- 1Department of Orthopedics, Sixian Hospital of Traditional Chinese Medicine, Suzhou, Anhui, China
- 2Department of Spinal Surgery, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
Background: Iatrogenic lumbosacral nerve bowstringing disease (ILNBD) is a severe complication of spinal surgery. The clinical presentation is often insidious and may be misdiagnosed as nerve root compression. Heightened diagnostic vigilance supported by characteristic radiological findings is essential.
Case presentation: This case report a 63-year-old woman who underwent L3–L5 interbody fusion and internal fixation surgery for vertebral slippage combined with lumbar spinal stenosis. Her symptoms improved significantly on the second day after surgery, but she suddenly developed lower limb neurological dysfunction on the fifth day after surgery. Imaging studies showed that the internal fixation and interbody fusion devices were well positioned, with increased spinal canal volume and no space-occupying lesions. However, axial MRI revealed high tension of the cauda equina and positive nerve descent signs, consistent with the characteristics of bowstring disease.
Conclusion: Excessive expansion of the intervertebral space can lead to sustained static traction on the nerve roots. The appropriate fusion device height should be determined through a stepwise expansion test to avoid increased axial tension on the nerves. Additionally, technical operational details can independently induce bowstring syndrome, and stepwise tension assessment is crucial for preventing traction-related nerve damage.
Background
Iatrogenic lumbosacral nerve bowstringing disease (ILNBD) is a rare but severe complication following spinal surgery (1). Its core pathology involves axial traction injury to the dura mater or nerve roots due to surgical interventions (Iaminectomy, fusion device implantation, and multilevel correction). This results in loss of physiological buffering capacity, generating a nerve axis hypertension state analogous to a taut bowstring. Consequently, pathological traction during spinal motion is exacerbated, ultimately causing neurological dysfunction. Clinical manifestations mimic persistent nerve root compression or spinal cord lesions, including refractory pain, sensory deficits, hyperreflexia, and in severe cases, foot drop or cauda equina syndrome, significantly impairing patients’ quality of life (2, 3). This report presents a case of intractable low back pain with radiating lower extremity pain, hyperreflexia, and sensory abnormalities following lumbar fusion with internal fixation. Postoperative imaging revealed no compressive lesions within the spinal canal, but MRI indicated cauda equina hypertension (4, 5). Based on clinical features and literature review, iatrogenic traction neuropathy was diagnosed. We herein analyze the clinical characteristics, key imaging findings, and pathogenesis to enhance awareness of this subtle postoperative complication.
Case presentation
A 63 year old female patient came to our hospital for treatment due to bilateral lower limb pain accompanied by intermittent claudication for over six months. She was diagnosed with L4 vertebral body slippage, L3–S1 lumbar disc herniation, and lumbar spinal stenosis. Her preoperative Visual Analogue Scale (VAS) pain score was 6 (Figure 1). After successful anesthesia, a midline incision is made around the L4 spinous process, exposing the L3–L5 vertebral plates, facet joints, and needle insertion points layer by layer. After confirming the gap, implant 65 * 4.5 mm universal pedicle screws, pre-bend the connecting rod and install it, appropriately widen each gap, and lock it. Bite off the spinous process of the lumbar spine, decompress the left vertebral plate, and bite the bitten vertebral plate and spinous process bone into granules for bone grafting. During the operation, spinal stenosis was observed, and decompression was performed. The compressed dura mater was restored, and the slipped vertebral body was reduced. The nerve root was protected by a rectangular incision of the posterior longitudinal ligament and annulus fibrosus. The nucleus pulposus was scraped off, the intervertebral space was treated, and the intervertebral cartilage plate was scraped off. An appropriate amount of autogenous bone was taken and placed in the intervertebral space. A 12 * 22 mm intervertebral fusion cage was placed in the L4–5 space, with the posterior edge of the cage about 5 mm away from the posterior edge of the vertebral body. The L4–5 space was appropriately compressed. Reprocess the L3–4 intervertebral space, protect the nerve root by rectangular incision of the posterior longitudinal ligament and annulus fibrosus, scrape off the nucleus pulposus, prepare the intervertebral space, scrape off the intervertebral cartilage plate, take an appropriate amount of autogenous bone and insert it into the intervertebral space, and insert a 12 * 22 mm intervertebral fusion device into the L3–4 space. Rinse the wound thoroughly, stop bleeding, and fix firmly. An x-ray shows that the pedicle screws are well positioned, the fusion device is in good position, and the slipped vertebral body has been reduced. After checking the dressing and instruments, suture layer by layer, insert a drainage tube into the incision, connect it to a negative pressure ball, close the incision, and cover it with a sterile dressing. The surgery went smoothly, with intraoperative bleeding of approximately 250 ml. The anesthesia was satisfactory, and the patient returned safely to the ward.
