- The Fifth Clinical Medical College of Henan University of Chinese Medicine (Zhengzhou People’s Hospital), Zhengzhou, Henan, China
Background: Accurate prediction of post-reduction mammoplasty drainage volume is critical for optimizing postoperative care and reducing complication risks in patients with macromastia. This study aimed to identify key predictors of total postoperative drainage volume. We further investigated whether these predictors demonstrate consistent effects across diverse populations or subgroups, thereby providing evidence to support personalized management of postoperative drainage.
Methods: Clinical data from 69 macromastia patients were analyzed, including preoperative and postoperative variables such as body mass index (BMI), neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and postoperative differential blood cell counts (e.g., postoperative neutrophils, lymphocytes, and monocytes). Data were summarized using descriptive statistics. Variables significantly associated with total drainage volume were screened via Spearman's correlation analysis. Univariate and multivariate regression analyses were subsequently performed to identify independent predictors. Additionally, stratified subgroup analyses based on BMI and age were conducted to assess the consistency of predictor effects.
Results: Univariate and correlation analyses revealed significant positive associations between total drainage volume and both BMI (Spearman's ρ = 0.564, P < 0.0001) and postoperative NLR (Spearman's ρ = 0.506, P < 0.0001). Multivariate regression confirmed BMI (P < 0.001) and postoperative NLR (P = 0.033) as independent and significant predictors of postoperative drainage volume. Furthermore, stratified analyses demonstrated consistent predictive effects for BMI and postoperative NLR across all BMI and age subgroups (P < 0.05), with no significant heterogeneity observed.
Conclusion: This study identifies BMI and postoperative NLR as independent predictors of total postoperative drainage volume, highlighting their clinical utility. The consistent predictive performance of these factors across BMI and age subgroups supports their broad applicability. These findings provide evidence-based support for personalized drainage management strategies and offer critical insights for clinical practice.
Introduction
Macromastia is a common breast developmental disorder characterized by excessive breast enlargement that causes physical and psychological symptoms (e.g., dorsal/neck pain, skin irritation). The diagnostic criteria for macromastia in this study were based on clinical manifestations (symptoms caused by breast weight) and imaging evaluation (breast volume exceeding 800 mL or glandular tissue resection weight ≥200 g per side). This condition frequently causes physical and psychological discomfort, including dorsal/neck pain, skin irritation, and psychosocial distress. While its etiology remains incompletely elucidated, genetic factors, endocrine dysregulation, and obesity are implicated contributors (1–4).
Surgical intervention is the only definitive treatment to alleviate symptoms and significantly improve both physical complaints (e.g., shoulder/back pain, headaches) and psychosocial well-being (5–7). Reduction mammoplasty involves resection of excess glandular tissue, fat, and skin to achieve proportionate breast size and contour. Common techniques include: Free nipple-areola complex (NAC) grafting for severe ptosis or macromastia, though it may compromise aesthetic outcomes (8); Superomedial pedicle technique to correct NAC displacement in high-BMI patients (9); Inferior pedicle technique, employed in 80.3% of cases with favorable aesthetic ratings (>78.4% achieving “good” or better outcomes) (10).
Postoperative care, particularly wound and drain management, remains critical. Controversy persists regarding routine drain placement following reduction mammoplasty. Some studies argue against routine drainage; a comparative analysis of 50 bilateral reduction mammoplasty patients reported lower complication rates without drains than with historical drained controls, suggesting re-evaluation of this practice (11). Similar conclusions exist in other surgical fields, such as anterior cervical surgery (12). Conversely, divergent findings support drain use in specific scenarios: A prospective study of 111 bilateral reduction mammoplasty patients found superior pedicle techniques significantly correlated with increased drainage needs, advocating drains to prevent seroma, infection, and wound breakdown in such cases (13). A large cohort analysis (n = 1,442) demonstrated that early drain removal coupled with weight control measures effectively reduced complications (14).
Given that reduction mammoplasty is pivotal for symptom relief and quality-of-life improvement in macromastia, optimized drainage management directly impacts wound healing and mitigates complications (e.g., seroma, flap necrosis). This study analyzed 69 reduction mammoplasty patients through integrated statistical approaches to investigate associations between drainage volume and anatomic (resection weight), metabolic (BMI), inflammatory (NLR, LMR), and operative (duration) parameters. These findings aim to establish an evidence-based algorithm for individualized drain removal decisions.
