- 1Department of Medical and Surgical Sciences, University of Foggia, Foggia, Italy
- 2Department of Experimental Medicine, University of Salento, Lecce, Italy
- 3Department of Precision and Regenerative Medicine and Ionian Area (DiMePRe-J), University of Bari Aldo Moro, Bari, Italy
Background: Real-time fluorescence-guided surgery using intraoperative indocyanine green (ICG) has gained increasing popularity in colorectal procedures. This study aims to assess the effectiveness of ICG fluorescence imaging in enhancing the intraoperative identification of lymph nodes and in reducing the rate of anastomotic leakage.
Methods: A retrospective single-center study was conducted between September 2020 and December 2024 at a tertiary colorectal cancer surgery center. Patients with rectal cancer who underwent minimally invasive anterior rectal resection were included. They were divided into two groups: Group A received both preoperative peritumoral and intraoperative intravenous ICG injections, while Group B did not receive ICG. The intraoperative and short-term outcomes, including the number of harvested lymph nodes and the rate of anastomotic leakage, were compared between the groups.
Results: A total of 40 patients (22 males) were included in the study. Operative time, hospital stay, intraoperative blood loss, and transfusion rates were similar between the two groups. Although the ICG group had a higher number of harvested lymph nodes (19 vs. 18), positive nodes, and lymph node ratio (LNR), these differences were not statistically significant on univariate analysis. Intraoperative changes were made in 30% of ICG cases to extend lymphadenectomy and in 25% to modify resection margins. Notably, no anastomotic leaks occurred in the ICG group (Group A), compared to a 10% leak rate in the control group (Group B). Linear regression analysis demonstrated that ICG use was significantly associated with increased lymph node yield (β = 3.65, p = 0.002), a higher number of positive nodes (β = 0.85, p = 0.028), and a greater LNR (β = 0.061, p = 0.034), indicating improved oncologic accuracy.
Conclusions: This study demonstrates the feasibility and safety of using ICG fluorescence imaging in minimally invasive rectal cancer surgery. Its use could enhance lymph node mapping, support faster bowel recovery, and potentially reduce the risk of anastomotic leaks.
Introduction
Minimally invasive surgery has become the standard approach for the treatment of rectal cancer, offering improved short-term outcomes and oncologic efficacy comparable to open procedures (1). However, challenges remain in achieving optimal lymphadenectomy and reducing postoperative complications, particularly anastomotic leakage, which continues to be a significant source of morbidity and mortality.
Indocyanine green (ICG) fluorescence imaging has emerged as a valuable tool in various surgical disciplines (2), enabling real-time visualization of vascular perfusion and lymphatic pathways. In colorectal surgery, ICG has shown promise in improving the assessment of bowel perfusion and facilitating more accurate lymph node mapping, both of which are critical for oncologic precision and surgical safety (3).
Despite growing interest in the application of ICG during rectal cancer surgery, the evidence supporting its routine use remains limited and heterogeneous (4).
When injected into peritumoral tissues, ICG migrates via the lymphatic pathways and accumulates in the regional lymph nodes, thereby enabling real-time mapping of tumor-specific lymphatic drainage (5). This method has shown promise in colorectal cancer surgery for improving the accuracy of lymphadenectomy.
Furthermore, the use of near-infrared fluorescence (NIRF) imaging with ICG enables real-time assessment of intestinal perfusion, contributing to improved anastomotic safety and a reduced risk of anastomotic leak (6).
Nowadays, robotic platforms such as the Da Vinci Xi system offer high-definition 3D visualization and enhanced tissue differentiation (7). When combined with ICG, these technologies may facilitate detailed intraoperative imaging of both vascular and lymphatic structures, without significantly extending operative time or disrupting workflow.
This study aims to evaluate the effectiveness of NIRF imaging with ICG in enhancing intraoperative identification of lymph nodes in patients undergoing minimally invasive surgery for rectal cancer.
Methods
A retrospective study was conducted between September 2020 and December 2024 at the University Unit of General Surgery, Policlinico of Foggia. Patients diagnosed with rectal adenocarcinoma who underwent minimally invasive anterior rectal resection—either laparoscopic or robotic—were included.
