- 1General Surgery, Yongchuan Hospital Affiliated with Chongqing Medical University, Chongqing, China
- 2Chongqing Medical University, Chongqing, China
Objective: To analyze the risk factors for the complications of access and to construct and validate a nomogram prediction model for their occurrence.
Methods: Patients undergoing endovascular intervention via femoral artery access between January 2020 and April 2025 were enrolled in the study. Related clinical data were retrospectively collected and analyzed. Patients were divided into complication (n = 19) and non-complication (n = 488) groups based on the occurrence of postoperative complications associated with femoral artery puncture site. The general cohort characteristics were compared between the two groups, and the risk factors for the postoperative complications were identified based on univariate and multivariate logistic regression analyses. A nomogram prediction model was constructed and its performance was evaluated using the area under the receiver operating characteristic (ROC) curve, the Hosmer-Lemeshow test, calibration curve, and decision curve analyses.
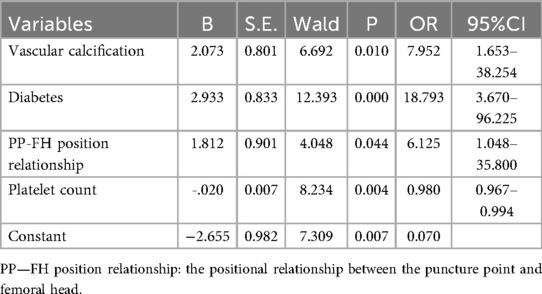
Results: Four potential predictors were identified based on the multivariate logistic regression analysis results: vascular calcification [odds ratio (OR) = 7.952, 95% confidence interval (CI): 1.653–38.254], history of diabetes (OR = 18.793, 95% CI: 3.670–96.225), platelet count (OR = 0.980, 95% CI: 0.967–0.994), and positional relationship between the puncture point and femoral head (OR = 6.125, 95% CI: 1.048–35.800). The nomogram model incorporating these factors demonstrated strong performance, with an area under the ROC curve of 0.924 (95% confidence interval: 0.839–1.000), sensitivity of 81.80%, specificity of 95.20%, and overall accuracy of 94.70%.The Hosmer-Lemeshow test yielded χ2 = 12.535 and P = 0.8184, indicating a good model fit. Calibration curves showed strong agreement between the nomogram predictions and observed outcomes. Both the ROC and decision curve analysis confirmed the nomogram's robust predictive performance.
Conclusions: Platelet count, history of diabetes, vascular calcification, and positional relationship between the puncture point and the femoral head are independent risk factors for the complications of femoral artery access. The nomogram model established based on these indicators demonstrated a high accuracy in predicting the risk of complications.
1 Introduction
Over 7 million percutaneous vascular interventions are performed worldwide annually. The common femoral artery is the most commonly used target vessel for puncture due to its large diameter, superficial location, and anatomical position anterior to the femoral head. However, clinical data show that approximately 5%–10% of patients experience access site complications, such as puncture site bleeding, hematoma, pseudoaneurysm, and even retroperitoneal hematoma (1, 2). These complications severely impact patients' quality of life and increase healthcare costs. With the rising adoption of technologies, such as transcatheter aortic valve replacement and thoracic endovascular aortic repair, the growing demand for larger-diameter devices for transfemoral access has substantially heightened the potential risk of vascular complications (3). Scientific strategies for the prevention and control of vascular access complications require accurate identification of relevant risk factors. Numerous clinical studies have demonstrated that the relative position of the femoral head access site, chronic hyperglycemia, and calcification at the access site are key factors influencing the incidence of postoperative complications (4–6). However, research on risk prediction models for femoral artery access complications remains limited. The present study aimed to systematically analyze the independent risk factors for femoral artery intervention and construct and validate a prediction model that can identify high-risk groups early in order to reduce the incidence of postoperative complications.
