- Department of Plastic and Hand Surgery and Laboratory for Tissue Engineering and Regenerative Medicine, University Hospital Erlangen, Erlangen, Germany
Breast cancer remains the most commonly diagnosed malignancy among women worldwide, with surgical intervention, ranging from breast-conserving procedures to total mastectomy, representing a cornerstone of curative treatment. In this context, breast reconstruction has become an essential component of comprehensive cancer treatment, addressing not only physical restoration but also playing a vital role in psychosocial rehabilitation and body image. Among the various reconstructive options, autologous tissue transfer has emerged as the preferred method for many patients, offering durable and natural-feeling results. In particular, abdominal-based free flaps such as the Deep Inferior Epigastric Perforator (DIEP) flap and the muscle-sparing Transverse Rectus Abdominis Myocutaneous (ms-TRAM) flap offer excellent results with reduced donor side morbidity. As the global number of breast cancer continues to rise, the demand for safe, individualized, and functionally superior reconstructive options rises as well. This article aims to provide a general overview of current surgical approaches and to highlight perspectives for future innovations in improving autologous breast reconstruction and patient satisfaction.
From observation to precision: the evolution of preoperative imaging in autologous breast reconstruction
Since the pioneering days of free flap surgery, the approach to autologous breast reconstruction has undergone remarkable transformation (1, 2). Due to advanced wound care tools, wound bed preparation has made it possible to transplant free flaps to the chest in contaminated and irradiated areas at an earlier time point (3). The seminal introduction of his first “free abdominoplasty flap”, which basically was a free Transverse Rectus Abdominis Myocutaneous (TRAM) flap by Holmström (4), followed by the refinement of perforator flap techniques such as the Deep Inferior Epigastric Perforator (DIEP) flap introduced by Koshima (5), marked milestones that revolutionized reconstructive options after mastectomy. In the early phases of these techniques, flap planning relied heavily on tactile surgical experience and clinical acumen—primarily through direct observation of skin perfusion and rudimentary Doppler ultrasound to locate suitable perforators (6, 7). However, with growing recognition of the complexity of abdominal vascular anatomy and the critical importance of optimizing flap perfusion while minimizing donor site morbidity, a paradigm shift occurred (8, 9). The field has steadily moved toward increasingly sophisticated and standardized preoperative imaging protocols (10).
Today, Computed Tomography Angiography (CTA) has emerged as the clinical gold standard for preoperative mapping of the perforators of the inferior epigastric artery (11) (see Figure 1). With near-perfect sensitivity, CTA offers unparalleled spatial resolution and precise anatomical visualization, dramatically improving surgical planning and intraoperative confidence (12). It represents a major leap forward from earlier tools such as handheld Doppler and Duplex sonography, which—though still valuable—lack the depth and clarity required for consistently reliable results in complex cases. Cinematic rendering of data acquired by CT-angiography allows for completely three-dimensional depicting of vessels and could well become an integrated part of the imaging algorithm (13, 14).
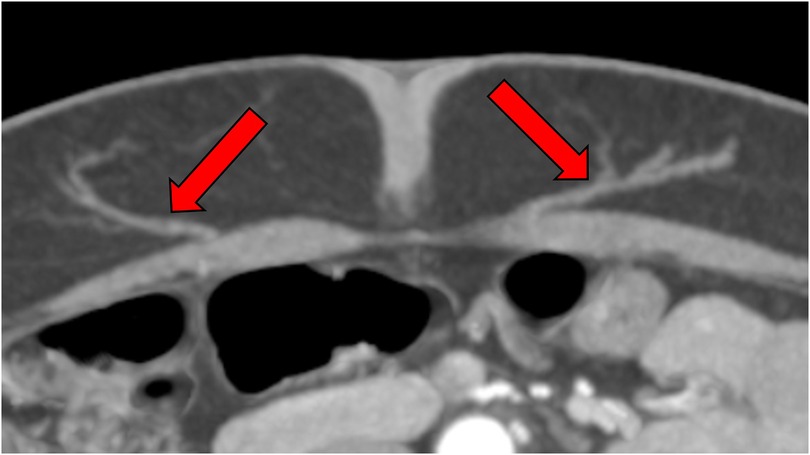
Figure 1. CTA-based perforator mapping enabling precise identification of vessel course and caliber, which facilitates tailored flap design and minimizes intraoperative dissection time.
