- 1Department of Plastic and Aesthetic Surgery, The Second Affiliated Hospital of Guilin Medical University, Guilin, Guangxi Zhuang Autonomous Region, China
- 2Department of Anesthesiology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
- 3Neurobiology of Pain Research Group, Institute of Neurosciences, School of Medicine, University of Barcelona, Barcelona, Spain
- 4Department of Anesthesiology, The Second Affiliated Hospital of Hainan Medical University, Haikou, China
- 5Department of Anesthesiology, The Affiliated Changde Hospital, Hengyang Medical School, University of South China, Hengyang, China
Objective: To develop and internally validate a nomogram for early postoperative prediction of acute kidney injury (AKI) within 7 days after orthotopic liver transplantation (LT).
Methods: We retrospectively analyzed 500 orthotopic liver transplants at the First Affiliated Hospital of Sun Yat-sen University (January 1, 2016–April 30, 2022). Patients were randomly split into training (n = 352) and validation (n = 148) cohorts for same-center internal validation using a random-split design. AKI within 7 postoperative days was defined by KDIGO serum-creatinine criteria only (KDIGO-SCr) because urine-output data were incomplete. Candidate predictors were screened using least absolute shrinkage and selection operator (LASSO) and entered into multivariable logistic regression to build a parsimonious nomogram for early postoperative (first 6–12 h) risk stratification and monitoring. Performance was assessed by AUC and calibration; decision-curve analysis illustrated relative net benefit without prespecified thresholds or actions.
Results: BMI, operation time, intraoperative urine volume, and postoperative levels of urea nitrogen, blood ammonia, and procalcitonin were identified as independent risk factors for AKI after LT (P < 0.05). The nomogram demonstrated good discrimination, calibration, and clinical usefulness in both the training and validation cohorts, with an AUC of 0.769 (95% CI: 0.715–0.823) in the training cohort and 0.704 (95% CI: 0.618–0.790) in the validation cohort.
Conclusion: The nomogram predictive model developed in this study shows good accuracy and can be conveniently applied for early identification and risk prediction of acute kidney injury following liver transplantation.
Introduction
Liver transplantation is the most effective treatment for patients with advanced liver failure, significantly improving overall survival rates and quality of life (1). However, liver transplantation can lead to various complications. One complication is the postoperative acute kidney injury which has many researches done on this topic (2). A meta-analysis in 2019 reported that in recent years, the incidence rates of AKI post-liver transplantation and severe AKI requiring RRT were 40.8% and 7% (3), respectively. AKI after liver transplantation is closely associated with delayed renal function recovery, increased transplantation failures, extended mechanical ventilation time, prolonged postoperative ICU stay, increased 30-day postoperative mortality, and decreased long-term survival rates of patients (4–6).
Preoperative, intraoperative, and postoperative factors all contribute to the occurrence of AKI after transplantation. Preoperative factors include patient's own conditions. Pre-existing conditions such as viral hepatitis, hepatic encephalopathy, cerebrovascular disease, overweight, and diabetes increase the risk of AKI (7–9). The duration of the surgery (10) and the surgical technique used also influence the occurrence of AKI. Some studies indicate that the piggyback technique significantly reduces the likelihood of acute renal failure after liver transplantation (11, 12). Postoperative factors include postoperative hypotension, postoperative infections, among others (13, 14).
Currently, there are many research on novel biological markers related to renal function and kidney injury such as NGAL, KIM-1, and Cystatin C (15). However, for ESLD patients, there's a lack of biochemical indicators with high sensitivity, strong specificity, and broad clinical applicability for renal function assessment. Therefore, early identification and diagnosis of postoperative AKI and immediate therapeutic measures can be crucial to improve patient prognosis (16). Therefore, to aid clinicians in early identification and risk stratification during the perioperative period, and enable effective intervention targeting various high-risk factors, it is essential to develop an accurate model for predicting the occurrence of AKI. This study aims to investigate the risk factors of OLT-AKI through a retrospective analysis of the clinical data of 500 OLT patients, based on the KDIGO diagnostic criteria. The objective of the prediction model was to achieve early individualized assessment of the risk of developing OLT-AKI.
Patients and methods
Patients selection
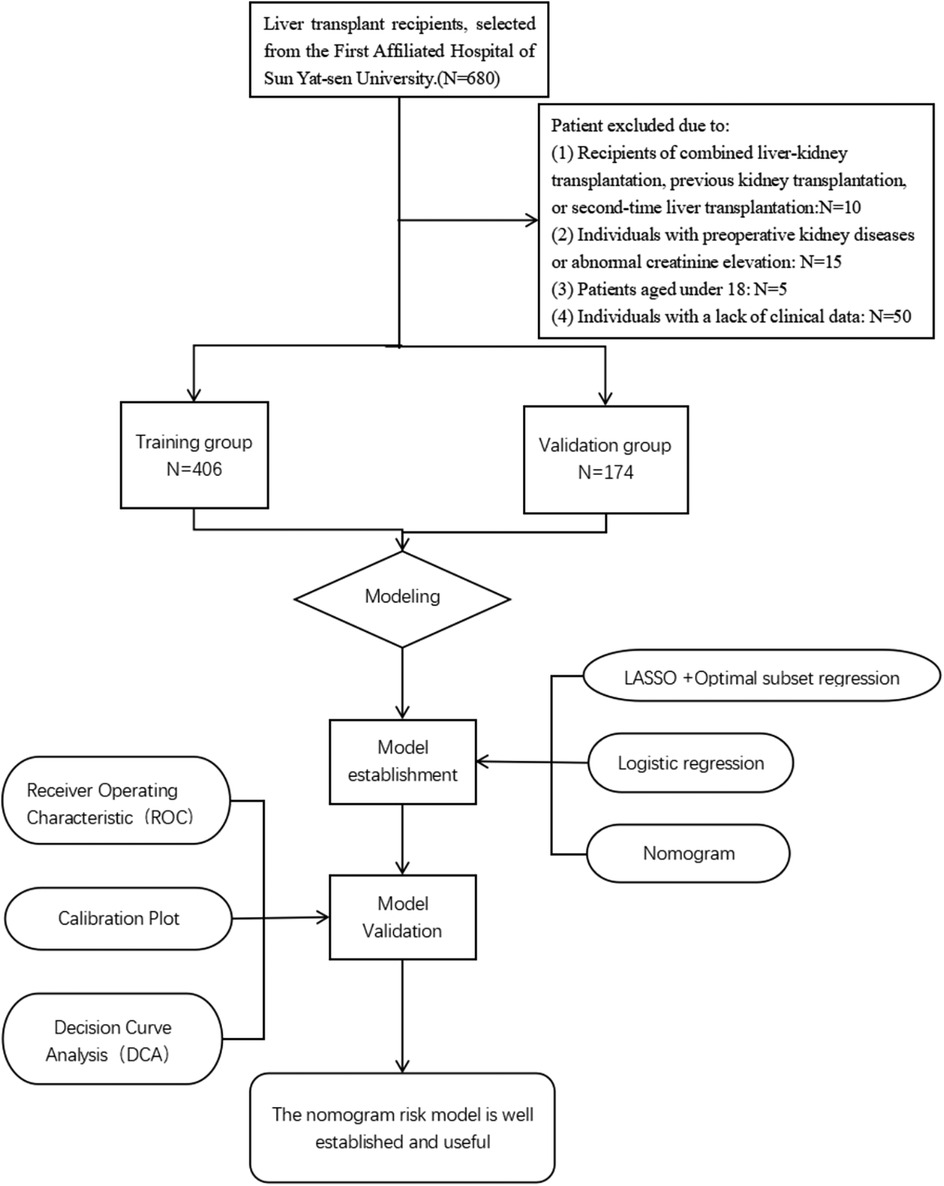
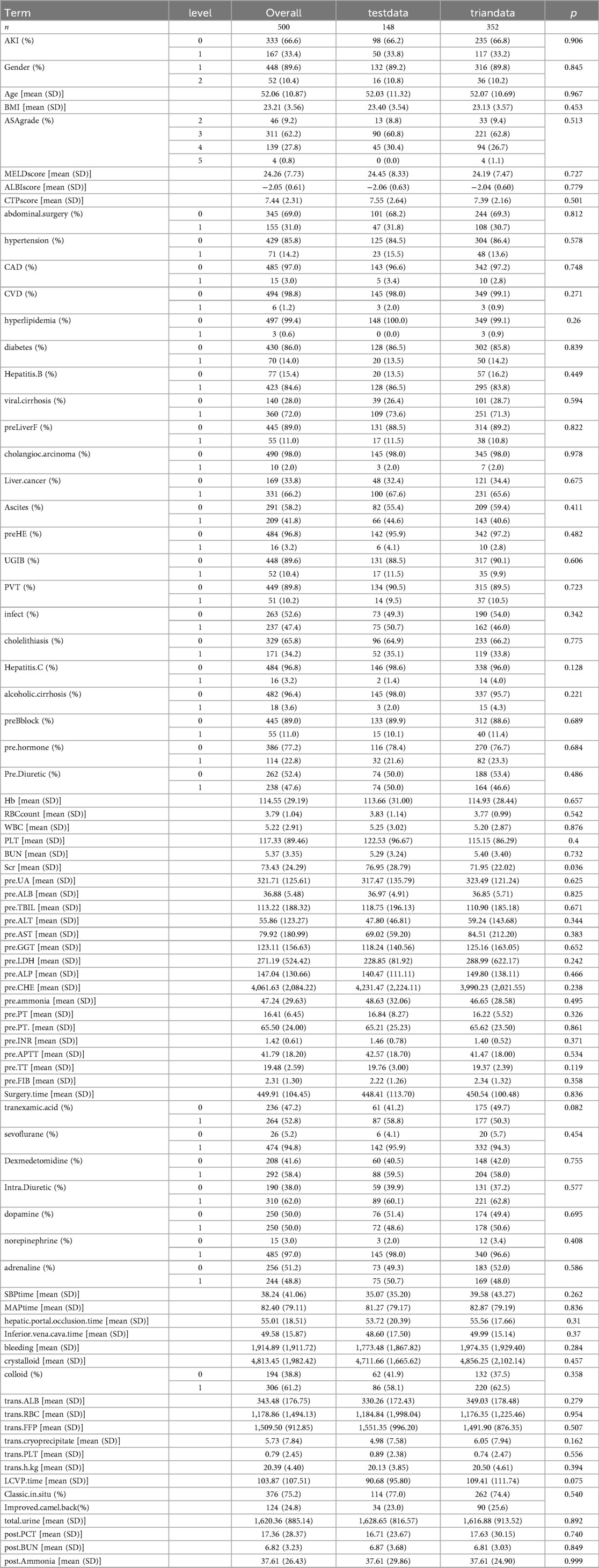
