- 1Department of Gastroenterology, Lu'an Hospital of Anhui Medical University, Lu'an, Anhui, China
- 2Department of Gastroenterology, Lu'an People's Hospital of Anhui Province, Lu'an, Anhui, China
- 3Department of Pathology, Lu'an Hospital of Anhui Medical University, Lu'an, Anhui, China
- 4Department of Gastroenterology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, China
Background: A gastric hamartomatous inverted polyp (GHIP) is an uncommon submucosal neoplasm that is histopathologically defined by a submucosal inverted growth of cystically dilated hyperplastic gastric glands.
Case presentation: A 74-year-old Chinese man presented with a submucosal tumor (SMT) in the cardia, identified through electronic gastroscopy. This report presents a case of cardia hamartomatous inverted polyp (CHIP), which represent a rare histological variant of gastric polyps that pose diagnostic challenges. The endoscopic examination revealed the presence of a submucosal tumor, and endoscopic ultrasonography indicated a heterogeneous tumor predominantly situated within the third (submucosal) layer. Immunohistochemistry outcomes indicated MUC5AC (+), MUC6 (+), Syn (+), Ki-67 (+, approximately 5%), Desmin (+), SMA (+), as well as MUC2 (−). To achieve en bloc resection for lesions >1.0 cm, endoscopic submucosal dissection (ESD) was performed. The pathological evaluation confirmed the diagnosis of CHIP. The patient was discharged without experiencing any complications.
Conclusion: Therefore, the ESD approach may be particularly suitable for the management of SMT-type hamartomatous inverted polyps.
Background
An atypical form of gastric polyp, which resembles a submucosal tumor, has been identified as a hamartomatous polyp (1). These polyps are composed of the gastric mucosa and a submucosal component characterized by the proliferation of pseudo-pyloric glands, cystic glands, and bundles of smooth muscle (2). Despite being classified as hamartomatous or ectopic, the histogenetic origins of these polyps remain unresolved. The preoperative diagnosis of gastric hamartomatous inverted polyp (GHIP) poses significant challenges due to its infrequency. In this report, we present a case of a hamartomatous inverted polyp located in the cardia, exhibiting characteristics of a submucosal tumor (SMT), which was successfully excised using the endoscopic submucosal dissection (ESD) technique without any complications.
Case report
A 74-year-old male patient was admitted to Lu'an People's Hospital for the evaluation and management of a SMT located in the cardia, measuring over 1.0 cm in diameter, which had been identified through upper gastrointestinal endoscopy. The primary complaint of the patient was abdominal bloating persisting for nearly 3 years, and no other familial conditions were detected, including cancers. The patient had not undergone gastroscopy or abdominal surgery before. The patient had irregularly taken proton pump inhibitors (PPIs) in the past due to abdominal bloating. The patient's abdominal bloating had shown minimal to no improvement with intermittent PPIs use over the 3-year period, which was a contributing factor in the decision to proceed with a comprehensive endoscopic evaluation to rule out an underlying structural cause. The patient had a history of hypertension for 2 years, with irregular administration of nifedipine sustained-release tablets, and his blood pressure was well-controlled. The patient denied having any other diseases or a history of previous surgeries. The patient is a farmer and has no mental or psychological issues.
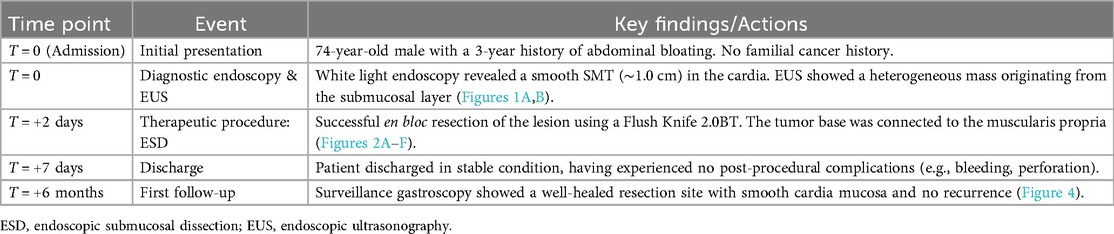
Upon examination, no pigmentation was observed in the oral cavity or on the lips. The patient's abdomen was flat and soft, without varicose veins on the abdominal wall. Vital signs such as respiratory rate and heart rate were within normal range, and there were no murmurs on auscultation of the heart and lungs. The physical examination and laboratory results upon admission did not reveal any abnormalities. Upper gastrointestinal endoscopy confirmed the presence of an SMT in the cardia, which exhibited no apparent erosion (see Figure 1A). Endoscopic ultrasonography indicated a heterogeneous tumor predominantly situated within the third (submucosal) layer (see Figure 1B). These observations raised the suspicion of a malignant neoplasm; however, a definitive diagnosis could not be established based solely on these findings. Consequently, it was deemed necessary to perform resection to obtain an accurate diagnosis and determine the most appropriate treatment. Given the challenges associated with conventional endoscopic mucosal resection (EMR) techniques, such as strip biopsy and EMR using a cap-fitted endoscope, we opted to utilize the ESD technique to achieve complete tumor resection in a single en bloc.