Magnetic Resonance Angiography (MRA) has become a valuable alternative for patients with contraindications to iodinated contrast or ionizing radiation, such as those with renal impairment or contrast allergies. While more resource-intensive and not yet universally available, MRA provides high-quality vascular imaging and continues to gain ground as an adjunct or substitute in selected scenarios (15). This method is promising but needs further validation.
Beyond vascular imaging, the field is rapidly embracing next-generation visualization tools. Techniques such as cinematic rendering and the creation of 3D-printed models of individual patients' vascular anatomy offer surgeons a tangible, spatially accurate reference to guide dissection and flap elevation (16, 17). These innovations are not only enhancing operative planning but also serve as powerful tools in surgical training and patient education (18). Furthermore modern Artificial Intelligence (AI) driven modalities are rapidly being developed and optimized for clinical application (19). The field of tissue engineering (TE) and regenerative medicine (RM) is also using 3D printing techniques with the aim to once produce tissue repair from the laboratory without the hitherto unavoidable donor site for autologous tissue, but those have not yet entered the clinical stage (20). Potential side effects of cultured cells in such replacement tissue need further investigations (21). Further on the effect of pre- or postoperative irradiation and appropriate imaging or influencing the radiation effects will be another step to further advance breast reconstruction (22).
Although only a limited number of studies have investigated the application of dynamic infrared thermography (DIRT) in DIEP reconstruction exists, it has been proposed that use of DIRT during the operation could allow the tailoring of the surgery and postoperative use may potentially identify vascularization problems in an early stage (23). Nevertheless, up to date additional high-quality studies are needed to ensure the true value for the pre-, per- and postoperative phase of DIEP-flap reconstructions.
Three-dimensional surface imaging and volumetric simulation technologies have also entered clinical practice, providing a non-invasive means of assessing body contour, estimating flap volume, and simulating postoperative outcomes (9). These tools foster clearer communication between patient and surgeon and support shared decision-making by setting realistic expectations (24) (see Figure 2).
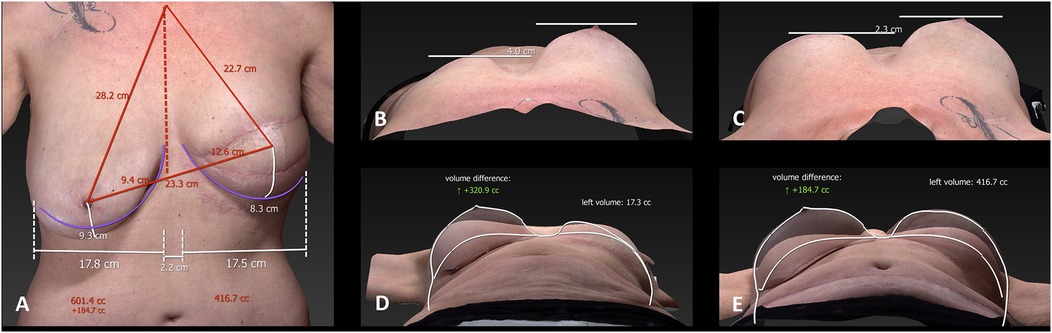
Figure 2. 3D surface imaging of a patient after mastectomy of the left breast and autologous breast reconstruction. This kind of imaging is used for preoperative planning, providing volumetric assessment and symmetry simulation to improve patient–surgeon communication before breast reduction on the right breast. The panels (A–E) show the different measurements of the breasts. With these measurements the volume difference is calculated.
During surgery, real-time assessment of flap vascularity has been significantly advanced by the use of fluorescence angiography with indocyanine green (ICG) (25). This technique provides immediate visual feedback on tissue perfusion, enabling surgeons to identify poorly perfused areas early and adjust the surgical plan accordingly (26). As a result, the risk of partial flap necrosis is substantially reduced, contributing to improved surgical outcomes and flap viability.
Advancements in surgical techniques and robotic integration
The landscape of reconstructive breast surgery is rapidly advancing toward less invasive, precision-driven methods, with a growing emphasis on robotic and microsurgical innovations. Robotic-assisted procedures, particularly with platforms like the Da Vinci Surgical System, have introduced the possibility of laparoscopic flap harvest with the aim to possibly reduce the length of the anterior rectus sheath fascial incision. It has also been speculated that this evolution could hold the promise of minimizing donor site morbidity and postoperative recovery times. However, these approaches often require transperitoneal access, which brings inherent risks such as bowel injury, adhesion formation, or postoperative ileus—factors that necessitate careful patient selection and surgical planning.
