- Orthopedic Surgery, Suzhou TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Suzhou, Jiangsu, China
This review explores the impact of osteoporosis on arthroscopic rotator cuff repair and subsequent tendon healing, focusing on challenges such as anchor fixation failure, intraoperative fractures, and limited surgical visibility. It examines how osteoporosis disrupts the tendon healing microenvironment post-surgery through mechanisms involving bone metabolism, growth factors, the immune system, sex hormones, oxidative stress, and adipose infiltration. Effective surgical planning is crucial to mitigate the adverse effects of osteoporosis on rotator cuff repair. This review offers recommendations for optimizing surgical strategies, including anchor selection, placement, and fixation techniques. In addition, it highlights the potential of anti-osteoporotic drugs and biological therapies to improve tendon-to-bone union and enhance clinical outcomes. For cases of inevitable repair failure, remedial strategies are proposed to inform clinical practice. A systematic literature search was conducted in the PubMed, Web of Science, and CNKI databases (2000–2025) using the following keywords: “osteoporosis,” “rotator cuff injury,” “shoulder arthroscopy,” “tendon-to-bone healing,” and “retear.” The inclusion criteria were as follows: (1) human or animal studies; (2) MRI-confirmed rotator cuff tears; and (3) full-text articles in English or Chinese. The exclusion criteria included case reports (n < 10).
1 Introduction
Rotator cuff injury is a prevalent shoulder pathology characterized by pain and functional impairment, particularly in middle-aged and elderly populations (1). Due to its limited capacity for self-healing, rotator cuff injuries frequently require surgical intervention. Arthroscopic shoulder surgery is widely used to treat such injuries, although the risk of postoperative retears remains significant (2). Osteoporosis, which is characterized by reduced bone mass and deteriorated bone microarchitecture, represents a metabolic imbalance in bone tissue, resulting in increased fragility and fracture risk. It is both a risk factor for the initial occurrence of rotator cuff injuries and a factor that adversely affects postoperative tendon-to-bone healing, thereby increasing the likelihood of retears (3, 4). Clinically, patients with rotator cuff injuries and osteoporosis face greater surgical challenges, an elevated risk of impaired tendon-to-bone healing, and subsequent retears that may necessitate revision surgery, significantly increasing the economic burden (5). Recent research has focused on improving surgical outcomes, optimizing the tendon-to-bone healing microenvironment, and reducing the postoperative retear rate. With advancements in medical technology, clinicians have explored new strategies in surgical techniques and bioengineering, offering promising pathways for enhanced prognosis. This review aims to analyze the impact of osteoporosis on arthroscopic rotator cuff repair and postoperative tendon-to-bone healing, while also discussing current clinical strategies for optimizing outcomes.
2 Relationship between osteoporosis and rotator cuff injury
Osteoporosis increases the risk of rotator cuff injury. In a matched cohort study by Hong et al. (4), 17,067 osteoporotic patients and 100,501 non-osteoporotic controls were followed for 7 years. The results revealed 166 and 89 cases of rotator cuff tears in the osteoporotic and non-osteoporotic groups, respectively, indicating a 1.79-fold higher risk of rotator cuff tear among osteoporotic patients compared with their non-osteoporotic counterparts. Age-related decreases in bone mineral density (BMD), particularly in the proximal humerus and greater tuberosity, complicate the surgical repair of rotator cuff injuries. Reduced BMD at these critical sites alters the tendon-to-bone healing microenvironment, increasing the risk of postoperative retear. In addition, decreased local mechanical stimulation from the rotator cuff injury may contribute to secondary osteoporosis (5, 6). Chen et al. (7) categorized 74 rotator cuff injury patients into three groups based on preoperative bone density (normal bone mass, osteopenia, and osteoporosis). After 12 months of postoperative follow-up, significantly higher retear rates were observed in the osteopenic and osteoporotic groups compared with the normal bone density group. Lee et al. (8) performed dual-energy x-ray absorptiometry (DEXA) on 87 patients with unilateral rotator cuff injuries and analyzed the BMD of various regions of interest (ROIs), including the humeral head, lesser tuberosity, medial greater tuberosity, middle greater tuberosity, lateral greater tuberosity, and the overall proximal humerus. Their results demonstrated significantly lower BMD in all ROIs on the injured side compared with the contralateral healthy side (all p < 0.05).
Thus, thorough osteoporosis screening is essential for middle-aged and elderly patients with rotator cuff injuries, and timely intervention should be implemented for those diagnosed with osteoporosis.
3 Influence of osteoporosis on rotator cuff repair surgery
3.1 Increased risk of anchor fixation failure
Patients with osteoporosis face greater challenges in achieving stable anchor fixation compared with those without osteoporosis. Localized osteoporosis frequently leads to early postoperative anchor loosening or dislodgement, resulting in long-term instability of the bone bed. This instability compromises the healing microenvironment and increases the risk of rotator cuff retears (6). Tingart et al. (9) conducted a cadaveric study to examine the relationship between proximal humeral BMD and anchor pull-out strength. They found a significant positive correlation, highlighting the need for thorough preoperative osteoporosis evaluation, optimization of surgical techniques, careful implant selection, and real-time intraoperative monitoring of anchor stability to mitigate the risk of postoperative retears.
3.2 Increased risk of intraoperative fracture
The risk of intraoperative fractures in osteoporotic patients primarily results from reduced bone strength combined with concentrated mechanical stress during surgery. Shoulder arthroscopy is typically performed with the patient in a lateral decubitus position, maintaining prolonged arm abduction to maximize the space between the humerus and glenoid (10). However, in osteoporotic patients, excessive abduction traction may predispose the proximal humerus to fractures. In addition, localized compression during cannula insertion can cause fractures at the entry site. Careful management of the footprint bone bed is also critical, as excessive abrasion in this area can disrupt the subchondral bone plate, potentially leading to localized humeral head collapse (5). Furthermore, careful drilling of anchor pilot holes and precise control of suture tension are essential, as excessive drilling speed and tension may contribute to further localized fractures.
3.3 Limited surgical visualization
Patients with osteoporosis often experience reduced bone density, which leads to weakened capsular attachment sites or humeral head joint surface collapse, resulting in joint misalignment. The passive stretching of the capsule required to maintain joint stability can cause capsular laxity, negatively affecting surgical visualization. Restricted visibility prolongs the operative time, increasing the risk of infection. In addition, diminished visibility compromises surgical precision, raising the risk of incorrect anchor placement or inadvertent damage to neurovascular structures and tendons. Higher irrigation pressures, necessary to maintain joint space expansion, also increase the risk of fluid extravasation, potentially causing localized limb swelling and adverse effects on peripheral vasculature (11). Therefore, continuous intraoperative monitoring of skin tension and limb swelling is essential to avoid excessive irrigation pressures and related complications.