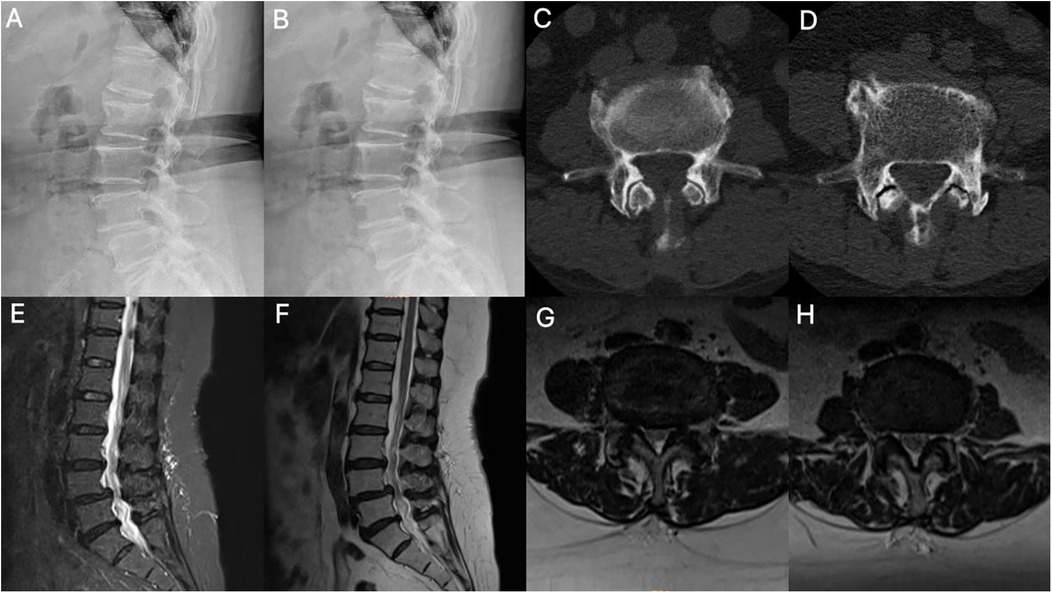
Figure 1. Imaging findings. (A,B) Dynamic lumbar radiographs demonstrate instability with spondylolisthesis. (C,D) Axial CT of the lumbar spine showed segments L3–4 and L4–5 with degenerative changes and lumbar disc herniation. (E-H) MRI shows disc herniation, folded ligamentum flavum causing pincer-type compression of the cauda equina, facet joint hypertrophy, and spinal canal stenosis.
Reviewing the surgical procedure, it is noteworthy that before implanting the fusion device, the method for expanding the intervertebral space relied on the surgeon's tactile experience with the flexibility of the space during surgery. No intervertebral spreader was used to perform objective, stepwise testing, nor was annular tension established as a quantifiable endpoint. This approach may introduce subjective error in the final determination of intervertebral space height, potentially increasing the risk of excessive expansion. Additionally, fusion cages of identical height were implanted in the L3–4 level. During multi-level fusion procedures, particular attention must be paid to restoring intervertebral space height while avoiding excessive expansion. Such practices may reduce the redundant volume within the spinal canal and neural foramina, resulting in persistent axial traction on the cauda equina and nerve root tails. This creates a potential risk for postoperative deterioration of neurological function.
