- 1Department of Neurosurgery, The Ganzhou Affiliated Hospital, Jiangxi Medical College, Nanchang University, Ganzhou, Jiangxi, China
- 2Department of Neurosurgery, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
- 3Department of Rehabilitation Medicine, The Ganzhou Affiliated Hospital, Jiangxi Medical College, Nanchang University, Ganzhou, Jiangxi, China
The simultaneous occurrence of intracranial aneurysms and intracranial arachnoid cysts is a rare clinical observation, with the majority of documented instances demonstrating ipsilateral presentation. In this report, we describe an atypical case involving the development of a secondary arachnoid cyst subsequent to the rupture of an intracranial aneurysm. Notably the cyst was situated contralaterally to the site of the aneurysm rupture and outside the surgical field. The patient's clinical history and imaging studies confirmedcorroborated the secondary nature of the cyst, which is postulated to have resulted from inflammatory responses triggered by a subarachnoid hemorrhage (SAH). The patient underwent neuroendoscopic partial resection of the cyst wall and lateral ventriculostomy, leading to a significant improvement in neurological dysfunction symptoms associated with the secondary arachnoid cyst. Follow-up cranial MRI demonstrated a substantial reduction in the cyst's volume, with no evidence of subsequent hydrocephalus or cyst enlargement. This case enhances the comprehension of the pathophysiological mechanisms underlying the formation of contralateral arachnoid cysts subsequent to aneurysm rupture and emphasizes the necessity of acknowledging arachnoid cysts as potential delayed complications associated with aneurysmal subarachnoid hemorrhage (aSAH).
Introduction
Intracranial aneurysms and intracranial arachnoid cysts are common conditions encountered in neurosurgical practice; nevertheless, their concurrent manifestation in a single patient is an infrequent phenomenon. In most documented instances, the arachnoid cyst is identified concurrently with the intracranial aneurysm during imaging procedures, with almost all previously reported cases exhibiting showing ipsilateral localization of both pathologies (1). The occurrence of an arachnoid cyst following surgical intervention for a ruptured cerebral aneurysm is rare, and the formation of a contralateral cyst distant from the ipsilateral surgical site, has not been reported in the literature (2–4). This study introduces a unique case of a contralateral arachnoid cyst emerging postoperatively after the rupture of an intracranial aneurysm and investigates the possible mechanisms responsible for contralateral cyst formation, potentially marking the first documented occurrence. Furthermore, it is crucial for clinicians to acknowledge that arachnoid cysts may represent delayed complications of aneurysmal subarachnoid hemorrhage (aSAH).
