in COPD and lung cancer through Mendelian randomization analysis and scRNA-seq data integration. BMC Cancer. (2024) 24(1):453. doi: 10.1186/s12885-024-12076-1
5. Young RP, Hopkins RJ. Chronic obstructive pulmonary disease (COPD) and lung cancer screening. Transl Lung Cancer Res. (2018) 7(3):347–60. doi: 10.21037/tlcr.2018.05.04
6. Kwak N, Park C-M, Lee J, Park YS, Lee S-M, Yim J-J, et al. Lung cancer risk among patients with combined pulmonary fibrosis and emphysema. Respir Med. (2014) 108(3):524–30. doi: 10.1016/j.rmed.2013.11.013
7. Ma D, Song X, Li S, Liu H, Cui Y, Huang C, et al. Video-assisted thoracoscopic surgery lobectomy performed satisfaction and complications of patients during hands-on training courses. J Laparoendosc Adv Surg Tech A. (2018) 28(7):804–10. doi: 10.1089/lap.2017.0661
8. Medbery RL, Perez SD, Force SD, Gillespie TW, Pickens A, Miller DL, et al. Video-assisted thoracic surgery lobectomy cost variability: implications for a bundled payment era. Ann Thorac Surg. (2014) 97(5):1686–92; discussion 1692–3. doi: 10.1016/j.athoracsur.2014.01.021
9. Fischel RJ, McKenna RJ Jr. Video-assisted thoracic surgery for lung volume reduction surgery. Chest Surg Clin N Am. (1998) 8(4):789–807, viii. doi: 10.1016/S1052-3359(25)00631-3
10. Beckers F, Lange N, Koryllos A, Picchioni F, Windisch W, Stoelben E. Unilateral lobe resection by video-assisted thoracoscopy leads to the most optimal functional improvement in severe emphysema. Thorac Cardiovasc Surg. (2016) 64(4):336–42. doi: 10.1055/s-0034-1395989
11. Gao X, Qi J, Du B, Weng X, Lai J, Wu R. Combined influence of nutritional and inflammatory status and breast cancer: findings from the NHANES. BMC Public Health. (2024) 24(1):2245. doi: 10.1186/s12889-024-19727-9
12. Uster A, Ruehlin M, Mey S, Gisi D, Knols R, Imoberdorf R, et al. Effects of nutrition and physical exercise intervention in palliative cancer patients: a randomized controlled trial. Clin Nutr. (2018) 37(4):1202–9. doi: 10.1016/j.clnu.2017.05.027
13. Pu CY, Batarseh H, Zafron ML, Mador MJ, Yendamuri S, Ray AD. Effects of preoperative breathing exercise on postoperative outcomes for patients with lung cancer undergoing curative intent lung resection: a meta-analysis. Arch Phys Med Rehabil. (2021) 102(12):2416–27.e4. doi: 10.1016/j.apmr.2021.03.028
14. Guo Y, Pan M, Xiong M, Liu M, Zhu N, Dong H, et al. Efficacy of preoperative pulmonary rehabilitation in lung cancer patients: a systematic review and meta-analysis of randomized controlled trials. Discov Oncol. (2025) 16(1):56. doi: 10.1007/s12672-025-01774-2
15. Wade-Mcbane K, King A, Urch C, Jeyasingh-Jacob J, Milne A, Boutillier CL. Prehabilitation in the lung cancer pathway: a scoping review. BMC Cancer. (2023) 23(1):747. doi: 10.1186/s12885-023-11254-x
16. Tewari KS, Monk BJ, Vergote I, Miller A, de Melo AC, Kim H-S, et al. Survival with cemiplimab in recurrent cervical cancer. N Engl J Med. (2022) 386(6):544–55. doi: 10.1056/NEJMoa2112187
17. Buchanan J, Shatila M, Menon A, Patel AJ. Small cell lung carcinoma presenting initially with recurrent pneumothoraces: a case report. J Cardiothorac Surg. (2024) 19(1):347. doi: 10.1186/s13019-024-02857-x
18. Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer. (2013) 13(4):233–45. doi: 10.1038/nrc3477
19. Boudoussier A, Larrouture I, Henrot P, Veillon R, Bardel C, Chautemps C, et al. COPD Patients with non-small cell lung cancer respond better to anti-PD-(L)1 immune checkpoint inhibitors. Sci Rep. (2025) 15(1):17145. doi: 10.1038/s41598-025-02251-0
20. Palma D, Lagerwaard F, Rodrigues G, Haasbeek C, Senan S. Curative treatment of Stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys. (2012) 82(3):1149–56. doi: 10.1016/j.ijrobp.2011.03.005
21. White A, Swanson SJ. Video-assisted thoracic surgery (VATS) segmentectomy: state of the art. Minerva Chir. (2016) 71(1):61–6. PMID: 26544908.26544908
22. Frasca L, Tacchi G, Longo F, Marziali V, De Peppo V, Moscardelli A, et al. Uniportal video-assisted thoracic surgery approach for simultaneous lung cancer and thymic carcinoma: case report and literature review. Thorac Cancer. (2022) 13(3):489–93. doi: 10.1111/1759-7714.14258
23. Halezeroğlu S. Single incision video-assisted thoracic surgery pneumonectomy for centrally located lung cancer. Future Oncol. (2018) 14(6s):41–5. doi: 10.2217/fon-2017-0422
24. Taioli E, Lee DS, Lesser M, Flores R. Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: a meta-analysis. Eur J Cardiothorac Surg. (2013) 44(4):591–7. doi: 10.1093/ejcts/ezt051