However, it needs to be mentioned that the robotic DIEP harvest technique involves entering the peritoneal cavity, unlike standard extraperitoneal techniques that preserve peritoneal integrity. This carries intraabdominal complications and potential risks of bowel injury, adhesions, seroma, especially in patients with prior surgeries (27). In these patients, adhesions may hinder robotic maneuverability and safe port placement. In addition, laparoscopy can lead to a loss of the peritoneal barrier and may increase postoperative discomfort and complications (28). The risk of multiple 8 mm–10 mm fascial defects by the port punctures needs to be taken into account together with the posterior rectus sheath violation by the intentional incision of the posterior rectus sheath (28). This may weaken abdominal wall integrity and should be considered. Robotic DIEP-flap harvest from the lower abdomen has been demonstrated to increase both ischemia time and total operative time. When considered in conjunction with the multiple port incisions, the claim that the technique is truly “minimally invasive” is called into question, despite the proposed reduction in fascial defects. Although the robotic-assisted DIEP flap technique therefore represents a notable advancement in microsurgical innovation, it is important to critically assess its limitations to avoid an overly favorable portrayal that may overlook significant surgical and logistical complexities. Future research should incorporate rigorous scientific evaluation, including data on hernia rates at both the fascial incision and robotic port sites, precise measurements of flap ischemia time (16, 29), and thorough cost–benefit analyses that address clinical outcomes, operative efficiency, and healthcare resource utilization.
In summary initial clinical data are encouraging, yet widespread adoption is tempered by longer operative times and the technical demands of setup and intraoperative coordination. The current state of development in robotic microsurgery needs to be further improved to definitely enter the daily clinical routine. While Wessel et al. suggest that the complete replacement of surgeons by robotic systems remains improbable, these technologies are expected to assume an increasingly impactful role in supporting and improving surgical performance. Continued technological advancement will necessitate rigorous research and well-designed clinical trials to optimize robotic platforms and substantiate their broader integration into routine surgical care (30).
A promising and increasingly studied approach in reconstructive breast microsurgery is vascularized lymph node transfer (VLNT), particularly when using abdominally based free flaps, as a therapeutic option for postmastectomy lymphedema (31). This method seeks to reestablish normal lymphatic drainage by transplanting viable lymphatic tissue along with its blood supply. Almadani demonstrated that simultaneous VLNT can be safely integrated with autologous breast reconstruction to treat or prevent breast cancer-related lymphedema. Nonetheless, they emphasize the need for further research to standardize protocols for data collection and to effectively report patient outcomes related to both lymphedema and immediate lymphatic reconstruction (32).
Preliminary patient-reported outcomes have been encouraging—many individuals report decreased reliance on compression garments, improved limb comfort, and a subjective sense of enhanced quality of life. However, when scrutinized under objective clinical parameters—such as limb circumference, bioimpedance, or volumetric reductions—the results from larger cohort studies have been modest and somewhat inconsistent.
These findings highlight the need for standardized outcome measures, better-defined surgical protocols, and improved patient selection criteria. Further prospective, controlled studies will be essential to clarify which patients are most likely to benefit, and under what clinical circumstances VLNT offers the most durable and clinically meaningful improvements in lymphatic function (33, 34).
Postoperative monitoring
Conventional flap monitoring involves clinical evaluation of a skin island (35). Non-invasive and reliable methods for early identification of postoperative complications of free flaps that allow higher rates of salvage rate and reduce the need for specific staff with continuous on-site presence for flap monitoring have been investigated and proposed ever since free flap surgery became a clinical routine procedure (36). Lindelauf et al. reported that tissue oximetry following DIEP flap breast reconstruction can potentially facilitate a decrease in hospital costs since its readings enable physicians to intervene in an early stage of tissue malperfusion, contributing to minimizing complications and that it may eliminate the need for specialized postoperative care (37). However, based on the current literature, no firm conclusions can yet be drawn regarding cost-effectiveness of standard implementation.