Case report
A 50-year-old male patient was admitted with a complaint of a persistent headache lasting for three hours. His medical history was notable for a 20-year history of smoking, with no evidence of hypertension or diabetes. Upon physical examination, the patient was found to be alert and oriented, with a Glasgow Coma Scale (GCS) score of 15 (E4V5M6). The pupils were bilaterally isocoric at 3.0 mm and exhibited intact light reflexes. Signs of meningeal irritation were absent, and no significant neurological deficits were detected. Cranial computed tomography (CT) and computed tomography angiography (CTA) revealed a subarachnoid hemorrhage and an aneurysm of the left internal carotid artery at the ophthalmic segment (Figures 1A,B). He was classified as Hunt-Hess grade II and modified Fisher grade III. The patient underwent a craniotomy for aneurysm clipping. The initial postoperative CT scan of the head demonstrated post-clipping alterations and a marked reduction in the intracranial hematoma relative to prior imaging (Figure 1C). Following this, the patient was administered continuous lumbar drainage of hemorrhagic cerebrospinal fluid (CSF) in conjunction with appropriate supportive care. Three weeks postoperatively, the patient demonstrated a favorable recovery and was discharged without complications. However, 10 days after discharge, the patient was readmitted in a state of sudden coma. The left pupil was noted to be dilated with an absent light reflex. An emergency cranial CT scan revealed the formation of a hematoma in the left frontotemporal lobe, indicating the possibility of rebleeding at the surgical site (Figure 1D), the patient underwent an emergency craniotomy for the evacuation of a hematoma. Intraoperative exploration revealed a recurrent and reruptured aneurysm located in the ophthalmic segment. Successful aneurysm clipping was achieved, along with hematoma evacuation and decompressive craniectomy (Figure 1E). The procedure was completed without complications. After an uncomplicated clinical recovery, the patient was discharged. The pre-discharge follow-up cranial CT scan showed postoperative changes in the brain, with no other significant abnormal findings observed (Figure 1F). Fifteen days post-discharge, the patient exhibited cognitive decline and left-sided weakness. A readmission cranial CT scan revealed a cystic lesion located dorsal to the right ambiens cistern and medial to the parahippocampal gyrus (Figure 2A). Serial imaging conducted at a three-month interval demonstrated progressive enlargement of the cystic lesion (Figure 2B). Subsequent cranial magnetic resonance imaging (MRI) excluded the presence of gliomas and