25. Bertolaccini L, Prisciandaro E, Uslenghi C, Chiari M, Cara A, Mazzella A, et al. The role of the surgical volume for clinical outcomes in VATS lobectomy for lung cancer: a national large database multicenter analysis. Updates Surg. (2024) 76(4):1475–82. doi: 10.1007/s13304-023-01723-0
26. Asakawa A, Ishibashi H, Matsuyama Y, Fujiwara T, Kobayashi M, Okubo K. Preoperative nutritional status is associated with the prognosis for lung cancer. Asian Cardiovasc Thorac Ann. (2021) 29(8):763–71. doi: 10.1177/02184923211014002
27. Templeton R, Greenhalgh D. Preoperative rehabilitation for thoracic surgery. Curr Opin Anaesthesiol. (2019) 32(1):23–8. doi: 10.1097/ACO.0000000000000668
28. Zhou T, Sun C. Effect of physical manipulation pulmonary rehabilitation on lung cancer patients after thoracoscopic lobectomy. Thorac Cancer. (2022) 13(3):308–15. doi: 10.1111/1759-7714.14225
29. Boden I, Skinner EH, Browning L, Reeve J, Anderson L, Hill C, et al. Preoperative physiotherapy for the prevention of respiratory complications after upper abdominal surgery: pragmatic, double blinded, multicentre randomised controlled trial. Br Med J. (2018) 360:j5916. doi: 10.1136/bmj.j5916
30. Benzo R, Wigle D, Novotny P, Wetzstein M, Nichols F, Shen RK, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. (2011) 74(3):441–5. doi: 10.1016/j.lungcan.2011.05.011
31. Okura K, Takahashi Y, Hatakeyama K, Saito K, Kasukawa Y, Imai K, et al. Preoperative inspiratory muscle training in a patient with lung cancer and comorbid chronic obstructive pulmonary disease and respiratory sarcopenia: a case report. Physiother Res Int. (2023) 28(2):e1987. doi: 10.1002/pri.1987
32. Lai Y, Su J, Yang M, Zhou K, Che G. Impact and effect of preoperative short-term pulmonary rehabilitation training on lung cancer patients with mild to moderate chronic obstructive pulmonary disease: a randomized trial. Zhongguo Fei Ai Za Zhi. (2016) 19(11):746–53. Chinese. doi: 10.3779/j.issn.1009-3419.2016.11.05
33. Ichikawa T, Yokoba M, Horimizu Y, Yamaguchi S, Kawakami A, Oikawa S, et al. Recovery of respiratory muscle strength, physical function, and dyspnoea after lobectomy in lung cancer patients undergoing pulmonary rehabilitation: a retrospective study. Eur J Cancer Care (Engl). (2022) 31(6):e13663. doi: 10.1111/ecc.13663
34. Ji W, Kwon H, Lee S, Kim S, Hong JS, Park YR, et al. Mobile health management platform-based pulmonary rehabilitation for patients with non-small cell lung cancer: prospective clinical trial. JMIR Mhealth Uhealth. (2019) 7(6):e12645. doi: 10.2196/12645
35. Divisi D, Di Francesco C, Di Leonardo G, Crisci R. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg. (2013) 43(2):293–6. doi: 10.1093/ejcts/ezs257
36. Wang T, Li F, Wang X, Sang T, Wang M, Ma X, et al. Evaluation of recovery efficacy of inspiratory muscle training after lobectomy based on computed tomography 3D reconstruction. Respir Care. (2023) 69(1):42–9. doi: 10.4187/respcare.11037
37. Granger CL, McDonald CF, Berney S, Chao C, Denehy L. Exercise intervention to improve exercise capacity and health related quality of life for patients with non-small cell lung cancer: a systematic review. Lung Cancer. (2011) 72(2):139–53. doi: 10.1016/j.lungcan.2011.01.006
38. Laurent H, Aubreton S, Galvaing G, Pereira B, Merle P, Richard R, et al. Preoperative respiratory muscle endurance training improves ventilatory capacity and prevents pulmonary postoperative complications after lung surgery. Eur J Phys Rehabil Med. (2020) 56(1):73–81. doi: 10.23736/S1973-9087.19.05781-2
Keywords: nutritional intervention, preoperative lung rehabilitation training, COPD complicated with lung cancer, pulmonary function, surgical tolerance
Citation: Hu Y, Cheng Q and Guo Q (2025) The role of preoperative lung rehabilitation training combined with nutritional intervention on surgical tolerance and accelerated recovery indicators in patients with moderate to severe COPD complicated with lung cancer. Front. Surg. 12:1667085. doi: 10.3389/fsurg.2025.1667085
Received: 16 July 2025; Accepted: 29 October 2025;
Published: 19 November 2025.
Edited by:
Marco Scarci, Hammersmith Hospital, United KingdomReviewed by:
Xu-Heng Chiang, National Taiwan University Hospital, TaiwanDavide Piloni, University of Pavia, Italy
Copyright: © 2025 Hu, Cheng and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiucheng Guo, Z2NfMDY1MUAxNjMuY29t
†These authors have contributed equally to this work
 Yan Hu†
Yan Hu† Qiucheng Guo
Qiucheng Guo