Materials and methods
Data collection
Clinical data were collected from 69 patients with macromastia undergoing medial pedicle vertical breast reduction surgery between January 2023 and March 2025. The surgical procedure included: (1) marking the medial pedicle (width 4–5 cm, length 8–10 cm) to preserve nipple-areola complex (NAC) blood supply; (2) resecting excess glandular tissue and skin; (3) shaping the breast and suturing in layers. The average operative time (excluding preoperative preparation and postoperative arrangement) was 4.5 h. All patients received closed-incision negative pressure therapy (ciNPT) postoperatively. Variables included: (1) demographic and comorbidity data (age, gender, body weight, diabetes, hypertension); (2) perioperative factors (operative duration, intraoperative blood loss, use of hemostatic drugs); (3) laboratory indices (preoperative and postoperative BMI, complete blood counts including neutrophils, lymphocytes, monocytes, platelets, NLR, LMR); (4) surgical outcomes (total postoperative drainage volume, resection weight). Total drainage volume was defined as the sum of bilateral drainage outputs; unilateral data were used if contralateral data were missing. All data underwent structured curation to ensure completeness and accuracy.
Data preprocessing
Clinical records of all macromastia patients were structurally organized during preprocessing. Normality of total drainage volume was assessed using Shapiro–Wilk test; nonparametric statistical methods (Spearman correlation) were consequently selected for analysis. Data collection aligned with medical records and complied with Ethics Committee-approved standard operating procedures.
Correlation analysis
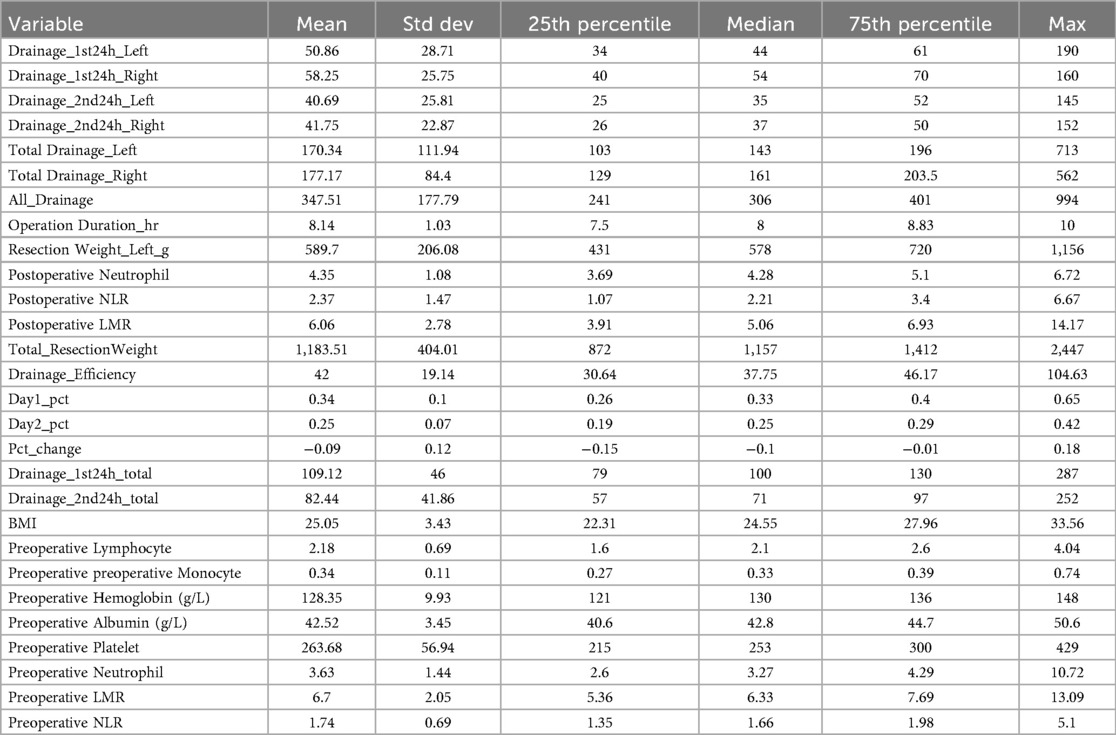
Spearman correlation was applied to evaluate associations between variables and total postoperative drainage volume. This nonparametric method assesses monotonic relationships. Correlation coefficients (Spearman ρ) and corresponding P-values were calculated to determine significance. Variables with P < 0.1 were selected for further analysis. Descriptive statistics are presented in Table 1.