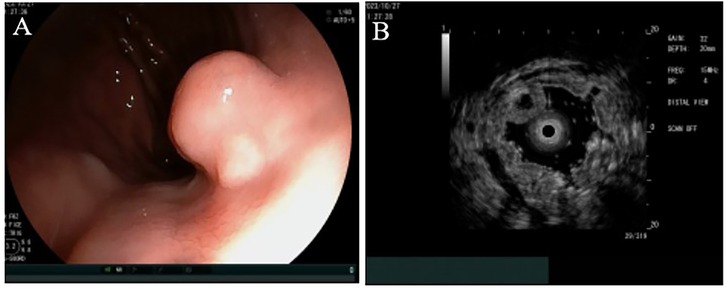
Figure 1. Endoscopic findings. (A) A hemispherical smooth submucosal mass was observed on white light endoscopy. (B) Endoscopic ultrasonography showed a hypoechoic mass with uniform internal echo originating from the third (submucosal) layer.
ESD procedure
The ESD procedure successfully resulted in the complete removal of the tumor, which measured 1.0 cm in diameter (see Figure 2). In details, The ESD procedure was performed under general anesthesia with the patient in the left lateral position. We used a standard single-channel gastroscope and a transparent cap attached to the tip. A mixed solution of indigo carmine, epinephrine (0.01%), and saline was injected into the submucosa around the lesion to create a cushion and lift the tumor. A circumferential mucosal incision was made around the lesion. Subsequent submucosal dissection was carefully performed using the same knife in Swift Coagulation mode, meticulously separating the tumor from the underlying muscularis propria layer to achieve en bloc resection. Hemostasis during the procedure was achieved using the knife in soft coagulation mode or with hemostatic forceps as needed. The resected specimen, measuring 1.0 cm × 1.0 cm, was retrieved with a Roth net and pinned flat on a foam board for formalin fixation and pathological assessment.
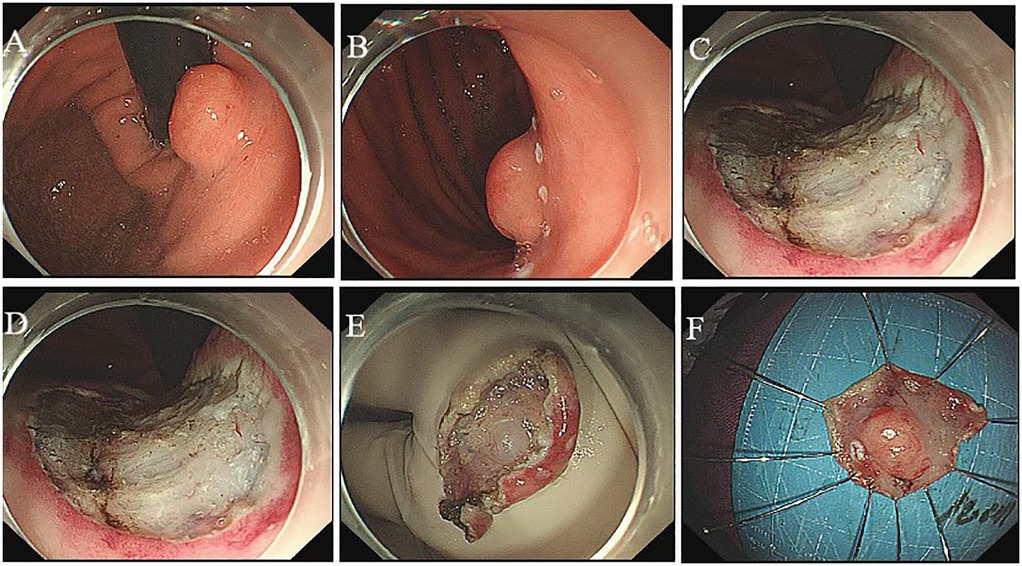
Figure 2. Endoscopic submucosal dissection. The flushing knife 2.0BT was used to cut along the margin to expose the submucosal white tumor (A,B), the base of which was connected to the muscular layer and completely peeled along the margin of the tumor (C–F).