4 Influence of osteoporosis on postoperative tendon-to-bone healing
The normal tendon-to-bone junction (enthesis) consists of a highly specialized tissue architecture, including bone, calcified fibrocartilage, non-calcified fibrocartilage, and tendon, which effectively distributes mechanical stress between the tendon and bone, thereby enhancing biomechanical properties (12). Surgical intervention is the primary treatment for injuries at the tendon-to-bone interface. However, clinical studies indicate that surgically repaired tendon-to-bone junctions typically heal as disorganized scar tissue, rather than restoring the original four-layer structure. The irregular extracellular matrix and reduced elasticity of scar tissue significantly impair biomechanical function (13). In patients with osteoporosis, the altered healing microenvironment often results in poor tendon-to-bone integration, significantly increasing the risk of postoperative retears (3). Key mechanisms by which osteoporosis affects tendon-to-bone healing (Figure 1) include the following.
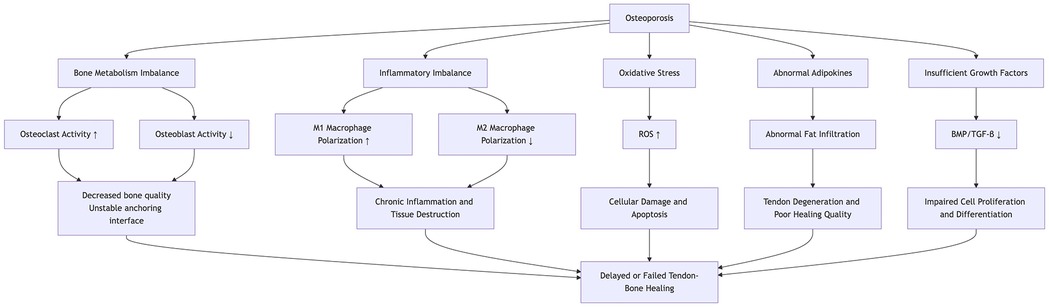
Figure 1. This diagram illustrates the mechanisms by which osteoporosis affects the healing process of the tendon-bone, involving bone metabolism, inflammation, oxidative stress, adipokines, and growth factors.
4.1 Imbalanced bone metabolism
Normal bone metabolism results from the precise coordination between osteoclasts and osteoblasts, maintaining skeletal integrity through the continuous resorption of old bone and formation of new bone. In osteoporosis, the dynamic balance between bone resorption and formation is disrupted; osteoclast activity is enhanced, accelerating bone resorption, while osteoblast function, particularly the differentiation and activity of mesenchymal stem cells (MSCs), is suppressed. This imbalance directly impacts the tendon-to-bone interface. Xu et al. (14), using an osteoporotic rat model, investigated the effect of osteoporosis on tendon-to-bone healing after rotator cuff repair. They found that early postoperative healing at the tendon-to-bone interface was impaired due to heightened osteoclast activity. MSCs exert multiple regulatory functions through exosome secretion. In a rat model of rotator cuff reconstruction, Huang et al. (15) demonstrated that bone marrow MSC-derived exosomes (BMSC-Exos) increased failure load and stiffness at the repair site, induced angiogenesis at the tendon-to-bone interface, and promoted healing. However, in osteoporosis, MSC function is often abnormal, with a shift toward enhanced adipogenic differentiation and reduced osteogenic and chondrogenic differentiation (16), thus hindering fibrocartilage regeneration at the interface and impairing effective tendon-to-bone healing.
4.2 Growth factor function is inhibited
Growth factors are cytokines or proteins that play pivotal roles in cell differentiation throughout the healing process. In tendon-to-bone healing, key growth factors such as bone morphogenetic proteins (BMPs), transforming growth factor (TGF), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF) regulate cell proliferation, differentiation, and extracellular matrix synthesis, promoting fibrocartilage regeneration and angiogenesis, which are essential for effective tendon-to-bone integration (17). In the pathological microenvironment of osteoporosis, the normal regulatory functions of growth factors may be impaired, or their associated signaling pathways may be disrupted (18, 19), potentially leading to delayed healing at the tendon-to-bone interface or reduced structural strength. Clinically, growth factors can be used as adjuncts to enhance repair; however, their therapeutic outcomes remain inconsistent, influenced by factors such as delivery method, dosage, and timing of application. Achieving precise, controlled local release of growth factors to optimize their effects in the microenvironment remains a key challenge for future research.
4.3 Decreased sex hormone levels
Previous studies have shown a strong association between sex hormones and tendon-to-bone healing. Tashjian et al. (20) investigated the effects of estrogen and testosterone supplementation in male mice following rotator cuff repair. Histological analysis at 8 weeks post-surgery revealed that supplementation with either estrogen or testosterone significantly improved tendon healing quality. Similarly, Tanaka et al. (21), using an ovariectomized rat model, examined the impact of estrogen deficiency on tendon-to-bone healing after rotator cuff repair. Their results indicated that insufficient estrogen impaired fibrocartilage-like tissue formation at the tendon-to-bone interface, thereby hindering the healing process.
Therefore, in postmenopausal women or elderly male patients, it may be beneficial to assess sex hormone levels and, when appropriate, consider hormone replacement therapy or other endocrine interventions to improve the tendon-to-bone healing microenvironment.
4.4 Persistent low-grade immune activation
Tendon-to-bone healing progresses through three overlapping phases: inflammation, repair, and remodeling. The inflammatory phase is crucial, with macrophages playing an active role throughout the process. During this phase, macrophages polarize into the M1 phenotype, secreting pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) to mediate inflammation, clear necrotic tissue, and promote fibroblast proliferation. In the repair and remodeling phases, macrophages shift toward the M2 phenotype, producing anti-inflammatory cytokines such as interleukin-10 (IL-10) and TGF-β to suppress inflammation and facilitate tissue repair (22, 23). Osteoporosis is often associated with persistent low-grade immune activation, characterized by a higher M1/M2 macrophage ratio and a shift toward M1 polarization. This leads to the accumulation of pro-inflammatory cytokines, intensifying local inflammation, which hinders tendon-to-bone healing (24–26). In addition, excessive early postoperative inflammation may further impair the healing microenvironment. To mitigate the negative effects of excessive inflammation, non-steroidal anti-inflammatory drugs (NSAIDs), cryotherapy, laser therapy, and other physical modalities may help reduce local inflammation and improve postoperative outcomes.