cholesteatomas (Figures 2C,D). One year later, amidst ongoing neurological deterioration, follow-up cranial CT indicated further expansion of the cystic lesion, accompanied by a significant mass effect (Figures 3A,B). Neuroendoscopic exploration was conducted under general anesthesia, during which a cortical fenestration of the temporal lobe was created to facilitate access to the cystic cavity. Intraoperative observations revealed a thickened cyst wall under high tension with evidence of vascular proliferation. The cystic fluid was colorless and transparent, lacking hemosiderin deposition (Figures 4A–C). An arachnoid cyst was suspected intraoperatively, prompting partial resection of the cyst wall and the creation of a lateral ventriculostomy. The fistulous opening measured approximately 2.5 cm in diameter, facilitating communication with the lateral ventricle (Figure 4C). Histopathological analysis of the cyst wall confirmed the diagnosis of an arachnoid cyst (Figure 5). Following the surgical procedure, the patient demonstrated a gradual enhancement in left-sided motor weakness and cognitive abilities. At the three-month follow-up, both motor and cognitive functions had nearly returned to baseline levels. The GCS score was recorded at 15 (E4V5M6). The latest cranial MRI revealed a substantial reduction in cyst volume, accompanied by a complete resolution of the mass effect (Figures 3C,D).
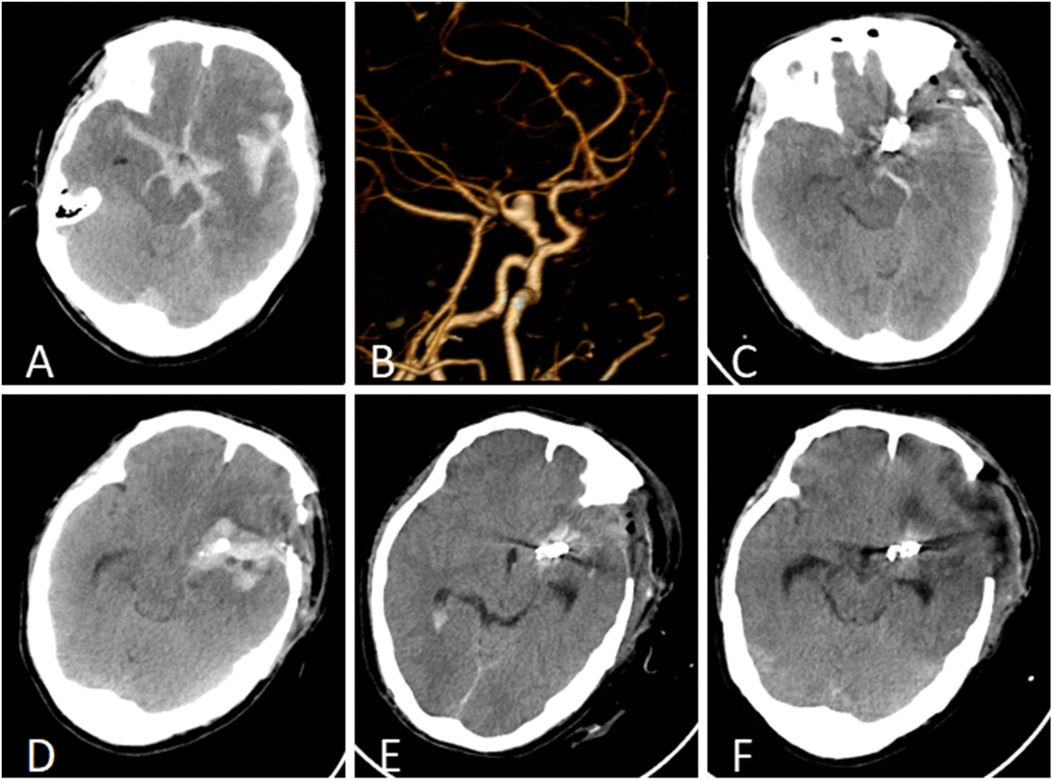
Figure 1. (A) Cranial CT identified a subarachnoid hemorrhage and a hematoma within the left fissure cistern. (B) CTA revealed an aneurysm in the ophthalmic segment of the left internal carotid artery. (C) The initial postoperative CT follow-up indicated a reduction in intracranial hemorrhage following cerebral aneurysm clipping. (D) Rebleeding was detected 10 days post-discharge, with an emergent cranial CT scan revealing a hematoma in the left frontotemporal region. (E) The initial follow-up CT after the second surgical intervention confirmed successful clipping of the cerebral aneurysm and substantial resolution of the temporal hematoma. (F) At the time of discharge, postoperative cranial CT demonstrated postsurgical changes without signs of hydrocephalus or cyst formation.
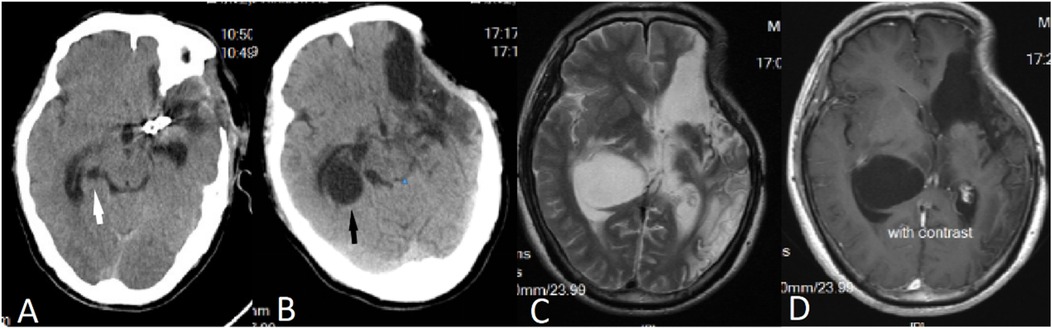
Figure 2. (A) Fifteen days post-surgery, a CT scan revealed the presence of an arachnoid cyst located dorsal to the right ambient cistern and medial to the parahippocampal gyrus (indicated by a white arrow). (B) A follow-up CT scan conducted three months later demonstrated an enlargement of the arachnoid cyst (indicated by a black arrow). (C,D) Subsequent MRI findings indicated that the cystic fluid exhibited a signal identical to that of CSF, with no enhancement observed on the contrast-enhanced scan.