2 Methods
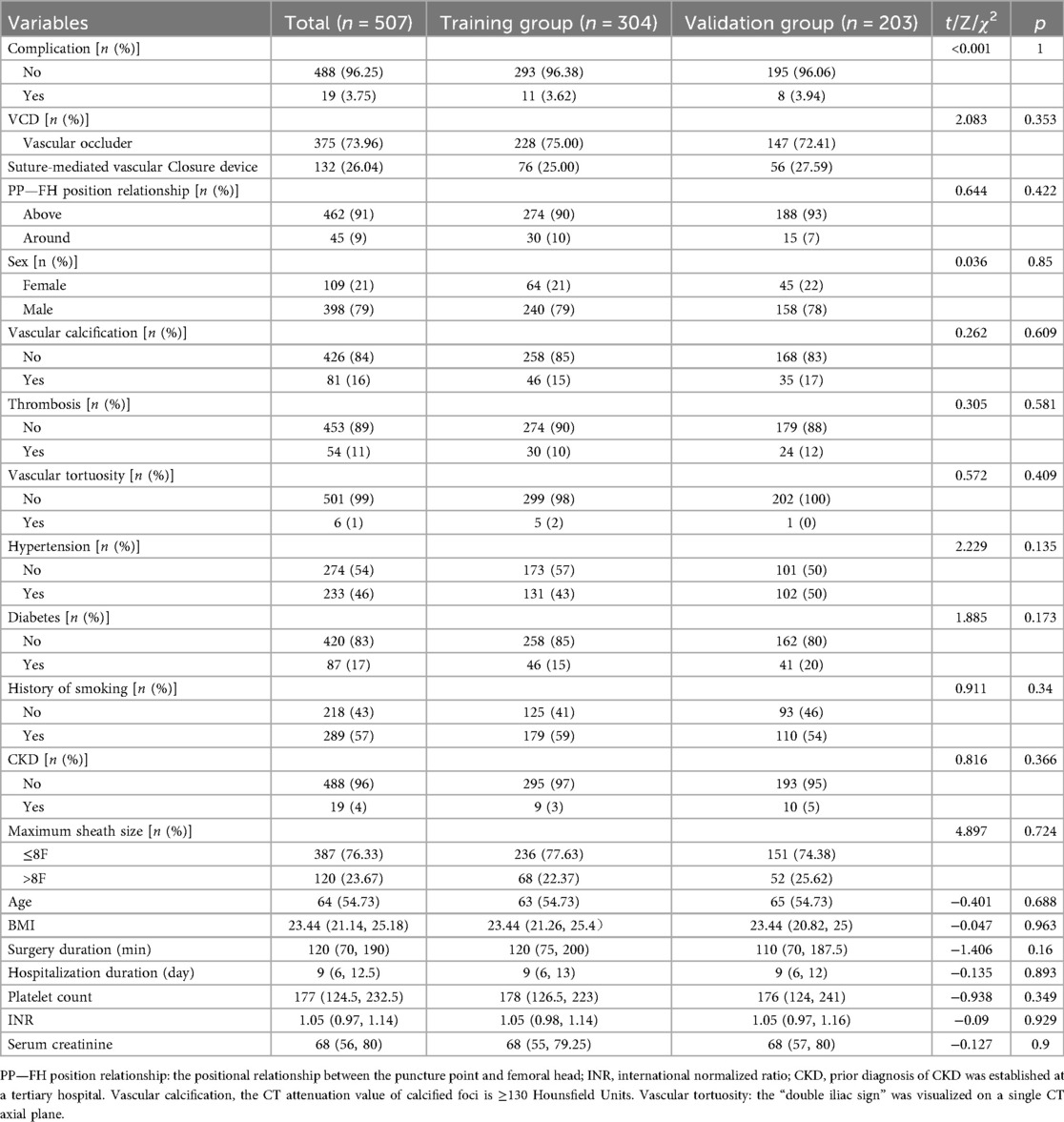
1. The clinical records of 507 patients who underwent femoral artery puncture at Yongchuan Hospital Affiliated with Chongqing Medical University between January 2020 and April 2025 were retrospectively analyzed. The cohort included 398 male and 109 female patients. A flowchart of the patient selection process is shown in Figure 1. The 507 patients were randomly divided into training (n = 304) and validation (n = 203) sets at a 6:4 ratio to ensure the reproducibility of the model's training and validation processes. The demographic and clinical characteristics of all patients in the training and validation sets are represented in Table 1. This clinical study adhered to the Declaration of Helsinki and met relevant ethical requirements. The study was approved by the Medical Ethics Committee of Yongchuan Hospital Affiliated with Chongqing Medical University.