4.5 Oxidative stress
Oxidative stress occurs when there is an imbalance between the production of reactive oxygen species (ROS) and the body's antioxidant defense systems, leading to excessive ROS accumulation, cellular damage, apoptosis, and impaired cellular function (27). Clinical studies have shown that patients with osteoporosis exhibit significantly lower total serum antioxidant capacity and higher serum peroxide levels (28). In another study, postmenopausal women were classified into normal bone mass, osteopenia, and osteoporosis groups based on BMD. Serum measurements revealed that catalase, superoxide dismutase 2 (SOD2), and peroxiredoxin 2 (PRX2) levels were significantly reduced in the abnormal bone mass groups compared with the normal group (29). This pathological imbalance between oxidative and antioxidative systems negatively impacts tendon-to-bone healing. Itoigawa et al. (2) found that oxidative stress and superoxide dismutase (SOD) levels were significantly higher in patients with postoperative retears compared with those with successful tendon healing after arthroscopic rotator cuff repair. Uehara et al. (30), using a rat rotator cuff repair model, demonstrated that antioxidant treatments, such as N-acetylcysteine (NAC) and vitamin C (VC), reduced oxidative stress at the repair site and accelerated the healing process.
4.6 Fatty infiltration
Studies have shown that osteoporosis is often accompanied by fatty infiltration (31–33). Fatty infiltration of shoulder musculature can impair fibrocartilage formation at the tendon-to-bone interface, leading to poor healing outcomes (3, 34). Yang et al. (35) reported that fatty infiltration of the rotator cuff muscles, particularly the infraspinatus, significantly increased the risk of postoperative retears and was associated with lower shoulder function scores. Li et al. (36), using a rat rotator cuff injury model, demonstrated that overexpression or knock-out of the ubiquitin ligase NEDD4 could regulate adipocyte differentiation and lipid metabolism, thereby reducing fatty infiltration and promoting tendon-to-bone healing. Therefore, prolonged immobilization of the shoulder joint after a rotator cuff injury should be avoided to prevent disuse osteoporosis and fatty infiltration. For patients already experiencing fatty infiltration, pharmacological interventions targeting lipid metabolism may be considered.
4.7 Vitamin D deficiency
Vitamin D, particularly its active form 1,25-dihydroxyvitamin D₃, enhances the intestinal absorption of calcium and phosphorus, providing essential substrates for bone mineralization, and works synergistically with parathyroid hormone (PTH) to regulate bone metabolism and maintain serum calcium homeostasis. Vitamin D deficiency is detrimental to tendon-to-bone healing. Chen et al. (37) included 89 patients with full-thickness rotator cuff tears undergoing arthroscopic repair, categorizing them into control and deficiency groups based on serum vitamin D levels. The results showed that the deficiency group had a significantly higher retear rate compared with the control group and was more prone to early postoperative pain. As osteoporosis is frequently accompanied by vitamin D deficiency, combined supplementation with calcium and vitamin D during treatment may help improve bone quality and promote tendon-to-bone healing.
5 Strategies to improve prognosis
To mitigate the impact of osteoporosis on rotator cuff repair and improve outcomes, current approaches focus on two main strategies: first, optimizing surgical techniques to prevent anchor pull-out, and second, enhancing bone quality at fixation sites through osteoporosis medication or biological therapies to promote tendon-to-bone union (Figure 2).
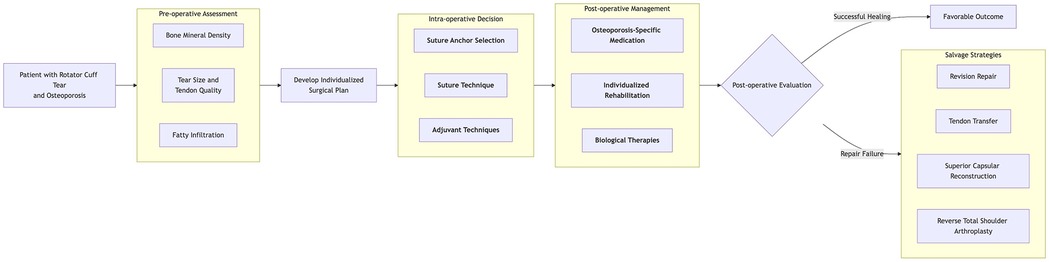
Figure 2. This diagram illustrates the clinical decision pathway for patients with rotator cuff injuries complicated by osteoporosis.
5.1 Enhancing stability of internal fixation
5.1.1 Increase the number of anchor points
Increasing the number of fixation points can effectively distribute stress across the bone–anchor interface, reducing stress concentration at the tendon-to-bone junction, lowering the risk of anchor loosening or failure, and improving anchor pull-out strength (5). However, the number of anchors is limited by the tear size and footprint area. Research suggests a minimum distance of 6 mm between anchors to maintain pull-out strength; therefore, anchors should be spaced at least 6 mm apart during placement (38). Clinicians should carefully evaluate tendon tear size, footprint dimensions, and local bone quality, as excessive anchor placement may prolong surgery, increase the risk of infection, and raise the patient's economic burden.
5.1.2 Single-row, double-row, and suture bridge techniques
The suture technique in rotator cuff repair is crucial for reattaching the tendon to the greater tuberosity. Common methods include single-row, double-row, and suture bridge techniques. Gu et al. (39) reported significantly lower retear rates with double-row techniques compared with single-row techniques. However, they also found no significant difference in outcomes between single- and double-row techniques for tears smaller than 3 cm. The suture bridge technique, an enhanced variation of double-row suturing, has demonstrated improved clinical outcomes in previous studies (40).
5.1.3 Appropriate anchor specification and type
The mechanical fixation strength (pull-out strength) of suture anchors depends on factors such as thread pitch, thread count, length, dimensions, and anchor geometry. Various anchor designs, materials, and dimensions have been developed, each exhibiting different biomechanical performances (41). Chae et al. (42) identified that increasing anchor length, thread count, thread height, and the contact area between anchor threads and surrounding bone enhances anchor pull-out strength. Clinically, commonly used anchors include metal anchors, polymer anchors [e.g., polyetheretherketone (PEEK)], bioabsorbable anchors, biocomposite anchors, and all-suture anchors. Yang et al. (43) conducted biomechanical comparisons of different anchor types used in rotator cuff repairs. They found that PEEK anchors exhibited the highest ultimate failure load, while biocomposite anchors had the lowest. All-suture anchors demonstrated the highest stiffness, while PEEK anchors had the lowest stiffness. Regarding displacement, metal anchors exhibited minimal displacement, followed by all-suture anchors. Importantly, they noted that BMD significantly influences anchor performance. All-suture anchors performed superiorly in osteoporotic bone models. This is primarily due to their superior stress dispersion capabilities, lower bone volume requirements, and reduced bone intrusion. Unlike traditional anchors, the full-thread anchor evenly distributes tensile forces through high-strength suture material, minimizing stress concentration in fragile bone areas, thereby reducing the risk of anchor loosening or fracture. Simultaneously, its compact size and design result in minimal bone intrusion, reducing the risk of bone damage. Moreover, it does not rely on substantial bone volume for stability. Instead, it capitalizes on the relatively well-preserved mechanical properties of cortical bone in osteoporotic skeletons, ensuring effective fixation even in osteoporotic patients and guaranteeing the stability of the repair. Therefore, in osteoporotic patients, the use of all-suture anchors should be considered if local bone conditions permit.