Histological examination
Histological examination of the tumor revealed a proliferation of the mucosal layer characterized by glandular and cystic structures, which exhibited no cytological atypia. The surface of the polyp was lined with gastric mucosa resembling either fundic or pyloric gland types. The glandular formations comprised a variety of epithelial cell types, including pyloric or mucous-neck cells, surface mucous (foveolar) cells, and parietal-like cells. Immunohistochemistry results showed: MUC5AC (+), MUC6 (+), Syn (+), Ki-67 (+, approximately 5%), Desmin (+), SMA (+), as well as MUC2 (−) (Figure 3). Based on the endoscopic findings and histopathological features, the diagnosis of an inverted polyp associated with a cardia hamartoma was established. The patient was discharged 7 days post-ESD without any complications.
Figure 3. Pathology findings. Immunohistochemistry: (A) MUC5AC (+), (B) MUC6 (+), (C) Syn (+), (D) Ki-67 (+, approximately 5%), (E) Desmin (+), (F) SMA (+), as well as (G) MUC2 (−). HE staining: Microscopy of the endoscopic resection specimen revealed hypertrophy of the polyp component in the submucosal layer (H) Bar, 200 μm.
Clinical timeline and follow up
The clinical timeline was as follows: T = 0 (initial presentation); T = 2 days (ESD procedure); T = 7 days (discharge). A follow-up visit was conducted at T = 6 months, during which the patient underwent gastroscopy in the outpatient department (Table 1). The results indicated that the patient's cardia mucosa was smooth and without protrusions (see Figure 4). Given the documented, albeit rare, association between GHIP and the development of gastric adenocarcinoma (3), a long-term monitoring plan was established. The patient is scheduled for repeat annual endoscopic surveillance for a minimum of 5 years. Furthermore, due to the known correlation with chronic mucosal inflammation, the patient's status for H. pylori infection and atrophic gastritis will be monitored closely; if present, more frequent biannual endoscopy would be recommended (4).
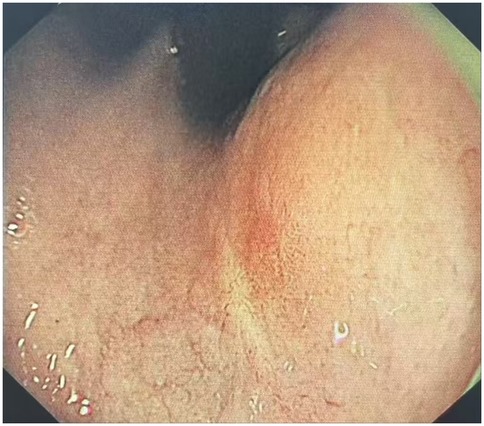
Figure 4. Endoscopic performance at 6 months post-ESD. The results indicated that the patient's cardia mucosa was smooth and without protrusions.
Brief patient perspective
The patient provided perspective on his experience, stating: The constant bloating that bothered me for years is finally gone after the procedure. I feel relieved to have a clear diagnosis and am happy with the result. This highlights the significant improvement in quality of life following the successful endoscopic treatment.
Discussion
Gastric lesions associated with familial colonic polyposis, referred to as GHIP, constitute less than 1% of all gastric polyps (5). Typically, GHIP is asymptomatic; however, some patients may experience abdominal discomfort without distinct clinical features. In certain instances, it may manifest as intestinal obstruction or anemia resulting from chronic blood loss (6, 7). Additionally, the condition has been linked to deep cystic gastritis (3). In the case presented, the patient exhibited no specific clinical symptoms, aside from abdominal bloating. Previous studies indicate that there is no significant gender bias in the occurrence of these lesions, which are considered to be acquired rather than congenital, as they predominantly arise in older adults (2).