Inclusion criteria comprised adults aged 18–80 years with histologically confirmed non-metastatic (M0) primary rectal cancer. Exclusion criteria included emergency procedures, pregnancy or breastfeeding, and known hypersensitivity to ICG. All participants provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Patients were divided into two groups:
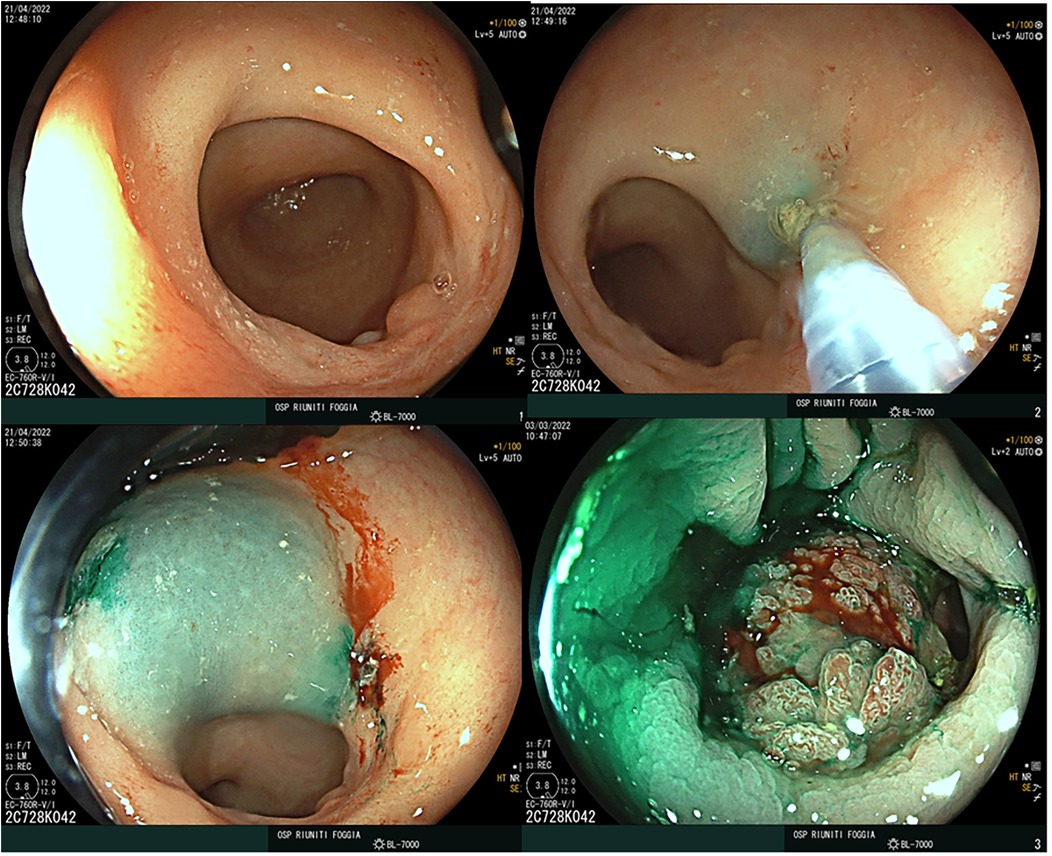
• Group A underwent ICG-guided surgery, which included a preoperative endoscopic peritumoral injection of 3 mg of ICG (0.5 mg/mL dilution) into the four quadrants surrounding the tumor, administered 12–16 h before surgery (Figure 1), as well as intraoperative intravenous ICG administration.
• Group B underwent standard surgery without the use of ICG.
Patient allocation to Group A (ICG-guided) and Group B (non-ICG) was balanced throughout the study period, with both groups including patients from the early and later phases. All procedures were performed by a dedicated colorectal surgical team, with comparable expertise and experience throughout the study, ensuring consistency in surgical technique and perioperative management.
