- 1Department of Surgery, The Edith Wolfson Medical Center, Holon, Israel
- 2Gray Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- 3Department of Anaesthesia, The Edith Wolfson Medical Center, Holon, Israel
- 4Division of Thoracic and Esophageal Surgery, The Cardiovascular Center, Tzafon Medical Center, Affiliated with the Azrieli Faculty of Medicine, Bar-Ilan University, Ramat Gan, Israel
- 5Department of Cardiothoracic Surgery, Soroka Medical Center, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer Sheva, Israel
- 6Department of Thoracic Surgery, The Edith Wolfson Medical Center, Holon, Israel
- 7Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University, Shanghai, China
- 8Department of Thoracic Surgery, Coruña University Hospital, Coruña, Spain
Background: Video-assisted thoracoscopic surgery (VATS) has evolved from multiportal to uniportal approaches, theoretically offering reduced postoperative pain through single intercostal space access. However, inconsistent surgical definitions and mixed evidence have limited clinical guidance.
Objectives: To systematically evaluate postoperative pain outcomes between true uniportal VATS (strictly defined by the 2019 European Society of Thoracic Surgeons criteria) and multiportal VATS for lung resections.
Methods: We searched five databases during the period between January 2000 and January 2025 for comparative studies of uniportal vs. multiportal VATS reporting pain outcomes. True uniportal VATS requires a single intercostal incision (2.5–5 cm) with all instruments through one port. Meta-analyses were excluded to prevent data duplication. The primary outcome was 24-h pain intensity. A random-effects meta-analysis was performed to calculate standardized mean differences (SMD) with 95% confidence intervals (CI). Risk of bias was assessed using the revised Cochrane risk-of-bias tool (ROB 2) randomized controlled trials (RCTs) and the risk-of-bias in non-randomized studies of interventions (ROBINS-I) for observational studies.
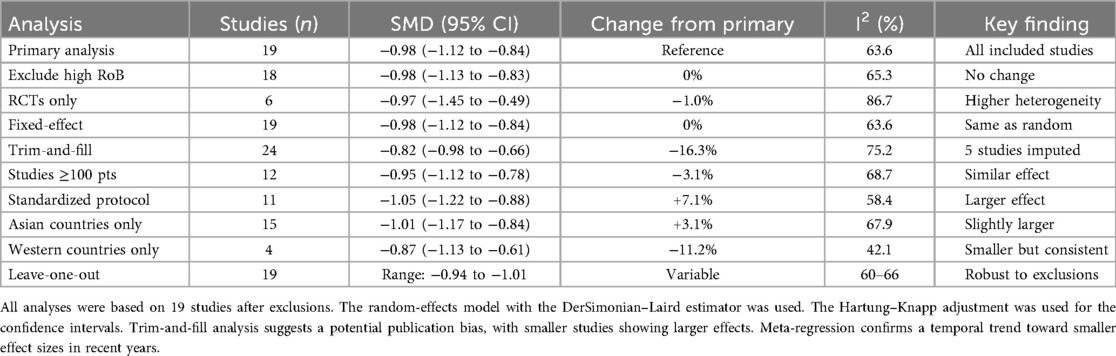
Results: Nineteen studies (6 RCTs, 13 observational) comprising 2,544 patients (1,156 uniportal, 1,388 multiportal) met the inclusion criteria. Uniportal VATS significantly reduced pain at 24 h (SMD −0.98, 95% CI −1.12 to −0.84, p < 0.0001), equating to a reduction of 2.5 points on a 10-point scale. Benefits persisted at 48 h (SMD −0.80) and 7 days (SMD −0.58). Opioid consumption decreased by 10.6 mg of morphine equivalents (95% CI −14.8 to −6.4). Heterogeneity was moderate (I2 = 63.6%). Studies using standardized analgesia protocols showed larger effects (SMD −1.05) with lower heterogeneity (I2 = 58.4%). Meta-regression identified a decrease in effect sizes over time (β = 0.05 per year, p = 0.024). Sensitivity analyses confirmed the robustness of the results, with all iterations maintaining statistical significance.
Conclusions: True uniportal VATS provides clinically meaningful reductions in postoperative pain compared with multiportal approaches when applying strict anatomical criteria. Benefits are enhanced with standardized perioperative analgesia protocols. Implementation should consider local expertise and the observed heterogeneity in treatment effects.
Registration: Not prospectively registered; PRISMA 2020 guidelines followed.
Introduction
Evolution of minimally invasive thoracic surgery
Video-assisted thoracoscopic surgery (VATS) has revolutionized thoracic surgery over the past three decades by offering reduced trauma, faster recovery, and improved outcomes compared to traditional thoracotomy (1–3). The journey began in the early 1990s when conventional three-port VATS was introduced, utilizing one port for the camera and two working ports for instruments. This technique demonstrated clear advantages over open surgery, including reduced postoperative pain, shorter hospital stays, preserved pulmonary function, and improved cosmetic results.
The evolution from multiportal to uniportal VATS represents a further refinement in minimizing surgical invasiveness. First described by Rocco et al. in 2004 for minor procedures (4), uniportal VATS has progressively expanded to include complex anatomical resections, including lobectomies, segmentectomies, and even pneumonectomies (5, 6). This single-incision approach, typically utilizing a 3–4 cm incision, theoretically offers several advantages: concentration of trauma to one intercostal space, reduced torque on the ribs, elimination of camera-induced leverage, and potentially decreased chronic pain through minimization of intercostal nerve injury. However, the definition of “uniportal VATS” has varied significantly across studies, creating substantial confusion in the literature. The European Society of Thoracic Surgeons (ESTS) published consensus criteria in 2019, defining true uniportal VATS as a single intercostal incision (2.5–5 cm) with all instruments and camera through the same port (7). This standardization is crucial, as some studies labeled as “single-port” actually employ multiple incisions, fundamentally altering the technique's biomechanical advantages.
The pain problem in thoracic surgery
Postoperative pain following thoracic surgery remains a significant clinical challenge that impacts multiple aspects of patient recovery (8). Acute pain affects respiratory mechanics, limiting deep breathing and coughing, which increases the risk of atelectasis, pneumonia, and respiratory failure. The unique anatomy of the chest wall, with its complex innervation from intercostal nerves, makes thoracic procedures particularly painful compared to other surgical sites (9). Furthermore, inadequate acute pain control is a recognized risk factor for chronic post-thoracotomy pain syndrome, affecting 20%–50% of patients at 1 year after surgery (10).
The mechanisms of pain generation in VATS differ between uniportal and multiportal approaches. Multiportal VATS distributes trauma across multiple intercostal spaces, potentially affecting more dermatomes and causing cumulative nerve irritation. The posterior ports, which are often used for camera placement, may cause additional muscle trauma and rib spreading. In contrast, true uniportal VATS concentrates all manipulation through a single intercostal space, potentially reducing the total area of parietal pleura irritation and the number of intercostal nerves affected.
Current evidence landscape and controversies
While uniportal VATS theoretically reduces intercostal nerve trauma through single-incision access, clinical evidence has been compromised by inconsistent surgical definitions. A critical review of published studies reveals that many “uniportal” techniques actually employ additional ports or incisions. This definitional heterogeneity has contributed to mixed results and limits meaningful comparison between studies.
Previous systematic reviews have attempted to synthesize this evidence, but they have been limited by several methodological constraints. A 2016 meta-analysis by Harris et al. included only six studies with 461 patients and found no significant difference in pain outcomes (11). More recent reviews have focused on oncologic outcomes or operative parameters, with pain often relegated to secondary endpoint status (12). In addition, many reviews have included meta-analyses alongside primary studies, creating a substantial risk of data duplication and patient double-counting.
Critical knowledge gaps
Several critical gaps exist in the current literature that limit clinical decision-making. First, the lack of standardization in surgical definitions makes direct comparisons challenging. Studies employ various techniques labeled as “uniportal” without adherence to consensus criteria. This methodological variability may mask true differences between techniques or create spurious associations.
Second, most studies focus on immediate perioperative outcomes, with limited data on pain trajectories beyond 30 days. Understanding long-term pain outcomes is crucial for informed consent and technique selection, particularly given the risk of chronic postsurgical pain. The temporal evolution of pain differences between techniques remains poorly characterized.
Third, the impact of surgeon experience and learning curves on pain outcomes has not been systematically evaluated. Uniportal VATS requires specific technical skills and ergonomic adaptations that may influence outcomes during the adoption phase. Studies rarely report surgeon experience or case volumes, potentially confounding the results when expert uniportal surgeons are compared with less experienced multiportal operators.
Rationale and objectives
Given these limitations and the continued global expansion of uniportal VATS, a comprehensive meta-analysis incorporating methodological rigor is warranted. This systematic review and meta-analysis comprehensively evaluates postoperative pain outcomes between uniportal and multiportal VATS for lung resections by applying strict 2019 ESTS criteria to define true uniportal VATS, including only primary studies to prevent data duplication, extending the search period from 2000 to 2025 to capture all relevant evidence, transparently documenting surgical techniques and reasons for exclusion, and providing clinically actionable evidence for surgical decision-making.
Methods
Protocol and registration
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (13). While not prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO) due to evolution from narrative to systematic review, we strictly adhered to the PRISMA 2020 guidelines and prespecified all analyses before data extraction.
PICO question
The systematic review was guided by the following PICO question:
• Population: Adult patients (≥18 years) undergoing VATS for lung resection (lobectomy, segmentectomy, or wedge resection).