On postoperative day 2, the patient reported significant relief of bilateral lower limb pain, with the VAS score improving to 3 points, representing a marked improvement compared to preoperative levels. By day 5, the VAS score worsened to 7 points, and new-onset bilateral lower limb numbness (particularly around the knees) accompanied by shooting pain emerged. These symptoms showed no significant improvement after treatment with corticosteroids and neurotropic agents. On day 7, numbness progressed with the development of saddle anesthesia, and bilateral lower limb muscle strength decreased to grade IV (Table 1). Repeat imaging demonstrated satisfactory reduction of spondylolisthesis with appropriate cage positioning and screw placement on lumbar radiographs. Lumbar MRI revealed no spinal stenosis or nerve root compression, showing instead expanded spinal canal volume without space-occupying lesions or foraminal obstruction. However, axial MRI sequences displayed positive nerve sedimentation signs, and the cauda equina exhibited a hyperintense, high-tension state (Figure 2).
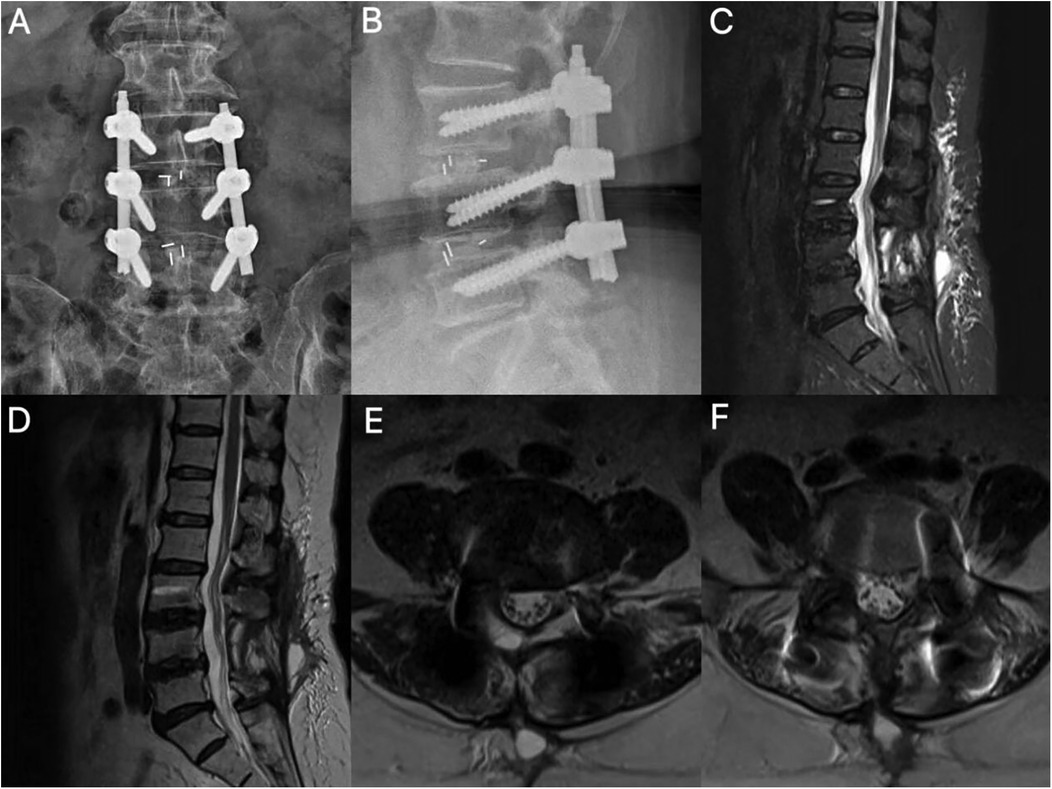
Figure 2. Postoperative imaging. (A,B) Lumbar radiographs demonstrate accurate positioning of L3–L5 pedicle screws and optimal interbody cage placement. (C,D) Sagittal MRI reveals spinal canal expansion and localized epidural fibrosis. (E,F) Segments L3–4 and L4–5 are shown with no space-occupying lesions; note the ventral cauda equina apposed to the dural sac in a hypertension state with a positive sedimentation sign (+).