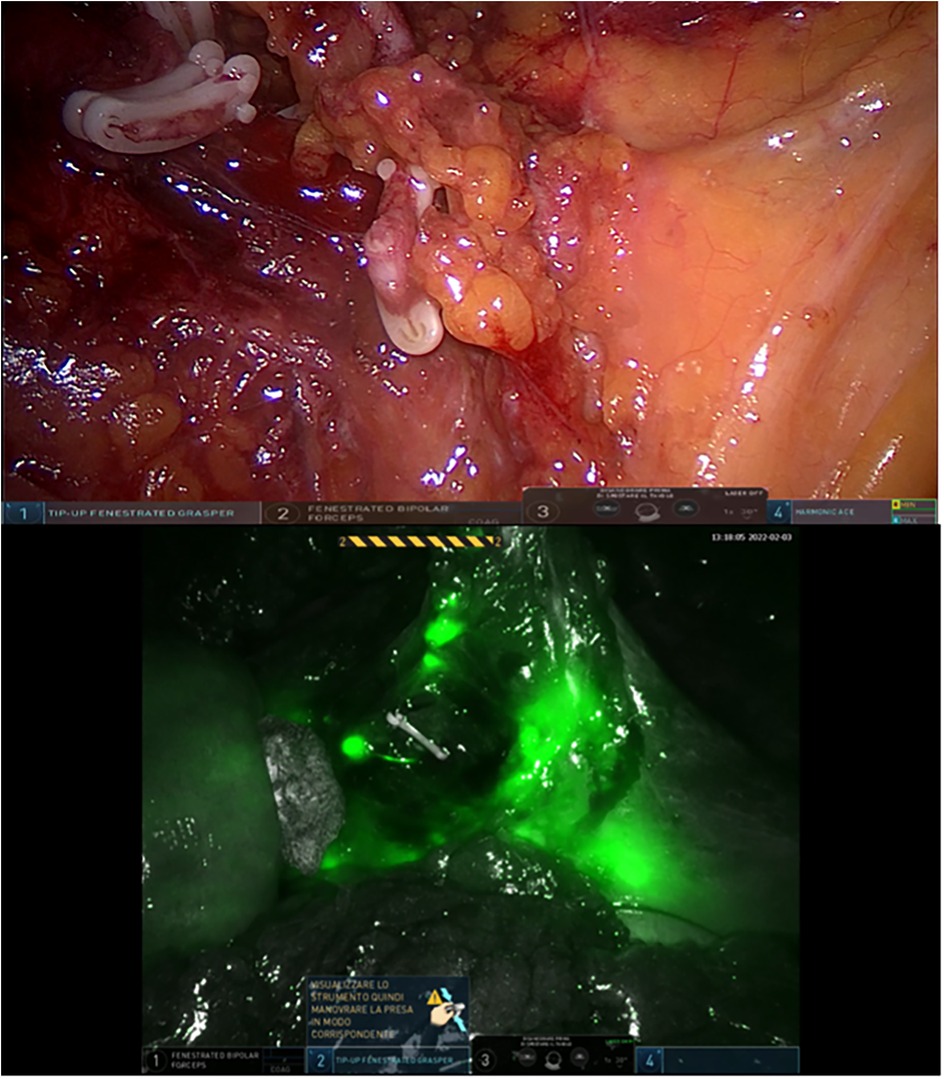
Preoperative staging for all patients included total colonoscopy with biopsy, chest and abdominal CT scans, pelvic MRI, and endoscopic ultrasound. For patients who underwent neoadjuvant chemoradiotherapy (CRT), restaging was performed following completion of treatment. Intraoperatively, near-infrared fluorescence imaging was employed in Group A to visualize lymphatic drainage pathways and to assess perfusion at the anastomotic site following intravenous administration of ICG (Verdye®, 5 mg/mL).