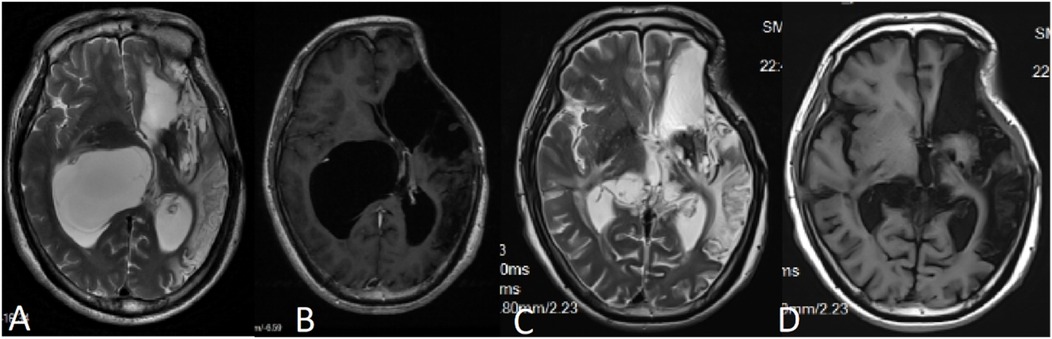
Figure 3. (A,B) MRI conducted 12 months post-craniotomy revealed a further enlargement of the arachnoid cyst accompanied by a significant mass effect, resulting in noticeable compression of the thalamus. (C,D) Six months following the partial resection of the cyst wall, MRI findings indicated a reduction in the mass effect exerted by the cyst, with no evidence of recurrence.
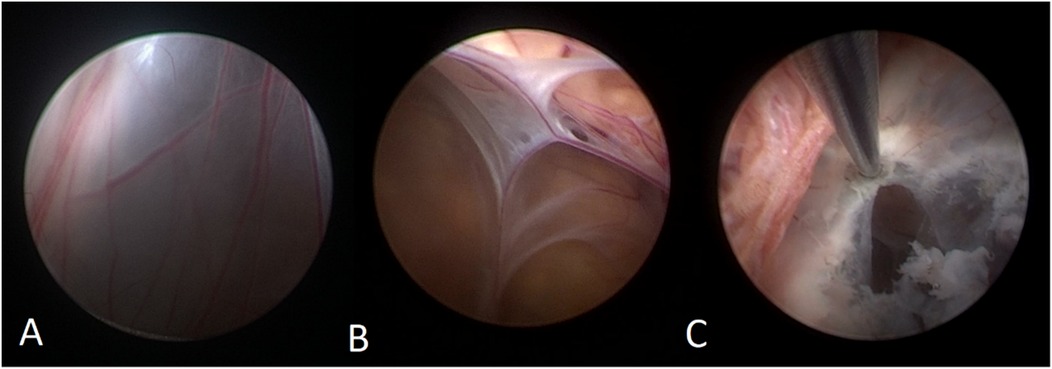
Figure 4. (A) The neuroendoscopic image illustrates vascular hyperplasia within the cyst wall, accompanied by tension in the cyst wall. (B) The internal perspective reveals that the cyst is closely adherent to the cerebral ventricular wall, with blood vessels situated between the arachnoid membranes. (C) The thick-walled cyst is positioned adjacent to the choroid plexus, and it exhibited a reduction in size following cauterization of the cyst wall.
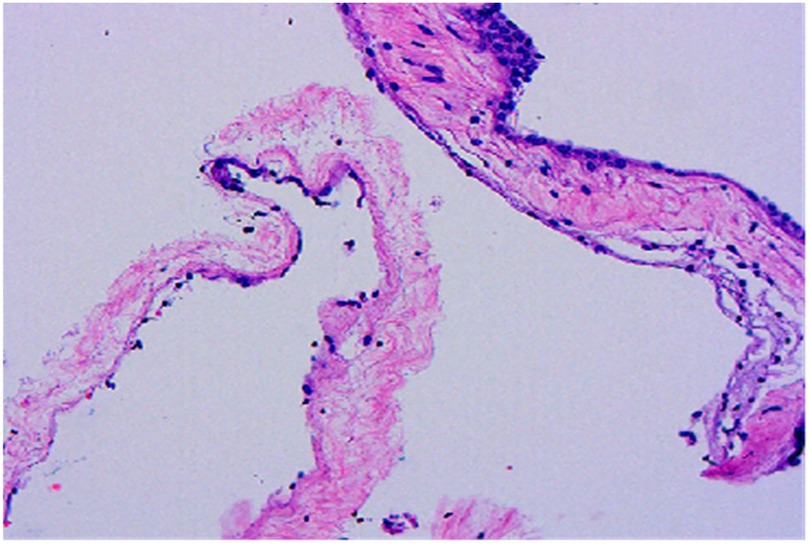
Figure 5. Histopathological examination reveals cyst walls composed of proliferative fibrous tissue lined with cuboidal or flattened epithelial cells (HE × 100).