The angle of anchor implantation is also crucial. Liu et al. (44) reported that, at equivalent bone densities, anchors implanted perpendicularly (at a 90° angle) significantly enhanced biomechanical stability. In addition, perpendicular implantation facilitates suture knotting during surgery and improves postoperative recovery of the supraspinatus muscle.
5.1.4 Bone grafting and cement augmentation
During arthroscopic rotator cuff repair, bone grafting or cement augmentation can effectively address defects caused by osteoporotic bone resorption or subchondral cystic lesions. Levy et al. (45) compressed cancellous allograft bone into proximal humeral cystic defects, creating a robust bone bed for anchor fixation and enhancing fixation strength. Fang et al. (46) demonstrated that autologous osteochondral tissue and periosteal grafts significantly promoted fibrocartilage regeneration at the tendon-to-bone interface. However, arthroscopic bone grafting remains technically challenging and is unsuitable for osteoporosis without significant bone defects. Therefore, clinical preference often favors injectable bone cement for bone augmentation and improved anchor fixation strength. Aziz et al. (47) demonstrated in cadaveric studies that polymethylmethacrylate (PMMA) cement significantly enhanced anchor pull-out strength. However, the non-absorbable nature of PMMA complicates revision surgeries and poses a risk of intra-articular extravasation during injection. In addition, thermal effects during cement curing may lead to osteonecrosis. Novel bioabsorbable fiber-reinforced bone cements offer similar tensile strength to PMMA without thermal effects and are absorbable, representing promising enhancement materials (48). Further research into the biocompatibility and safety of the degradation products from these new cements is needed.
5.1.5 Patch augmentation techniques
Patch augmentation involves placing graft materials around torn rotator cuff tendons to provide mechanical support, distribute stress, and promote tissue healing. Both synthetic and biological patches are currently used clinically. Wang et al. (49) demonstrated improved biomechanical properties and tendon-to-bone healing using decellularized amniotic membrane (DAM) in a rat supraspinatus tendon tear model. Similarly, another study (50) showed that acellular amniotic membrane (AAM) facilitated tendon-to-bone healing more effectively when interposed between the tendon and bone, rather than when merely overlaid. Synthetic patches may induce chronic inflammation or infections due to poor tissue compatibility, potentially leading to repair failure (51). Biological patches carry the risk of immunological rejection, and mismatched degradation rates may negatively affect repair outcomes. In addition, decellularized tendon grafts face unresolved issues, such as inferior biomechanical properties compared with normal tendons post-decellularization (52). Therefore, further research is needed to enhance the properties of these biomaterials for improved tendon repair.
5.1.6 Optimization of footprint management
Proper management of the tendon footprint is crucial for ensuring anchor stability and promoting favorable tendon-to-bone healing. Hyatt et al. (53) studied human humeral specimens to examine the biomechanical impact of cortical bone decortication on anchor fixation. The results showed significantly reduced anchor pull-out strength in decorticated specimens (62.84 ± 38.04 N/mm) compared with non-decorticated specimens (244.04 ± 89.06 N/mm; P < 0.0001). Therefore, excessive cortical bone decortication at the footprint should be avoided, particularly in osteoporotic patients. In addition, Sun et al. (54) demonstrated in a rabbit rotator cuff tear model that preserving remnant tendon tissues significantly enhanced biomechanical and histological outcomes, improving overall rotator cuff healing.
5.2 Mitigating the effects of osteoporosis on tendon-to-bone healing
5.2.1 Application of anti-osteoporosis medications
Postoperative administration of anti-osteoporosis medications can enhance tendon-to-bone healing following rotator cuff repair. Current medications for osteoporosis include foundational treatments (e.g., calcium and vitamin D), antiresorptive agents (e.g., bisphosphonates, RANKL inhibitors), anabolic agents (e.g., PTH analogues), and alternative treatments (e.g., strontium salts, traditional Chinese medicines, and their extracts) (55). Zhao et al. (56) demonstrated in a retrospective study that intravenous zoledronic acid significantly reduced retear rates after rotator cuff repair in elderly osteoporotic patients. Xu et al. (57) explored the effects of abaloparatide (ABL) and denosumab (Dmab) on tendon-to-bone healing using an osteoporotic rat model with chronic rotator cuff tears, finding both drugs beneficial, with ABL's anabolic effects yielding superior outcomes compared with Dmab's antiresorptive effects. However, discontinuing anti-osteoporosis medication may lead to deterioration in bone quality. This “bone deterioration” refers not to an absolute decline in bone quality, but to the gradual weakening or even disappearance of the benefits provided by the medication—specifically, the increase in bone density and reduction in fracture risk. Therefore, determining an appropriate treatment duration and implementing regular monitoring is crucial. Treatment cycles are not indefinite but are planned based on pharmacokinetics and the patient's fracture risk. For example, bisphosphonates enter an evaluation phase after an initial 3–5 years of treatment. During this period, regular bone density monitoring and biomarker testing can reflect drug efficacy and bone metabolic status, helping clinicians adjust treatment regimens. Reports have also indicated that osteoporosis medications can cause adverse reactions such as osteonecrosis of the jaw and hypocalcemia. Thus, individualized treatment, considering patient-specific bone quality and overall health status, is essential.
5.2.2 Biological therapies
Biological therapies include cellular treatments [platelet-rich plasma (PRP), stem cell therapy], growth factors, scaffolds, and gene therapy. PRP contains multiple growth factors that can enhance tendon-to-bone healing after rotator cuff repair. Peng et al. (58) reported reduced retear rates and improved clinical outcomes with PRP during arthroscopic rotator cuff repairs. However, further exploration is needed regarding optimal PRP concentration, bioactive component mechanisms, and the timing of application. Scaffold technologies, which serve as carriers for growth factors and stem cells, can activate repair potential at the injury site, providing temporary mechanical support and promoting organized regeneration through biomimetic structures (17). Advances in 3D printing have enhanced scaffold fabrication, allowing the creation of bioactive scaffolds that replicate natural tendon structure, maintain mechanical strength, and improve cellular communication and tendon-to-bone integration (59). Ni et al. (60) developed a 3D-printed polycaprolactone (PCL) scaffold loaded with basic FGF and bone marrow mesenchymal stem cells (BMSCs), significantly improving biomechanical strength, histological scores, and local bone density 2 weeks post-surgery in a rat rotator cuff tear model. Nevertheless, precise control of scaffold degradation rates to match tissue regeneration remains challenging, and immunological reactions requiring prolonged immunosuppression are potential drawbacks. Gene therapy involves targeting osteogenic genes (e.g., Runx2 and Osterix) to the tendon-to-bone interface via adenovirus or liposomes. Xie et al. (61) used an adenoviral vector carrying the Runx2 gene to transfect human amniotic mesenchymal stem cells (hAMSCs), directing differentiation toward ligament fibroblasts and enhancing tendon-to-bone healing in a rabbit anterior cruciate ligament (ACL) reconstruction model. However, the risk of viral integration into host genomes, leading to uncontrolled gene expression, remains a concern.