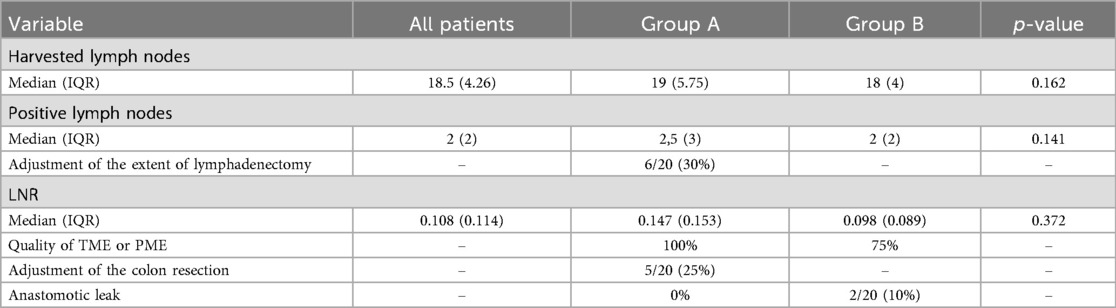
Clinical data, intraoperative observations, and postoperative outcomes were prospectively recorded in a dedicated Excel database. The primary outcomes assessed were the feasibility and safety of ICG use for lymph node mapping and perfusion evaluation. Comparative analyses between the two groups focused on total and positive lymph nodes retrieved, lymph node ratio (LNR), frequency of intraoperative modifications to lymphadenectomy, and incidence of clinically evident anastomotic leakage (Figures 2, 3).
Statistical analysis
Statistical analyses were performed to evaluate differences in clinical, surgical, and pathological outcomes between the ICG group (Group A) and the non-ICG group (Group B). The distribution of continuous variables was assessed using the Shapiro–Wilk test. Continuous variables are expressed as median and interquartile range (IQR) and compared using the Wilcoxon signed-rank test. Categorical variables are reported as frequencies and percentages, and compared using the chi-square test.
To assess potential associations between the use of ICG and key surgical outcomes—including the number of harvested lymph nodes, number of positive nodes, and LNR—linear regression analyses were conducted. The level of statistical significance was set at p < 0.05. All analyses were performed using SPSS software, version 27.
Results
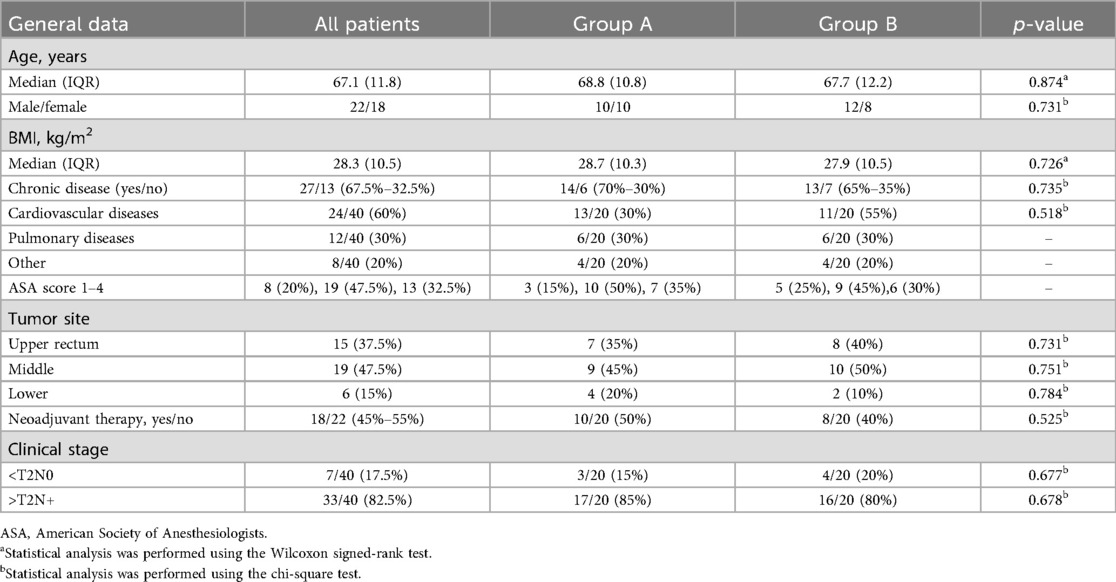
Forty patients (22 males and 18 females) were enrolled in the study. Patients were equally distributed between the two groups, with no significant differences in baseline characteristics. The baseline characteristics of all patients are reported in Table 1.
Table 2 shows a comparative analysis between intra- and postoperative data between Group A (ICG) and Group B (no ICG). The distribution of laparoscopic and robotic approaches was equal in both groups. The conversion rate to open surgery rate was 20% in Group A and 30% in Group B (p = 0.346). The median operative time was comparable between groups. Postoperative hospital stay was slightly shorter in Group A, but with no statistical significance (p = 0.128). Intraoperative blood transfusion was required in 15% of patients in Group A and in 30% in Group B (p = 0.451). Estimated blood loss was similar between the groups (p = 0.447). No 30-day mortality occurred in either group.