Univariate analysis
Univariate analysis using Spearman correlation tested relationships between continuous variables and total drainage volume. Variables with P < 0.1 were selected for multivariate regression. This identified variables exhibiting significant linear/monotonic relationships with drainage volume when considered individually, providing candidate variables for multivariate modeling.
Multivariate regression analysis
Multivariate regression employed backward stepwise elimination, sequentially removing variables with P ≥ 0.05 to identify significant independent predictors of total drainage volume. All continuous variables were standardized via Z-score before analysis. During model optimization, variable retention was strictly based on statistical significance. Final results provided regression coefficients for independent predictors and their clinical interpretations.
Stratified analysis
Stratified analysis evaluated the predictive effects of BMI and postoperative NLR across subgroups. Stratification was performed by BMI and age:
BMI subgroups
BMI < 25 and BMI ≥ 25 (assessing differences in BMI/postoperative NLR predictive effects between low-weight and overweight/obese individuals).
Age subgroups
<30 years and ≥30 years (testing predictor consistency across age groups).
Within each subgroup, linear regression models analyzed independent predictive effects of BMI and postoperative NLR on total drainage volume, with significance of regression coefficients tested.
Results
Correlation analysis
Total drainage volume exhibited a right-skewed distribution, with Shapiro–Wilk normality testing confirming significant deviation from normality (p < 0.05). Drainage ranges were 62.5–713 ml (left) and 64–562 ml (right), with a median volume of 193.5 ml. Bilateral drainage analysis revealed weak positive correlations between left and right total drainage volumes (r = 0.42, p = 0.0012). Correlation coefficients for postoperative drainage were r = 0.37 (p = 0.0067) at 0–24 h and r = 0.29 (p = 0.043) at 24–48 h. Right drainage volumes were significantly higher than left (p < 0.05).
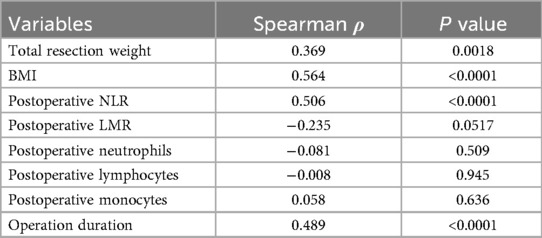
Spearman correlation analysis (Table 2) demonstrated significant positive associations between total drainage volume and both BMI (ρ = 0.564, p < 0.001) and postoperative NLR (ρ = 0.506, p < 0.001). Postoperative LMR showed a negative correlation (ρ = −0.235, p = 0.0517). No significant correlations were observed between drainage volume and postoperative neutrophils, lymphocytes, or monocytes (all p > 0.05). Consequently, BMI and postoperative NLR were selected as key variables for subsequent analyses.
Univariate analysis
Univariate analysis using Spearman correlation identified variables significantly associated with total drainage volume (Table 3). BMI and postoperative NLR demonstrated the strongest correlations (P < 0.0001). Total resection weight and operative duration also showed significant correlations with total drainage volume (P < 0.05). Based on these results, BMI and postoperative NLR were selected as key predictors for inclusion in multivariate regression analysis.
Multivariate regression analysis
In multivariate regression analysis using backward stepwise elimination, BMI and postoperative NLR were retained as independent significant predictors (Table 4). Regression results demonstrated a significant positive effect of BMI on total drainage volume (regression coefficient: 95.81, P < 0.001), indicating that for each unit increase in BMI, postoperative drainage increased by approximately 95.8 ml. Postoperative NLR also showed a significant positive effect (regression coefficient: 37.87, P = 0.033), confirming that higher NLR values correlate with greater drainage output. The model's R2 = 0.370 explained approximately 37% of the variability in total drainage volume. Other variables, including postoperative LMR and monocytes, were eliminated and did not achieve significance as independent predictors.
Stratified analysis
Stratified analyses by BMI and age groups consistently identified BMI and postoperative NLR as independent significant predictors across all subgroups.
BMI stratification
In both BMI < 25 and BMI ≥ 25 groups, BMI exhibited significant positive effects on drainage volume (regression coefficient: 95.81 ml, P < 0.001 for both), indicating a 95.8 ml drainage increase per BMI unit. Postoperative NLR remained significant in both groups (regression coefficient: 37.87 ml, P = 0.033).