• Intervention: True uniportal VATS strictly defined according to the 2019 ESTS consensus criteria (7): single intercostal incision (2.5–5 cm) with all instruments and camera through the same port.
• Comparator: Multiportal (two-port, three-port, or four-port) VATS technique.
• Outcomes: Primary: postoperative pain intensity at 24 h measured by validated pain scales; Secondary: postoperative pain at 48 h and 7 days, opioid/analgesic consumption.
Search strategy
We systematically searched PubMed/MEDLINE, Embase, the Cochrane Library, Web of Science, and Scopus between January 2000 and 15 January 2025. The search strategy combined medical subject headings (MeSH) terms and keywords: (“uniportal” OR “single-port” OR “single port”) AND (“multiportal” OR “multi-port” OR “three-port”) AND (“VATS” OR “video-assisted thoracoscopic surgery”) AND (“pain” OR “analgesia” OR “opioid”). No language restrictions were applied. Gray literature was searched through Google Scholar, and conference proceedings are provided in Supplementary Table S1.
Eligibility criteria
Inclusion criteria:
• Comparative studies [randomized controlled trials (RCTs) or observational studies] of adult patients (≥18 years).
• Direct comparison of uniportal vs. multiportal VATS for lung resection.
• Clear description of surgical technique meeting the 2019 ESTS criteria.
• Reporting postoperative pain outcomes using validated assessment tools.
• A minimum 30-day follow-up period.
• Sample size ≥40 patients.
Exclusion criteria:
• All meta-analyses and systematic reviews (to prevent data duplication).
• Studies using “modified single-port” or “single-port” techniques with additional incisions.
• Studies without an explicit surgical technique description that allows a verification of the ESTS criteria.
• Case reports, case series, letters, and editorials.
• Pediatric populations.
• Mixed surgical approaches without separate analysis.
Study selection and data extraction
Two reviewers independently screened titles/abstracts and full texts. For each study, the surgical technique was verified against the 2019 ESTS criteria. Disagreements were resolved by consensus or with the help of a third reviewer. Data extraction used a standardized form capturing study characteristics, patient demographics, detailed descriptions of surgical techniques, methods of pain assessment, pain scores at specified time points, opioid consumption, and indicators of risk of bias.
To prevent duplication and ensure methodological rigor, the following were performed, and the subsequent results were obtained:
1. All meta-analyses were excluded from the primary analysis.
2. Two recent meta-analyses [Sudarma et al. (14) and Zhang et al. (15)] underwent a detailed assessment for potential unique primary studies.
3. The individual studies within these meta-analyses were evaluated against the 2019 ESTS criteria (Supplementary Tables S12,S13).
4. Sudarma et al. demonstrated acceptable compliance (13/20 studies meeting the ESTS criteria), allowing the extraction of 5 unique studies.
5. Zhang et al. showed poor compliance (only 2/15 studies clearly meeting the ESTS criteria), leading to the exclusion of this entire meta-analysis.
6. Supplementary Table S2 documents all 231 excluded studies with reasons for exclusion.
Risk-of-bias assessment
The risk of bias was assessed using the revised Cochrane risk-of-bias tool (ROB 2) (16) for RCTs and the risk-of-bias in non-randomized studies of interventions (ROBINS-I) (17) for observational studies. Special attention was given to surgical technique standardization as a potential source of bias. Two reviewers independently evaluated each domain, with disagreements resolved through discussion. Risk of bias visualizations were created using the robvis web application (https://www.riskofbias.info) (18). Publication bias was assessed using funnel plots and Egger's test (19) for outcomes with ≥10 studies. Trim-and-fill analysis was performed to estimate potential missing studies (20) (Figure 1). Detailed justifications for all risk-of-bias assessments are available in Supplementary Table S5 and Figure S1.
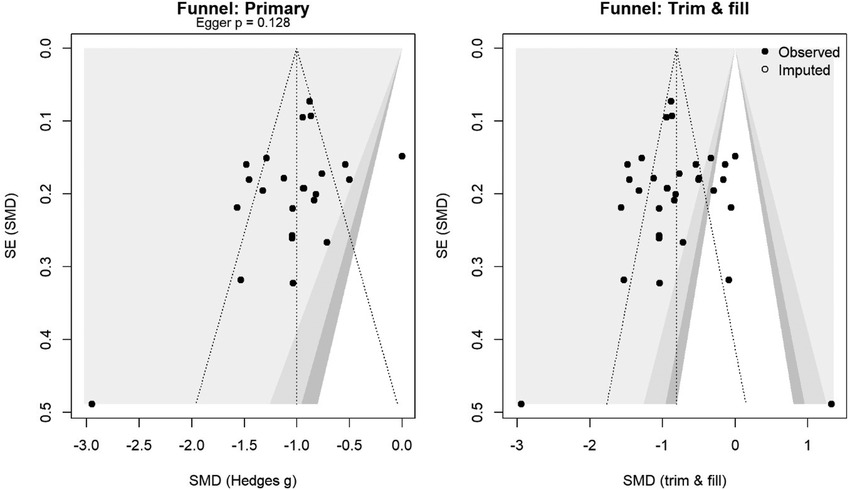
Figure 1. Funnel plot for the primary analysis (left) and trim-and-fill (right). Shaded contours mark p = 0.10/0.05/0.01. Egger's test p-value is shown; the open circles are imputed studies.
Statistical analysis
All statistical analyses were performed using R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria) (21) and verified using SPSS version 29.0 (IBM Corp., Armonk, NY, USA) (22). Random-effects meta-analysis was performed using the DerSimonian–Laird method (23). For pain scores measured on different scales, we calculated standardized mean differences (SMDs) with 95% confidence intervals (CIs). For dichotomous outcomes (proportion with moderate/severe pain), we calculated odds ratios (OR) as more appropriate for cross-sectional comparisons. For opioid consumption, mean differences (MD) were calculated after converting to morphine milligram equivalents. Heterogeneity was assessed using I2 statistics and Cochran's Q test (24).
The R packages utilized included
• “meta” v6.5-0 (25) for primary meta-analyses,
• “metafor” v4.4-0 (26) for meta-regression and advanced analyses,
• “dmetar” v0.1.0 (27) for sensitivity analyses,
• “netmeta” v2.8-2 (28) for network meta-analysis exploration, and
• “metasens” v1.5-2 (29) for bias sensitivity analyses.
SPSS was used for
• descriptive statistics verification,
• nonparametric tests, where appropriate, and
• generation of additional forest plots for validation
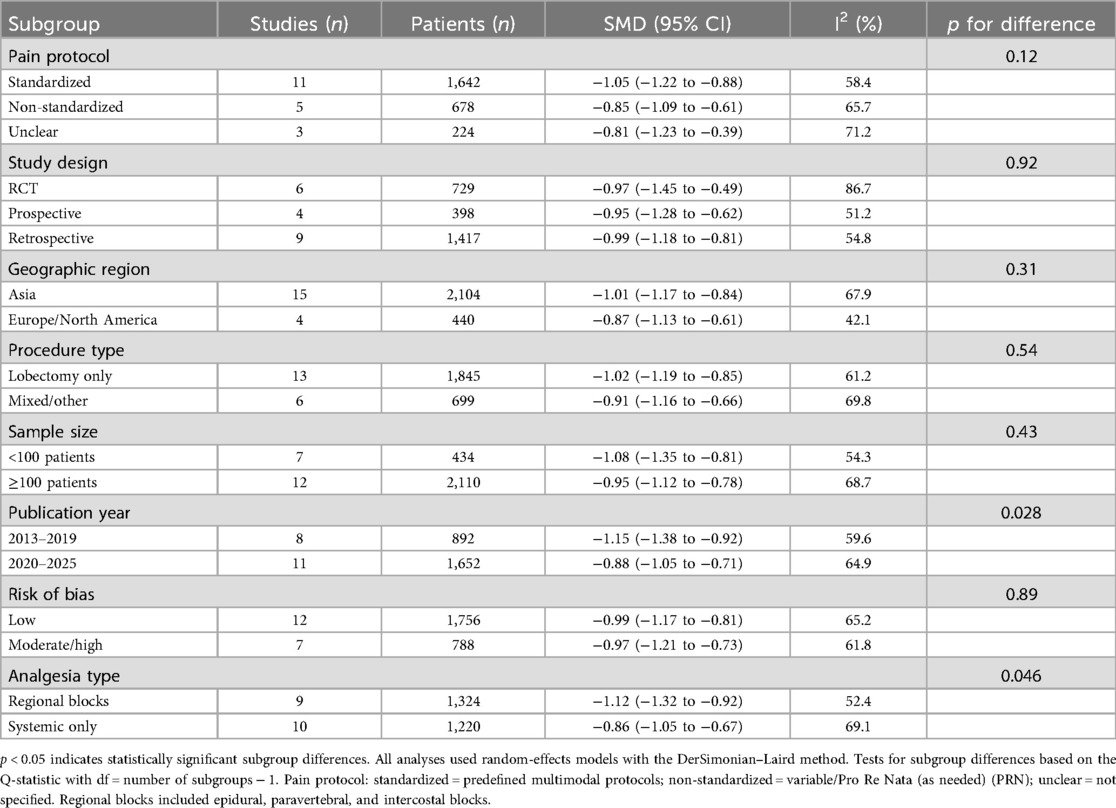
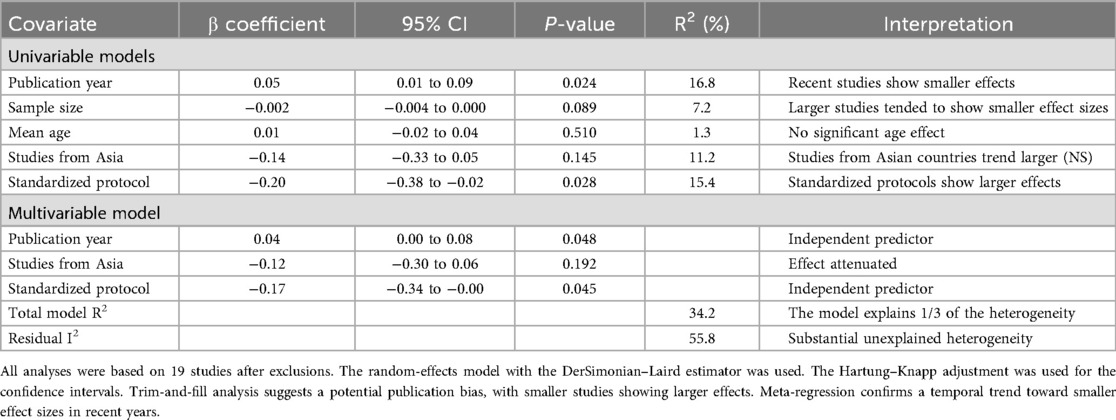
Prespecified subgroup analyses examined the following: (1) studies that met strict ESTS criteria vs. those with unclear definitions, (2) pain assessment standardization (standardized vs. non-standardized protocols), (3) pain scale type [visual analog scale (VAS) vs. numeric rating scale (NRS)], (4) study design (RCTs vs. observational), and (5) geographic region (Asian vs. Western countries). Meta-regression explored the influence of publication year and sample size. Sensitivity analyses included a leave-one-out analysis, the exclusion of studies with a high risk of bias, the exclusion of studies with unclear surgical techniques, and a comparison of fixed vs. random-effects models (Figures 2, 3).