Gastric hamartomatous polyps are classified as benign lesions from a pathological perspective; however, approximately 20% of these polyps are found to coexist with gastric adenocarcinoma (4). CHIP is a rare lesion (<1% of gastric polyps) with no unique symptoms (e.g., abdominal bloating only). This can lead to misdiagnosis as common gastrointestinal disorders. In some cultural contexts, non-specific symptoms like bloating may be dismissed as “indigestion” or treated with traditional remedies, delaying formal evaluation. Consequently, the diagnosis and management of these polyps present significant clinical challenges. The therapeutic approach for gastric hamartomatous polyps is influenced by the characteristics and dimensions of the tumors. There are two distinct categories of hamartomatous inverted polyps: those that are stalkless, referred to as the “submucosal tumor (SMT) type,” due to their inversion into the submucosal layer, and those that possess a stalk, known as the “polyp type.” Endoscopic resection is typically performed for all stalked polyps, whereas surgical resection is mandated for stalkless polyps (SMT type), as previous studies have indicated that endoscopic resection of these lesions often results in incomplete removal (2). A recent case study identified low-grade intraepithelial neoplasia in certain areas of postoperative pathology, indicating a potential association between gastric hamartomatous inverted polyps (GHIP) and the development of gastric cancer, thereby highlighting the risk of subsequent gastric cancer progression (8). Furthermore, a recent investigation of the adjacent mucosa in ten patients with GHIP revealed that six patients (60%) exhibited atrophic gastritis or gastritis associated with Helicobacter pylori, while four patients (40%) were diagnosed with non-specific gastritis (9).
GHIP is believed to arise from the infiltration of the mucosa through the muscularis layer, as well as from mucosal crevices or defects resulting from recurrent erosion (10). Typically, GHIP is identified as an isolated submucosal mass during endoscopic procedures (11). An endoscopic evaluation conducted by Dohi indicated that the lesion was enveloped by a normal mucous membrane, characterized by a distinct irregular depression at its apex (12). In a case documented by Takuma Okamura, the use of magnification endoscopy and narrow-band imaging revealed a reddened area surrounding the depression, along with an irregular arrangement of villi and pits, lacking a clear demarcation (13). Consequently, diagnosing GHIP based solely on endoscopic findings presents challenges. Endoscopic ultrasonography typically demonstrates heterogeneous tumors with cystic components. In the case reported by Okamura, endoscopic ultrasonography identified a nonuniform tumor with cystic areas situated within the third layer (13). Additionally, the case presented by Moyu Dohi exhibited a heterogeneous tumor originating from the second layer, characterized by small cystic hypoechoic spots observed via ultrasound endoscopy (12). In this instance, endoscopic ultrasonography confirmed the presence of a heterogeneous tumor, predominantly located within the third (submucosal) layer.
Histologically, the tumor is distinguished by submucosal gland hyperplasia and the presence of cystic structures. The polyp's surface exhibits characteristics of either gastric fundus gland type or pyloric gland type gastric mucosa. Furthermore, the glandular architecture comprises various types of epithelial cells, including pyloric or mucous neck cells, as well as surface mucous (foveolar) cells. The submucosal glands or cystic components are linked to the overlying gastric mucosa through defects in the mucosal muscularis (14). However, the pathological findings in previously documented cases typically reveal polycystic structures (15). Recent studies indicate that approximately three-quarters of cases of GHIP exhibit features resembling submucosal tumors, while the endoscopic characteristics, including findings from endoscopic ultrasonography, are often non-specific. Previous study indicated GHIP coexisted with gastric adenocarcinoma (4). Case reports documented adenocarcinoma arising within GHIPs (3, 13). Low-grade neoplasia has been identified in GHIP resection specimens (8). Potential mechanisms include chronic mucosal injury from inverted gland proliferation, inflammation-driven carcinogenesis (60% of GHIP patients have H. pylori/atrophy) (9), cystic dilation and glandular obstruction, et al. Consequently, achieving an endoscopic diagnosis of GHIP may pose challenges, necessitating complete endoscopic resection for definitive pathological evaluation (16). In the case presented herein, histological examination revealed that the GHIP was characterized by a singular cystic structure located in the submucosa, which was not connected to the surface mucosa. The cyst's surface was lined with foveolar cells and mucinous neck cells.