Age stratification
For both <34 years and ≥34 years groups, BMI and postoperative NLR were independent significant predictors, with consistent regression coefficients and P-values (P < 0.05).
These results demonstrate consistent predictive effects of BMI and postoperative NLR on postoperative drainage volume across all BMI and age subgroups, supporting their broad applicability.
Discussion
This study systematically analyzed clinical data from 69 patients undergoing reduction mammoplasty for macromastia, revealing complex interrelationships between postoperative drainage volume and anatomical reconstruction, metabolic inflammation, and surgical parameters.
Our results demonstrate that BMI, postoperative NLR, resection weight, body weight, and operative duration are significant predictors of drainage volume. Patients with BMI ≥25 kg/m2 exhibited significantly higher median drainage volumes than normal-weight counterparts (p < 0.05). These findings align with prior studies linking breast resection weight and BMI to drain-dependent complications such as seroma and infection (14, 15). Consequently, meticulous drain management is essential for macromastia patients to prevent drainage insufficiency leading to these complications.
The inclusion of postoperative neutrophil, lymphocyte, monocyte counts, and their ratios (NLR, LMR) was based on the core hypothesis that systemic inflammatory-immune imbalance regulates postoperative exudation. This hypothesis is supported by the distinct and coordinated roles of each cell type in modulating inflammation and exudation: Neutrophils promote early inflammatory exudation by releasing pro-inflammatory cytokines (e.g., IL-6, TNF-α) and increasing vascular permeability—consistent with findings in ankylosing spondylitis (AS) patients undergoing total hip replacement (THA), where elevated preoperative neutrophil levels correlated with increased 24 h postoperative drainage, attributed to cytokine-mediated vascular hyperpermeability (16); similar effects were observed in Fontan surgery patients, where neutrophil-driven pro-inflammatory cytokines (IL-8, TNF-α) in pleural drainage fluid were linked to prolonged drainage duration (17). Lymphocytes maintain immune homeostasis to prevent excessive inflammation—disruption of this function, as seen in total knee replacement patients with postoperative lymphocyte apoptosis and reduced T/B cell counts, leads to uncontrolled inflammation and prolonged drainage (18); ovarian cancer postoperative drainage fluid also induces neutrophil-mediated T-cell immunoparalysis, further confirming that impaired lymphocyte homeostasis exacerbates exudation (19). Monocytes participate in tissue repair and phagocytosis of necrotic tissue—their regulatory role is evident in diabetic wound models, where reduced monocyte chemoattractant protein-1 (MCP-1) (a monocyte-derived cytokine) via combined negative-pressure wound therapy (NPWT) and autologous fat transplantation (AFT) accelerated wound healing and reduced exudation (20); abnormal monocyte activation in Fontan patients, however, contributed to pro-inflammatory cytokine imbalance and increased drainage (17).
However, single cell counts are easily affected by physiological fluctuations (e.g., surgical stress, dehydration), while ratios (NLR, LMR) integrate multiple inflammatory signals to reflect the ‘inflammation-immunity imbalance’ more accurately. For instance, in oral and maxillofacial space infection patients, single neutrophil/lymphocyte counts showed no correlation with drainage needs, but NLR (combining neutrophil elevation and lymphocyte reduction) strongly predicted surgical drainage requirements (21); similarly, pancreatic ductal adenocarcinoma (PDAC) patients demonstrated that MLR (not single monocyte counts) correlated with postoperative complications and drainage duration, as ratios mitigate the impact of physiological variability (22). In this study, individual postoperative neutrophil, lymphocyte, or monocyte counts showed no significant correlation with drainage volume, whereas NLR (combining elevated neutrophils and relatively decreased lymphocytes) exhibited a strong positive correlation—aligning with observations in pediatric laparoscopic appendectomy patients, where NLR (not single cell counts) correlated with postoperative length of stay (an indirect marker of drainage volume) (23), and in cholangiocarcinoma patients, where NLR ≥3.4 independently predicted drainage-related infectious complications (24). This confirms that NLR is a more robust marker of systemic inflammation than single cell counts in predicting postoperative drainage.