Discussion
Intracranial aneurysms and arachnoid cysts are relatively prevalent anomalies observed in neurosurgical practice. Intracranial aneurysms are found in approximately 3%–5% of the general population (5), whereas arachnoid cysts constitute about 1% of all intracranial space-occupying lesions (6). Despite their individual prevalence, the concurrent manifestation of both conditions in a single patient is uncommon (1, 2, 7). In most documented instances, arachnoid cysts are frequently identified alongside intracranial aneurysms during imaging studies. Aneurysms are predominantly located at the bifurcation of the middle cerebral artery or in the region of the carotid-posterior communicating artery, whereas arachnoid cysts are primarily situated in the middle cranial fossa. Importantly, the aneurysms are consistently positioned adjacent to or within these cysts (1). The anatomical colocalization within the subarachnoid space is a fundamental prerequisite for their coexistence. Moreover, the presence of an aneurysm can alter local hemodynamics, subsequently affecting CSF dynamics and pressure gradients within the subarachnoid space, potentially creating conditions favorable for the development of arachnoid cysts. However, the formation of arachnoid cysts secondary to ruptured intracranial aneurysms, particularly those occurring contralaterally at non-surgical sites, has not been documented in the existing literature. This case may represent the first recorded instance of this rare phenomenon. The precise pathogenesis underlying the concurrent development of intracranial aneurysms and arachnoid cysts remains poorly understood. Current evidence suggests that these lesions may be either primary (congenital), involving genetic factors (8, 9) or developmental anomalies (10), or secondary, typically induced by hemorrhage, infection, or trauma (11, 12). Congenital variants demonstrate a strong association with inheritable connective tissue disorders. In this regard, autosomal dominant polycystic kidney disease (ADPKD) is notably linked to both intracranial aneurysms and arachnoid cysts. Schievink reports that the prevalence of intracranial aneurysms in individuals with ADPKD is 10.8%, whereas the prevalence of arachnoid cysts is 8.1% (8). In the current case, the absence of a history of ADPKD, the lack of extracranial anomalies on preoperative imaging, and a negative family history collectively rule out the diagnosis of autosomal dominant polycystic kidney disease. Given the absence of intracranial cystic lesions preoperatively and the clinical presentation, it is postulated the arachnoid cyst is secondary, potentially associated with subarachnoid hemorrhage following the rupture of an intracranial aneurysm.
Current evidence indicates that the development of secondary arachnoid cysts may be linked to aseptic inflammatory responses at the leptomeningeal-arachnoid interface, triggered by subarachnoid hemorrhage. Postprocedural arachnoiditis commonly occurs following aneurysm clipping or coil embolization, with its chronic inflammatory process identified as a key factor in cystogenesis (13, 14). Studies suggest that recurrent subarachnoid hemorrhage and occult meningitis, resulting from lumbar drainage, may exacerbate arachnoiditis development (15, 16). Additionally, prolonged bed rest and the use of intraoperative fibrin sealants are acknowledged as risk factors for adhesive arachnoiditis (17). Although surgical intervention, trauma, and infection are well-known etiological factors for adhesive arachnoiditis, growing evidence suggests that blood products within the subarachnoid space-particularly erythrocyte degradation products-substantially increase the severity of arachnoiditis, leading to arachnoidal fibrosis, hyperplasia, and obliteration of the subarachnoid space (18–21). This inflammatory cascade has the potential to alter CSF dynamics, resulting in flow obstruction that ultimately facilitates cystogenesis. In the current case, the arachnoid cyst developed on the side opposite to the ruptured aneurysm, yet it was located at a distance from the ipsilateral surgical site. This unusual localization may be associated with the patient's postoperative decubitus position, which favored the contralateral side. This positioning could have promoted the gravitational accumulation of blood products in dependent regions, thereby creating conditions conducive tocyst formation. The precise mechanisms underlying the enlargement of arachnoid cysts remain incompletely understood. Several theories have been proposed, including the ball-valve mechanism (22), active fluid secretion by the cyst walls (23), and osmotic gradients between the cyst contents and the CSF (24).