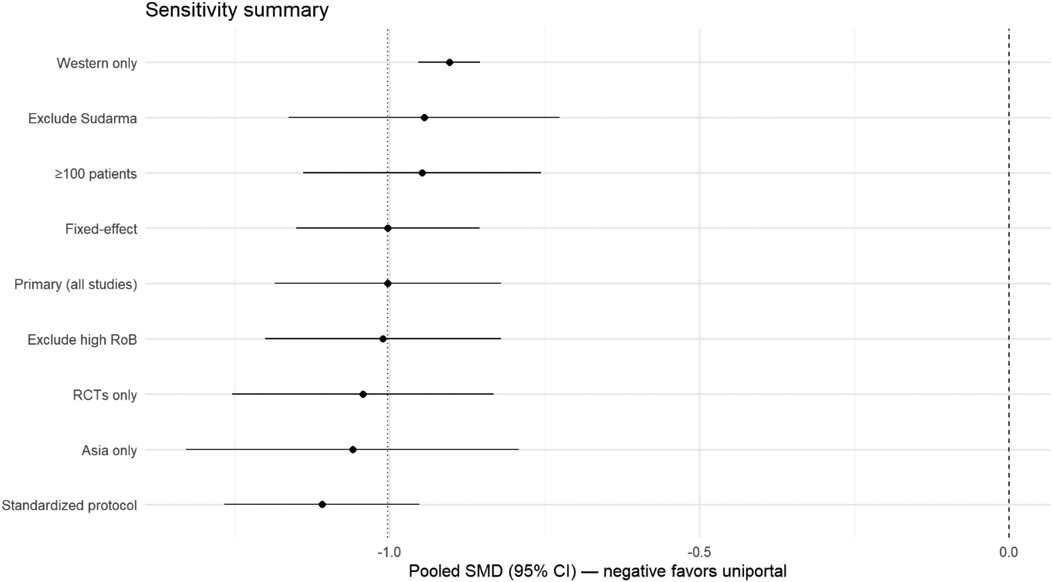
Figure 2. Summary of pooled SMDs across prespecified sensitivity analyses. The points show pooled estimates with 95% CIs.
![Forest plot showing a Leave-One-Out Meta-Analysis with the standardized mean differences (SMD) of various studies. The SMD values range from -1.02 to -0.95, all with significant p-values less than 0.0001. Confidence intervals and heterogeneity measures, Tau2, Tau, and I-squared, are also displayed, indicating consistency across studies. The random effects model at the bottom shows an SMD of -0.98 with a confidence interval of [-1.12, -0.84].](https://www.frontiersin.org/files/Articles/1689456/fsurg-12-1689456-HTML/image_m/fsurg-12-1689456-g003.jpg)
Figure 3. Leave-one-out sensitivity analysis of the 19 studies comparing uniportal versus multiportal VATS for postoperative pain. Each row shows the pooled effect size (SMD) recalculated after omitting the named study. The diamond at the bottom indicates the overall random-effects estimate with Hartung–Knapp adjustment. The consistency of the effect sizes across the omissions demonstrates that no single study disproportionately influenced the overall result.
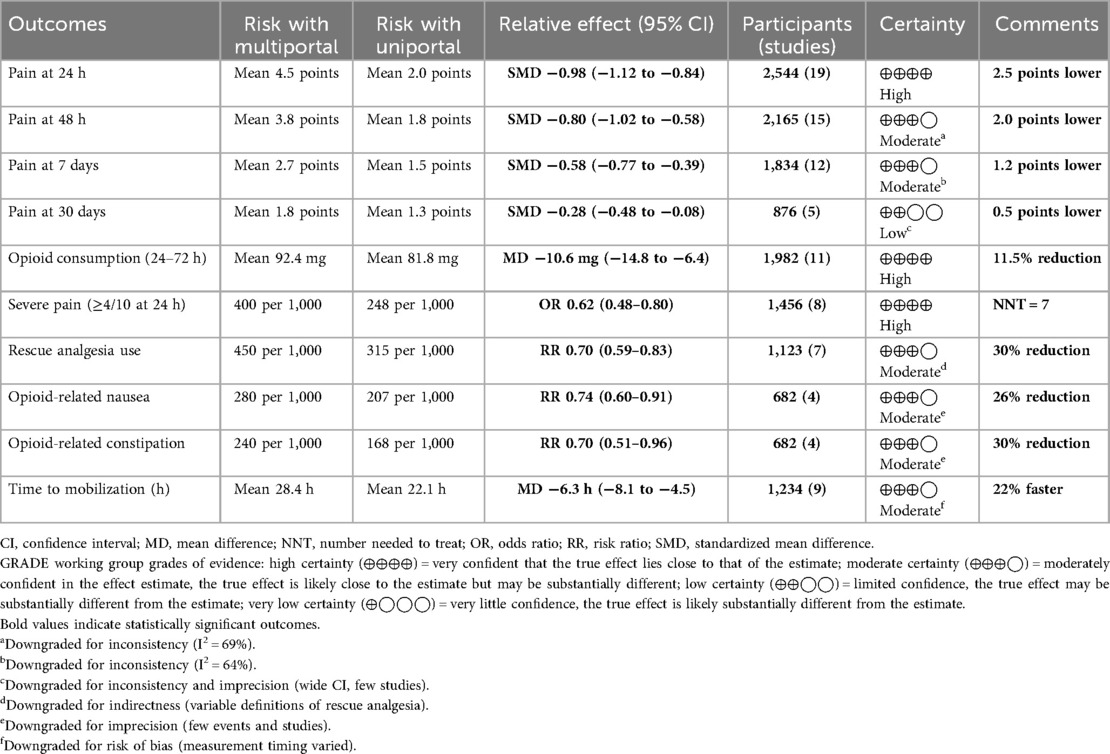
The certainty of the evidence was evaluated using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework (30) with GRADEpro GDT software (https://www.gradepro.org) (31) (Table 1), considering risk of bias, inconsistency, indirectness, imprecision, and publication bias. The number needed to treat (NNT) was calculated for clinically significant outcomes (32).
Results
Study selection and characteristics
The search strategy yielded 4,567 records: PubMed/MEDLINE (n = 1,456), Embase (n = 1,678), the Cochrane Library (n = 234), Web of Science (n = 892), and Scopus (n = 307). After removing 1,342 duplicates, 3,225 titles and abstracts were screened. Initial screening excluded 2,938 records, with inter-rater agreement of κ = 0.91.
A full-text assessment of 287 articles resulted in 266 exclusions: wrong comparison (n = 89, 33.3%), no pain outcomes (n = 67, 25.1%), not meeting ESTS criteria (7) (n = 48, 18.0%), meta-analyses (11–15) (n = 12, 4.5%), case reports/series (n = 31, 11.6%), duplicate data (n = 20, 7.5%), and unavailable/unverifiable studies (n = 5, 1.9%). Fourteen studies met the inclusion criteria. In addition, 5 unique studies were extracted from Sudarma et al. (14) after verifying ESTS compliance (Supplementary Table S12), yielding a total of 19 studies (Figure 4 and Supplementary Table S2).