The association between NLR and postoperative drainage/infection has been established across multiple surgical disciplines (25–28). Our study uniquely identifies postoperative NLR as an independent predictor of drainage volume in reduction mammoplasty (p < 0.0001). Though postoperative neutrophil and lymphocyte counts showed only marginal individual increases (clinically often overlooked), their ratio (postoperative NLR) demonstrated robust correlation with drainage output. This elevates NLR as a critical warning indicator for surgeons.
Mechanistically, elevated NLR reflects systemic inflammation that may increase exudate production through enhanced vascular permeability and tissue edema. While no prior studies directly link NLR to drainage in reduction mammoplasty, analogous evidence exists: post-cardiac surgery patients with high NLR require prolonged mechanical ventilation and ICU stays (29), and elevated NLR correlates with increased D-dimer levels suggesting hypercoagulability that may impede drainage (30). Furthermore, NLR significantly correlates with prolonged hospitalization and extended ICU stays across surgical specialties, potentially through inflammation-mediated vascular changes, hypercoagulability, and infection risk (31). Thus, NLR monitoring facilitates early identification of patients needing intensive drain management. Regarding NLR threshold and clinical applicability, the median value of 2.2 of the postoperative neutrophil - to—lymphocyte ratio (NLR) was chosen as the cutoff value to predict high drainage volume (≥ 401.0 mL, defined as the 75th percentile of the total drainage volume in the cohort). The findings revealed a sensitivity of 61.1%, suggesting that this cutoff value can identify over 60% of patients with high drainage volume, indicating a certain level of identification efficacy. Nevertheless, some patients with high drainage volume may still be missed. The specificity was 52.9%, just above 50%, which implies that the accuracy in identifying non—high drainage volume patients is mediocre and may lead to a relatively large number of false positive results. The area under the curve (AUC) was 0.548, slightly higher than the random chance level, suggesting relatively limited predictive performance. Overall, while this cutoff value has some reference value, its reliability is inadequate and necessitates further research with a larger sample size and in—depth exploration for validation.
Notably, right-sided drainage volume was significantly higher than left-sided (P < 0.05), which may be related to the right-handed dominance of the surgeon (leading to slightly more tissue traction on the right side). However, this finding is hypothesis-generating and requires verification in multicenter cohorts with multiple surgeons, so it cannot be used as a routine clinical reference.
Limitations include potential surgeon-technique bias from single-center design and absence of dynamic drain fluid biomarkers (e.g., protein, chylomicrons). Future multicenter studies integrating intraoperative real-time imaging (e.g., indocyanine green lymphography) and metabolomic profiling could elucidate spatiotemporal mechanisms of exudate regulation.
Conclusion
BMI and postoperative NLR are independent significant predictors of total postoperative drainage volume. Their predictive consistency across BMI (<25 vs. ≥25 kg/m2) and age (<34 vs. ≥34 years) subgroups confirms broad clinical applicability. These biomarkers provide objective criteria for personalized drain management strategies in reduction mammoplasty patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
CW: Conceptualization, Writing – original draft, Writing – review & editing. LZ: Data curation, Formal analysis, Writing – original draft. JW: Investigation, Supervision, Writing – review & editing. HL: Investigation, Methodology, Writing – review & editing. YT: Software, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Massey GG, Firriolo JM, Nuzzi LC, Pramanick T, Malloy SM, DiVasta AD, et al. Risk factors associated with severe macromastia among adolescents and young women. Plast Reconstr Surg. (2022) 150(6):1212–8. doi: 10.1097/PRS.0000000000009719