While novel technologies such as surface probes (38), implantable Doppler probes (39), and flow couplers represent promising advancements in the intraoperative and postoperative monitoring of free flap anastomotic patency (40), their clinical implementation is still accompanied by important limitations and areas of uncertainty. These tools are designed to offer alternatives to traditional external skin paddles for monitoring, aiming to enhance early detection of complications, particularly venous insufficiency, without compromising the aesthetic outcome. For example, implantable Doppler probes provide continuous auditory signals indicating flow at the anastomotic site, while flow couplers integrate a Doppler sensor into the venous coupler ring, potentially allowing for non-invasive assessment of venous outflow. While surface and implantable monitoring systems hold substantial promise for improving flap surveillance, their clinical utility remains partially validated. Until larger, prospective, and ideally randomized studies confirm their efficacy and cost-effectiveness, these technologies should be considered as adjuncts, not replacements, to clinical judgment and traditional monitoring protocols.
Another innovation is the O2C (Oxygen to See) system, which noninvasively measures tissue oxygenation, hemoglobin concentration, and blood flow, offering real-time perfusion diagnostics to distinguish between arterial and venous complications (41, 42). Also, hyperspectral imaging alone or together with thermography has been propagated as another promising tool for perfusion controls in DIEP flaps (43, 44), similar to other application in reconstructive and hand surgery (45, 46). However, at the moment a lack of standardization hampers a more widespread clinical use and solid prospective studies are warranted.
Artificial intelligence in reconstructive breast surgery
The integration of AI is rapidly advancing within the field of reconstructive surgery, offering transformative potential across the entire perioperative continuum. AI-driven tools are being developed and validated for a range of applications, from preoperative planning to intraoperative guidance and postoperative monitoring. Ozmen and coauthors developed a machine learning model to predict 30-day readmission risk using a large national surgical quality database. They reported a stacked machine learning approach that demonstrates a strong predictive capability for post-DIEP flap readmissions, with high sensitivity for identifying at-risk patients. The model's performance suggests clinical utility in preoperative risk stratification and resource allocation (47). Implementation could enable targeted intervention strategies to potentially reduce readmission rates in high-risk populations.
In the preoperative phase, machine learning algorithms trained on radiologic datasets -particularly from CTA and MRI scans—have demonstrated the ability to identify and rank perforators, thereby streamlining perforator flap planning and reducing both time and interobserver variability. These tools may enhance the precision of DIEP and ms-TRAM flap surgeries, optimizing donor site selection and potentially improving outcomes.
AI-based predictive modeling is also being explored to forecast patient-specific risk profiles (48, 49). By incorporating multivariate data such as body mass index (BMI), comorbidities, surgical technique, smoking behavior, and prior interventions, these models may assist surgeons in personalizing risk stratification, surgical decision-making, and patient counseling.
Intraoperatively, AI holds promise for real-time decision support. Applications under investigation include aesthetic outcome prediction, augmented surgical navigation, and integration with robotic platforms for automated or semi-automated tissue dissection. Combined with augmented reality (AR), AI could enable surgeons to visualize subsurface vascular anatomy or highlight critical structures dynamically, enhancing operative precision and safety (50, 51).
In the postoperative setting, AI-based platforms are being tested for automated flap monitoring. By analyzing serial photographs captured via smartphone or tablet, these systems could potentially detect early signs of vascular compromise or wound complications and alert the clinical team. While these approaches are promising—especially for outpatient follow-up or remote care—they rely on the availability of high-quality training datasets and must be critically evaluated for algorithmic bias, particularly those arising from sociodemographic, ethnic, or geographic disparities.
While emerging technologies in imaging, robotics, and AI hold significant promise, their current clinical integration is limited by heterogeneous study designs, small sample sizes, and short follow-up periods. High costs, restricted availability, and the need for specialized training also constrain widespread adoption. Furthermore, many AI tools have not undergone robust external validation, and their performance in diverse patient populations remains unclear. Future research should prioritize large-scale, multi-center trials, standardized outcome measures, and cost-effectiveness analyses to ensure these innovations can be safely and equitably implemented.
Additionally one obstacle to clinical implementation of the latest AI-driven technology is the question of lega liablity in case complications occur which harm the patient. This issue needs to be solved in the future to allow the introduction of AI technology into routine clinical practice. Evidence base for certain innovations is therefore at the moment naturally restricted to small series or early feasibility studies.
In summary, AI stands to redefine many aspects of reconstructive surgery by enhancing precision, efficiency, and personalization. However, its integration must be approached with methodological rigor, robust validation, and ethical consideration to ensure equitable and safe implementation in clinical practice.
Conclusion
Autologous breast reconstruction stands at the forefront of innovation in reconstructive surgery, driven by rapid advancements in preoperative imaging, microsurgical techniques, robotic assistance, and the integration of artificial intelligence. The evolution from basic clinical assessment to high-resolution vascular imaging and dynamic intraoperative visualization has dramatically refined surgical planning and execution. Simultaneously, the emergence of robotics and microsurgical platforms enables greater precision, reduced invasiveness, and the potential for decreased donor-site morbidity.