A neuroendoscopic evaluation of the arachnoid cyst revealed a bilaminar arachnoid wall characterized by intervening vascular proliferation. The cyst maintained its structural integrity under tension and was situated adjacent to the choroid plexus and the medial wall of the lateral ventricle, with no observable slit-like formations. The cystic fluid was colorless and transparent, resembling CSF, and comparative analysis confirmed its biochemical similarity to CSF. Immunohistochemical analysis demonstrated immunopositivity for epithelial membrane antigen (EMA). Definitive histological examination confirmed a fibrotic cyst wall devoid of epithelial cellular components. In this case, we propose that cyst enlargement may not primarily involve ball-valve mechanisms or osmotic gradients. Instead, it is plausible that unrestricted communication between the cyst and the subarachnoid space facilitates the transmission of CSF pulsations into the cyst cavity, or that active fluid secretion by the cyst wall itself may contribute to the expansion. Additionally, intraoperative observations suggest that the pronounced vascular proliferation within the cyst wall may expedite this enlargement process.
Arachnoid cysts are typical benign entities, with clinical presentations that vary depending on their anatomical location and size. These cysts may result in increased intracranial pressure, seizures, cerebral compression, and localized mass effects, including cognitive decline, cerebellar ataxia, and hemiparesis. Instances of spontaneous regression have been documented in isolated case reports. Currently, there is no standardized management protocol for arachnoid cysts. Common surgical interventions include cystoperitoneal shunting, cyst fenestration, and complete or partial cystectomy (25). Shunt procedures carry risks such as infection and shunt obstruction, while fenestration is prone to recurrence. Although complete cystectomy is theoretically the most effective strategy, the presence of dense adhesions between the cyst walls and normal neurovascular structures often renders total resection unfeasible. Given the secondary nature and contralateral non-surgical location of this rare cyst, we preformed a neuroendoscopic partial cystectomy alongside a ventriculostomy. This approach led to: (1) effective alleviation of the mass effect; (2) restoration of patency in the proximal cistern and Sylvian fissure; (3) reestablishment of construction of CSF circulation; and (4) symptomatic relief with a diminished risk of recurrence. The postoperative resolution of cyst-induced neurological deficits highlights the minimally invasive nature, favorable outcomes, and low complication rate associated with neuroendoscopic management.