SMT-type CHIP (stalkless, >1 cm) requires ESD for complete resection, while incomplete resection risks recurrence or missed malignancy. Meanwhile, ESD may be unavailable in rural/underfunded hospitals, forcing referral delays. Previous studies have advocated for surgical resection as the preferred approach for the complete removal of gastric hamartomatous polyps of the SMT type. In the case presented herein, the submucosal tumor in the stomach measured greater than 1.0 cm in diameter. Traditional EMR techniques, however, necessitate advanced skills and have proven reliable primarily for lesions measuring 10 mm or less, as they can be resected in a single piece (17). Consequently, achieving complete resection of larger tumors via conventional EMR methods is deemed challenging. The advent of a novel technique employing an insulation-tipped diathermic knife has demonstrated that performing circumferential mucosal incisions around lesions prior to snaring can enhance en bloc resection rates, thereby broadening the applicability of EMR (18). Numerous reports indicate that for lesions measuring 10 mm or greater, the ESD method is preferred to avoid piecemeal resection, which may yield insufficient tissue for accurate pathological evaluation (19). Moreover, endoscopic resection offers several advantages over traditional surgical methods, including reduced invasiveness and cost-effectiveness (20). Notably, recent research has highlighted the efficacy of ESD for gastric hamartomatous inverted polyps, utilizing a clip with a line attachment prior to incision (21).
Repeat endoscopy at 3–6 months post-ESD was need to assess resection site healing. Annual endoscopy for ≥5 years should be conducted, given reports of adenocarcinoma developing during follow-up (13). Those with H. pylori infection or atrophic gastritis require biannual endoscopy (4). For this case, a follow-up visit was conducted for the patient (T = 6-month: follow up), who underwent a gastroscopy in the outpatient department. The results indicated that the patient's cardia mucosa was smooth and without protrusions.
Some single-case limitations should be noted. This case introduced an elderly male patient, while CHIP presentation may differ by age, ethnicity, or geography. No long-term follow-up data provided for this patient. ESD success at a tertiary center may not reflect outcomes in low-resource settings. Single cystic structure in this case instead of typical polycystic GHIPs and the heterogeneity may affect behavior.
Accordingly, we successfully employed the ESD technique to treat the tumor in this case. We recommend that SMT-type hamartomatous inverted polyps larger than 1.0 cm in diameter be managed using the ESD method.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval were waived for this study because it was a single case report. All procedures were conducted based on the ethical standards and the Helsinki Declaration. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ: Funding acquisition, Investigation, Writing – original draft, Data curation. H-nP: Data curation, Writing – original draft, Investigation. X-zW: Methodology, Resources, Supervision, Validation, Writing – original draft. ML: Formal analysis, Methodology, Resources, Validation, Writing – original draft. JL: Conceptualization, Writing – original draft, Validation, Methodology. X-yL: Formal analysis, Writing – review & editing, Methodology, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Lu'an Science and Technology Plan Project (No. 2022lakj040).
Acknowledgments
The authors thank all participants in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sipponen P, Siurala M. Cystic “hamartomatous” epithelial polyps of the stomach. Acta Hepatogastroenterol. (1978) 25(5):380–3.
2. Aoki M, Yoshida M, Saikawa Y, Otani Y, Kubota T, Kumai K, et al. Diagnosis and treatment of a gastric hamartomatous inverted polyp: report of a case. Surg Today. (2004) 34:532–6. doi: 10.1007/s00595-004-2761-1
3. Lee SJ, Park JK, Seo HI, Han KH, Kim YD, Jeong WJ, et al. A case of gastric inverted hyperplastic polyp found with gastritis cystica profunda and early gastric cancer. Clin Endosc. (2013) 46(5):568–71. doi: 10.5946/ce.2013.46.5.568