Moreover, AI-powered tools offer new dimensions in personalized risk assessment, aesthetic outcome prediction, and automated postoperative monitoring, marking a paradigm shift toward data-driven, patient-specific surgical strategies. Collectively, these technological breakthroughs aim to improve surgical safety, reproducibility, and functional and aesthetic outcomes, while enhancing patient satisfaction and quality of life.
Nevertheless, the clinical integration of these innovations must proceed with methodological rigor and ethical oversight. Large-scale, multi-center trials are necessary to validate emerging techniques and technologies to ensure their equitable applicability across diverse patient populations. Challenges such as cost-effectiveness, training requirements, regulatory approval, and algorithmic transparency must be addressed proactively to facilitate responsible and sustainable implementation.
In summary, while autologous breast reconstruction has already made significant strides, it continues to evolve as a dynamic field at the intersection of surgical artistry and technological innovation. The path forward lies in harmonizing these advances with evidence-based practice and patient-centered care, thereby expanding access to safe, individualized, and high-quality reconstructive solutions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
IS: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing. RH: Writing – original draft, Writing – review & editing. DP: Writing – original draft, Writing – review & editing. TP: Writing – original draft, Writing – review & editing. EE: Writing – original draft, Writing – review & editing. AA: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Heidekrueger PI, Moellhoff N, Horch RE, Lohmeyer JA, Marx M, Heitmann C, et al. Overall complication rates of DIEP flap breast reconstructions in Germany-a multi-center analysis based on the DGPRÄC prospective national online registry for microsurgical breast reconstructions. J Clin Med. (2021) 10(5):1016. doi: 10.3390/jcm10051016
2. Thiessen FEF, Vermeersch N, Tondu T, Verhoeven V, Bersenji L, Sinove Y, et al. The evolution of breast reconstructions with free flaps: a historical overview. Acta Chir Belg. (2023) 123(4):454–62. doi: 10.1080/00015458.2023.2199497
3. Gruener JS, Horch RE, Geierlehner A, Mueller-Seubert W, Cai A, Arkudas A, et al. Is instillational topical negative pressure wound therapy in peri-prosthetic infections of the breast effective? A pilot study. J Pers Med. (2022) 12(12):2054. doi: 10.3390/jpm12122054
4. Holmström H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg. (1979) 13(3):423–27. doi: 10.3109/02844317909013092
5. Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg. (1989) 42(6):645–8. doi: 10.1016/0007-1226(89)90075-1
6. Chae MP, Hunter-Smith DJ, Rozen WM. Comparative analysis of fluorescent angiography, computed tomographic angiography and magnetic resonance angiography for planning autologous breast reconstruction. Gland Surg. (2015) 4(2):164–78. doi: 10.3978/j.issn.2227-684X.2015.03.06
7. Beier JP, Horch RE, Arkudas A, Dragu A, Schmitz M, Kneser U. Decision-making in DIEP and ms-TRAM flaps: the potential role for a combined laser Doppler spectrophotometry system. J Plast Reconstr Aesthet Surg. (2013) 66(1):73–9. doi: 10.1016/j.bjps.2012.08.040
8. Hauck T, Horch RE, Schmitz M, Arkudas A. Secondary breast reconstruction after mastectomy using the DIEP flap. Surg Oncol. (2018) 27(3):513. doi: 10.1016/j.suronc.2018.06.006
9. Promny T, Huberth P, Müller-Seubert W, Promny D, Cai A, Horch RE, et al. The impact of technical innovations and donor-site mesh repair on autologous abdominal-based breast reconstruction-A retrospective analysis. J Clin Med. (2024) 13(8):2165. doi: 10.3390/jcm13082165