Clinical data of patients undergoing their first liver transplantation at the First Affiliated Hospital of Sun Yat-sen University from January 1, 2016 to April 30, 2022 was collected. Post-liver transplantation AKI was diagnosed based on the diagnostic criteria established by the Kidney Disease: Improving Global Outcomes (KDIGO) in 2012. Exclusion criteria: (1) recipients of combined liver-kidney transplantation, previous kidney transplantation, or second-time liver transplantation; (2) preoperative kidney diseases or abnormal creatinine elevation; (3) patients aged under 18; (4) lack of clinical data. A total of 500 patients were included in this study. Donor characteristics such as age, sex, and graft quality were recorded when available. However, information regarding extended-criteria donor (ECD) status and graft steatosis was not uniformly documented in the database and was therefore not analyzed.Candidates were divided into a training group (n = 352) and a validation group (n = 148). The research flow chart is shown in Figure 1. Based on the C-statistic of 0.8, an estimated 10 risk factors were projected. According to previous literature, the incidence rate of AKI is 36.1% (17), resulting in an estimated sample size of 355. Due to the limitations of retrospective studies which prevent accurate collection of urine volume for each patient, this study only used Scr as the diagnostic marker for AKI which still aligns to the KDIGO diagnostic standards. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (Approval Number: 2022-468).
Data collection
Preoperative data included age, gender, body mass index (BMI), ASA classification, primary disease and comorbidities, preoperative medication, and laboratory tests. Intraoperative data encompassed the surgical method, duration of low central venous pressure, duration of hypotension, duration of surgery and anesthesia, intraoperative medication, volume of various fluids administered, total intraoperative blood loss, and total urine output. “Inferior vena cava (IVC) time” was defined as the duration of temporary occlusion of the recipient's inferior vena cava during the anhepatic phase of liver transplantation. Postoperative data covered postoperative AST and ALT peaks, coagulation parameters, and infection indicators. Prognostic data included postoperative hepatic insufficiency, incidence of hepatic encephalopathy, ICU stay duration, duration of postoperative mechanical ventilation, reintubation rate, secondary laparotomy, postoperative CRRT requirement, and mortality.
Definition and outcome
The surgical approach utilized either the classic orthotopic liver transplantation (OLT) or the modified piggyback OLT. In the classic OLT, the recipient's retrohepatic inferior vena cava (IVC) was completely removed and replaced with the donor IVC, requiring temporary suprahepatic and infrahepatic caval clamping. In contrast, the modified piggyback OLT preserved the recipient's IVC by anastomosing the donor hepatic vein cuff to the sidewall of the recipient IVC, thereby maintaining partial venous return and avoiding total caval interruption. All procedures were performed by the same experienced surgical team.
AKI was diagnosed using KDIGO criteria based on serum creatinine changes within 7 days post-transplantation. Because urine output data were not consistently available, the KDIGO urine output criterion was not applied; thus, intraoperative urine volume was analyzed as a perioperative variable rather than part of the AKI definition.MELD, ALBI, and CTP scores were utilized to evaluate end-stage liver disease (18). The ALBI (Albumin–Bilirubin) score is an objective indicator reflecting liver synthetic and excretory function, calculated from serum albumin and total bilirubin, and is considered a simple and reproducible index for grading liver function without subjective parameters (19). The Child–Turcotte–Pugh (CTP) score, which incorporates serum bilirubin, albumin, INR, ascites, and encephalopathy, has been widely used to assess hepatic reserve and predict postoperative outcomes (20). In addition to the MELD score, which mainly reflects liver disease severity and short-term mortality risk, the inclusion of ALBI and CTP scores provides a more comprehensive assessment of hepatic functional reserve, thereby improving the accuracy of preoperative evaluation (21). MELD = 3.8 × Ln(TBIL) + 9.6 × Ln(SCr) + 11.2 × Ln(INR) + 6.4 × etiology (0 for alcoholic and cholestatic liver disease, 1 for others), ALBI = (log10 bilirubin × 0.66) + (albumin × −0.085), CTP = 0.957 × loge(total bilirubin) + 0.378 × loge(albumin) + 1.12 × loge(PT-INR) + 0.643 (1 point if ascites present, otherwise 0) + 0.871 (graded from 0–3 based on encephalopathy level). However, the diagnostic criteria for early postoperative liver dysfunction (EAD) lacks a unified standard. According to Olthoff et al. (22), EAD diagnosis is determined by the following: (1) Total bilirubin (TBIL) ≥ 10 mg/dl on postoperative day 7; (2) International Normalized Ratio (INR) ≥ 1.6 on postoperative day 7; (3) Peak aspartate aminotransferase (AST) or peak alanine aminotransferase (ALT) > 2,000 U/L within 7 days post-surgery. Any of the above criteria confirms an EAD diagnosis.