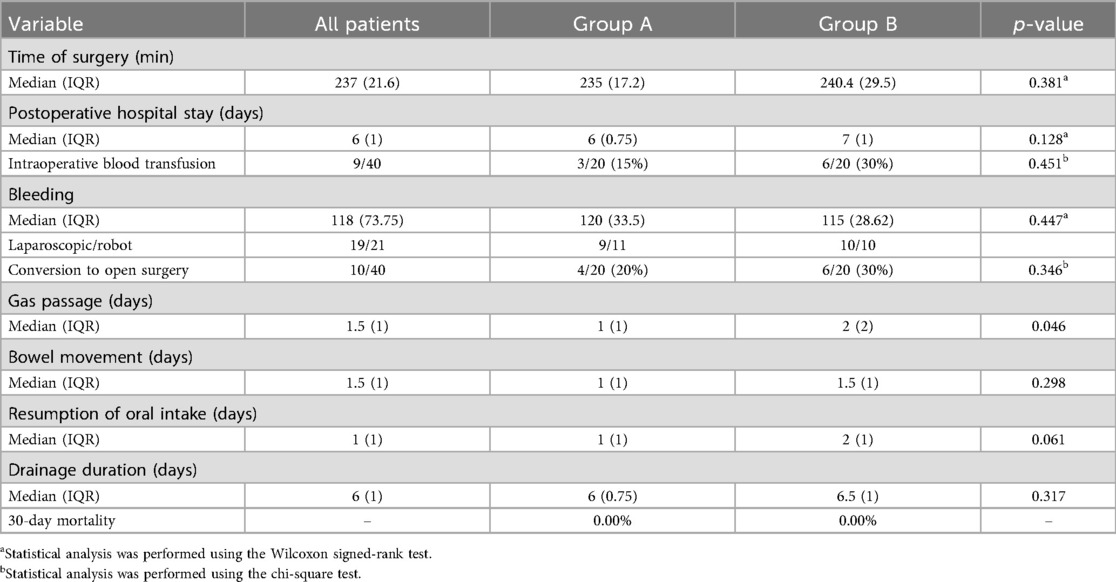
The median number of harvested and positive lymph nodes was slightly higher in Group A (19) than that in Group B (18), although the difference was not statistically significant (p = 0.162). Notably, in 30% of cases in Group A, the extent of lymphadenectomy was adjusted intraoperatively based on ICG fluorescence guidance. No difference in LNR was found between the groups (p = 0.372). The quality of total mesorectal excision (TME) or partial mesorectal excision (PME) was optimal in 100% of patients in Group A, compared to 75% in Group B. In the remaining five cases from Group B, the quality of excision was classified as grade 2 according to the Quirke classification (8). Oncologic margins were also assessed in both groups. The circumferential resection margin (CRM) and distal resection margin were free of tumor involvement in all cases, with no significant differences observed between Group A and Group B.
Intraoperative adjustments to the colonic resection margin were made in 25% of cases in Group A, whereas no such adjustments were reported in Group B. No anastomotic leak occurred in Group A, whereas Group B experienced a 10% leak rate, which was treated conservatively Table 3).
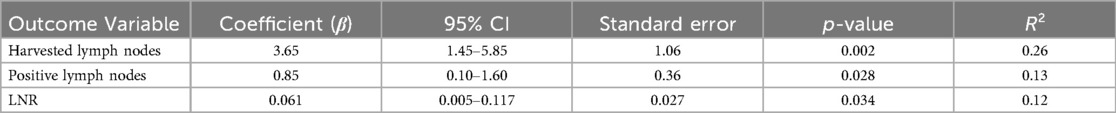
To assess the potential association between the use of ICG and surgical and pathological outcomes, a linear regression analysis was performed. The primary objective was to assess whether ICG administration influenced the number of harvested lymph nodes, the number of positive lymph nodes, and the LNR (Table 4).