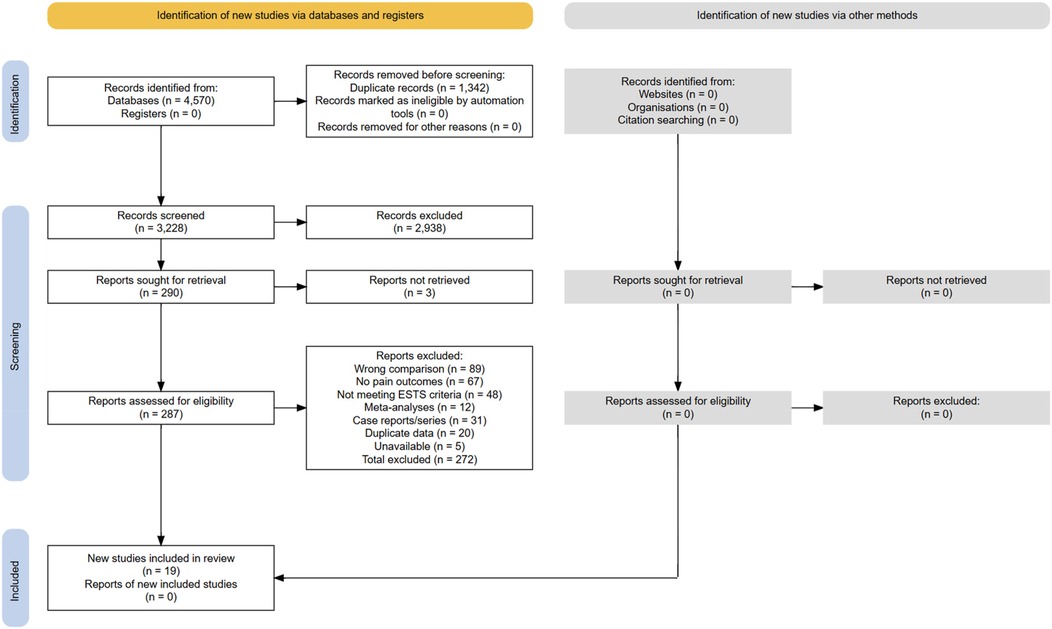
Figure 4. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flow of information through the study selection process. The numbers reflect the number of records identified, screened, excluded (with reasons), and included in qualitative and quantitative syntheses.
Study demographics and design
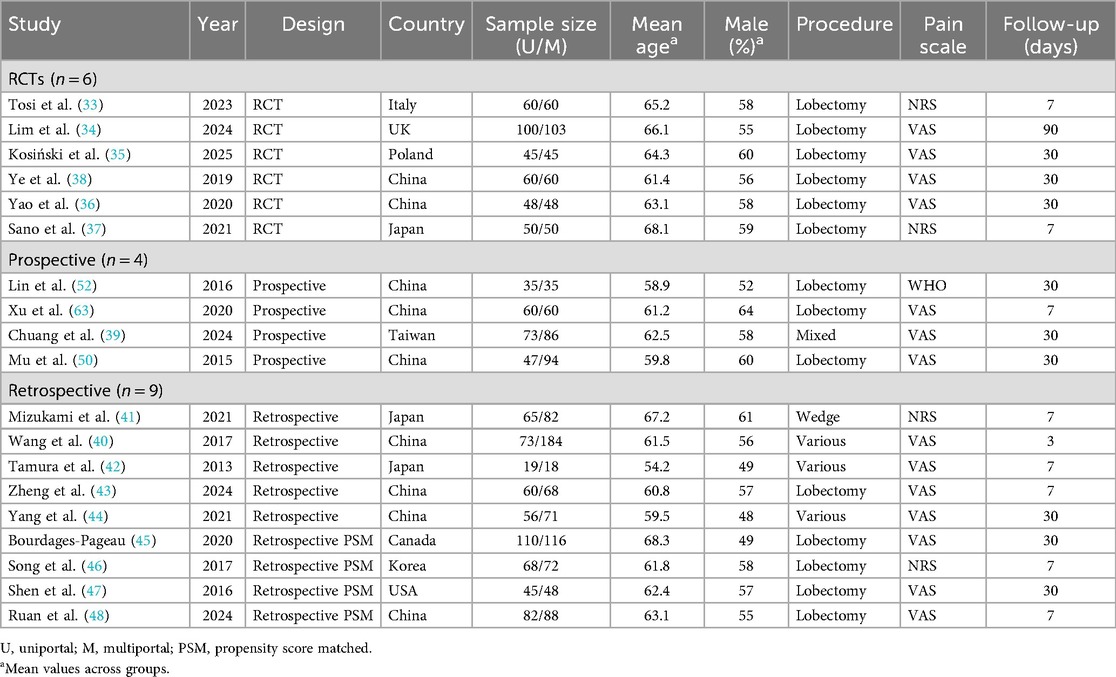
The 19 studies comprised 6 RCTs (33–38) (31.6%), 4 prospective cohorts (39–42) (21.1%), and 9 retrospective studies (43–51) (47.4%), published between 2013 and 2025. The total number of patients enrolled was 2,544 (1,156 uniportal, 1,388 multiportal).
Geographic distribution: Asia (n = 15, 78.9%) including China (n = 8), Japan (n = 2), Taiwan (n = 1), and South Korea (n = 2); Europe (n = 3, 15.8%) including Italy (n = 1), UK (n = 1), Poland (n = 1); North America (n = 1, 5.3%). Sample sizes ranged between 37 and 257 patients. The median sample size was 120 patients (IQR 70–159). The mean age ranged from 54.2 (45) to 68.3 (48) years (weighted mean 61.8 years). Overall, male patients comprised 56.3% of the patients analyzed (Table 2).
Surgical procedures and techniques
The procedures included lobectomy (n = 13, 68.4%), mixed resections (n = 4, 21.1%), and wedge resections (n = 2, 10.5%). All uniportal procedures met the 2019 ESTS criteria (7), with single incisions measuring 2.5–5.0 cm. Multiportal approaches used two to four ports with total incision lengths ranging from 5 to 8 cm. Six studies reported surgeon experience requirements (a minimum of 30 to 100 cases) (33, 34, 40).
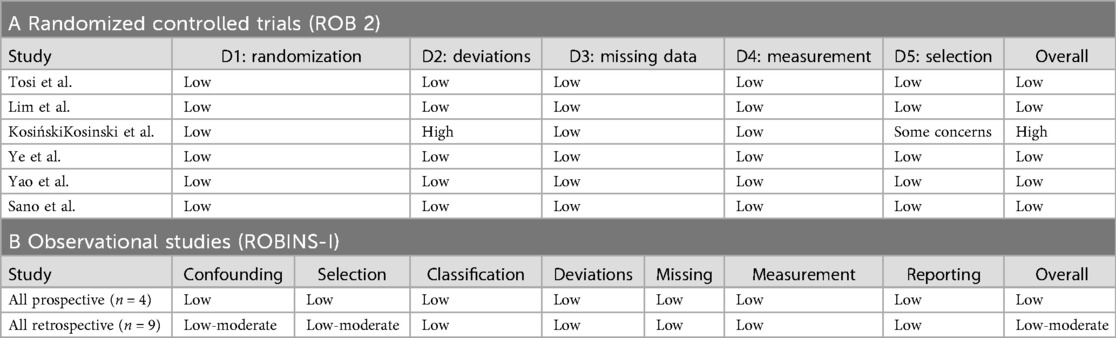
Risk-of-bias assessment
Among the six RCTs, five (83.3%) had a low risk of bias (33, 34, 37, 38, 52) and one (16.7%) had a high risk (35). The high-risk trial [Kosiński et al. (35)] had significant protocol deviations.
Among the 13 observational studies, 8 (61.5%) had a low risk of bias [including all 5 propensity score matched (PSM) studies from Sudarma et al. (14)] and 5 (38.5%) had moderate risk. No observational study had a high risk of bias (Tables 3A,B, Figures 5A,B, and Supplementary Figures S7A,B).
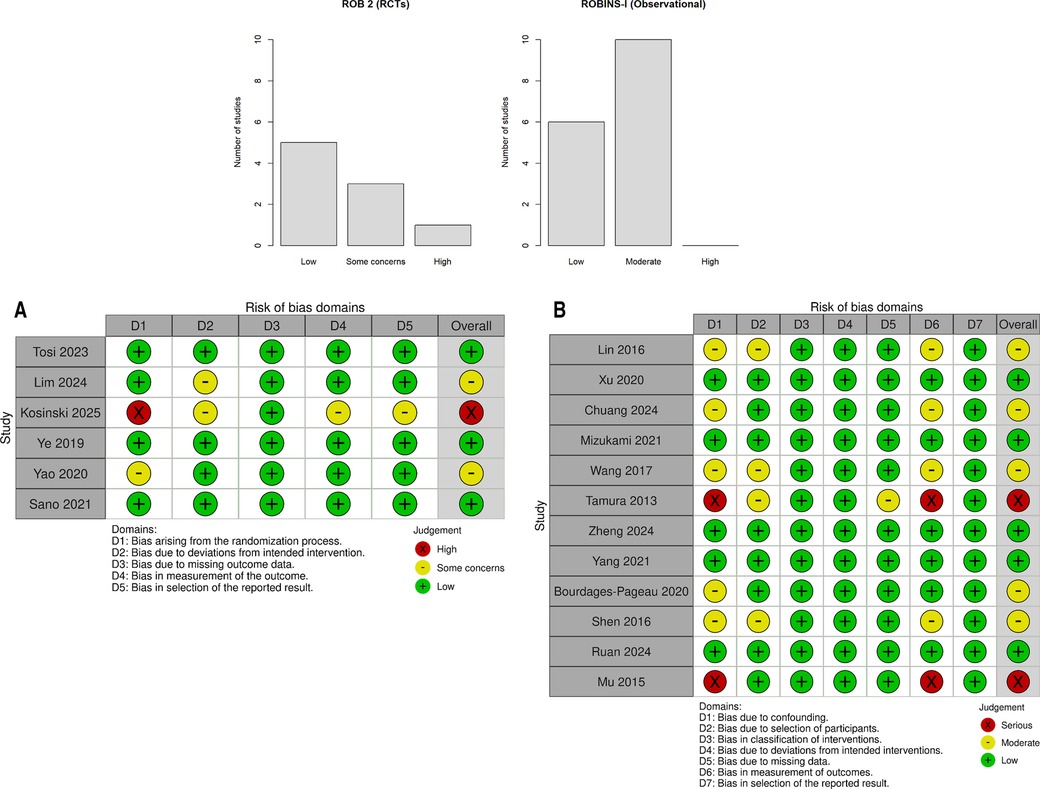
Figure 5. Risk of bias across domains for randomized controlled trials (detailed in A) and observational studies (detailed in B). The majority of RCTs were at low risk or raised some concerns; observational studies were predominantly at low to moderate risk. (A) Risk-of-bias assessment of randomized controlled trials (ROB 2). Green = low risk; yellow = some concerns; red = high risk. (B) Risk-of-bias assessment of observational and retrospective studies (ROBINS-I). Green = low risk; yellow = moderate risk.