5.3 Appropriate postoperative rehabilitation
Postoperative rehabilitation is critical following rotator cuff repair. Yoo et al. (62) randomly assigned 75 patients to early or delayed rehabilitation protocols and found no significant differences between the groups in postoperative range of motion, functional outcomes, muscle strength recovery, or tendon healing during short- and midterm follow-ups. Therefore, rehabilitation strategies should balance the risk of fixation failure associated with early rehabilitation against the risk of joint stiffness from delayed rehabilitation. Patient-specific factors, such as bone quality, tear size, and fixation technique, are crucial. In addition, combining extracorporeal shockwave therapy with rehabilitation exercises has shown superior outcomes in reducing early postoperative shoulder pain and accelerating tendon healing at anchor sites compared with rehabilitation alone (63).
6 Salvage strategies following failed rotator cuff repair
When anchor fixation failure or retear occurs postoperatively, timely salvage strategies should be implemented. For patients with good tendon quality, if significant anchor loosening is observed, the loose anchors should be removed, and new anchor points should be selected for refixation. For anchors with minimal loosening, they may be retained and supplemented with additional anchors for reinforced fixation (64). Intraoperatively, adjustments to suture techniques, alternative anchor types, or cement augmentation can be considered to reattach the tendon. However, revision surgery carries a higher risk of infection, and scar tissue or adhesions from the initial procedure may complicate surgery.
For poor tendon quality, where re-suturing is not feasible, alternative options include tendon transfer procedures such as latissimus dorsi transfer (LDT) or pectoralis major transfer (PMT), superior capsular reconstruction (SCR), or reverse total shoulder arthroplasty (RTSA). LDT is primarily indicated for irreparable posterosuperior rotator cuff tears, with studies showing that arthroscopy-assisted LDT can restore flexion and abduction comparable to the asymptomatic contralateral shoulder and healthy controls (65). PMT is effective for irreparable subscapularis tears, improving anterior shoulder stability and internal rotation strength. SCR, suitable for massive irreparable tears without severe glenohumeral arthritis, uses autograft or allograft tissue to reconstruct the superior capsule, restoring humeral head stability, reducing superior migration, and improving shoulder function (66). Mihata et al. (67) followed patients for 10 years after SCR using autologous fascia lata grafts, reporting high rates of return to sports and work with sustained clinical and structural improvements.
Subacromial balloon spacer implantation has emerged as a novel approach, involving the insertion of an absorbable balloon in the subacromial space. This device cushions the humeral head from the acromion during deltoid activation and arm abduction, potentially improving biomechanics and reducing pain (68, 69). However, clinical results have been mixed. Verma et al. (70) randomized 184 patients with massive irreparable posterosuperior tears to balloon implantation or partial repair, finding that the balloon could substitute for partial repair, offering superior early functional recovery and pain relief. In contrast, Haque et al. (71) reported no additional benefit of balloon implantation over arthroscopic debridement alone in a randomized controlled trial. Therefore, the clinical effectiveness of subacromial balloon spacers warrants further investigation.
For irreparable rotator cuff tears with concurrent joint degeneration, RTSA is often the preferred option. While it does not restore the rotator cuff, it effectively relieves pain and, through the “ball-and-socket reversal” design, medializes and lowers the center of rotation, enabling the deltoid muscle-particularly its anterior and middle fibers to compensate for lost abduction function. Even in the complete absence of supraspinatus and infraspinatus function, this allows restoration of arm elevation. However, osteoporosis-related risks, such as prosthesis loosening and periprosthetic fractures, remain important considerations.
7 Conclusion
Arthroscopic treatment of rotator cuff injuries complicated by osteoporosis presents dual challenges: osteoporosis not only increases the risk of injury but also disrupts the postoperative tendon-to-bone healing microenvironment, leading to anchor loosening, high retear rates, and suboptimal functional recovery. Current strategies, including optimization of anchor fixation techniques, the use of anti-osteoporosis medications, and the application of biological therapies, have improved repair stability and healing quality. In addition, salvage options such as revision repair and tendon transfer provide solutions for failed cases. However, systemic anti-osteoporosis therapies lack precise regulation of the local healing microenvironment, and biomaterials still face challenges related to compatibility and degradation rate matching. Moreover, individualized surgical planning requires more objective guidance from reliable biomarkers.
To address these challenges, future research should focus on several key areas aimed at precisely regulating the local microenvironment and optimizing therapeutic strategies. First, locally targeted drug delivery systems will become a major research direction. For example, developing injectable hydrogels loaded with bisphosphonates or teriparatide will enable precise local drug release, synergistically inhibiting bone resorption and promoting regeneration to improve tendon healing. In addition, novel composite materials hold immense potential, particularly biomimetic mineralized collagen scaffolds and nanobioceramic composites. These materials offer superior osseointegration and mechanical strength, while also modulating local bioactivity to accelerate postoperative healing. Beyond material innovations, precision medicine will play a pivotal role in future research. Integrating imaging techniques with serum biomarkers will enable the development of personalized postoperative prognosis prediction models, guiding the formulation of tailored treatment and rehabilitation plans.