2. Inclusion criteria: (1) Age of ≥18 years; (2) femoral artery puncture access; and 3. Complete medical records; Exclusion criteria: (1) Total occlusion of the femoral artery on the planned puncture side; (2) Patients who received concurrent thrombolysis; (3) Patients with pseudoaneurysm or dissection on the puncture side; (4) Patients who received femoral artery puncture under direct visualization through a local incision; (5) Incomplete medical records; and (6) Age of <18 years.
3. The study preliminarily formulated 18 risk factors based on expert consultation and literature review. The following patient information was collected: age, sex, body mass index (BMI), Vascular calcification, thrombosis, and Vascular tortuosity, history of hypertension, history of diabetes and smoking, operation duration, Hospitalization duration, platelet count, international normalized ratio, serum creatinine level, Chronic Kidney Disease (CKD), maximum sheath size, Surgery duration, vascular closure device (VCD), and positional relationship between the puncture site and the femoral head. The patient information recorded at discharge was considered the observation endpoint. Based on the model sample size calculation formula (4), 5–10 cases were required to validate each variable.
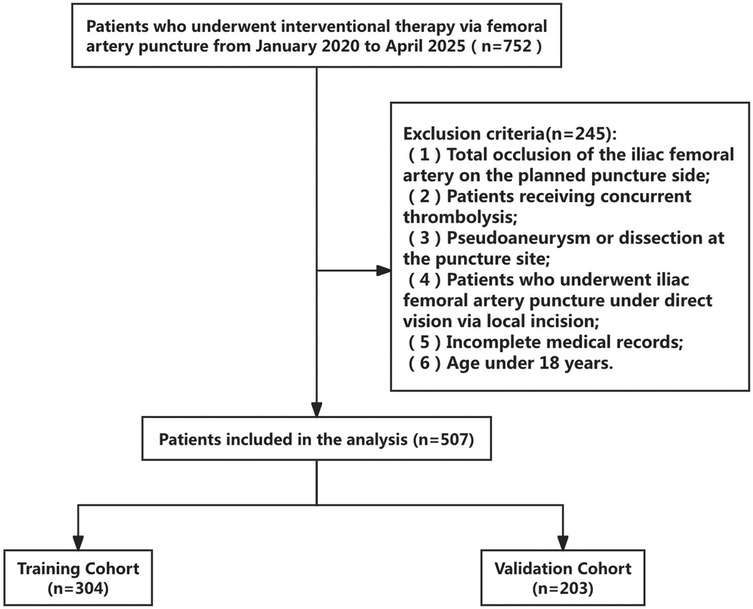
Figure 1. Flowchart of a screening process. A total of 507 participants were included in the analysis: 304 in the training and 203 in the validation cohorts.
2.1 Statistical methods
Data analysis and graphs were generated using SPSS 23.0 and R packages. Continuous data were evaluated for normality using the Kolmogorov–Smirnov test. Normally distributed continuous data were expressed as ‘x ± s, and inter-group comparisons were performed using the independent sample t-test. Continuous data that did not follow a normal distribution were expressed as M (Q1, Q3), and the Mann–Whitney U test was used for inter-group comparisons. Count data were expressed as percentages (%), and the chi-square test or Fisher's exact test was used for inter-group comparisons. Univariate and multivariate logistic regression model was used to analyze the risk factors of complications. A nomogram prediction model was constructed based on the identified independent risk factors. The receiver operating characteristic (ROC) curve was utilized to determine the efficacy of the nomogram model in predicting complications. The calibration curve was employed to evaluate the model's calibration rate. The clinical decision curve was used to assess the clinical benefit of the model. A two-sided P < 0.05 was considered statistically significant.