10. Mohan AT, Saint-Cyr M. Advances in imaging technologies for planning breast reconstruction. Gland Surg. (2016) 5(2):242–54. doi: 10.3978/j.issn.2227-684X.2016.01.03
11. Frank K, Ströbel A, Ludolph I, Hauck T, May MS, Beier JP, et al. Improving the safety of DIEP flap transplantation: detailed perforator anatomy study using preoperative CTA. J Pers Med. (2022) 12(5):701. doi: 10.3390/jpm12050701
12. Masia J, Clavero JA, Larrañaga JR, Alomar X, Pons G, Serret P. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg. (2006) 59(6):594–9. doi: 10.1016/j.bjps.2005.10.024
13. Hauck T, Arkudas A, Horch RE, Ströbel A, May MS, Binder J, et al. The third dimension in perforator mapping-comparison of cinematic rendering and maximum intensity projection in abdominal-based autologous breast reconstruction. J Plast Reconstr Aesthet Surg. (2022) 75(2):536–43. doi: 10.1016/j.bjps.2021.09.011
14. Shaheen MS, Necker FN, Momeni A. ASO author reflections: reevaluating the role of preoperative CT-angiography in autologous breast reconstruction. Ann Surg Oncol. (2025) 32(9):6640–1. doi: 10.1245/s10434-025-17579-0
15. Thimmappa ND. MRA for preoperative planning and postoperative management of perforator flap surgeries: a review. J Magn Reson Imaging. (2024) 59(3):797–811. doi: 10.1002/jmri.28946
16. Jablonka EM, Wu RT, Mittermiller PA, Gifford K, Momeni A. 3-DIEPrinting: 3D-printed models to assist the intramuscular dissection in abdominally based microsurgical breast reconstruction. Plast Reconstr Surg Glob Open. (2019) 7(4):e2222. doi: 10.1097/GOX.0000000000002222
17. Cholok DJ, Fischer MJ, Leuze CW, Januszyk M, Daniel BL, Momeni A. Spatial fidelity of microvascular perforating vessels as perceived by augmented reality virtual projections. Plast Reconstr Surg. (2024) 153(2):524–34. doi: 10.1097/PRS.0000000000010592
18. Necker FN, Cholok DJ, Fischer MJ, Shaheen MS, Gifford K, Januszyk M, et al. HoloDIEP-faster and more accurate intraoperative DIEA perforator mapping using a novel mixed reality tool. J Reconstr Microsurg. (2025) 41(4):318–29. doi: 10.1055/s-0044-1788548
19. Necker FN, Cholok DJ, Shaheen MS, Fischer MJ, Gifford K, Le Castillo C, et al. Suture packaging as a marker for intraoperative image alignment in augmented reality on mobile devices. Plast Reconstr Surg Glob Open. (2024) 12(6):e5933. doi: 10.1097/GOX.0000000000005933
20. Schmid R, Schmidt SK, Hazur J, Detsch R, Maurer E, Boccaccini AR, et al. Comparison of hydrogels for the development of well-defined 3D cancer models of breast cancer and melanoma. Cancers (Basel). (2020) 12(8):2320. doi: 10.3390/cancers12082320
21. Promny T, Scherrer I, Kadam S, Schmid R, Jost T, Distel LV, et al. The effect of ionizing irradiation on the autotaxin-lysophasphatidic acid axis and interleukin-6/8 secretion in different breast cancer cell lines. J Pers Med. (2024) 14(9):968. doi: 10.3390/jpm14090968
22. Müller-Seubert W, Ostermaier P, Horch RE, Distel L, Frey B, Erber R, et al. The influence of different irradiation regimens on inflammation and vascularization in a random-pattern flap model. J Pers Med. (2023) 13(10):1514. doi: 10.3390/jpm13101514
23. Thiessen FEF, Tondu T, Cloostermans B, Dirkx YAL, Auman D, Cox S, et al. Dynamic InfraRed Thermography (DIRT) in DIEP-flap breast reconstruction: a review of the literature. Eur J Obstet Gynecol Reprod Biol. (2019) 242:47–55. doi: 10.1016/j.ejogrb.2019.08.008
24. Chrobot N, Alfertshofer M, Frank K, Smolka W, Anker A, Taxis J, et al. Advancing digital anthropometry in plastic surgery: comparing smartphone 3D surface imaging to Vectra H2 in breast reconstruction. J Plast Reconstr Aesthet Surg. (2025) 104:398–406. doi: 10.1016/j.bjps.2025.03.039