Beyond the well-documented acute complications of aSAH, such as cerebral vasospasm, hydrocephalus, and rebleeding, clinical evidence suggests that the delayed onset of arachnoid cysts may constitute a rare yet clinically significant late complication. While arachnoid cysts are typically regarded as congenital anomalies, several case reports and retrospective studies have identified instances of acquired cystic lesions developing months to years post-aSAH. In conclusion, the formation of arachnoid cysts following aSAH represents a clinically underrecognized phenomenon with potentially profound neurological implications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Ganzhou People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
KH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. YYu: Data curation, Methodology, Resources, Writing – original draft, Writing – review & editing. YYa: Investigation, Methodology, Writing – original draft, Writing – review & editing. HL: Data curation, Methodology, Writing – original draft. JL: Conceptualization, Investigation, Writing – review & editing. HZ: Investigation, Resources, Software, Writing – review & editing. QJ: Investigation, Methodology, Supervision, Writing – review & editing. JL: Data curation, Formal analysis, Writing – original draft, Writing – review & editing, Conceptualization, Investigation, Software. SZ: Data curation, Methodology, Writing – original draft, Writing – review & editing. NY: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. WZ: Data curation, Formal analysis, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Igarashi Y, Murai Y, Yamada O, Shirokane K, Hironaka K, Sato S, et al. Cerebral aneurysm associated with an arachnoid cyst: 3 case reports and a systematic review of the literature. World Neurosurg. (2018) 109:e203–9. doi: 10.1016/j.wneu.2017.09.139
2. Zanini MA, Faleiros AT, Rondinelli G, Gabarra RC, Resende LA. A form of dysplasia or a fortuitous association? A cerebral aneurysm inside an arachnoid cyst: case report. Neurosurgery. (2007) 61:E654–5. discussion E655. doi: 10.1227/01.Neu.0000290917.70717.39
3. Ishizaka S, Hayashi K, Otsuka M, Fukuda S, Tsunoda K, Ushijima R, et al. Syringomyelia and arachnoid cysts associated with spinal arachnoiditis following subarachnoid hemorrhage. Neurol Med Chir (Tokyo). (2012) 52:686–90. doi: 10.2176/nmc.52.686
4. Abhinav K, Bradley M, Aquilina K, Patel NK. Spinal arachnoiditis and cyst formation with subarachnoid haemorrhage. Br J Neurosurg. (2012) 26:574–5. doi: 10.3109/02688697.2011.651512
5. Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. (2013) 44:3613–22. doi: 10.1161/strokeaha.113.002390
6. Rabiei K, Jaraj D, Marlow T, Jensen C, Skoog I, Wikkelsø C. Prevalence and symptoms of intracranial arachnoid cysts: a population-based study. J Neurol. (2016) 263:689–94. doi: 10.1007/s00415-016-8035-1
7. Aguiar GB, Santos RGD, Paiva ALC, Silva JMA, Silva RCD, Veiga JCE. Intracranial aneurysm and arachnoid cyst: just a coincidence? A case report. Sao Paulo Med J. (2019) 137:92–5. doi: 10.1590/1516-3180.2017.0083290517
8. Schievink WI, Huston J 3rd, Torres VE, Marsh WR. Intracranial cysts in autosomal dominant polycystic kidney disease. J Neurosurg. (1995) 83:1004–7. doi: 10.3171/jns.1995.83.6.1004
9. Alehan FK, Gürakan B, Ağildere M. Familial arachnoid cysts in association with autosomal dominant polycystic kidney disease. Pediatrics. (2002) 110:e13. doi: 10.1542/peds.110.1.e13
10. Yeaton-Massey A, Monteagudo A. Intracranial cysts. Am J Obstet Gynecol. (2020) 223:B42–b46. doi: 10.1016/j.ajog.2020.08.185
11. E. Öcal, understanding intracranial arachnoid cysts: a review of etiology, pathogenesis, and epidemiology. Childs Nerv Syst. (2023) 39:73–8. doi: 10.1007/s00381-023-05860-0