Statistical analysis
All statistical analyses were performed using Stata/SE 17.0 (College Station, TX, USA) and R version 4.2.2 software (https://www.r-project.org/). Continuous variables were expressed as mean ± standard deviation (SD) and compared using Student's t test when approximately normally distributed, as assessed by the Shapiro–Wilk test. Non-normally distributed variables were presented as median [interquartile range (IQR, P25–P75)] and compared using the Mann–Whitney U test. Categorical variables were expressed as counts (percentages) and compared using the χ² test or Fisher's exact test. Differences were considered statistically significant at P < 0.05. The use of mean ± SD for continuous variables followed previous liver transplantation studies and facilitated comparability across cohorts with approximately normal data distributions.
The LASSO regression model allowed for the risk factors (non-zero coefficients) to be obtained from the clinical data characteristics. The penalty term was determined by 10-fold cross-validation, selecting the penalty that yielded the smallest mean square error (“glmnet” package). The main tuning parameter was set as follows: type. Measure (loss to use for cross-validation) = “default”, family = “binomial”, nfolds = 10. The optimal model, with the fewest variables, was identified based on λ1 se as the criterion. The multifactorial logistic regression method was then used for further screening of independent risk factors. A nomogram was also established by the final model through the “rms” package of R software. The area under the curve (AUC) of the receiver operating characteristic curve (ROC) alongside calibration curves and decision curves were used to internally and externally validate the nomogram prediction model to judge its' discriminative ability, calibration, and clinical usefulness.In addition to the AUC, the model's sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were also calculated to further evaluate its discriminative performance.
Results
Patient baseline characteristics and prognosis
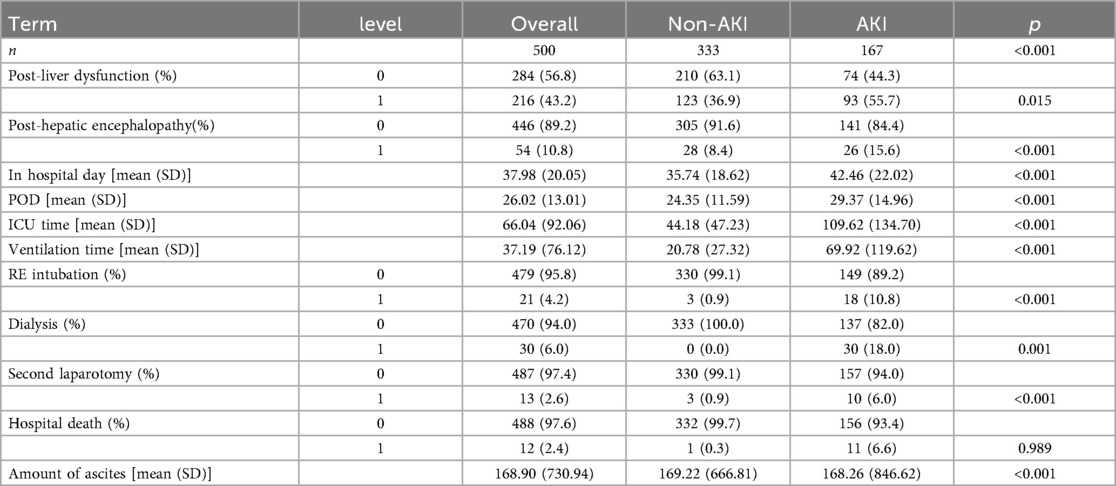
From January 1, 2016 to April 30, 2022, our hospital had a total of 500 patients undergoing LT. Based on the inclusion criteria, a total of 500 patients were added into the analysis, with 352 in the training group and 148 in the validation group. The detailed flowchart is shown in Figure 1. The overall characteristics of the two groups of patients are summarized in Table 1. In the training and validation cohorts, postoperative AKI occurred in 117 patients (33.2%) and 50 patients (33.8%) respectively. For analysis of postoperative characteristics and outcomes, patients were divided into two groups: AKI group and non-AKI group (Table 2). Compared to the non-AKI group, patients in the AKI group were more likely to undergo dialysis, had higher chances of reintubation, a higher mortality rate, prolonged hospital stay, prolonged postoperative ICU stay, longer duration of mechanical ventilation after surgery, and prolonged postoperative hospitalization. The likelihood of postoperative liver dysfunction and the incidence of hepatic encephalopathy had also increased. (P < 0.05).
Selection of variables for AKI post LT
Using the LASSO algorithm, 6 potential risk factors were selected from 80 variables, with the chosen Lambda.1se being 0.0201 (Figures 2A,B). After multivariate logistic regression analysis, the 6 factors remained independently associated with the risk of AKI in LT patients (Table 3). Although variables such as hepatic portal occlusion time, inferior vena cava time, MELD score, and pre-FIB were statistically significant in the univariate analysis, they lost significance after LASSO selection and multivariable logistic regression due to collinearity with other predictors (e.g., operation time, postoperative BUN, and PCT) or overlapping explanatory power. Preoperative variables (BMI), intraoperative variables (surgical time, total urine output during surgery), and postoperative variables (postoperative PCT, postoperative BUN, postoperative blood ammonia) were identified to be independent risk factors for AKI after LT.
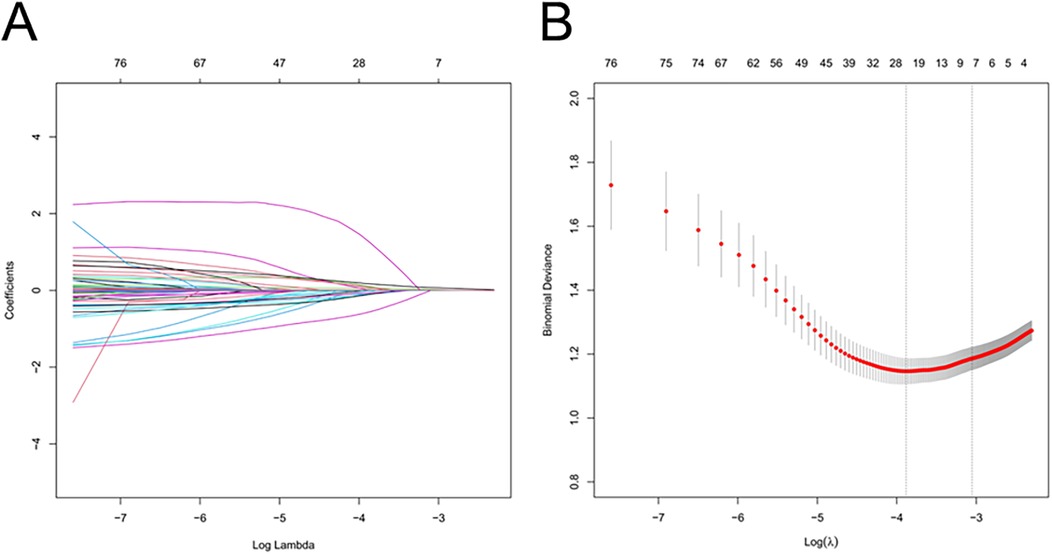
Figure 2. Feature selection of LT patients using the LASSO logistic regression model. (A) A Lasso coefficient profile plot was built for the prediction of AKI after LT. (B) The optimal parameter (λ) was selected by the LASSO model using 10-fold cross-validation via 1 standard error of the minimum criteria.