Discussion and conclusions
Traditionally, postoperative neurological complications following spinal surgery have been attributed to direct mechanical compression (e.g., hematoma, fusion device displacement) or ischemic injury. However, the pathological core of ILNBD lies in the pathological increase in neural axial tension, which can independently cause disease even in the absence of obvious space-occupying compression (6, 7). In this case, the patient's symptoms improved early postoperatively, suggesting that the initial decompression was effective (8). However, on the fifth day, the patient suddenly developed progressive worsening of sensory-motor deficits in both lower limbs and numbness in the saddle region. Imaging studies ruled out common causes such as hematoma, shunt displacement, or residual compression, but axial MRI showed a positive nerve descent sign (9, 10). This finding strongly suggests that the cauda equina has lost its normal floating relaxation state and is in an axial traction state, consistent with the characteristic imaging findings of bowstring disease.
Upon reviewing the surgical procedure and relevant literature, we believe that the occurrence of iatrogenic lumbosacral neural arch string disease in this case was most likely due to technical errors in the highly controlled intervertebral fusion technique. Although the spinal cord and nerve roots possess a certain degree of elasticity, the surgeon relied on experience to select the size of the fusion device, neglecting the stepwise testing of the intervertebral spreader, which led to excessive expansion of the intervertebral space. This maneuver directly caused sustained static traction on the nerve roots and cauda equina at the nerve root exit point, resulting in progressively increasing axial tension and ultimately leading to neural conduction blockage (11–13). From a neurobiomechanical perspective, the pathological process is not merely simple mechanical traction. Although the spinal cord and nerve roots possess some elasticity, neural tissue has a critical threshold for tolerating traction injury. When axial tension applied to the cauda equina and nerve roots exceeds their physiological range of extension, this persistent static traction first compresses the microvascular system within the nerves, obstructing blood flow. This leads to ischemia and hypoxia of the nerve fascicle sheath, causing ischemic damage to the nerves. These injuries ultimately result in neurological dysfunction, triggering symptoms such as pain and sensory abnormalities (12, 14). The diagnosis of ILNBD is often insidious because it lacks specific clinical manifestations and is easily confused with common postoperative conditions such as nerve root compression, epidural fibrosis, or even early inflammatory reactions (3). Symptoms often appear several days or even later after surgery and progressively worsen. Conventional dehydration and hormone therapy often yield poor results, further complicating diagnosis. Imaging is key to differential diagnosis. The diagnostic turning point in this case was the axial MRI: although sagittal and coronal views showed enlarged spinal canal volume, no mass lesions, and unobstructed nerve root exit, the axial plane clearly captured the characteristic “positive sedimentation sign” (15, 16). The sedimentation sign (Lack of Nerve Root Sedimentation Sign) refers to the failure of the cauda equina nerve bundle to settle to the dorsal side of the dural sac under the influence of gravity in the supine axial MRI, instead tightly adhering to the ventral sac wall, strongly suggesting that the nerves are in a state of axial high tension and have lost their normal floating relaxation (17). This sign is of significant value in identifying nerve traction injuries without obvious compression. Therefore, for patients who develop unexplained, progressive neurological dysfunction postoperatively, it is essential to carefully analyze axial MRI sequences to identify features of cauda equina syndrome, such as high tension and the sedimentation sign, to avoid misdiagnosis or missed diagnosis (18).