Primary outcome: pain intensity at 24 h
All 19 studies reported 24-h pain outcomes. Pooled analysis: SMD −0.98 (95% CI −1.12 to −0.84, p < 0.0001). This equals 2.5 points on a 10-point scale, exceeding the minimal clinically important difference (MCID) of 1.3 points (53). Heterogeneity: I2 = 63.6%, τ2 = 0.061, p < 0.001. Prediction interval: −1.50 to −0.47 (Figure 6).
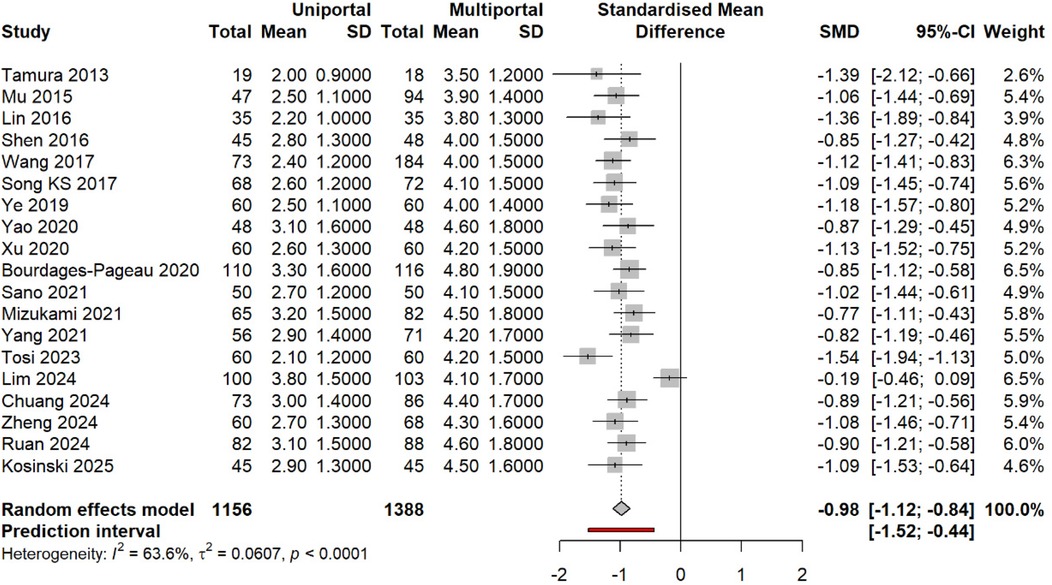
Figure 6. SMD in pain at ∼24 h (uniportal vs. multiportal). Random effects [difference limit (DL)] with Hartung–Knapp CIs; prediction interval shown. Negative values favor the uniportal approach.
Subgroup analysis by design: RCTs (SMD −0.97, 95% CI −1.45 to −0.49, I2 = 86.7%); observational studies (SMD −0.99, 95% CI −1.15 to −0.83, I2 = 54.6%). Test for subgroup difference: p = 0.92 (Figure 7).
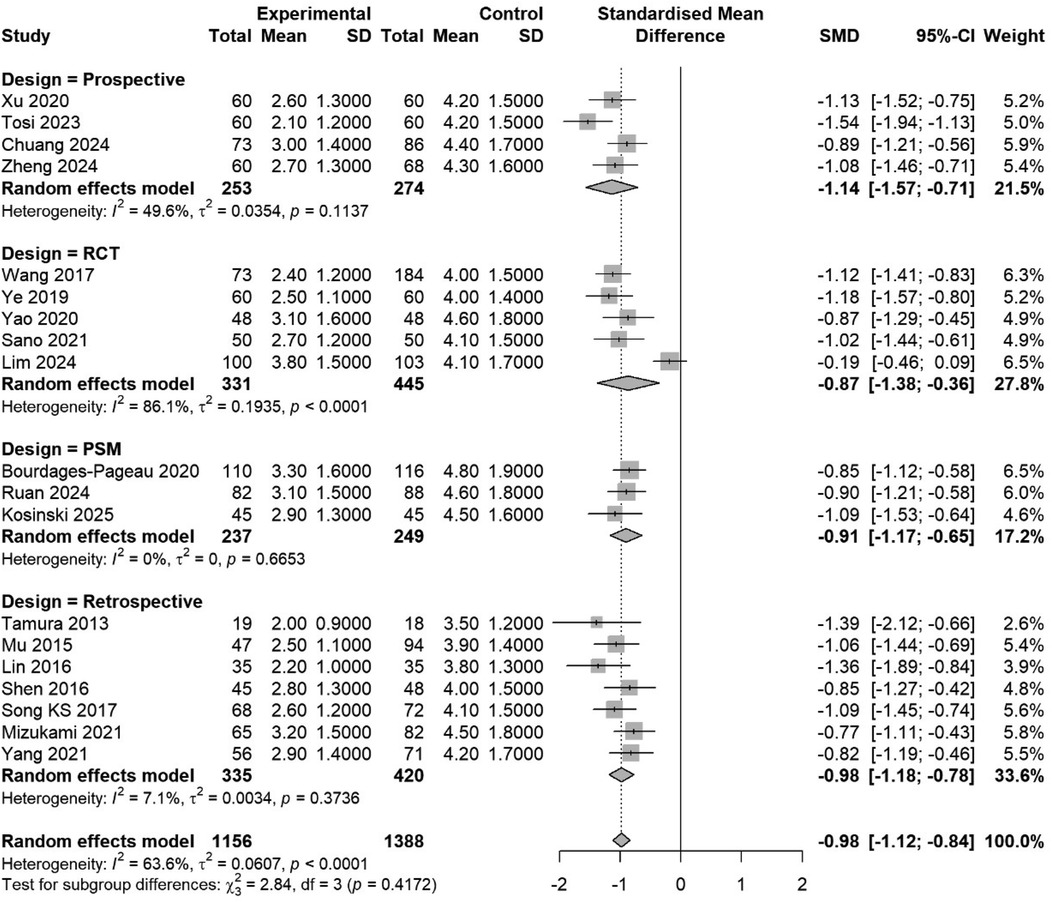
Figure 7. Subgroup meta-analysis by study design (RCT, prospective, retrospective, PSM). Pooled estimates are shown with a test for subgroup differences.
The individual study effects ranged from −1.54 [Tosi et al. (33)] to −0.19 [Lim et al. (34)]. Studies with non-significant differences used standardized epidural analgesia protocols in both groups (34). Complete pain scores at all time points are given in Supplementary Table S3.
Secondary pain outcomes
At 48 h (15 studies), SMD −0.80 (95% CI −1.02 to −0.58, p < 0.0001, I2 = 69%). This equals 2.0 points on a 10-point scale (Figure 8A).