2.2 Data collection
Two team members completed all data collection between January 2025 and April 2025. All researchers underwent standardized training and assessment to ensure the standardization of data collection and research methods and to prevent data bias or errors due to human factors. Only those who passed the assessment were allowed to participate in the study. Furthermore, data entry was performed by two persons and was double-checked to ensure its accuracy, completeness, and authenticity. Any errors or discrepancies were reported to the researchers for prompt evaluation and correction.
3 Results
We conducted eligibility assessment for 752 patients who underwent endovascular intervention via femoral artery puncture between January 2020 and April 2025 using the hospital's electronic medical record system. After excluding 245 patients who met the exclusion criteria, 507 patients (398 males and 109 females) were ultimately enrolled in this study. Using RStudio 4.5.1, these 507 patients were randomly divided into a training set (n = 304) and a validation set (n = 203) at a 6:4 ratio. The demographic and clinical characteristics of all patients in both the training and validation sets are presented in Table 1. Among these patients, a total of 19 cases developed postoperative complications, including three cases of postoperative bleeding (0.6%), 15 cases of hematoma (3%), and one case of pseudoaneurysm (0.2%). Four potential predictors were identified based on the multivariate logistic regression analysis results (Table 2): vascular calcification [odds ratio (OR) = 7.952, 95% confidence interval (CI): 1.653–38.254], history of diabetes (OR = 18.793, 95% CI: 3.670–96.225), platelet count (OR = 0.980, 95% CI: 0.967–0.994), and positional relationship between the puncture point and femoral head (OR = 6.125, 95% CI: 1.048–35.800). The four independent predictors were then employed to develop a nomogram associated with femoral artery access complications (Figure 2).
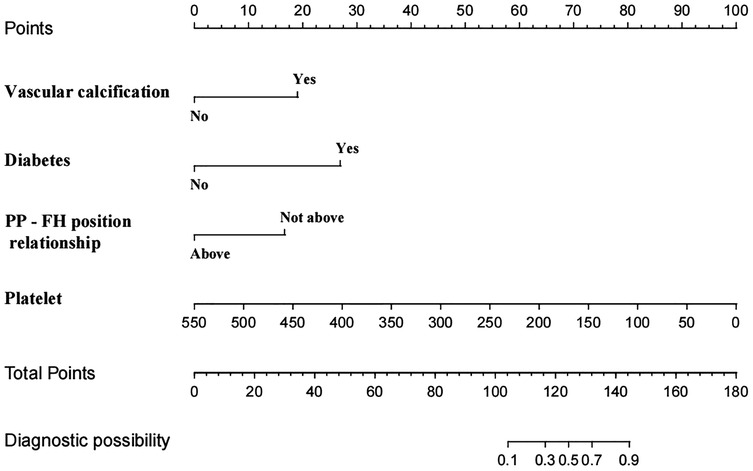
Figure 2. Nomogram model of femoral artery interventional access complications. PP—FH position relationship: the positional relationship between the puncture point and femoral head.
The ROC curves were plotted and the area under the ROC curve (AUC) values were calculated in order to test the discriminatory ability of the nomogram. The AUC values for the training and validation sets were 0.924 (95% CI: 0.839–1.000) and 0.946 (95% CI: 0.896–0.997), respectively, demonstrating good diagnostic performance (Figure 3). The sensitivity, specificity, and accuracy under the optimal cutoff value were 81.80%, 95.20%, and 94.70%, respectively. Nomogram calibration was evaluated based on the calibration curves and the Hosmer-Lemeshow goodness-of-fit test (Figure 4). The calibration curves demonstrated that the nomogram's predictions of femoral artery access complications were consistent with the actual incidence. The Hosmer-Lemeshow test yielded a p-value of 0.8184 for the training set and 0.9983 for the validation set. Overall, the nomogram showed strong agreement between the observed outcomes and predictions.