12. Rogers AJ, Kuppermann N, Thelen AE, Stanley RM, Maher CO. Children with arachnoid cysts who sustain blunt head trauma: injury mechanisms and outcomes. Acad Emerg Med. (2016) 23:358–61. doi: 10.1111/acem.12887
13. Patsouris V, Blecharz-Lang KG, Nieminen-Kelhä M, Schneider UC, Vajkoczy P. Resolution of cerebral inflammation following subarachnoid hemorrhage. Neurocrit Care. (2023) 39:218–28. doi: 10.1007/s12028-023-01770-w
14. Khey KMW, Huard A, Mahmoud SH. Inflammatory pathways following subarachnoid hemorrhage. Cell Mol Neurobiol. (2020) 40:675–93. doi: 10.1007/s10571-019-00767-4
15. Caplan LR, Norohna AB, Amico LL. Syringomyelia and arachnoiditis. J Neurol Neurosurg Psychiatry. (1990) 53:106–13. doi: 10.1136/jnnp.53.2.106
16. de Carvalho S, Almeida A, Reis C. Intracranial arachnoiditis and hydrocephalus. World Neurosurg. (2023) 179:26–7. doi: 10.1016/j.wneu.2023.07.106
17. Taguchi Y, Suzuki R, Okada M, Sekino H. Spinal arachnoid cyst developing after surgical treatment of a ruptured vertebral artery aneurysm: a possible complication of topical use of fibrin glue. Case Report. J Neurosurg. (1996) 84:526–9. doi: 10.3171/jns.1996.84.3.0526
18. Sajanti J, Majamaa K. Detection of meningeal fibrosis after subarachnoid haemorrhage by assaying procollagen propeptides in cerebrospinal fluid. J Neurol Neurosurg Psychiatry. (1999) 67:185–8. doi: 10.1136/jnnp.67.2.185
19. Motohashi O, Suzuki M, Yanai N, Umezawa K, Shida N, Yoshimoto T. Thrombin and TGF-beta promote human leptomeningeal cell proliferation in vitro. Neurosci Lett. (1995) 190:105–8. doi: 10.1016/0304-3940(95)11513-v
20. Motohashi O, Suzuki M, Shida N, Umezawa K, Ohtoh T, Sakurai Y, et al. Subarachnoid haemorrhage induced proliferation of leptomeningeal cells and deposition of extracellular matrices in the arachnoid granulations and subarachnoid space. Immunhistochemical Study. Acta Neurochir (Wien). (1995) 136:88–91. doi: 10.1007/bf01411441
21. Marshman LA, David KM, King A, Chawda SJ. Delayed fibrotic obliteration of the spinal subarachnoid space after cerebral aneurysmal subarachnoid hemorrhage: case report. Neurosurgery. (2007) 61:E659–60. discussion E660. doi: 10.1227/01.Neu.0000290920.55470.Ec
22. Halani SH, Safain MG, Heilman CB. Arachnoid cyst slit valves: the mechanism for arachnoid cyst enlargement. J Neurosurg Pediatr. (2013) 12:62–6. doi: 10.3171/2013.4.Peds12609
23. Go KG, Houthoff HJ, Blaauw EH, Havinga P, Hartsuiker J. Arachnoid cysts of the sylvian fissure. Evidence of Fluid Secretion. J Neurosurg. (1984) 60:803–13. doi: 10.3171/jns.1984.60.4.0803
24. Cincu R, Agrawal A, Eiras J. Intracranial arachnoid cysts: current concepts and treatment alternatives. Clin Neurol Neurosurg. (2007) 109:837–43. doi: 10.1016/j.clineuro.2007.07.013
Keywords: intracranial aneurysm, arachnoid cyst, surgery, endoscopic techniques, diagnosis
Citation: Hu K, Yu Y, Yang Y, Li H, Liao J, Zhong H, Jiang Q, Liu J, Zeng S, Yang N and Zhang W (2025) Postoperative secondary contralateral arachnoid cyst following rupture and bleeding of intracranial aneurysm: a case report. Front. Surg. 12:1672623. doi: 10.3389/fsurg.2025.1672623
Received: 25 July 2025; Accepted: 8 September 2025;
Published: 23 September 2025.
Edited by:
Sunil Manjila, Insight Institute of Neurosurgery and Neuroscience (IINN), United StatesReviewed by:
Shankar Ayyappan Kutty, NMC Healthcare, United Arab EmiratesBaylis Joseph, Christian Medical College & Hospital, India
Copyright: © 2025 Hu, Yu, Yang, Li, Liao, Zhong, Jiang, Liu, Zeng, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Liu, bGl1anVuNDQ4MTUzNzk4QDEyNi5jb20=; Shuiying Zeng, emVuZ3NodWl5aW5nMTk4MUAxNjMuY29t; Nan Yang, eW5hbjEwMDExMkAxMjYuY29t; Wenjun Zhang, endqYWNoYmRAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Kun Hu1,†
Kun Hu1,† Qiuhua Jiang
Qiuhua Jiang Jun Liu
Jun Liu