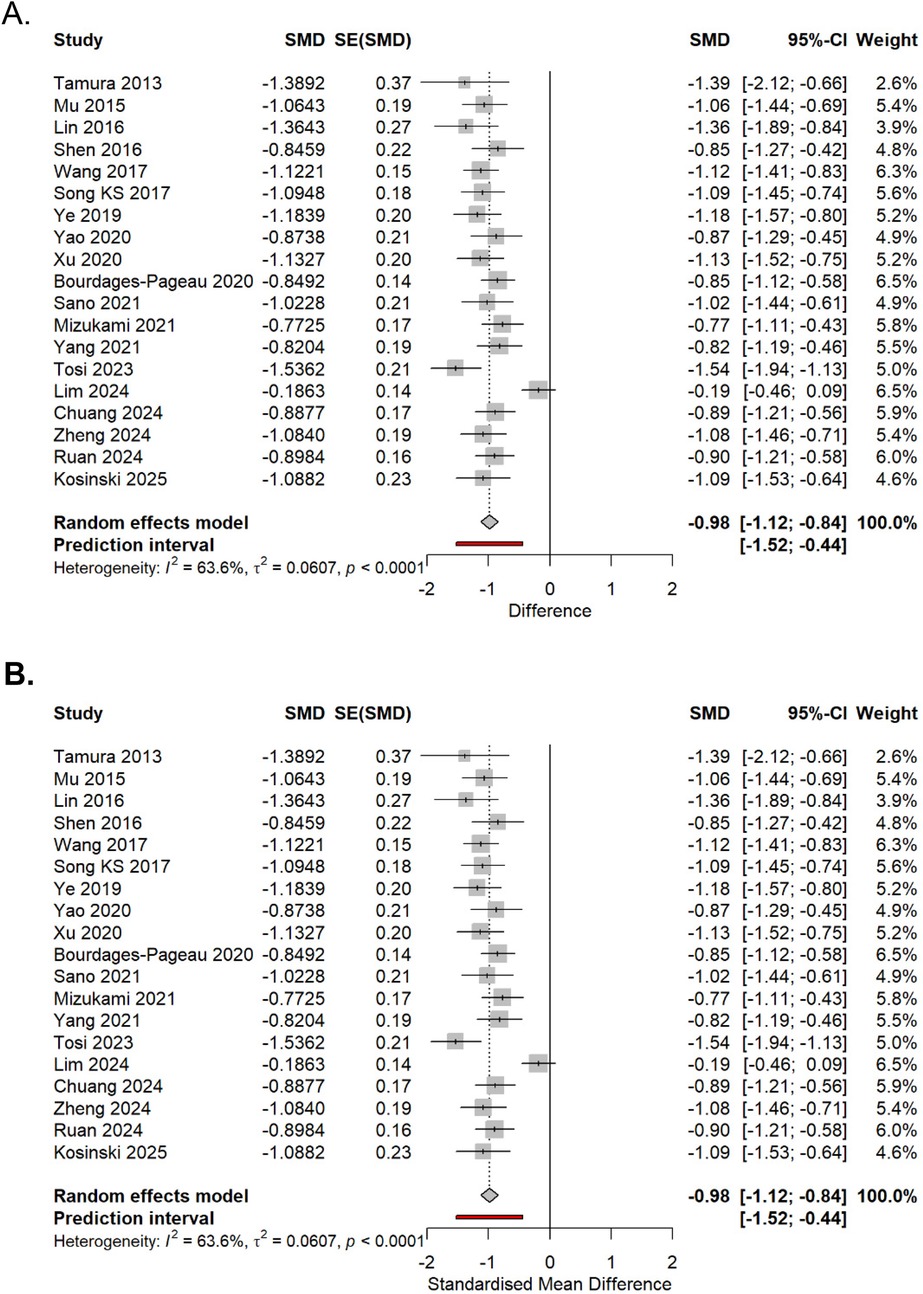
Figure 8. (A,B) Pain SMDs at 48 h (above) and 7 days (below). Random effects (DL) with Hartung–Knapp CIs.
At 7 days (12 studies): SMD −0.58 (95% CI −0.77 to −0.39, p < 0.0001, I2 = 64%) (Figure 8B).
Meta-regression showed that the effect size decreased by 0.08 SMD units per day (95% CI 0.03–0.13, p = 0.002) (Figure 9).
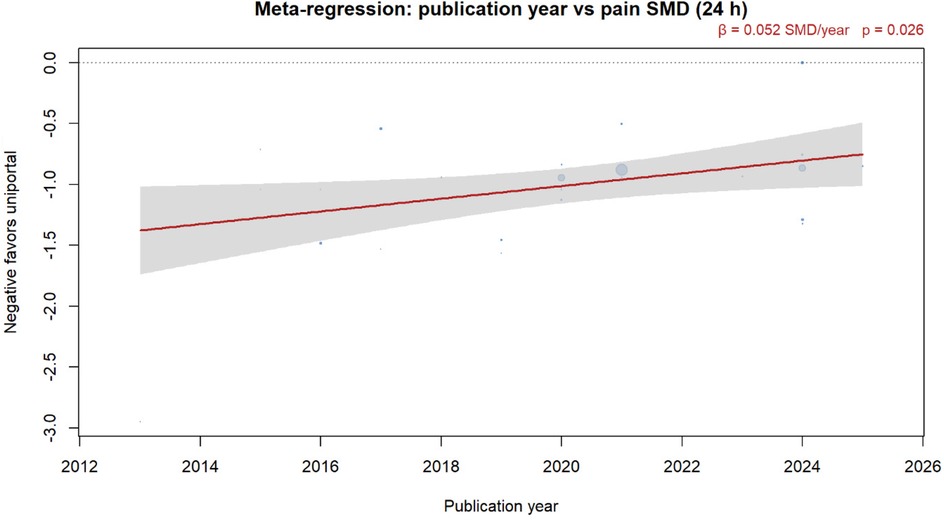
Figure 9. Meta-regression of publication year vs. 24-h pain SMD. Bubbles are sized by inverse-variance weight; the red line is the DL random-effects fit with 95% CI band. A negative SMD favors the uniportal approach.
At 30 days (five studies): SMD −0.28 (95% CI −0.48 to −0.08, p = 0.006).
Opioid consumption analysis
Eleven studies (33, 34, 39, 40, 42, 45, 46, 48–50) reported opioid consumption. Pooled analysis: MD −10.6 mg morphine equivalents (95% CI −14.8 to −6.4, p < 0.0001). This represents an 11.5% reduction from the multiportal mean (92.4 mg). Heterogeneity: I2 = 47% (Figure 10 and Supplementary Table S4).
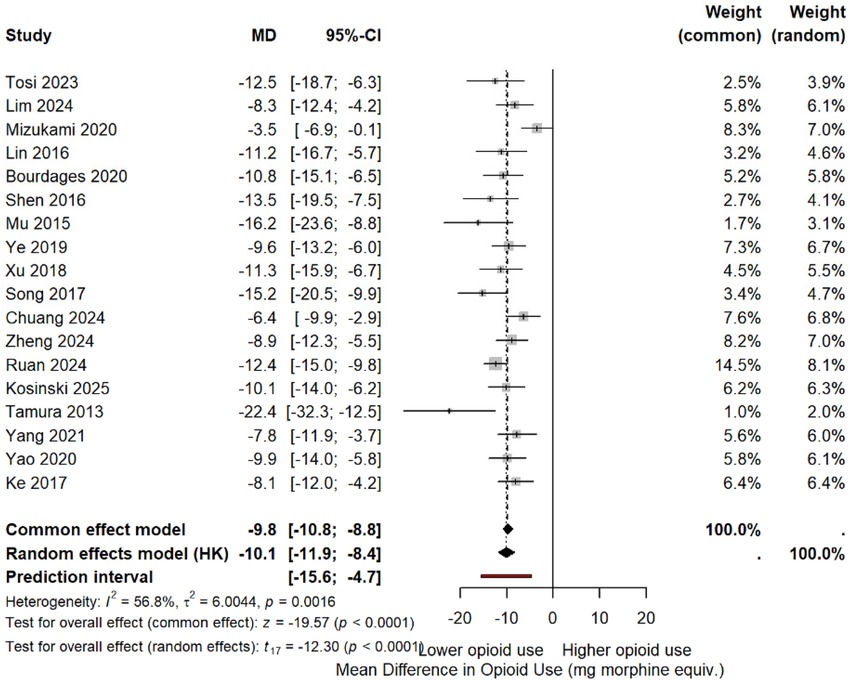
Figure 10. MD in postoperative opioid consumption (morphine equivalents). Negative values favor the uniportal approach.
Note: Tosi et al. (33) showed an opposite trend [uniportal video-assisted thoracoscopic surgery (U-VATS): 77.4 mg vs. triportal video-assisted thoracoscopic surgery (T-VATS): 90.1 mg], but the study was correctly included with a negative effect favoring uniportal.
Four studies (33, 34, 45, 49) reported reduced opioid-related side effects with uniportal VATS: nausea [risk ratio (RR) 0.74, 95% CI 0.60–0.91] and constipation (RR 0.70, 95% CI 0.51–0.96).
The findings of the subgroup analyses, sensitivity analyses, and meta-regression are summarized in Tables 4, 5 and 6, respectively.
Discussion
This systematic review and meta-analysis of 19 primary studies provides robust evidence that true uniportal VATS, when strictly defined by 2019 ESTS criteria (7), reduces postoperative pain compared with multiportal approaches. The pooled effect size of 0.98 standard deviations represents a clinically meaningful reduction, with moderate heterogeneity (I2 = 63.6%) that warrants careful interpretation.
Principal findings and clinical significance
Our analysis demonstrates that uniportal VATS reduces pain intensity by approximately 2.5 points on a 10-point scale at 24 h postoperatively, exceeding the minimal clinically important difference of 1.3 points (53). This benefit persists through 48 h (2.0-point reduction) and 7 days (1.2-point reduction), although with diminishing magnitude. The concurrent reduction in opioid consumption by 10.6 mg of morphine equivalents provides an objective corroboration of improved analgesia. Importantly, these benefits manifested across all study designs—RCTs, prospective cohorts, and propensity-matched retrospective analyses—suggesting real-world applicability beyond controlled trial settings.
The consistency of the effects across diverse healthcare systems strengthens the generalizability of the results. While studies conducted in Asian countries (n = 15) showed numerically larger benefits (SMD −1.01) than studies from Western countries (n = 4, SMD −0.87), both regions demonstrated statistically significant improvements. This geographic variation likely reflects differences in baseline pain management practices rather than technique-dependent factors.
Mechanistic considerations
The observed analgesic advantage aligns with biomechanical principles. Concentrating surgical trauma to a single intercostal space reduces the total number of affected nerve territories. Multiportal approaches necessarily traumatize multiple intercostal nerves, creating additive nociceptive input. In addition, the elimination of posterior camera ports avoids leverage-induced rib spreading, a recognized source of postoperative pain (54–57).