The key lesson from this case is that while restoring intervertebral disc height is an important goal of fusion surgery, it should not be pursued blindly. Overemphasizing height restoration while ignoring individual differences and neural tolerance is the primary technical risk leading to ILNBD. We should use annular tension as an important reference for individualized fusion. After endplate preparation, the spreader should be incrementally increased from the minimum height until the annulus reaches appropriate tension—where the instrument can slide freely without elastic recoil. Additionally, a fusion device 1–2 mm smaller than the test value may be selected to compensate for postoperative subsidence and account for residual intervertebral soft tissue space. This case highlights that iatrogenic manipulation can independently trigger ILNBD. Spinal surgeons must thoroughly understand the pathophysiological mechanisms, clinical manifestations, and imaging characteristics of ILNBD and incorporate them into the important considerations for postoperative neurological dysfunction.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Sixian Hospital of traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HG: Conceptualization, Formal analysis, Writing – original draft. LT: Conceptualization, Formal analysis, Writing – original draft. YT: Supervision, Writing – original draft. HaZ: Funding acquisition, Writing – original draft. HeZ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shi JG, Xu XM, Sun JC, Wang Y, Guo YF, Yang HS, et al. Lumbosacral nerve bowstring disease. Zhonghua Yi Xue Za Zhi. (2017) 97(11):852–6. doi: 10.3760/cma.j.issn.0376-2491.2017.11.012
2. Barz T, Melloh M, Staub LP, Lord SJ, Lange J, Röder CP, et al. Nerve root sedimentation sign: evaluation of a new radiological sign in lumbar spinal stenosis. Spine (Phila Pa 1976). (2010) 35(8):892–7. doi: 10.1097/BRS.0b013e3181c7cf4b
3. Kong QJ, Xu XM, Sun JC, Wang Y, Shi GD, Guo YF, et al. Analysis of correlation between spinal nerve high tension and disc degeneration in 100 cases. Zhonghua Yi Xue Za Zhi. (2017) 97(43):3416–20. doi: 10.3760/cma.j.issn.0376-2491.2017.43.012
4. Ishak B, Pierre CA, Ansari D, Lachkar S, von Glinski A, Unterberg AW, et al. A novel anatomo-physiologic high-grade spondylolisthesis model to evaluate L5 nerve stretch injury after spondylolisthesis reduction. Neurosurg Rev. (2022) 45(2):1741–6. doi: 10.1007/s10143-021-01721-z
5. Yang J, Feng Z, Chen N, Hong Z, Zheng Y, Yang J, et al. Gravity determines the direction of nerve roots sedimentation in the lumbar spinal canal. BMC Musculoskelet Disord. (2021) 22(1):154. doi: 10.1186/s12891-021-04032-y
6. Elkahla G, Trifa A, Darmoul M. The validity of posterior approach in thoracolumbar spine fracture surgery: a study of 104 cases with literature review. J Craniovertebr Junction Spine. (2025) 16(2):212–7. doi: 10.4103/jcvjs.jcvjs_50_25
7. Sai Reddy Munugala C, Biradar R. A prospective study on the functional outcomes of surgical management of lumbar and lumbosacral spondylolisthesis using pedicle screw fixation and posterolateral fusion. Cureus. (2025) 17(5):e83934. doi: 10.7759/cureus.83934
8. Sailhan F, Gollogly S, Roussouly P. The radiographic results and neurologic complications of instrumented reduction and fusion of high-grade spondylolisthesis without decompression of the neural elements: a retrospective review of 44 patients. Spine (Phila Pa 1976). (2006) 31(2):161–9. doi: 10.1097/01.brs.0000194780.17528.6b