4. Yamashita M, Hirokawa M, Nakasono M, Kiyoku H, Sano N, Fujii M, et al. Gastric inverted hyperplastic polyp. Report of four cases and relation to gastritis cystica profunda. APMIS. (2002) 110(10):717–23. doi: 10.1034/j.1600-0463.2002.1101005.x
5. Iida M, Yao T, Watanabe H, Itoh H, Iwashita A. Fundic gland polyposis in patients without familial adenomatosis coli: its incidence and clinical features. Gastroenterology. (1984) 86(6):1437–42. doi: 10.1016/S0016-5085(84)80156-0
6. Yun JT, Lee SW, Kim DP, Choi SH, Kim S-H, Park JK, et al. Gastric inverted hyperplastic polyp: a rare cause of iron deficiency anemia. World J Gastroenterol. (2016) 22(15):4066. doi: 10.3748/wjg.v22.i15.4066
7. Taori K, Kirnake V, Daswani R, Padwale V, Acharya S. Endoscopic findings of oozing gastric hamartomatous polyps: a rare cause of upper gastrointestinal bleeding. J Clin Diagn Res. (2024) 18(6):1. doi: 10.7860/JCDR/2024/64124.19468
8. Han YP, Min CC, Li YB, Chen Y-Q, Liu H, Tian Z-B, et al. Diagnosis and treatment of gastric hamartomatous inverted polyp (GHIP) by endoscopic submucosal dissection: a case report. Medicine. (2023) 102(13):e33443. doi: 10.1097/MD.0000000000033443
9. Dong N, Meng F, Yue B, Hou J. Clinicopathologic and endoscopic characteristics of ten patients with gastric hamartomatous inverted polyp: a single center case series. BMC Gastroenterol. (2024) 24(1):139. doi: 10.1186/s12876-024-03233-8
10. Yamagiwa H, Matsuzaki O, Ishihara A, Yoshimura H. Heterotopic gastric glands in the submucosa of the stomach. Acta Pathol Jpn. (1979) 29(3):347–50. doi: 10.1111/j.1440-1827.1979.tb00192.x
11. Vyas M, Yang X, Zhang X. Gastric hamartomatous polyps-review and update. Clin Med Insights Gastroenterol. (2016) 9:3–10. doi: 10.4137/CGast.S38452
12. Dohi M, Gen Y, Yoshioka M. A case of gastric hamartomatous inverted polyp resected endoscopically. Endosc Int Open. (2016) 4(6):E608–9. doi: 10.1055/s-0042-105201
13. Okamura T, Iwaya Y, Nagaya T, Muranaka F, Ota H, Umemura T. Gastric adenocarcinoma arising from hamartomatous inverted polyp during 8-year follow-up. DEN Open. (2021) 2(1):e16. doi: 10.1002/deo2.16
14. Hayase S, Sakuma M, Chida S, Saito M, Ami H, Koyama Y, et al. Diagnosis and treatment of gastric hamartomatous inverted polyp (GHIP) using a modified combination of laparoscopic and endoscopic approaches to neoplasia with a non-exposure technique (modified CLEAN-NET): a case report. Surg Case Rep. (2020) 6(1):200. doi: 10.1186/s40792-020-00951-5
15. Mori H, Kobara H, Tsushimi T, Fujihara S, Nishiyama N, Matsunaga T, et al. Two rare gastric hamartomatous inverted polyp cases suggest the pathogenesis of growth. World J Gastroenterol. (2014) 20(19):5918–23. doi: 10.3748/wjg.v20.i19.5918
16. Ohtsu T, Takahashi Y, Tokuhara M, Tahara T, Ishida M, Miyasaka C, et al. Gastric hamartomatous inverted polyp: report of three cases with a review of the endoscopic and clinicopathological features. DEN Open. (2023) 3(1):e198. doi: 10.1002/deo2.198
17. Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. (1993) 39(1):58–62. doi: 10.1016/S0016-5107(93)70012-7
18. Gotoda T, Kondo H, Ono H, Saito Y, Yamaguchi H, Saito D, et al. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc. (1999) 50(4):560–3. doi: 10.1016/S0016-5107(99)70084-2
19. Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. (2001) 48(2):225–9. doi: 10.1136/gut.48.2.225
20. Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. (2007) 10:1–11. doi: 10.1007/s10120-006-0408-1
Keywords: cardia hamartomatous inverted polyp, submucosal tumor, endoscopic submucosal dissection, gastric cancer, endoscopic treatment
Citation: Zhang X, Pan H-n, Wang X-z, Li M, Liu J and Liu X-y (2025) The successful management of a cardia hamartomatous inverted polyp via endoscopic submucosal dissection: a case report. Front. Surg. 12:1682095. doi: 10.3389/fsurg.2025.1682095
Received: 8 August 2025; Accepted: 18 September 2025;
Published: 9 October 2025.
Edited by:
Wenbin Yu, Shandong University, ChinaReviewed by:
Zhenhua Li, Brigham and Women's Hospital and Harvard Medical School, United StatesPencho Tonchev, Medical University Pleven, Bulgaria
Copyright: © 2025 Zhang, Pan, Wang, Li, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-yan Liu, bHh5eTIwMjRAMTI2LmNvbQ==
 Xu Zhang1,2
Xu Zhang1,2 Jie Liu
Jie Liu Xiao-yan Liu
Xiao-yan Liu