2. Alhindi N, Mortada H, Alzaid W, Al Qurashi AA, Awan B. A systematic literature review of the clinical presentation, management, and outcome of gestational macromastia in the 21st century. Aesthetic Plast Surg. (2023) 47(1):10–29. doi: 10.1007/s00266-022-03003-5
3. Kikuchi DS, Mustin DE, Ghanouni A, Walsh MD. A review of pediatric macromastia etiology and indications for reduction mammaplasty. J Plast Reconstr Aesthet Surg. (2023) 77:209–17. doi: 10.1016/j.bjps.2022.12.003
4. Antoszewski B, Kasielska-Trojan A, Jones TE, Danilewicz M, Jones MW. The immunohistochemical profile of mammary tissue in women with macromastia and its potential clinical implications. Endocrinology. (2024) 165(4):bqae026. doi: 10.1210/endocr/bqae026
5. White AG, McNamara CT, Nuzzi LC, Hwang CD, Labow BI. Reduction mammaplasty in younger patients: an evidence-based approach to treatment. Plast Aesthet Nurs. (2023) 43(4):203–9. doi: 10.1097/PSN.0000000000000521
6. Bragina L, Koehl P, Dietrich M, Schuh A. Verbessern brustverkleinernde operationen nackenschmerzen und die lebensqualität? [does reduction mammoplasty improve neck pain and quality of life?]. Schmerz. (2023) 37(2):134–40. doi: 10.1007/s00482-022-00635-z
7. Can B. Frequency of headaches in macromastia patients and relief after reduction mammoplasty. Aesthetic Surg J. (2021) 41(6):NP322–6. doi: 10.1093/asj/sjaa330
8. Selamioğlu E, Agdoğan Ö. Mammoplasty using modified superomedial pedicle technique in severely macromastia and ptotic breasts. Breast J. (2024) 2024:7635485. doi: 10.1155/2024/7635485
9. Hudson DA, Lelala NB. Dealing with the displaced nipple-areola complex in macromastia using a superomedial pedicle and inverted T pattern. Plast Reconstr Surg Glob Open. (2022) 10(2):e4105. doi: 10.1097/GOX.0000000000004105
10. Xia TY, Scomacao I, Duraes E, Cakmakoglu C, Schwarz G. Aesthetic, quality-of-life, and clinical outcomes after Inferior pedicle oncoplastic reduction mammoplasty. Aesthetic Plast Surg. (2023) 47(3):905–11. doi: 10.1007/s00266-023-03257-7
11. Matarasso A, Wallach SG, Rankin M. Reevaluating the need for routine drainage in reduction mammaplasty. Plast Reconstr Surg. (1998) 102(6):1917–21. doi: 10.1097/00006534-199811000-00016
12. Liu Y, Meng Y, Liu H, Shuai WB, Ding C, Wang BY, et al. Routinely placing drainage tube in patients with anterior cervical surgery: is it really necessary? Chin Med J. (2021) 134(5):521–3. doi: 10.1097/CM9.0000000000001253
13. Anzarut A, Edwards DC, Calder K, Guenther CR, Tsuyuki R. Superior pedicle breast reduction techniques increase the risk of postoperative drainage. Ann Plast Surg. (2008) 60(4):367–71. doi: 10.1097/SAP.0b013e31812f7ba7
14. Boccara D, Chaouat M, Mimoun M, Kaplan J, Serror K, Couteau C. Reduction mammoplasties: risk factors and early complications-about 1442 cases. Aesthetic Plast Surg. (2025) 49(1):211–23. doi: 10.1007/s00266-024-04239-z
15. Kuruoglu D, Nguyen MT, Antezana LA, Curiel D, Vijayasekaran A, Martinez-Jorge J, et al. Predictors of seroma after breast reduction: when should drains be considered? J Plast Reconstr Aesthet Surg. (2025) 103:374–9. doi: 10.1016/j.bjps.2025.02.011
16. Liu ZJ, Yu XR, Dong Y, Zhou Q, Zou XS, Weng XS, et al. Anesthesia strategies and perioperative optimization for patients with ankylosing spondylitis undergoing total hip replacement surgery. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. (2016) 38(3):305–11. doi: 10.3881/j.issn.1000-503X.2016.03.011
17. Goldstein SA, Beshish AG, Bush LB, Lowery RE, Wong JH, Schumacher KR, et al. Analysis of inflammatory cytokines in postoperative fontan pleural drainage. Pediatr Cardiol. (2019) 40(4):744–52. doi: 10.1007/s00246-019-02059-6
18. Kosel J, Rusak M, Gołembiewski Ł, Dąbrowska M, Siemiątkowski A. Total knee replacement induces peripheral blood lymphocytes apoptosis and it is not prevented by regional anesthesia—a randomized study. Br J Anesthesiol. (2016) 66(2):133–9. doi: 10.1016/j.bjane.2014.07.008