Author contributions
JH: Writing – original draft. TC: Writing – review & editing. CW: Writing – review & editing. KZ: Writing – review & editing, Funding acquisition. PY: Writing – review & editing. QL: Writing – review & editing. HJ: Writing – review & editing. GL: Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Suzhou Science and Technology Strengthening Health Project (No. MSXM2024026); the Suzhou Gusu Health Talent Research Project (No. GSWS2022085); and the Suzhou Applied Basic Research Medical and Health Project (No. SYW2024130).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rossi LA, Chahla J, Verma NN, Millett PJ, Ranalletta M. Rotator cuff retears. JBJS Rev. (2020) 8(1):e0039. doi: 10.2106/JBJS.RVW.19.00039
2. Itoigawa Y, Yoshida K, Nojiri H, Morikawa D, Kawasaki T, Wada T, et al. Association of recurrent tear after arthroscopic rotator cuff repair and superoxide-induced oxidative stress. Am J Sports Med. (2021) 49(8):2048–55. doi: 10.1177/03635465211014856
3. Chung SW, Oh JH, Gong HS, Kim JY, Kim SH. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. (2011) 39(10):2099–107. doi: 10.1177/0363546511415659
4. Hong JP, Huang SW, Lee CH, Chen HC, Charoenpong P, Lin HW. Osteoporosis increases the risk of rotator cuff tears: a population-based cohort study. J Bone Miner Metab. (2022) 40(2):348–56. doi: 10.1007/s00774-021-01293-4
5. Entezari V, Lazarus M. Surgical considerations in managing osteoporosis, osteopenia, and vitamin D deficiency during arthroscopic rotator cuff repair. Orthop Clin North Am. (2019) 50(2):233–43. doi: 10.1016/j.ocl.2018.10.006
6. Zhou JP, Zhang GR, Liu JX, Wu D, An LP, Zhang MT, et al. Progress on effect of osteoporosis on rotator cuff repair. Zhongguo Gu Shang. (2020) 33(10):982–5. doi: 10.12200/j.issn.1003-0034.2020.10.019
7. Chen S, Wei HW, Zheng WP, Liu ZJ, Liao ZH. A clinical study on the relationship between osteoporosis and rotator cuff retear. J Pract Orthop. (2021) 27(11):966–970, 987. doi: 10.13795/j.cnki.sgkz.2021.11.002
8. Lee WY, Jeon YS, Kim KC, Shin HD, Joo YB, Chung HJ. Comparative analysis of bone mineral density of the lumbar spine, hip, and proximal humerus in patients with unilateral rotator cuff tears. Clin Orthop Surg. (2024) 16(5):751–60. doi: 10.4055/cios24015
9. Tingart MJ, Apreleva M, Zurakowski D, Warner JJ. Pullout strength of suture anchors used in rotator cuff repair. J Bone Joint Surg Am. (2003) 85(11):2190–8. doi: 10.2106/00004623-200311000-00021
10. Batra AK, Brusalis CM, Jawanda H, Jackson G, Verma NN. Lateral decubitus positioning for shoulder arthroscopy. Video J Sports Med. (2023) 3(3):26350254231169488. doi: 10.1177/26350254231169488
11. Zhuang C, Yang R, Xu Y, Song Y, Zhang Y, Liu J, et al. The safety assessment of irrigation fluid management for shoulder arthroscopy and its effect on postoperative efficacy. Orthop Surg. (2023) 15(8):2016–24. doi: 10.1111/os.13619
12. Wang D, Zhang X, Huang S, Liu Y, Fu BS, Mak KK, et al. Engineering multi-tissue units for regenerative medicine: bone-tendon-muscle units of the rotator cuff. Biomaterials. (2021) 272:120789. doi: 10.1016/j.biomaterials.2021.120789
13. Golman M, Abraham AC, Kurtaliaj I, Marshall BP, Hu YJ, Schwartz AG, et al. Toughening mechanisms for the attachment of architectured materials: the mechanics of the tendon enthesis. Sci Adv. (2021) 7(48):eabi5584. doi: 10.1126/sciadv.abi5584
14. Xu J, Su W, Chen J, Ye Z, Wu C, Jiang J, et al. The effect of antiosteoporosis therapy with risedronate on rotator cuff healing in an osteoporotic rat model. Am J Sports Med. (2021) 49(8):2074–84. doi: 10.1177/03635465211011748
15. Huang Y, He B, Wang L, Yuan B, Shu H, Zhang F, et al. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. (2020) 11(1):496. doi: 10.1186/s13287-020-02005-x
16. Pino AM, Rosen CJ, Rodríguez JP. In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol Res. (2012) 45(3):279–87. doi: 10.4067/S0716-97602012000300009
17. Wang X, Yang TY, Xiong BH, Zhang YZ, Lu XJ, Long D, et al. Regeneration mechanism and problems of tissue engineering in rotator cuff tendon–bone healing. Chin J Tissue Eng Res. (2023) 27(18):2928–34.
18. Gao Y, Chen N, Fu Z, Zhang Q. Progress of Wnt signaling pathway in osteoporosis. Biomolecules. (2023) 13(3):483. doi: 10.3390/biom13030483
19. Ren LJ, Zhu XH, Tan JT, Lv XY, Liu Y. MiR-210 improves postmenopausal osteoporosis in ovariectomized rats through activating VEGF/Notch signaling pathway. BMC Musculoskelet Disord. (2023) 24(1):393. doi: 10.1186/s12891-023-06473-z
20. Tashjian RZ, Zitnay J, Kazmers NH, Veerabhadraiah SR, Zelada AC, Honeggar M, et al. Estrogen and testosterone supplementation improves tendon healing and functional recovery after rotator cuff repair. J Orthop Res. (2024) 42(2):259–66. doi: 10.1002/jor.25695
21. Tanaka K, Kanazawa T, Gotoh M, Tanesue R, Nakamura H, Ohzono H, et al. Effects of estrogen-deficient state on rotator cuff healing. Am J Sports Med. (2019) 47(2):389–97. doi: 10.1177/0363546518815869
22. Li J, Zhen D, Guo CF. Research progress of macrophage polarization in tendon–bone healing. Chin J Bone Jt. (2023) 12(10):796–800. CNKI:SUN:GZGL.0.2023-10-016
23. Chen Z, Jin M, He H, Dong J, Li J, Nie J, et al. Mesenchymal stem cells and macrophages and their interactions in tendon-bone healing. J Orthop Translat. (2023) 39:63–73. doi: 10.1016/j.jot.2022.12.005
24. Wang W, Liu H, Liu T, Yang H, He F. Insights into the role of macrophage polarization in the pathogenesis of osteoporosis. Oxid Med Cell Longev. (2022) 2022:2485959. doi: 10.1155/2022/2485959
25. Dou C, Ding N, Zhao C, Hou T, Kang F, Cao Z, et al. Estrogen deficiency-mediated M2 macrophage osteoclastogenesis contributes to M1/M2 ratio alteration in ovariectomized osteoporotic mice. J Bone Miner Res. (2018) 33(5):899–908. doi: 10.1002/jbmr.3364
26. Chisari E, Rehak L, Khan WS, Maffulli N. Tendon healing is adversely affected by low-grade inflammation. J Orthop Surg Res. (2021) 16(1):700. doi: 10.1186/s13018-021-02811-w