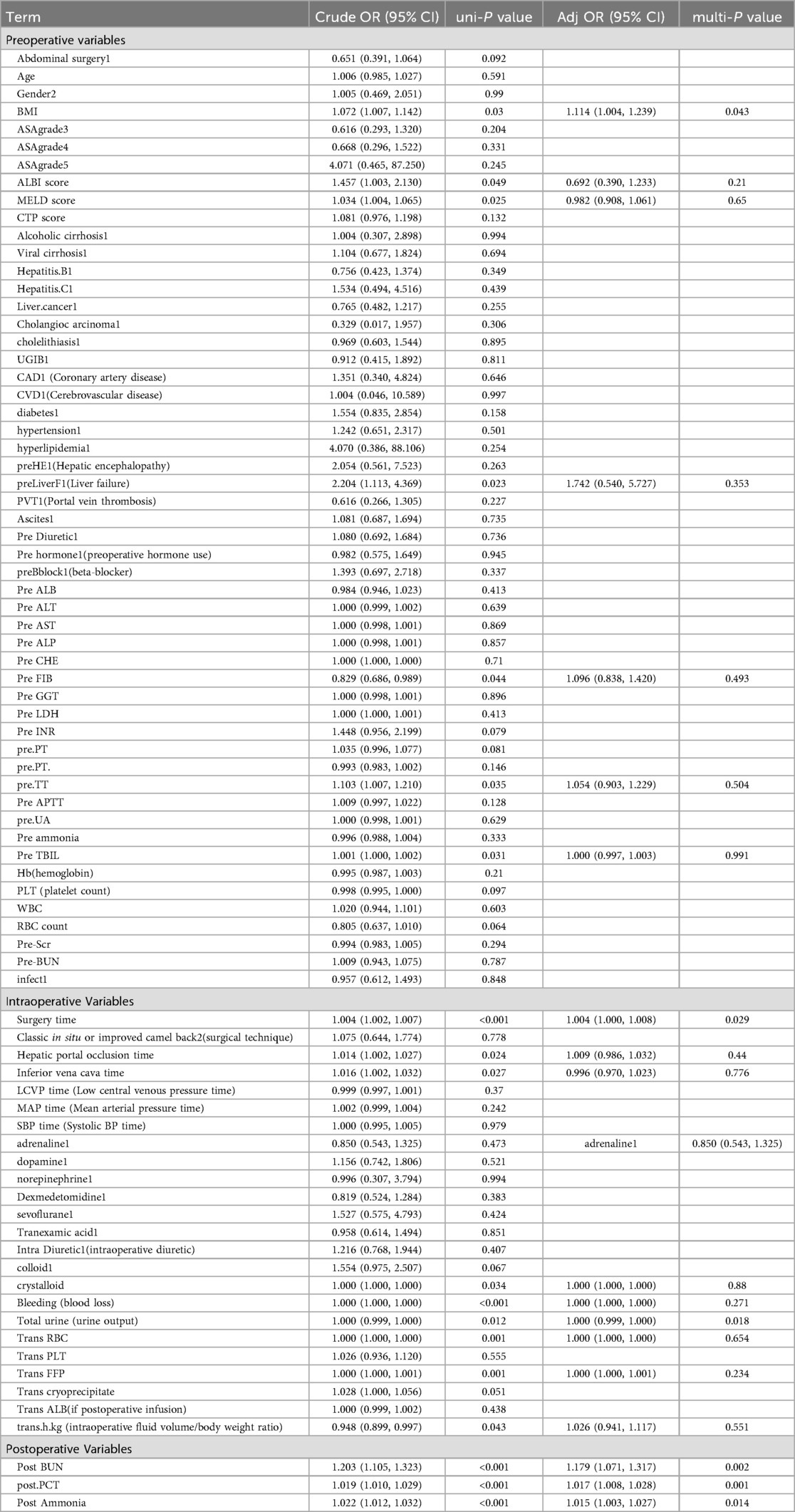
Table 3. Multivariable logistic regression analysis of predicting AKI after LT in the training cohort.
Construction of nomogram model for predicting AKI in patients
Taking the development of AKI as the dependent variable, the variables determined in the multivariate logistic regression analysis were used as predictive variables, and the total scores of each factor in the nomogram predicting the risk of AKI were calculated. The risk of AKI is represented by the total score, with a scoring range from 0–280. The scores of each risk factor are shown in Figure 3. By combining the scores of BMI, surgery duration, total urine volume, post-operative PCT, post-operative BUN, and post-operative blood ammonia, the corresponding AKI risk can be determined.
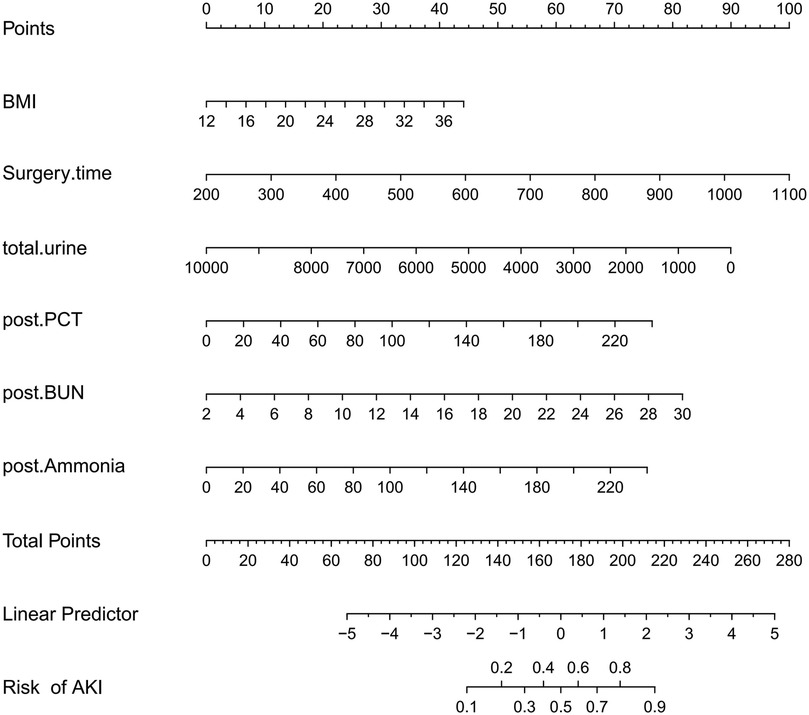
Figure 3. A risk factors of BMI, surgery time, total urine, postoperative PCT, postoperative BUN, postoperative ammonia for nomogram prediction model.
Performance and validation of the AKI nomogram model post-LT
The AUC of the ROC analysis for the training group and validation group were 0.769 (95% CI: 0.715–0.823) and 0.704 (95% CI: 0.618–0.790), respectively (Figures 4A,B) indicating that the model demonstrated excellent accuracy in estimating the probability of AKI following LT. Additionally, the sensitivity, specificity, PPV, and NPV of the model were calculated to complement the AUC results, all of which demonstrated consistent and acceptable predictive performance (see Supplementary Table S1). The calibration curve of the nomogram is presented in Figures 5A,B, and revealing good agreement between the predicted and observed outcomes.
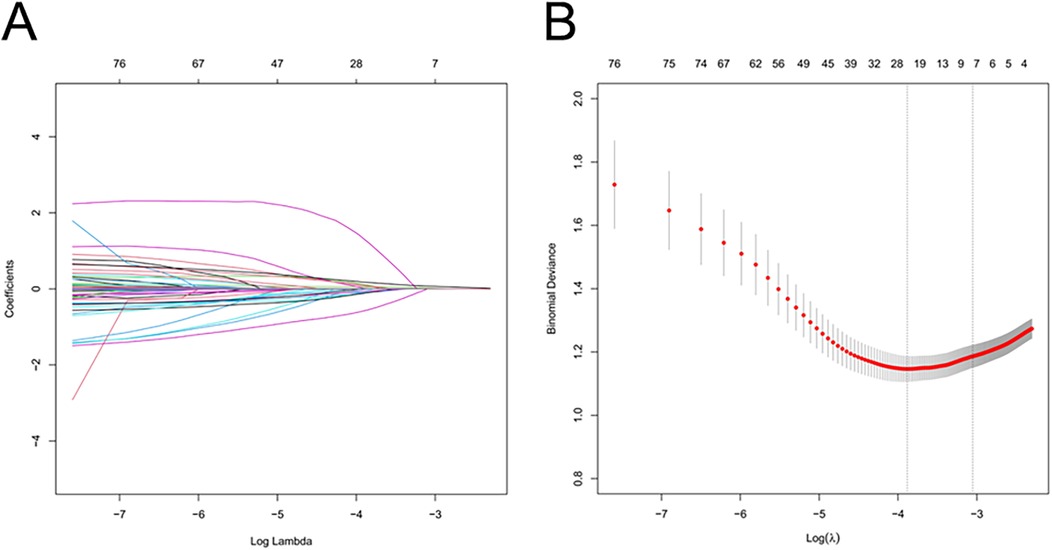
Figure 4. Receiver operating characteristic (ROC) curve validates the nomogram prediction for the risk of acute kidney injury post liver transplantation. The blue curve (A) represents the performance of the nomogram in the training group, while the red curve (B) demonstrates its performance in the validation group.