Linear regression analyses demonstrated that ICG use was significantly associated with an increased number of harvested lymph nodes (β = 3.65, 95% CI: 1.45–5.85, p = 0.002), a higher number of positive nodes (β = 0.85, p = 0.028), and a greater LNR (β = 0.061, p = 0.034). These findings support the hypothesis that ICG-guided mapping may enhance nodal yield and detection (Table 4).
Discussion
This study evaluated the impact of ICG with NIRF imaging on short-term surgical and oncologic outcomes in minimally invasive rectal cancer surgery. Our findings demonstrate that ICG use is associated with enhanced lymph node retrieval and more frequent intraoperative adaptations to both resection margins and lymphadenectomy.
Although the overall lymph node yield between groups was not statistically significant, linear regression analysis confirmed a significant association between ICG use and increased lymph node harvest. Furthermore, both the number of positive lymph nodes and the LNR were significantly higher in the ICG group, suggesting improved sensitivity for detecting N+ disease. This aligns with previous reports indicating that peritumoral ICG injection enables more accurate mapping of individual lymphatic drainage patterns, potentially revealing aberrant nodal basins that are not routinely dissected during standard oncologic resections (9, 10).
Importantly, 30% of patients in the ICG group required intraoperative modifications to the extent of lymphadenectomy based on fluorescence guidance, highlighting the dynamic utility of ICG imaging for tailoring surgery in real time. A study by Watanabe et al. (11) similarly observed that ICG mapping enabled more targeted lymphadenectomies without compromising resection margins or increasing operative times.
No anastomotic leaks occurred in Group A, compared to a 10% leak rate in the control group. Although the absolute numbers were small, the difference is clinically meaningful and mirrors the trend observed in larger prospective trials such as PILLAR II, where perfusion assessment using ICG reduced leak-related reinterventions (12). In our cohort, 25% of ICG cases required intraoperative modification of the resection margin, likely contributing to the improved anastomotic integrity.
The quality of TME/PME was also superior in the ICG group, with 100% achieving optimal dissection, compared to only 75% in the non-ICG group. The ability of ICG to enhance visual discrimination of vascular and lymphatic structures may support more precise dissection, particularly when combined with robotic platforms that offer stable, magnified visualization (13, 14).
Despite these encouraging outcomes, the study has some limitations. This was a retrospective and single-center study with potential selection bias. Although baseline characteristics were balanced, unmeasured confounders may have influenced surgical decision-making. The sample size was relatively small, and although statistical associations were demonstrated, larger multicenter studies are necessary to validate these findings. In addition, long-term oncologic outcomes such as disease-free and overall survival were not assessed.
In conclusion, the integration of ICG fluorescence imaging into minimally invasive rectal cancer surgery enhances intraoperative visualization, enabling more accurate lymph node mapping and safer anastomotic construction. While these results are promising, larger prospective studies are warranted to confirm the oncologic benefits and to standardize the use of ICG in routine colorectal practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for this study because it involved a retrospective analysis of anonymized data collected during standard clinical care, without any intervention outside routine surgical practice. No identifiable personal information was used, and the study complied with the principles of the Declaration of Helsinki. The study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MP: Writing – original draft, Writing – review & editing. GP: Writing – original draft. EL: Writing – original draft. AP: Writing – original draft, Writing – review & editing. MDF: Writing – review & editing. AA: Writing – review & editing. NT: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This article has been published with the financial support of the Department of Medical and Surgical Sciences of the University of Foggia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Garcia LE, Taylor J, Atallah C. Update on minimally invasive surgical approaches for rectal cancer. Curr Oncol Rep. (2021) 23(10):117. doi: 10.1007/s11912-021-01110-1