27. Wang Q, Qian YH, Huang HQ, Qiang PK, Xu F. Research progress of oxidative stress in the pathogenesis of osteoporosis and rotator cuff injury. Chin J Osteoporos. (2023) 29(5):776–80. CNKI:SUN:ZGZS.0.2023-05-029
28. Yilmaz N, Eren E. Homocysteine oxidative stress and relation to bone mineral density in post-menopausal osteoporosis. Aging Clin Exp Res. (2009) 21(4-5):353–7. doi: 10.1007/BF03324927
29. Azizieh FY, Shehab D, Jarallah KA, Gupta R, Raghupathy R. Circulatory levels of RANKL, OPG, and oxidative stress markers in postmenopausal women with normal or low bone mineral density. Biomark Insights. (2019) 14:1177271919843825. doi: 10.1177/1177271919843825
30. Uehara H, Itoigawa Y, Morikawa D, Koga A, Tsurukami H, Maruyama Y, et al. The effect of vitamin C and N-acetylcysteine on tendon-to-bone healing in a rodent model of rotator cuff repair. Am J Sports Med. (2023) 51(6):1596–607. doi: 10.1177/03635465231160772
31. Ozer FF, Güler E. Relation of bone mineral density with fat infiltration of paraspinal muscles: the goutallier classification. Osteoporos Sarcopenia. (2024) 10(2):84–8. doi: 10.1016/j.afos.2024.04.002
32. Li Z, Chen J, Yang J, Wang R, Wang W. Relationship between paraspinal muscle properties and bone mineral density based on QCT in patients with lumbar disc herniation. BMC Musculoskelet Disord. (2024) 25(1):360. doi: 10.1186/s12891-024-07484-0
33. Li X, Zhang Y, Xie Y, Lu R, Tao H, Chen S. Correlation between bone mineral density (BMD) and paraspinal muscle fat infiltration based on QCT: a cross-sectional study. Calcif Tissue Int. (2022) 110(6):666–73. doi: 10.1007/s00223-022-00944-6
34. Park JS, Park HJ, Kim SH, Oh JH. Prognostic factors affecting rotator cuff healing after arthroscopic repair in small to medium-sized tears. Am J Sports Med. (2015) 43(10):2386–92. doi: 10.1177/0363546515594449
35. Yang Z, Zhang M, Liu T, Zhang B, Wang X, Liang J, et al. Does the fatty infiltration influence the re-tear rate and functional outcome after rotator cuff repair? A systematic review and meta-analysis. Indian J Orthop. (2023) 57(2):227–37. doi: 10.1007/s43465-022-00807-0
36. Li J, Peng Y, Zhen D, Guo C, Peng W. NEDD4 enhances bone-tendon healing in rotator cuff tears by reducing fatty infiltration. Mol Med Rep. (2025) 31(3):55. doi: 10.3892/mmr.2024.13420
37. Chen J, Lou J, Wang W, Xu G. Association of preoperative vitamin D deficiency with retear rate and early pain after arthroscopic rotator cuff repair: a retrospective cohort study. Orthop J Sports Med. (2022) 10(10):23259671221130315. doi: 10.1177/23259671221130315
38. Kawakami J, Yamamoto N, Nagamoto H, Itoi E. Minimum distance of suture anchors used for rotator cuff repair without decreasing the pullout strength: a biomechanical study. Arthroscopy. (2018) 34(2):377–85. doi: 10.1016/j.arthro.2017.07.022
39. Gu Z, Wu S, Yang Y, Ren T, Zhang KW. Comparison of arthroscopic single-row and double-row repair for rotator cuff injuries with different tear sizes: a systematic review and meta-analysis. Orthop J Sports Med. (2023) 11(8):23259671231180854. doi: 10.1177/23259671231180854
40. Ren YM, Zhang HB, Duan YH, Sun YB, Yang T, Tian MQ. Comparison of arthroscopic suture-bridge technique and double-row technique for treating rotator cuff tears: a PRISMA meta-analysis. Medicine (Baltimore). (2019) 98(20):e15640. doi: 10.1097/MD.0000000000015640
41. Li X, Xiao Y, Shu H, Sun X, Nie M. Risk factors and corresponding management for suture anchor pullout during arthroscopic rotator cuff repair. J Clin Med. (2022) 11(22):6870. doi: 10.3390/jcm11226870
42. Chae SW, Kang JY, Lee J, Han SH, Kim SY. Effect of structural design on the pullout strength of suture anchors for rotator cuff repair. J Orthop Res. (2018) 36(12):3318–27. doi: 10.1002/jor.24135
43. Yang YS, Shih CA, Fang CJ, Huang TT, Hsu KL, Kuan FC, et al. Biomechanical comparison of different suture anchors used in rotator cuff repair surgery-all-suture anchors are equivalent to other suture anchors: a systematic review and network meta-analysis. J Exp Orthop. (2023) 10(1):45. doi: 10.1186/s40634-023-00608-w
44. Liu WT, Gu XL, Lai WG, Xiao SS. Influences from implant angle of suture anchors on the maximum pullout force. J Med Biomech. (2020) 35(4):455–60. doi: 10.16156/j.1004-7220.2020.04.010
45. Levy DM, Moen TC, Ahmad CS. Bone grafting of humeral head cystic defects during rotator cuff repair. Am J Orthop (Belle Mead NJ). (2012) 41(2):92–4.22482095
46. Fang S, Zhang M, Yang X, Daoji C, Li M, Nian Z, et al. Effect of autologous osteochondral tissue and periosteum transplantation on tendon-bone healing of rotator cuff in rabbits. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2025) 39(2):187–92. doi: 10.7507/1002-1892.202409094
47. Aziz KT, Shi BY, Okafor LC, Smalley J, Belkoff SM, Srikumaran U. Pullout strength of standard vs. Cement-augmented rotator cuff repair anchors in cadaveric bone. Clin Biomech (Bristol). (2018) 54:132–6. doi: 10.1016/j.clinbiomech.2018.03.016
48. Postl LK, Ahrens P, Beirer M, Crönlein M, Imhoff AB, Foehr P, et al. Pull-out stability of anchors for rotator cuff repair is also increased by bio-absorbable augmentation: a cadaver study. Arch Orthop Trauma Surg. (2016) 136(8):1153–8. doi: 10.1007/s00402-016-2484-y
49. Wang J, Li C, Zhang J, An M, Zhao G, Stark SD, et al. Effect of decellularized amniotic membrane on the tendon-bone integration in rotator cuff repair: a comparative rat model study. Orthop Surg. (2025) 17(2):575–82. doi: 10.1111/os.14316
50. Wang JT, Li CB, Zhang JT, An MY, Zhao G, Liu YJ. Interposition of acellular amniotic membrane at the tendon to bone interface would be better for healing than overlaying above the tendon to bone junction in the repair of rotator cuff injury. Chin J Traumatol. (2025) 28(3):187–92. doi: 10.1016/j.cjtee.2024.04.001
51. Kimura A, Aoki M, Fukushima S, Ishii S, Yamakoshi K. Reconstruction of a defect of the rotator cuff with polytetrafluoroethylene felt graft. Recovery of tensile strength and histocompatibility in an animal model. J Bone Joint Surg Br. (2003) 85(2):282–7. doi: 10.1302/0301-620x.85b2.12823