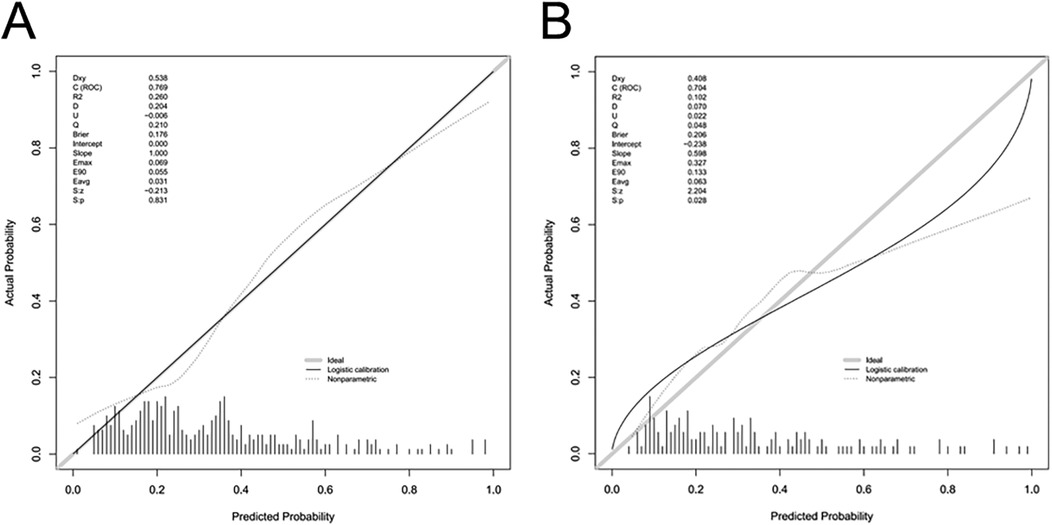
Figure 5. Calibration curves of the predictive nomogram for AKI risk. The y-axis represents the actual diagnosed cases of AKI, and the x-axis represents the predicted risk of AKI. The diagonal dotted line denotes a perfect prediction by an ideal model. The solid lines represent the performance of the nomogram in the training group (A) and validation group (B), with a closer alignment to the diagonal dotted line indicating a more accurate prediction. I am creating a legend title; please assist me with the translation.
Clinical application of the AKI nomogram model post-LT
Decision curve analysis (DCA) was used to evaluate the clinical usefulness of the nomogram by quantifying the net benefit across a range of threshold probabilities. When the threshold probability values were within 10%–82% in the training cohort and 18%–50% in the validation cohort, the nomogram provided a higher net benefit compared with the “treat-all” or “treat-none” strategies, indicating good potential for assisting individualized clinical decision-making in predicting postoperative AKI risk (Figures 6A,B).
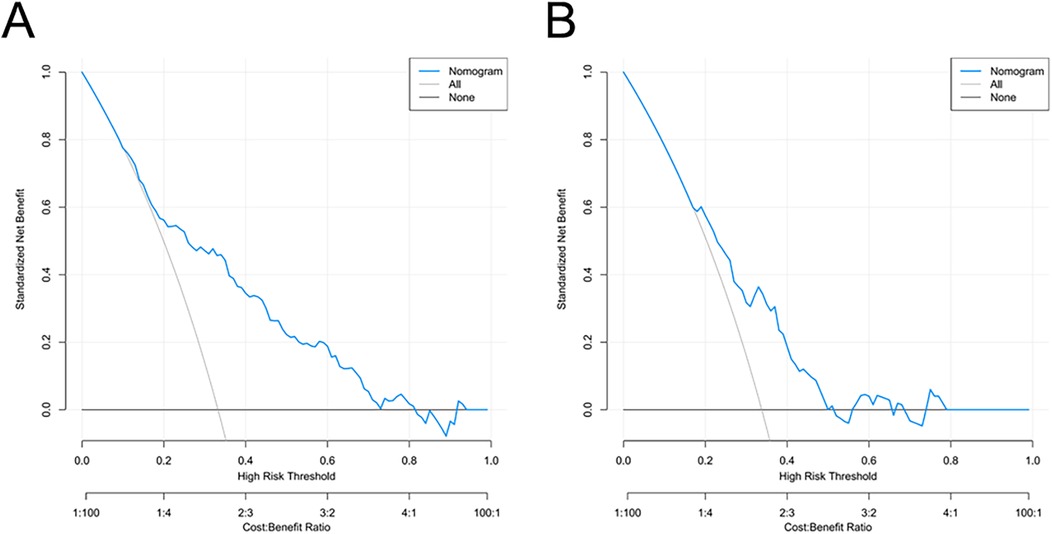
Figure 6. Decision curve analysis for the AKI risk nomogram. The y-axis measures the net benefit. The thick solid line represents the assumption that all patients do not have AKI, the thin solid line represents the assumption that all patients have AKI, and the blue line represents the risk nomogram. (A) originates from the training group, and (B) originates from the validation group.
Discussion
OLT is the ultimate effective treatment for various categories of liver failure which include acute, sub-acute, or chronic liver failure. In recent years, there has been an upward trend in liver transplantation (23). However, various short-term and long-term complications after LT remain to be critical issues and they needed to be addressed in current clinical practice. AKI is one of the common complications after LT surgery (24). In this study, the independent risk factors for OLT-AKI include: BMI, surgical duration, total urine volume during surgery, postoperative PCT, postoperative BUN, and postoperative blood ammonia. Based on the results of the Logistic regression, a risk prediction model for OLT-AKI was constructed and a nomogram was plotted. The results indicate that the prediction model has good calibration and discrimination capabilities, allowing risk stratification and earlier identification. It further allows the clinicians to implement effective interventions improving the patients' prognosis.
In this study, the incidence of AKI after liver transplantation was 32.4%. The morbidity rate previously reported by Zhu and others was 61.1%. The higher AKI incidence reported in other clinical studies may be due to the adoption of the newly released KDIGO criteria. Recent reports on AKI after liver transplantation have uniformly adopted this criterion with incidence rates ranging from 12.3%–56.6% (2, 3) which is consistent with the findings of this study. Meanwhile, this study indicates that early AKI post-transplantation not only increases in-hospital mortality, prolongs discharge time, and postoperative mechanical ventilation duration, but it is also closely associated with adverse outcomes such as secondary laparotomy and re-intubation which is again, consistent with the previous research findings.