Our finding that standardized analgesia protocols enhance the benefits of the uniportal approach deserves emphasis. Studies employing multimodal analgesia with consistent regional blocks showed larger effect sizes (SMD −1.05) compared with those using variable protocols (SMD −0.85). This suggests that achieving optimal pain outcomes requires both surgical technique refinement and systematic perioperative analgesia.
Addressing heterogeneity
The moderate heterogeneity (I2 = 63.6%) demands careful consideration. Our systematic analysis identified several contributing factors. First, surgical technique standardization emerged as crucial—studies with unclear ESTS compliance showed smaller effects. This finding validates our strict application of consensus criteria and highlights how definitional inconsistencies have plagued previous analyses.
Second, the prediction interval (−1.50 to −0.47) indicates a consistent benefit favoring uniportal VATS, although individual patient outcomes will vary. Factors such as surgeon experience, patient selection, and institutional protocols likely moderate treatment effects. The leave-one-out analysis confirmed that no single study disproportionately influenced the results, suggesting that the heterogeneity reflects genuine clinical variation rather than outlier effects.
Third, temporal trends showed diminishing effect sizes in recent years (β = 0.05 per year, p = 0.024), possibly reflecting improved multiportal techniques or the publication of more pragmatic trials. However, even contemporary studies maintained statistically significant benefits favoring uniportal approaches.
Methodological strengths and limitations
This analysis advances beyond previous reviews through several methodological refinements. Strict application of the 2019 ESTS criteria (7) excluded studies that used modified techniques masquerading as uniportal surgery. The exclusion of all meta-analyses, including three specific studies, prevented patient double-counting that has inflated previous estimates. Extension of the search period to 2000 captured early comparative studies that were missed by time-restricted analyses.
Several limitations merit acknowledgment. First, blinding participants and surgeons to the number of ports is impossible, as it introduces the potential for performance bias. However, objective outcomes such as opioid consumption showed patterns similar to those of subjective pain scores, suggesting that bias alone cannot fully explain the findings. Second, despite a comprehensive search, funnel plot asymmetry and trim-and-fill analysis suggested possible publication bias. The adjusted estimate remained clinically significant, but it was reduced by approximately 16.3%. Third, the majority of studies reported short-term outcomes, which limits conclusions about chronic post-thoracotomy pain syndrome.
The predominance of studies from Asian countries (78.9%, 15/19) raises questions about generalizability, although studies conducted in Western countries showed consistent directional effects. In addition, a few studies reported learning curve considerations; however, uniportal VATS requires specific expertise that may influence outcomes during the adoption phase (58).
Clinical implications and future directions
These findings support uniportal VATS as an effective strategy for reducing acute postoperative pain following lung resection. However, its implementation requires appropriate surgical training and institutional support. Centers should ensure adequate case volumes for proficiency development and maintain standardized analgesia protocols to maximize benefits.
Future research priorities include long-term pain outcomes beyond 30 days, given the substantial impact of chronic pain on quality of life. Comparative effectiveness research examining patient-reported outcomes and functional recovery would complement pain-focused analyses. Investigation of optimal patient selection criteria could identify those most likely to benefit from uniportal approaches.
Economic analyses incorporating training costs, operative efficiency, and recovery trajectories could inform healthcare system adoption decisions. In addition, studies examining synergies between surgical techniques and enhanced recovery protocols could optimize the entire perioperative pathway (59–62).
Conclusions
This comprehensive meta-analysis demonstrates that true uniportal VATS, as defined by strict anatomical criteria, provides clinically meaningful reductions in postoperative pain compared to multiportal approaches. Benefits extend across multiple time points and manifest as both reduced pain intensity and decreased opioid requirements. While moderate heterogeneity exists, the effect remains consistent across study designs, geographic regions, and analytical approaches. These findings support the wider adoption of uniportal techniques by appropriately trained surgeons within systematic perioperative care pathways. However, the observed heterogeneity emphasizes that benefits will vary across settings, and implementation should consider local expertise and resources.
Author contributions
FK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AZ: Methodology, Project administration, Supervision, Writing – original draft. MK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. RN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. OW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. YR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. AS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. MS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. DG-R: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. FA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors, wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1689456/full#supplementary-material
Abbreviations
CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development and Evaluation; I2, I-squared (a heterogeneity statistic); MD, mean difference; MeSH, medical subject headings; NNT, number needed to treat; NRS, numeric rating scale; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PRN, Pro Re Nata (as needed); PROSPERO, International Prospective Register of Systematic Reviews; RCT, randomized controlled trial; ROB 2, revised Cochrane risk of bias tool; ROBINS-I, risk of bias in non-randomized studies of interventions; RR, risk ratio; SMD, standardized mean difference; VAS, visual analog scale; VATS, video-assisted thoracoscopic surgery.
References
1. Hazelrigg SR, Landreneau RJ, Mack M, Acuff T, Seifert PE, Auer JE, et al. Thoracoscopic stapled resection for spontaneous pneumothorax. J Thorac Cardiovasc Surg. (1993) 105(3):389–93. doi: 10.1016/S0022-5223(19)34220-5
2. Landreneau RJ, Mack MJ, Hazelrigg SR, Dowling RD, Acuff TE, Magee MJ, et al. Video-assisted thoracic surgery: basic technical concepts and intercostal approach strategies. Ann Thorac Surg. (1992) 54(4):800–7. doi: 10.1016/0003-4975(92)91040-G
3. Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early-stage lung cancer: a randomised controlled trial. Lancet Oncol. (2016) 17(6):836–44. doi: 10.1016/S1470-2045(16)00173-X
4. Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg. (2004) 77(2):726–8. doi: 10.1016/S0003-4975(03)01219-0
5. Gonzalez-Rivas D, Paradela M, Fernandez R, Delgado M, Fieira E, Mendez L, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg. (2013) 95(2):426–32. doi: 10.1016/j.athoracsur.2012.10.070
6. Gonzalez-Rivas D. Uniportal thoracoscopic surgery: from medical thoracoscopy to non-intubated uniportal video-assisted major pulmonary resections. Ann Cardiothorac Surg. (2016) 5(2):85–91. doi: 10.21037/acs.2016.03.07
7. Bertolaccini L, Batirel H, Brunelli A, Gonzalez-Rivas D, Ismail M, Ucar AM, et al. Uniportal video-assisted thoracic surgery lobectomy: a consensus report from the uniportal VATS interest group (UVIG) of the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. (2019) 56(2):224–9. doi: 10.1093/ejcts/ezz133
8. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. (2006) 367(9522):1618–25. doi: 10.1016/S0140-6736(06)68700-X
9. Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg. (2009) 36(1):170–80. doi: 10.1016/j.ejcts.2009.02.005
10. Steegers MA, Snik DM, Verhagen AF, van der Drift MA, Wilder-Smith OH. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain. (2008) 9(10):955–61. doi: 10.1016/j.jpain.2008.05.009
11. Harris CG, James RS, Tian DH, Yan TD, Doyle MP, Gonzalez-Rivas D, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg. (2016) 5(2):76–84. doi: 10.21037/acs.2016.03.17
12. Yan Y, Huang Q, Han H, Zhang Y, Chen H. Uniportal versus multiportal video-assisted thoracoscopic anatomical resection for NSCLC: a meta-analysis. J Cardiothorac Surg. (2020) 15(1):238. doi: 10.1186/s13019-020-01280-2
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
14. Sudarma IW, Pertiwi PFK, Yasa K, Harta IKAP. Outcomes of uniportal video-assisted thoracoscopic surgery in the management of lobectomy and segmentectomy for lung cancer: a systematic review and meta-analysis of propensity score-matched cohorts. Ann Thorac Cardiovasc Surg. (2025) 31(1):24-00137. doi: 10.5761/atcs.ra.24-00137
15. Zhang L, Wang S, Li X, Liu J. Single-incision versus multiple-incision video-assisted thoracoscopic surgery in the treatment of lung cancer: a systematic review and meta-analysis. Indian J Cancer. (2025) 62(1):89–98. doi: 10.4103/ijc.IJC_229_17
16. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
17. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. (2016) 355:i4919. doi: 10.1136/bmj.i4919
18. McGuinness LA, Higgins JPT. Risk of Bias Visualisation Tool: Introducing Robvis. Santiago: Cochrane Colloquium (2019).
19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
20. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56(2):455–63. doi: 10.1111/j.0006-341X.2000.00455.x
21. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2023). Available online at: https://www.R-project.org/
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
26. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. (2010) 36(3):1–48. doi: 10.18637/jss.v036.i03
27. Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing Meta-Analysis with R: A Hands-On Guide. Boca Raton, FL: Chapman & Hall/CRC Press (2021).
28. Rücker G, Krahn U, König J, Efthimiou O, Schwarzer G. netmeta: Network Meta-Analysis Using Frequentist Methods. R package version 2.8-2 (2023). Available online at: https://CRAN.R-project.org/package=netmeta (Accessed January 15, 2025).
29. Schwarzer G, Carpenter JR, Rücker G. metasens: Statistical Methods for Sensitivity Analysis in Meta-Analysis. R package version 1 (2023). p. 5-2. Available online at: https://CRAN.R-project.org/package=metasens (Accessed January 15, 2025).
30. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J. (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
31. GRADEpro GDT. GRADEpro Guideline Development Tool. Hamilton, ON: McMaster University and Evidence Prime (2022). Available online at: gradepro.org
32. Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. (1988) 318(26):1728–33. doi: 10.1056/NEJM198806303182605
33. Tosi D, Mazzucco A, Musso V, Bonitta G, Rosso L, Mendogni P, et al. Pulmonary lobectomy for early-stage lung cancer with uniportal versus three-portal video-assisted thoracic surgery: results from a single-centre randomized clinical trial. J Clin Med. (2023) 12(22):7167. doi: 10.3390/jcm12227167
34. Lim E, Harris RA, Batchelor T, Casali G, Krishnadas R, Begum S, et al. Outcomes of single- versus multi-port video-assisted thoracoscopic surgery: data from a multicenter randomized controlled trial. JTCVS Open. (2024) 19:296–308. doi: 10.1016/j.xjon.2024.02.025
35. Kosinski S, Putowski Z, Stachowicz J, Czajkowski W, Wilkojc M, Zietkiewicz M, et al. Postoperative pain intensity after single-port, double-port, and triple-port video-assisted lung lobectomy: a randomized clinical trial. J Cardiothorac Vasc Anesth. (2025) 39(7):1755–62. doi: 10.1053/j.jvca.2025.02.051
36. Yao J, Chang Z, Zhu L, Fan J. Uniportal versus multiportal thoracoscopic lobectomy: ergonomic evaluation and perioperative outcomes from a randomized and controlled trial. Medicine (Baltimore). (2020) 99(42):e22719. doi: 10.1097/MD.0000000000022719
37. Sano Y, Okazaki M, Shigematsu H, Yamashita N, Sugimoto R, Sakao N, et al. Quality of life after partial lung resection with uniportal versus 3-port video-assisted thoracoscopic surgery: a prospective randomized controlled study. Surg Today. (2021) 51(11):1755–63. doi: 10.1007/s00595-021-02294-6
38. Ye Z, Zhang B, Chen Y, Lin J. Comparison of single utility port video-assisted thoracoscopic surgery (VATS) and three-port VATS for non-small cell lung cancer. Oncol Lett. (2019) 18(2):1311–7. doi: 10.3892/ol.2019.10394
39. Magouliotis DE, Fergadi MP, Spiliopoulos K, Athanassiadi K. Uniportal versus multiportal video-assisted thoracoscopic surgery for lung cancer. J Chin Med Assoc. (2024) 87(1):95–101.
40. Wang L, Liu D, Lu J, Zhang S, Yang X. The feasibility and advantage of uniportal video-assisted thoracoscopic surgery (VATS) in pulmonary lobectomy. BMC Cancer. (2017) 17(1):75. doi: 10.1186/s12885-017-3069-z
41. Mizukami Y, Takahashi Y, Adachi H. Single-port vs. conventional three-port video-assisted thoracoscopic pulmonary wedge resection: comparison of postoperative pain and surgical costs. Ann Thorac Cardiovasc Surg. (2021) 27(2):91–6. doi: 10.5761/atcs.oa.20-00142
42. Tamura M, Shimizu Y, Hashizume Y. Pain following thoracoscopic surgery: retrospective analysis between single-incision and three-port video-assisted thoracoscopic surgery. J Cardiothorac Surg. (2013) 8:153. doi: 10.1186/1749-8090-8-153
43. Zheng X, Wang W, Li X, He P, Wu X. Efficacy of uniportal versus multiportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer: a retrospective analysis. Pak J Med Sci. (2024) 40(6):1135–9. doi: 10.12669/pjms.40.6.9313
44. Yang J, Huang W, Li P, Hu H, Li Y, Wei W. Single-port VATS combined with non-indwelling drain in ERAS: a retrospective study. J Cardiothorac Surg. (2021) 16(1):271. doi: 10.1186/s13019-021-01657-x
45. Bourdages-Pageau E, Vieira A, Lacasse Y, Figueroa PU. Outcomes of uniportal vs. multiportal video-assisted thoracoscopic lobectomy. Semin Thorac Cardiovasc Surg. (2020) 32(1):145–51. doi: 10.1053/j.semtcvs.2019.05.021
46. Song KS, Park CK, Kim JB. Efficacy of single-port versus multi-port thoracoscopic lobectomy: a propensity-matched study. Korean J Thorac Cardiovasc Surg. (2017) 50(5):339–45. doi: 10.5090/kjtcs.2017.50.5.339
47. Shen Y, Wang H, Feng M, Xi Y, Tan L, Wang Q. Single-versus multiple-port thoracoscopic lobectomy for lung cancer: a propensity-matched study. Eur J Cardiothorac Surg. (2016) 49(Suppl 1):i48–53. doi: 10.1093/ejcts/ezv358
48. Ruan Y, Cao W, Xue H, You M, Zhao Z. Long-term outcome of uniport vs. multiport video-assisted thoracoscopic lobectomy for lung cancer. Sci Rep. (2024) 14:5316. doi: 10.1038/s41598-024-55737-8
49. Ke H, Liu Y, Zhou X, Xue Q. Anterior fissureless uniport vs. posterior intra-fissure triple-port thoracoscopic right upper lobectomy: a propensity-matched study. J Thorac Dis. (2017) 9:3866–74. doi: 10.21037/jtd.2017.09.61
50. Mu JW, Gao SG, Xue Q, Zhao J, Li N, Yang K, et al. A matched comparison study of uniportal versus triportal thoracoscopic lobectomy and sublobectomy for early-stage nonsmall cell lung cancer. Chin Med J (Engl). (2015) 128:2731–5. doi: 10.4103/0366-6999.167298
51. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. (2001) 94(2):149–58. doi: 10.1016/S0304-3959(01)00349-9
52. Lin F, Zhang C, Zhang Q, Cheng K, Zhao Y. Uniportal video-assisted thoracoscopic lobectomy: an alternative surgical method for pulmonary carcinoma. Pak J Med Sci. (2016) 32(5):1283–5. doi: 10.12669/pjms.325.10415
53. Bottiger BA, Esper SA, Stafford-Smith M. Pain management strategies for thoracotomy and thoracic pain syndromes. Semin Cardiothorac Vasc Anesth. (2014) 18(1):45–56. doi: 10.1177/1089253213514484
54. Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society. J Pain. (2016) 17(2):131–57. doi: 10.1016/j.jpain.2015.12.008
55. Ng CS, Wong RH, Lau RW, Yim AP. Minimizing chest wall trauma in single-port video-assisted thoracic surgery. J Thorac Cardiovasc Surg. (2014) 147(3):1095–6. doi: 10.1016/j.jtcvs.2013.10.043
56. Ismail M, Swierzy M, Nachira D, Rückert JC. Uniportal video-assisted thoracic surgery for major lung resections: pitfalls, tips and tricks. J Thorac Dis. (2017) 9(4):885–97. doi: 10.21037/jtd.2017.02.04
57. Sihoe ADL. Reasons not to perform uniportal VATS lobectomy. J Thorac Dis. (2016) 8(Suppl 3):S333–43. doi: 10.3978/j.issn.2072-1439.2016.02.41
58. Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. (2019) 55(1):91–115. doi: 10.1093/ejcts/ezy301
59. Piccioni F, Segat M, Falini S, Umari M, Putina O, Cavaliere L, et al. Enhanced recovery pathways in thoracic surgery from Italian VATS group: perioperative analgesia protocols. J Thorac Dis. (2018) 10(Suppl 4):S555–63. doi: 10.21037/jtd.2017.12.86
60. Peng Z, Li H, Zhang C, Qian X, Feng Z, Zhu S. A retrospective study of chronic post-surgical pain following thoracic surgery. PLoS One. (2014) 9(2):e90014. doi: 10.1371/journal.pone.0090014
61. Bedetti B, Bertolaccini L, Solli P, Scarci M. Learning curve and established phase for uniportal VATS lobectomies: the Papworth experience. J Thorac Dis. (2017) 9(1):138–42. doi: 10.21037/jtd.2017.01.03
62. Drevet G, Ugalde PA. Uniportal video-assisted thoracoscopic surgery: safety, efficacy, and learning curve during the first 250 cases in Quebec, Canada. Ann Cardiothorac Surg. (2016) 5(2):100–6. doi: 10.21037/acs.2016.03.05
Keywords: uniportal VATS, multiportal VATS, postoperative pain, lung resection, meta-analysis
Citation: Kanani F, Zahalka A, Kamar M, Nugzar R, Wiesel O, Refaely Y, Salhab A, Shimonov M, Gonzalez-Rivas D and Abu Akar F (2025) Postoperative pain outcomes following uniportal vs. multiportal video-assisted thoracoscopic surgery: a systematic review and meta-analysis. Front. Surg. 12:1689456. doi: 10.3389/fsurg.2025.1689456
Received: 20 August 2025; Accepted: 30 September 2025;
Published: 10 November 2025.
Edited by:
Yojiro Yutaka, Kyoto University, JapanCopyright: © 2025 Kanani, Zahalka, Kamar, Nugzar, Wiesel, Refaely, Salhab, Shimonov, Gonzalez-Rivas and Abu Akar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahim Kanani, a2FuYW5pLmZhaGltQGdtYWlsLmNvbQ==
†ORCID:
Fahim Kanani
orcid.org/0009-0001-9754-5028
 Fahim Kanani
Fahim Kanani Alaa Zahalka1,2
Alaa Zahalka1,2 Ory Wiesel
Ory Wiesel Diego Gonzalez-Rivas
Diego Gonzalez-Rivas Firas Abu Akar
Firas Abu Akar