52. Ide J, Kikukawa K, Hirose J, Iyama K, Sakamoto H, Mizuta H. Reconstruction of large rotator-cuff tears with acellular dermal matrix grafts in rats. J Shoulder Elbow Surg. (2009) 18(2):288–95. doi: 10.1016/j.jse.2008.09.004
53. Hyatt AE, Lavery K, Mino C, Dhawan A. Suture anchor biomechanics after rotator cuff footprint decortication. Arthroscopy. (2016) 32(4):544–50. doi: 10.1016/j.arthro.2015.08.034
54. Sun Y, Kwak JM, Qi C, Kholinne E, Wang Y, Koh KH, et al. Remnant tendon preservation enhances rotator cuff healing: remnant preserving versus removal in a rabbit model. Arthroscopy. (2020) 36(7):1834–42. doi: 10.1016/j.arthro.2020.03.012
55. Wang Q, Huang HQ, Qian PK, Xu F. Effect of anti-osteoporosis drugs on tendon–bone healing and re-tear after rotator cuff repair. Chin J Bone Jt. (2023) 12(6):471–5. CNKI:SUN:GZGL.0.2023-06-012
56. Zhao Y, Shang D, Zhang Y, Geng Z, Li D, Song Q, et al. The effectiveness of intravenous zoledronic acid in elderly patients with osteoporosis after rotator cuff repair: a retrospective study. Sci Rep. (2024) 14(1):20891. doi: 10.1038/s41598-024-68246-5
57. Xu J, Ye Z, Chen C, Zhang X, Han K, Wu X, et al. Abaloparatide improves rotator cuff healing via anabolic effects on bone remodeling in a chronic rotator cuff tear model of rat with osteoporosis: a comparison with denosumab. Am J Sports Med. (2022) 50(6):1550–63. doi: 10.1177/03635465221079651
58. Peng Y, Du L, Yang B, Fan D, Jia S, Zheng C. Efficacy of platelet-rich plasma and platelet-rich fibrin in arthroscopic rotator cuff repair: a systematic review and meta-analysis. PM R. (2023) 15(12):1643–53. doi: 10.1002/pmrj.13049
59. Balestri W, Hickman GJ, Morris RH, Hunt JA, Reinwald Y. Triphasic 3D in vitro model of bone-tendon-muscle interfaces to study their regeneration. Cells. (2023) 12(2):313. doi: 10.3390/cells12020313
60. Ni Y, Tian B, Lv J, Li D, Zhang M, Li Y, et al. 3D-printed PCL scaffolds loaded with bFGF and BMSCs enhance tendon-bone healing in rat rotator cuff tears by immunomodulation and osteogenesis promotion. ACS Biomater Sci Eng. (2025) 11(2):1123–39. doi: 10.1021/acsbiomaterials.4c02340
61. Xie T, Zhong H, Jin Y, Liu X, Chen F, Xiang K, et al. Research on Runx2 gene induced differentiation of human amniotic mesenchymal stem cells into ligament fibroblasts in vitro and promotion of tendon-bone healing in rabbits. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2023) 37(12):1523–32. doi: 10.7507/1002-1892.202306010
62. Yoo SJ, Kang H, Kim B, Lee CH, Song J, Choi S. Which is better? Early versus delayed rehabilitation after arthroscopic rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. (2024) 32(4):1049–57. doi: 10.1002/ksa.12129
63. Shao H, Zhang S, Chen J, Wen A, Wu Z, Huang M, et al. Radial extracorporeal shockwave therapy reduces pain and promotes proximal tendon healing after rotator cuff repair: randomized clinical trial. Ann Phys Rehabil Med. (2023) 66(4):101730. doi: 10.1016/j.rehab.2023.101730
64. Wang MX, Liu YJ, Wang YT, Wu YD, Li CB. Factors related to failure of arthroscopic rotator cuff repair with anchor suture and corresponding revision arthroscopies. Orthopedic J Chin. (2023) 31(02):101–5. CNKI:SUN:ZJXS.0.2023-02-002
65. Galasso O, Mantovani M, Muraccini M, Berardi A, De Benedetto M, Orlando N, et al. The latissimus dorsi tendon functions as an external rotator after arthroscopic-assisted transfer for massive irreparable posterosuperior rotator cuff tears. Knee Surg Sports Traumatol Arthrosc. (2020) 28(7):2367–76. doi: 10.1007/s00167-019-05819-2
66. Li H, Yang M, Li Y, Zhou B, Tang K. Research progress of indication and treatment of graft in shoulder superior capsular reconstruction for rotator cuff tear. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2021) 35(2):252–7. doi: 10.7507/1002-1892.202006015
67. Mihata T, Lee TQ, Hasegawa A, Fukunishi K, Fujisawa Y, Ohue M. Long-term clinical and structural outcomes of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears: 10-year follow-up. Am J Sports Med. (2025) 53(1):46–56. doi: 10.1177/03635465241298898
68. Marigi EM, Alder KD, Morrey MM, Sanchez-Sotelo J. Subacromial balloon implantation for the treatment of irreparable posterosuperior rotator cuff tears. Arthrosc Tech. (2023) 12(8):e1297–304. doi: 10.1016/j.eats.2023.03.021
69. Lobao MH, Canham RB, Melvani RT, Abboud JA, Parks BG, Murthi AM. Biomechanics of biodegradable subacromial balloon spacer for irreparable superior rotator cuff tears: study of a cadaveric model. J Bone Joint Surg Am. (2019) 101(11):e49. doi: 10.2106/JBJS.18.00850
70. Verma N, Srikumaran U, Roden CM, Rogusky EJ, Lapner P, Neill H, et al. Inspace implant compared with partial repair for the treatment of full-thickness massive rotator cuff tears: a multicenter, single-blinded, randomized controlled trial. J Bone Joint Surg Am. (2022) 104(14):1250–62. doi: 10.2106/JBJS.21.00667
71. Haque A, Parsons H, Parsons N, Mason J, Khan I, Stallard N, et al. Two-year follow-up of a group-sequential, multicenter randomized controlled trial of a subacromial balloon spacer for irreparable rotator cuff tears of the shoulder (START:REACTS). Am J Sports Med. (2025) 53(6):1291–8. doi: 10.1177/03635465251326891
Keywords: osteoporosis, rotator cuff injury, shoulder arthroscopy, tendon-to-bone healing, retear
Citation: He J, Chen T, Wu C, Zhang K, You P, Lai Q, Jiang H and Liu G (2025) The impact of osteoporosis on arthroscopic rotator cuff repair and postoperative tendon-to-bone healing. Front. Surg. 12:1683843. doi: 10.3389/fsurg.2025.1683843
Received: 11 August 2025; Accepted: 7 October 2025;
Published: 24 October 2025.
Edited by:
Siyu Shen, Cincinnati Children's Hospital Medical Center, United StatesCopyright: © 2025 He, Chen, Wu, Zhang, You, Lai, Jiang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanhong Liu, bGl1Z3VhbjU4QDEyNi5jb20=
†These authors have contributed equally to this work
 Junlong He
Junlong He Tao Chen†
Tao Chen† Peijie You
Peijie You