25. Ludolph I, Bettray D, Beier JP, Horch RE, Arkudas A. Leaving the perfusion zones? Individualized flap design in 100 free DIEP and ms-TRAM flaps for autologous breast reconstruction using indocyanine green angiography. J Plast Reconstr Aesthet Surg. (2022) 75(1):52–60. doi: 10.1016/j.bjps.2021.08.002
26. Geierlehner A, Horch RE, Ludolph I, Arkudas A. Intraoperative blood flow analysis of DIEP vs. ms-TRAM flap breast reconstruction combining transit-time flowmetry and microvascular indocyanine green angiography. J Pers Med. (2022) 12(3):482. doi: 10.3390/jpm12030482
27. Huntington CR, Prince J, Hazelbaker K, Lopes B, Webb T, LeMaster CB, et al. Safety first: significant risk of air embolism in laparoscopic gasketless insufflation systems. Surg Endosc. (2019) 33(12):3964–9. doi: 10.1007/s00464-019-06683-4
28. de Beaux AC, East B. Thoughts on trocar site hernia prevention. A narrative review. J Abdom Wall Surg. (2022) 1:11034. doi: 10.3389/jaws.2022.11034
29. Arellano JA, Comerci AJ, Liu HY, Alessandri Bonetti M, Nguyen VT, Parent B, et al. Complications in prolonged intraoperative ischemia time in free flap breast reconstruction: a systematic review and meta-analysis. Aesthetic Plast Surg. (2025) 49(5):1262–70. doi: 10.1007/s00266-024-04382-7
30. Wessel KJ, Dahmann S, Kueckelhaus M. Expanding applications and future of robotic microsurgery. J Craniofac Surg. (2025) 36(1):367–71. doi: 10.1097/SCS.0000000000010860
31. Ciudad P, Manrique OJ, Bustos SS, Vargas MI, Reynaga C, Agko M, et al. Combined microvascular breast and lymphatic reconstruction with deep inferior epigastric perforator flap and gastroepiploic vascularized lymph node transfer for postmastectomy lymphedema patients. Gland Surg. (2020) 9(2):512–20. doi: 10.21037/gs.2020.01.14
32. Almadani H, Lu J, Bokhari S, How-Volkman C, Brazio PS. Simultaneous vascularized lymph node transfer and breast reconstruction: a systematic review. J Clin Med. (2025) 14(5):1694. doi: 10.3390/jcm14051694
33. Kappos EA, Haas Y, Schulz A, Peters F, Savanthrapadian S, Stoffel J, et al. The LYMPH trial: comparing microsurgical with conservative treatment for chronic breast cancer-associated lymphoedema - study protocol of a pragmatic randomised international multicentre superiority trial. BMJ Open. (2025) 15(2):e090662. doi: 10.1136/bmjopen-2024-090662
34. Haas Y, Williams OP, Masia J, Pons G, Taylor EM, Katapodi MC, et al. Microsurgical versus complex physical decongestive therapy for chronic breast cancer-related lymphoedema. Cochrane Database Syst Rev. (2025) 2(2):Cd016019. doi: 10.1002/14651858.CD016019
35. Nagel SS, Thomas B, Bigdeli AK, Hirche C, Kneser U, Radu CA. Postoperative monitoring of free muscle flaps using perforator-based adipocutaneous skin paddles: economy, quality of care and aesthetics. Handchir Mikrochir Plast Chir. (2022) 54(2):139–48. doi: 10.1055/a-1655-9135
36. Malagón P, Taghizadeh R, Torrano L, González J. A new protocol for improving immediate monitoring of skin-island free flap with near-infrared spectroscopy and ultrasound. J Plast Reconstr Aesthet Surg. (2023) 83:334–42. doi: 10.1016/j.bjps.2023.04.029
37. Lindelauf A, Vranken NPA, Rutjens VGH, Schols RM, Heijmans JH, Weerwind PW, et al. Economic analysis of noninvasive tissue oximetry for postoperative monitoring of deep inferior epigastric perforator flap breast reconstruction: a review. Surg Innov. (2020) 27(5):534–42. doi: 10.1177/1553350620942985
38. Ooms M, Winnand P, Heitzer M, Peters F, Bock A, Katz M, et al. Flap perfusion monitoring with an attached surface probe in microvascular reconstruction of the oral cavity. Clin Oral Investig. (2023) 27(9):5577–85. doi: 10.1007/s00784-023-05177-x
39. Fischer JC, Parker PM, Shaw WW. Comparison of two laser doppler flowmeters for the monitoring of dermal blood flow. Microsurgery. (1983) 4(3):164–70. doi: 10.1002/micr.1920040304