BMI, identified as the sole preoperative independent risk factor in this study, has been previously reported (9). High BMI predisposes individuals to cardiovascular diseases, hypertension, and diabetes (25). Overweight patients, particularly those undergoing liver transplantation, are prone to postoperative complications (26). However, some studies have shown that obesity is associated with a higher long-term postoperative survival rate. Underweight patients significantly suffer from worse postoperative complications and higher mortality rates (27). Several epidemiological studies suggest that obesity provides a protective effect for critically ill patients. In this context, Sleeman et al. observed in an established extracorporeal circulation model of pigs that obese pigs showed reduced kidney functions and tissue apoptosis after surgery. The animal models and clinical studies have yielded contradictory findings to each other (28). Our findings revealed that individuals with a high BMI undergoing orthotopic liver transplant are at a higher risk of postoperative AKI. This susceptibility may be attributed to the association between obesity, heightened oxidative stress, elevated proinflammatory cytokine levels, and potential endothelial dysfunction, all of which could contribute to the development of AKI (29, 30). From a clinical perspective, patients who are obese or overweight can pose challenges in the surgical setting, leading to increased complexity of the procedure, potentially prolonging the operation time, and raising the risk of postoperative complications. Moreover, fluid resuscitation in obese patients presents a challenge due to the uncertainty surrounding whether fluid infusion should be adjusted based on actual weight or formula weight, and this ambiguity can impact renal perfusion (9).
In our research, we identified two intraoperative variables as risk factors: operation time and the total urine output during surgery. In this model, operation time played the most significant role in predicting postoperative AKI. Operation time was determined by various factors, such as the difficulty of the operation, the technical proficiency of the operator, the pathophysiology of the patient, and the management by the anesthesiologist during the perioperative period. This might indirectly result in varying threshold values obtained in previous studies when operation time is considered as an independent risk factor (10, 31). Although operative time was identified as an independent risk factor in our multivariable analysis, it may act as a surrogate marker for intraoperative physiological stress or surgical complexity rather than a direct cause of postoperative AKI. Factors such as massive blood loss, intraoperative hemorrhagic shock, ischemia–reperfusion injury, and the use or dose of vasopressors could partially explain the observed association between longer surgery duration and renal dysfunction (32, 33). Furthermore, urine output is a macroscopic representation of renal perfusion, and intraoperative urine output is influenced by various factors such as blood volume and hemodynamics. Previous studies (10) have shown that intraoperative urine output of <2 ml/(kg·h) is an independent risk factor for postoperative AKI in liver transplant patients.
Previous studies have used the Framingham Risk-scheme to develop a risk prediction model for AKI occurrence after deceased donor LT. However, this study did not include relevant indicators after LT (34). In our model, three post-operative risk factors were established as standalone predictive indicators which include PCT after surgery, BUN, and blood ammonia. PCT is a regular diagnostic measure for sepsis due to its' excellent specificity and sensitivity, and PCT is also closely associated with sepsis (35). It is a marker of inflammation with high sensitivity and precision which aids in detecting infectious diseases earlier, assessing inflammation, evaluating disease prognosis, and guiding accurate drug use. A recent meta-analysis on PCT labeled it as a potential predictive factor for AKI (36). For conditions like traumatic brain injury, septic shock, acute pancreatitis, primary percutaneous coronary interventions post-coronavirus infection, and acute Type A aortic dissection, PCT holds substantial predictive value for AKI (37–39, 42). However, the forecasting capability of PCT for post-liver transplant AKI hasn't been discussed. In our research, PCT demonstrates potential predictive capacity for AKI following liver transplantation, which might be associated with its low molecular weight. Under normal circumstances, the kidneys can efficiently remove (PCT) from the bloodstream. However, when kidney function is compromised, the clearance rate of PCT from the plasma decreases, resulting in the accumulation of this substance in the bloodstream. Since this topic hasn't been extensively explored in previous research, it is imperative to collect more cases and even consider conducting multicenter collaborative studies to corroborate the association between PCT and AKI. Furthermore, determining the ideal threshold for predicting post-liver transplant AKI introduces a novel perspective and avenue for investigation.
Postoperative blood ammonia levels are significantly correlated with the occurrence of AKI after liver transplantation. Research has found that high blood ammonia can damage the kidneys through multiple mechanisms. For instance, elevated blood ammonia during chronic liver disease can directly interact with renal glomerular cells, leading to glomerular damage (40), and ammonia also plays a crucial role on renal tubulointerstitial fibrosis. Other studies have demonstrated that hyperammonemia can facilitate the progression of kidney injury through the activation of the complement cascade and the stimulating impact of ammonia on kidney growth (41). Yoon Sook Lee (42) et al.'s research suggested that preoperative blood ammonia can serve as a predictor for AKI after liver transplantation, which contradicted the findings from our study. These discrepancies may arise from factors such as sample size in the research and the timing of preoperative blood ammonia collection. Hence, additional research may be necessary to investigate the reasons behind the discrepancies with previous study findings and to delve more deeply into the relationship between blood ammonia levels and AKI following liver transplantation under various clinical scenarios.
Previous models predicting post-operative AKI often included preoperative urea nitrogen, but there is limited research on whether postoperative urea can also be a predictor. A previous study from Portugal reported that postoperative blood urea nitrogen can be a strong predictor for acute kidney injury after pediatric cardiac surgery (43). Lu Haiyang et al. found that after liver transplantation, the AUC analysis showed that BUN had a good distinction for Stage 3 AKI. However, there were significant differences in the preoperative urea values between the AKI group and non-AKI group, which might lead to biased results (44). In our study, a significant correlation between elevated postoperative urea levels and postoperative AKI was observed, further supporting the importance of closely monitoring renal function in patients after liver transplantation, especially those with elevated postoperative urea levels. Timely renal-protective measures, including optimized fluid management and appropriate pharmacologic interventions, may help reduce the risk of postoperative AKI and improve recovery outcomes.
Furthermore, some predictors identified in this study—such as operation time and intraoperative urine volume—are potentially modifiable. Future studies should investigate whether optimizing these perioperative factors can causally reduce AKI risk, possibly through advanced causal inference frameworks such as target trial emulation (45).
The potential heterogeneity of the study population should also be acknowledged. Variations in baseline characteristics, perioperative management, and comorbidities among liver transplant recipients may influence the development of postoperative AKI. Although strict inclusion criteria and internal validation were applied, unmeasured heterogeneity might still have affected the observed associations. Future studies should perform subgroup analyses stratified by demographic or clinical factors (e.g., age, BMI, MELD score, or liver disease etiology) to verify the model's consistency across populations (46).
This study has several limitations. First, serum creatinine was used to define AKI, which may underestimate renal injury due to perioperative hemodilution or fluid shifts. Because urine-output data were unavailable, AKI was defined by KDIGO-SCr rather than full KDIGO; SCr-only ascertainment may misclassify AKI in post-LT high-fluid states and affect observed associations, so incidence estimates and predictor effects should be interpreted cautiously. We did not quantify the impact of excluding urine output; future work should externally validate the model in cohorts with complete urine-output capture and, where feasible, compare SCr-only with full KDIGO ascertainment. Second, this was a single-center retrospective analysis, so causal inference cannot be fully established. Third, detailed donor information such as extended-criteria donor (ECD) status and graft steatosis was not consistently available in the dataset, which may have introduced minor residual confounding (47). Fourth, the decision-curve analysis (DCA) serves only to visualize relative net benefit; no implementation inferences are made because decision thresholds and linked clinical actions were not prespecified. For future translation, thresholds should be prospectively defined with clinical stakeholders and explicitly mapped to actions relevant to transplant care (e.g., increased monitoring frequency and nephrotoxin avoidance at lower thresholds; early nephrology consultation; CRRT readiness; ICU resource prioritization at higher thresholds) and then evaluated in independent cohorts to confirm utility. Additionally, in line with TRIPOD, we report measurement timing, candidate-predictor rationale, modeling choices, and the full model specification with coefficients/intercept to enable independent use; calibration is shown graphically, and numerical calibration slope/intercept and the Brier score are not reported here and represent a reporting limitation to be addressed—together with optimism-corrected performance—in future temporal/multicenter external validation. Finally, although the nomogram showed good internal validation performance, external validation using independent cohorts was not available; thus, potential overfitting and limited generalizability cannot be excluded. Future multicenter studies are warranted to confirm the robustness of this model.