9. Stuart MJ, Burnett A, Webster JD. Cauda equina syndrome and severe lumbar-sacral radiculopathy in general practice: finding the needle in the haystack. Aust J Gen Pract. (2025) 54(7):439–43. doi: 10.31128/AJGP-08-24-7389
10. Tawa N, Rhoda A, Diener I. Accuracy of clinical neurological examination in diagnosing lumbo-sacral radiculopathy: a systematic literature review. BMC Musculoskelet Disord. (2017) 18(1):93. doi: 10.1186/s12891-016-1383-2
11. Singh A, Kallakuri S, Chen C, Cavanaugh JM. Structural and functional changes in nerve roots due to tension at various strains and strain rates: an in vivo study. J Neurotrauma. (2009) 26(4):627–40. doi: 10.1089/neu.2008.0621
12. Kobayashi S, Suzuki Y, Asai T, Yoshizawa H. Changes in nerve root motion and intraradicular blood flow during intraoperative femoral nerve stretch test. Report of four cases. J Neurosurg. (2003) 99(3 Suppl):298–305. doi: 10.3171/spi.2003.99.3.0298
13. Nishida N, Kanchiku T, Ohgi J, Ichihara K, Chen X, Taguchi T. Mechanical properties of nerve roots and rami radiculares isolated from fresh pig spinal cords. Neural Regen Res. (2015) 10(11):1869–73. doi: 10.4103/1673-5374.170319
14. Takamori Y, Arimizu J, Izaki T, Naito M, Kobayashi T. Combined measurement of nerve root blood flow and electrophysiological values: intraoperative straight-leg-raising test for lumbar disc herniation. Spine (Phila Pa 1976). (2011) 36(1):57–62. doi: 10.1097/BRS.0b013e3181ccb1d4
15. Ogikubo O, Forsberg L, Hansson T. The relationship between the cross-sectional area of the cauda equina and the preoperative symptoms in central lumbar spinal stenosis. Spine (Phila Pa 1976). (2007) 32(13):1423–8. doi: 10.1097/BRS.0b013e318060a5f5
16. Schizas C, Theumann N, Burn A, Tansey R, Wardlaw D, Smith FW, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976). (2010) 35(21):1919–24. doi: 10.1097/BRS.0b013e3181d359bd
17. Chen J, Xu Z, Wu H, Feng Z, Zhao F. Diagnostic efficacy of nerve root sedimentation sign in post-lumbar interbody fusion patients: an imaging evaluation and modification. J Orthop Surg Res. (2025) 20(1):258. doi: 10.1186/s13018-025-05672-9
18. Kanno H, Ozawa H, Koizumi Y, Morozumi N, Aizawa T, Kusakabe T, et al. Dynamic change of dural sac cross-sectional area in axial loaded magnetic resonance imaging correlates with the severity of clinical symptoms in patients with lumbar spinal canal stenosis. Spine (Phila Pa 1976). (2012) 37(3):207–13. doi: 10.1097/BRS.0b013e3182134e73
Keywords: iatrogenic lumbosacral nerve bowstringing disease, axial nerve tension, lumbarfusion with internal fixation surgery, nerve damage, intervertebral space height
Citation: Gao H, Tian L, Tong Y, Zhang H and Zhang H (2025) Revised understanding of iatrogenic lumbosacral nerve bowstringing disease: a case report and literature review. Front. Surg. 12:1681708. doi: 10.3389/fsurg.2025.1681708
Received: 7 August 2025; Accepted: 29 September 2025;
Published: 14 October 2025.
Edited by:
Siying Song, MD Anderson Cancer Canter, United StatesReviewed by:
Chenglong Wang, Southwest Medical University, ChinaShaocheng Liu, Capital Medical University, China
Copyright: © 2025 Gao, Tian, Tong, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heling Zhang, NjQ2MDgwNTM1QHFxLmNvbQ==
†These authors have contributed equally to this work
 Haoran Gao
Haoran Gao Lei Tian1†
Lei Tian1† Heling Zhang
Heling Zhang