2. Peltrini R, Podda M, Castiglioni S, Di Nuzzo MM, D'Ambra M, Lionetti R, et al. Intraoperative use of indocyanine green fluorescence imaging in rectal cancer surgery: the state of the art. World J Gastroenterol. (2021) 27(38):6374–86. doi: 10.3748/wjg.v27.i38.6374
3. Liu G, Zhang S, Zhang Y, Fu X, Liu X. Robotic surgery in rectal cancer: potential, challenges, and opportunities. Curr Treat Options Oncol. (2022) 23(7):961–79. doi: 10.1007/s11864-022-00984-y
4. Litchinko A, Meyer J, Buhler L, Ris F, Adamina M. Fluorescence indocyanine green (ICG) for sentinel-lymph-node mapping in colorectal cancer: a systematic review. Langenbecks Arch Surg. (2025) 410(1):202. doi: 10.1007/s00423-025-03786-6
5. Khalafi S, Botero Fonnegra C, Reyes A, Hui VW. Developments in the use of indocyanine green (ICG) fluorescence in colorectal surgery. J Clin Med. (2024) 13(14):4003. doi: 10.3390/jcm13144003
6. Faber RA, Meijer RPJ, Droogh DHM, Jongbloed JJ, Bijlstra OD, Boersma F, et al. Indocyanine green near-infrared fluorescence bowel perfusion assessment to prevent anastomotic leakage in minimally invasive colorectal surgery (AVOID): a multicentre, randomised, controlled, phase 3 trial. Lancet Gastroenterol Hepatol. (2024) 9(10):924–34. doi: 10.1016/S2468-1253(24)00198-5
7. Chen E, Chen L, Zhang W. Robotic-assisted colorectal surgery in colorectal cancer management: a narrative review of clinical efficacy and multidisciplinary integration. Front Oncol. (2025) 15:1502014. doi: 10.3389/fonc.2025.1502014
8. Maslekar S, Sharma A, Macdonald A, Gunn J, Monson JR, Hartley JE. Mesorectal grades predict recurrences after curative resection for rectal cancer. Dis Colon Rectum. (2007) 50(2):168–75. doi: 10.1007/s10350-006-0756-2
9. Cahill RA, Anderson M, Wang LM, Lindsey I, Cunningham C, Mortensen NJ. Near-infrared (NIR) laparoscopy for intraoperative lymphatic road-mapping and sentinel node identification during definitive surgical resection of early-stage colorectal neoplasia. Surg Endosc. (2012) 26(1):197–204. doi: 10.1007/s00464-011-1854-3
10. Park SY, Park JS, Kim HJ, Choi GS. Feasibility of indocyanine green fluorescence lymph node mapping for radical colectomy of mid-transverse and left-sided colon cancer. Ann Robot Innov Surg. (2022) 3(1):1–8. doi: 10.37007/aris.2022.3.1.1
11. Watanabe J, Ota M, Suwa Y, Ishibe A, Masui H, Nagahori K. Real-time indocyanine green fluorescence imaging-guided complete mesocolic excision in laparoscopic flexural colon cancer surgery. Dis Colon Rectum. (2016) 59(7):701–5. doi: 10.1097/DCR.0000000000000608
12. Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DA, et al. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg. (2015) 220(1):82–92.e1. doi: 10.1016/j.jamcollsurg.2014.09.015
13. Boni L, David G, Mangano A, Dionigi G, Rausei S, Spampatti S, et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc. (2015) 29(7):2046–55. doi: 10.1007/s00464-014-3895-x
Keywords: rectal cancer, minimally invasive surgery, indocyanine green, fluorescence, lymph node mapping
Citation: Pacilli M, Pavone G, Lamanna E, Picciariello A, De Fazio M, Ambrosi A and Tartaglia N (2025) Impact of preoperative indocyanine green injection on intraoperative decision-making and lymph node harvest in rectal cancer surgery. Front. Surg. 12:1683663. doi: 10.3389/fsurg.2025.1683663
Received: 11 August 2025; Accepted: 8 September 2025;
Published: 26 September 2025.
Edited by:
Gianpiero Gravante, ASL Lecce, ItalyReviewed by:
Alfredo Annicchiarico, University of Parma, ItalyYuliia Medkova, I.M. Sechenov First Moscow State Medical University, Russia
Copyright: © 2025 Pacilli, Pavone, Lamanna, Picciariello, De Fazio, Ambrosi and Tartaglia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mario Pacilli, bWFyaW8ucGFjaWxsaUB1bmlmZy5pdA==
 Mario Pacilli
Mario Pacilli Giovanna Pavone
Giovanna Pavone Ennio Lamanna
Ennio Lamanna Arcangelo Picciariello
Arcangelo Picciariello Michele De Fazio
Michele De Fazio Antonio Ambrosi
Antonio Ambrosi Nicola Tartaglia
Nicola Tartaglia