19. Emmons TR, Giridharan T, Singel KL, Khan ANH, Ricciuti J, Howard K, et al. Mechanisms driving neutrophil-induced T-cell immunoparalysis in ovarian cancer. Cancer Immunol Res. (2021) 9(7):790–810. doi: 10.1158/2326-6066.CIR-20-0922
20. Zhang H, Zhou M, Wang Y, Zhang D, Qi B, Yu A. Role of autologous fat transplantation combined with negative-pressure wound therapy in treating rat diabetic wounds. Plast Reconstr Surg. (2023) 152(3):561–70. doi: 10.1097/PRS.0000000000010226
21. Liu Y, Zhu H, Bao X, Qin Y, He Z, Zheng L, et al. When is surgical intervention needed in oral and maxillofacial space infection patients? A retrospective case control study in 46 patients. BMC oral Health. (2024) 24(1):973. doi: 10.1186/s12903-024-04737-1
22. Jabłońska B, Pawlicki K, Mrowiec S. Associations between nutritional and immune Status and clinicopathologic factors in patients with pancreatic cancer: a comprehensive analysis. Cancers (Basel). (2021) 13(20):5041. doi: 10.3390/cancers13205041
23. Liu M, Gou Y, Yang P. Association between preoperative neutrophil-to-lymphocyte ratio and length of stay in pediatric patients undergoing laparoscopic appendectomy: a retrospective cohort study. BMC Pediatr. (2025) 25(1):668. doi: 10.1186/s12887-025-06043-3
24. Ruzzenente A, Alaimo L, Caputo M, Conci S, Campagnaro T, De Bellis M, et al. Infectious complications after surgery for perihilar cholangiocarcinoma: a single western center experience. Surgery. (2022) 172(3):813–20. doi: 10.1016/j.surg.2022.04.028
25. Feng L, Liang J, Wang N, Zhang Q. Systemic inflammatory markers and clinical outcomes of open versus biportal endoscopic transforaminal lumbar interbody fusion. Ther Clin Risk Manag. (2024) 20:249–59. doi: 10.2147/TCRM.S447394
26. Kaur M, Chandel K, Reddy P, Gupta P, Samanta J, Mandavdhare H, et al. Neutrophil-lymphocyte ratio predicts clinical response to percutaneous transhepatic biliary drainage in acute cholangitis. J Clin Exp Hepatol. (2023) 13(3):390–6. doi: 10.1016/j.jceh.2023.01.002
27. Lee SH, Lee TY, Jeong JH, Cheon YK. Clinical significance of the neutrophil-lymphocyte ratio as an early predictive marker for adverse outcomes in patients with acute cholangitis. Medicina. (2022) 58(2):255. doi: 10.3390/medicina58020255
28. Wang H, Hao C, Bai D. Risk factors of urinary tract infection in pediatric patients with ureteropelvic junction obstruction after primary unilateral pyeloplasty. Comput Math Methods Med. (2022) 2022:3482450. doi: 10.1155/2022/3482450
29. Zhou W, Wang H, Li C, Ma QM, Gu YH, Sheng SY, et al. Alterations in novel inflammatory biomarkers during perioperative cardiovascular surgeries involving cardiopulmonary bypass: a retrospective propensity score matching study. Front Cardiovasc Med. (2024) 11:1433011. doi: 10.3389/fcvm.2024.1433011
30. Zhang J, Bai W, Guo C, Liu L, Wang G, Huang C, et al. Postoperative short-term outcomes between sublobar resection and lobectomy in patients with lung adenocarcinoma. Cancer Manag Res. (2020) 12:9485–93. doi: 10.2147/CMAR.S266376
Keywords: reduction mammaplasty, postoperative drainage, neutrophil-to-lymphocyte ratio (NLR), obesity, precision monitoring
Citation: Wu C, Zhao L, Wang J, Liang H and Tao Y (2025) NLR and BMI are independent predictors of postoperative drainage volume in macromastia patients following reduction mammoplasty. Front. Surg. 12:1667856. doi: 10.3389/fsurg.2025.1667856
Received: 17 July 2025; Accepted: 30 September 2025;
Published: 10 October 2025.
Edited by:
Domenico Tripodi, Saint Camillus International University of Health and Medical Sciences, ItalyReviewed by:
Martin Kauke-Navarro, Yale-New Haven Hospital, United StatesCathy Henry, Penn State Health Milton S. Hershey Medical Center, United States
Copyright: © 2025 Wu, Zhao, Wang, Liang and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongwei Liang, MTUwMzcxNzEwMDJAMTYzLmNvbQ==; Ye Tao, dGFveWVxaW5nY2FvMTIzNEAxNjMuY29t
†These authors have contributed equally to this work
 Chong Wu
Chong Wu Lin Zhao†
Lin Zhao†