40. Um GT, Chang J, Louie O, Colohan SM, Said HK, Neligan PC, et al. Implantable Cook-Swartz Doppler probe versus Synovis Flow Coupler for the post-operative monitoring of free flap breast reconstruction. J Plast Reconstr Aesthet Surg. (2014) 67(7):960–6. doi: 10.1016/j.bjps.2014.03.034
41. Ooms M, Winnand P, Heitzer M, Bock A, Katz M, Bickenbach J, et al. Confounding effects of blood hemoglobin and hematocrit levels on flap perfusion measurement with the oxygen-to-see (O2C) analysis system in microvascular head and neck reconstruction- a retrospective study. BMC Surg. (2025) 25(1):145. doi: 10.1186/s12893-025-02888-8
42. Kneser U, Beier JP, Schmitz M, Arkudas A, Dragu A, Schmidt VJ, et al. Zonal perfusion patterns in pedicled free-style perforator flaps. J Plast Reconstr Aesthet Surg. (2014) 67(1):e9–e17. doi: 10.1016/j.bjps.2013.09.006
43. Kleiss SF, Michi M, Schuurman SN, de Vries JP, Werker PM, de Jongh SJ. Tissue perfusion in DIEP flaps using indocyanine green fluorescence angiography, hyperspectral imaging, and thermal imaging. JPRAS Open. (2024) 41:61–74. doi: 10.1016/j.jpra.2024.04.007
44. Pruimboom T, Lindelauf AA, Felli E, Sawor JH, Deliaert AE, van der Hulst RR, et al. Perioperative hyperspectral imaging to assess mastectomy skin flap and DIEP flap perfusion in immediate autologous breast reconstruction: a pilot study. Diagnostics (Basel). (2022) 12(1):184. doi: 10.3390/diagnostics12010184
45. Müller-Seubert W, Cai A, Horch RE. Application of near infrared spectroscopy to enhance safety and individualize distraction of severely contracted joints in far-advanced dupuytren’s disease. J Clin Med. (2024) 13(14):4025. doi: 10.3390/jcm13144025
46. Müller-Seubert W, Cai A, Arkudas A, Ludolph I, Fritz N, Horch RE. A personalized approach to treat advanced stage severely contracted joints in dupuytren’s disease with a unique skeletal distraction device-utilizing modern imaging tools to enhance safety for the patient. J Pers Med. (2022) 12(3):378. doi: 10.3390/jpm12030378
47. Ozmen BB, Phuyal D, Berber I, Schwarz GS. Artificial intelligence prediction model for readmission after DIEP flap breast reconstruction based on NSQIP data. J Plast Reconstr Aesthet Surg. (2025) 106:1–8. doi: 10.1016/j.bjps.2025.04.028
48. Chen J, Gabay A, Kim M, Amakiri U, Boe LA, Stern C, et al. AI risk prediction tools for alloplastic breast reconstruction. Plast Reconstr Surg. (2025). doi: 10.1097/PRS.0000000000012124 PMID: 40178533
49. Seth I, Lim B, Joseph K, Gracias D, Xie Y, Ross RJ, et al. Use of artificial intelligence in breast surgery: a narrative review. Gland Surg. (2024) 13(3):395–411. doi: 10.21037/gs-23-414
50. Timóteo R, Pinto D, Matono P, Mavioso C, Cardoso M, Gouveia P, et al. BREAST+: an augmented reality interface that speeds up perforator marking for DIEAP flap reconstruction surgery. Healthc Technol Lett. (2024) 11(6):301–6. doi: 10.1049/htl2.12095
Keywords: breast cancer, breast reconstruction, autologous breast reconstruction, 3D imaging, artificial intelligence
Citation: Scherrer I, Horch RE, Promny D, Promny T, Eschenbacher E and Arkudas A (2025) Pushing the boundaries in autologous breast reconstruction: innovations from imaging to artificial intelligence. Front. Surg. 12:1679524. doi: 10.3389/fsurg.2025.1679524
Received: 4 August 2025; Accepted: 4 September 2025;
Published: 26 September 2025.
Edited by:
Joseph M. Escandón, Wyckoff Heights Medical Center, United StatesReviewed by:
Gianluca Marcaccini, University of Siena, ItalyCopyright: © 2025 Scherrer, Horch, Promny, Promny, Eschenbacher and Arkudas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabell Scherrer, aXNhYmVsbC5zY2hlcnJlckB1ay1lcmxhbmdlbi5kZQ==
 Isabell Scherrer
Isabell Scherrer Raymund E. Horch
Raymund E. Horch Dominik Promny
Dominik Promny Theresa Promny
Theresa Promny Andreas Arkudas
Andreas Arkudas