In summary, our study suggests a strong link between AKI and higher mortality rates as well as adverse outcomes. After liver transplantation, the acute kidney injury can be attributed to several independent risk factors such as BMI, surgery duration, total urine volume, post-surgery PCT, post-surgery BUN, and post-surgery blood ammonia. These individual determinants are incorporated into a nomogram, allowing for personalized risk assessment of acute kidney injury following orthotopic liver transplantation. Overall, this nomogram demonstrated good discrimination and calibration performance, as reflected by the AUC and calibration plots.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by by the Medical Ethics and Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (Approval Number: 2022-468). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin.
Author contributions
DW: Writing – review & editing, Software, Writing – original draft, Formal analysis, Data curation. XC: Data curation, Software, Formal analysis, Writing – review & editing. YT: Data curation, Software, Writing – review & editing, Formal analysis. WX: Resources, Data curation, Writing – review & editing. KX: Writing – review & editing. JD: Writing – original draft, Investigation, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Dahui Wang holds a master's degree in anesthesiology and specializes in transplant surgery anesthesia. He is guided and mentored by Kangqing Xu, who are senior anesthesiology doctors with substantial experience in the field.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1683424/full#supplementary-material
Abbreviations
AKI, acute kidney injury; KDIGO, kidney disease: improving global outcomes; RRT, renal replacement therapy; BMI, body mass index; MELD, model for end-stage liver disease; RBC, red blood cell; SCr, serum creatinine; Na, sodium; TBIL, total bilirubin; PT, prothrombin time; INR, international normalized ratio; MAP, mean arterial pressure; FFP, fresh frozen plasma; ROC, receiver operating characteristic; OR, odds ratio; CI, confidence interval; AUC, area under the receiver operating characteristic curve.
References
1. Yang LS, Shan LL, Saxena A, Morris DL. Liver transplantation: a systematic review of long-term quality of life. Liver Int. (2014) 34(9):1298–313. doi: 10.1111/liv.12553
2. Zhu M, Li Y, Xia Q, Wang S, Qiu Y, Che M, et al. Strong impact of acute kidney injury on survival after liver transplantation. Transplant Proc. (2010) 42(9):3634–8. doi: 10.1016/j.transproceed.2010.08.059
3. Thongprayoon C, Kaewput W, Thamcharoen N, Bathini T, Watthanasuntorn K, Lertjitbanjong P, et al. Incidence and impact of acute kidney injury after liver transplantation: a meta-analysis. J Clin Med. (2019) 8(3):372. doi: 10.3390/jcm8030372
4. Hussaini T, Yoshida EM, Partovi N, Erb SR, Scudamore C, Chung S, et al. Early persistent progressive acute kidney injury and graft failure post liver transplantation. Transplant Direct. (2019) 5(3):e429. doi: 10.1097/TXD.0000000000000868
5. Bao B, Wang W, Wang Y, Chen Q. A prediction score model and survival analysis of acute kidney injury following orthotopic liver transplantation in adults. Ann Palliat Med. (2021) 10(6):6168–79. doi: 10.21037/apm-21-842
6. Arani RH, Abbasi MR, Mansournia MA, Nassiri Toosi M, Jafarian A, Moosaie F, et al. Acute kidney injury after liver transplant: incidence, risk factors, and impact on patient outcomes. Exp Clin Transplant. (2021) 19(12):1277–85. doi: 10.6002/ect.2021.0300
7. Barreto AGC, Daher EF, Junior GBS, Garcia JHP, Magalhães CBA, Lima JMC, et al. Risk factors for acute kidney injury and 30-day mortality after liver transplantation. Ann Hepatol. (2015) 14(5):688–94. doi: 10.1016/S1665-2681(19)30763-X
8. Chen H-P, Tsai Y-F, Lin J-R, Liu F-C, Yu H-P. Incidence and outcomes of acute renal failure following liver transplantation. Medicine (Baltimore). (2015) 94(52):e2320. doi: 10.1097/MD.0000000000002320
9. Zhou J, Lyu L, Zhu L, Liang Y, Dong H, Chu H. Association of overweight with postoperative acute kidney injury among patients receiving orthotopic liver transplantation: an observational cohort study. BMC Nephrol. (2020) 21(1):223. doi: 10.1186/s12882-020-01871-0
10. Zhou ZQ, Fan LC, Zhao X, Xia W, Luo AL, Tian YK, et al. Risk factors for acute kidney injury after orthotopic liver transplantation: a single-center data analysis. J Huazhong Univ Sci Technolog Med Sci. (2017) 37(6):861–3. doi: 10.1007/s11596-017-1818-5
11. Cabezuelo JB, Ramirez P, Acosta F, Torres D, Sansano T, Pons JA, et al. Does the standard vs piggyback surgical technique affect the development of early acute renal failure after orthotopic liver transplantation? Transplant Proc. (2003) 35(5):1913–4. doi: 10.1016/S0041-1345(03)00598-0
12. Nikeghbalian S, Dehghani M, Salahi H, Bahador A, Kazemi K, Kakaei F, et al. Effects of surgical technique on postoperative renal function after orthotopic liver transplant. Exp Clin Transplant. (2009) 7(1):25–7. PMID: 19364308.19364308
13. Lima EQ, Zanetta DMT, Castro I, Massarollo PCB, Mies S, Machado MM, et al. Risk factors for development of acute renal failure after liver transplantation. Renal Fail. (2003) 25(4):553–60. doi: 10.1081/JDI-120022546
14. Yuan CH, Xiu DR, Jiang B, Li ZF, Li L, Song SB, et al. The influential factors and clinical significance of acute renal failure complicated to orthotopic liver transplantation. Zhonghua Wai Ke Za Zhi. (2011) 49(11):1003–6. PMID: 22333421.22333421
15. Lim C, Audureau E, Salloum C, Levesque E, Lahat E, Merle JC, et al. Acute kidney injury following hepatectomy for hepatocellular carcinoma: incidence, risk factors and prognostic value. HPB (Oxford). (2016) 18(6):540–8. doi: 10.1016/j.hpb.2016.04.004
16. Duah A, Duah F, Ampofo-Boobi D, Addo BP, Osei-Poku F, Agyei-Nkansah A. Acute kidney injury in patients with liver cirrhosis: prevalence, predictors, and in-hospital mortality at a district hospital in Ghana. BioMed Res Int. (2022) 2022:4589767. doi: 10.1155/2022/4589767
17. Kim WH, Oh H-W, Yang S-M, Yu JH, Lee H-C, Jung C-W, et al. Intraoperative hemodynamic parameters and acute kidney injury after living donor liver transplantation. Transplantation. (2019) 103(9):1877–86. doi: 10.1097/TP.0000000000002584
18. Morandi A, Risaliti M, Montori M, Buccianti S, Bartolini I, Moraldi L. Predicting post-hepatectomy liver failure in HCC patients: a review of liver function assessment based on laboratory tests scores. Medicina. (2023) 59(6):1099. doi: 10.3390/medicina59061099
19. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. (2015) 33(6):550–8. doi: 10.1200/JCO.2014.57.9151
20. Durand F, Valla D. Assessment of the prognosis of cirrhosis: child-pugh versus MELD. J Hepatol. (2005) 42:S100–7. doi: 10.1016/j.jhep.2004.11.015
21. Ho S-Y, Liu P-H, Hsu C-Y, Hsia C-Y, Su C-W, Lee Y-H, et al. Comparison of twelve liver functional reserve models for outcome prediction in patients with hepatocellular carcinoma undergoing surgical resection. Sci Rep. (2018) 8(1):4773. doi: 10.1038/s41598-018-22923-4
22. Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. (2010) 16(8):943–9. doi: 10.1002/lt.22091
23. Kassel CA, Wilke TJ, Fremming BA, Brown BA. 2021 clinical update in liver transplantation. J Cardiothorac Vasc Anesth. (2022) 36(11):4183–91. doi: 10.1053/j.jvca.2022.05.027
24. Zeng J, Li Q, Wu Q, Li L, Ye X, Liu J, et al. A novel online calculator predicting acute kidney injury after liver transplantation: a retrospective study. Transpl Int. (2023) 36:10887. doi: 10.3389/ti.2023.10887
25. Kwakernaak AJ, Toering TJ, Navis G. Body mass index and body fat distribution as renal risk factors: a focus on the role of renal haemodynamics. Nephrol Dial Transplant. (2013) 28:iv42–9. doi: 10.1093/ndt/gft331
26. Barone M, Viggiani MT, Losurdo G, Principi M, Leandro G, Di Leo A. Systematic review with meta-analysis: post-operative complications and mortality risk in liver transplant candidates with obesity. Aliment Pharmacol Ther. (2017) 46(3):236–45. doi: 10.1111/apt.14139
27. Tjeertes EEKM, Hoeks SSE, Beks SSBJC, Valentijn TTM, Hoofwijk AAGM, Stolker RJRJ. Obesity–a risk factor for postoperative complications in general surgery? BMC Anesthesiol. (2015) 15:112. doi: 10.1186/s12871-015-0096-7
28. Hafner S, Hillenbrand A, Knippschild U, Radermacher P. The obesity paradox and acute kidney injury: beneficial effects of hyper-inflammation? Crit Care. (2013) 17(6):1023. doi: 10.1186/cc13152
29. Suneja M, Kumar AB. Obesity and perioperative acute kidney injury: a focused review. J Crit Care. (2014) 29(4):694.e1–6. doi: 10.1016/j.jcrc.2014.02.021
30. Billings FT, Pretorius M, Schildcrout JS, Mercaldo ND, Byrne JG, Ikizler TA, et al. Obesity and oxidative stress predict AKI after cardiac surgery. J Am Soc Nephrol. (2012) 23(7):1221–8. doi: 10.1681/ASN.2011090940
31. Park MH, Shim HS, Kim WH, Kim HJ, Kim DJ, Lee SH, et al. Clinical risk scoring models for prediction of acute kidney injury after living donor liver transplantation: a retrospective observational study. PLoS One. (2015) 10(8):e0136230. doi: 10.1371/journal.pone.0136230
32. Ariyarathna D, Bhonsle A, Nim J, Huang CKL, Wong GH, Sim N, et al. Intraoperative vasopressor use and early postoperative acute kidney injury in elderly patients undergoing elective noncardiac surgery. Renal Fail. (2022) 44(1):648–59. doi: 10.1080/0886022X.2022.2061997
33. Penev Y, Ruppert MM, Bilgili A, Li Y, Habib R, Dozic A-V, et al. Intraoperative hypotension and postoperative acute kidney injury: a systematic review. Am J Surg. (2024) 232:45–53. doi: 10.1016/j.amjsurg.2024.02.001
34. Kalisvaart M, Schlegel A, Umbro I, de Haan JE, Polak WG, IJzermans JN, et al. The AKI prediction score: a new prediction model for acute kidney injury after liver transplantation. HPB (Oxford). (2019) 21(12):1707–17. doi: 10.1016/j.hpb.2019.04.008
35. Lee JH, Kim S-H, Jang JH, Park JH, Jo KM, No T-H, et al. Clinical usefulness of biomarkers for diagnosis and prediction of prognosis in sepsis and septic shock. Medicine (Baltimore). (2022) 101(48):e31895. doi: 10.1097/MD.0000000000031895
36. Feng Y, He H, Jia C, Xu Z, Li Y, Liao D. Meta-analysis of procalcitonin as a predictor for acute kidney injury. Medicine (Baltimore). (2021) 100(10):e24999. doi: 10.1097/MD.0000000000024999
37. Huang H-L, Nie X, Cai B, Tang J-T, He Y, Miao Q, et al. Procalcitonin levels predict acute kidney injury and prognosis in acute pancreatitis: a prospective study. PLoS One. (2013) 8(12):e82250. doi: 10.1371/journal.pone.0082250
38. Nie X, Wu B, He Y, Huang X, Dai Z, Miao Q, et al. Serum procalcitonin predicts development of acute kidney injury in patients with suspected infection. Clin Chem Lab Med. (2013) 51(8):1655–61. doi: 10.1515/cclm-2012-0822
39. Kurtul A, Murat SN, Yarlioglues M, Duran M, Ocek AH, Celik IE, et al. Procalcitonin as an early predictor of contrast-induced acute kidney injury in patients with acute coronary syndromes who underwent percutaneous coronary intervention. Angiology. (2015) 66(10):957–63. doi: 10.1177/0003319715572218
40. Lang F, Leibrock C, Pelzl L, Gawaz M, Pieske B, Alesutan I, et al. Therapeutic interference with vascular calcification-lessons from klotho-hypomorphic mice and beyond. Front Endocrinol (Lausanne). (2018) 9:207. doi: 10.3389/fendo.2018.00207
41. Terker AS, Zhang Y, Arroyo JP, Cao S, Wang S, Fan X, et al. Kir4.2 mediates proximal potassium effects on glutaminase activity and kidney injury. Cell Rep. (2022) 41(12):111840. doi: 10.1016/j.celrep.2022.111840
42. Lee YS, Choi YJ, Park KH, Park BS, Son JM, Park JY, et al. Liver transplant patients with high levels of preoperative serum ammonia are at increased risk for postoperative acute kidney injury: a retrospective study. J Clin Med. (2020) 9(6):1629. doi: 10.3390/jcm9061629
43. Cardoso B, Laranjo S, Gomes I, Freitas I, Trigo C, Fragata I, et al. Acute kidney injury after pediatric cardiac surgery: risk factors and outcomes. Proposal for a predictive model. Rev Port Cardiol. (2016) 35(2):99–104. doi: 10.1016/j.repc.2015.06.006
44. Lu H-Y, Ning X-Y, Chen Y-Q, Han S-J, Chi P, Zhu S-N, et al. Predictive value of serum creatinine, blood urea nitrogen, uric acid, and β 2-microglobulin in the evaluation of acute kidney injury after orthotopic liver transplantation. Chin Med J. (2018) 131(9):1059–66. doi: 10.4103/0366-6999.230726
45. Yang J, Wang L, Chen L, Zhou P, Yang S, Shen H, et al. A comprehensive step-by-step approach for the implementation of target trial emulation: evaluating fluid resuscitation strategies in post-laparoscopic septic shock as an example. Laparosc Endosc Rob Surg. (2025) 8(1):28–44. doi: 10.1016/j.lers.2025.01.001
46. Yang J, Zhang B, Hu C, Jiang X, Shui P, Huang J, et al. Identification of clinical subphenotypes of sepsis after laparoscopic surgery. Laparosc Endosc Rob Surg. (2024) 7(1):16–26. doi: 10.1016/j.lers.2024.02.001
Keywords: liver transplantation, acute kidney injury, risk factors, nomogram, predictive model
Citation: Wang D, Chen X, Tang Y, Xiao W, Xu K and Deng J (2025) Development of a risk prediction model for acute kidney injury in liver transplant recipients. Front. Surg. 12:1683424. doi: 10.3389/fsurg.2025.1683424
Received: 11 August 2025; Accepted: 29 October 2025;
Published: 18 November 2025.
Edited by:
Nadia Mansour, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, ItalyReviewed by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaNassiba Beghdadi, Hôpital Beaujon, France
Copyright: © 2025 Wang, Chen, Tang, Xiao, Xu and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiehua Deng, amllaHVhZGVuZ0BnbG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Dahui Wang
Dahui Wang Xin Chen
Xin Chen Yanchen Tang5
Yanchen Tang5 Wei Xiao
Wei Xiao Jiehua Deng
Jiehua Deng