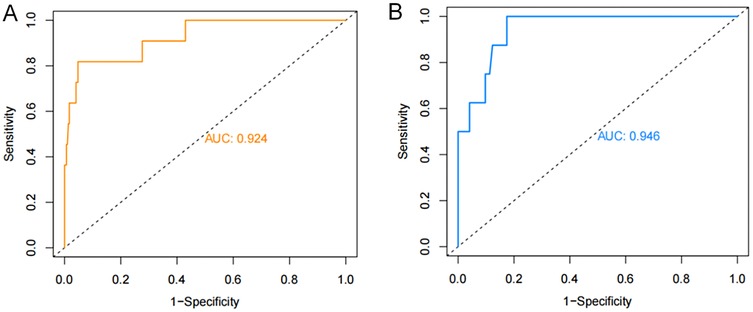
Figure 3. ROC validation of the nomogram prediction of femoral artery interventional access complications. The area under the curve represents the performance of the nomogram in the (A) training and (B) validation sets.
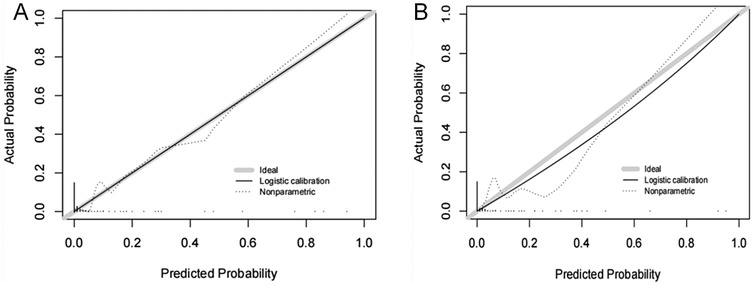
Figure 4. Calibration curves of femoral artery interventional access complications. The diagonal dotted line represents a perfect prediction by an ideal model. The solid line represents the performance of the (A) training and (B) validation sets, which indicated that a closer fit to the diagonal dotted line showed a better prediction.
Decision curves showed that the risk of femoral artery access complications was more accurately predicted using the nomogram when the risk threshold probability was between 3% and 100% in the present study and between 3% and 99% in the validation set (Figure 5).
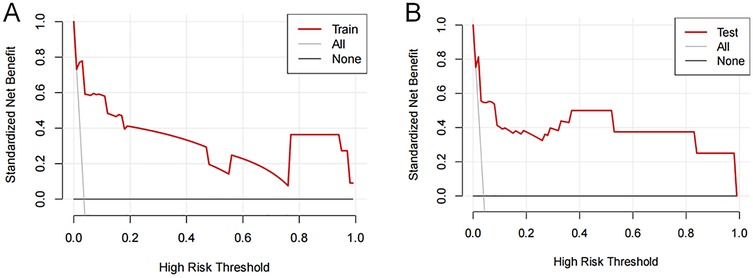
Figure 5. Decision curve analysis of femoral artery interventional access complications. The thick solid line represents the assumption that all patients did not develop complications after puncture. The thin solid line represents the assumption that all patients developed complications after puncture. The red line represents the risk nomogram for the (A) training and (B) validation sets.
4 Discussion
The femoral artery access complication prediction model developed in the present study is easy to implement and demonstrates strong predictive performance. It shifts the clinical approach from reactive intervention after symptom onset to proactive and targeted prevention before complications occur. ROC curves were used to evaluate the discrimination ability of the model. AUC values of 0.5–0.7 indicated low accuracy, AUC values of >0.7–0.9 represented moderate accuracy, and those ≥0.9 showed high accuracy (5).
The present study suggests that platelet count, vascular calcification, diabetes history, and relative position of the puncture point to the femoral head are independent risk factors for the complications of femoral artery access. The complication rate was 3.8% (19/507) in the 507 analyzed cases, which was lower than that reported in previous studies. The lower complication rate was primarily attributed to a systematic and precise order of operations, such as comprehensively assessing the target vessel calcification burden, thrombus distribution, and vascular tortuosity using preoperative computed tomography imaging and artificial intelligence analysis, precisely locating the spatial relationship between the puncture site and the femoral head based on intraoperative Digital Subtraction Angiography guidance to optimize the puncture path, and performing all procedures by the same experienced vascular interventional physician (6, 7). In this study, all three patients with post-procedural bleeding received continued manual compression at the femoral artery puncture site for 30 min, followed by the application of an elastic compression bandage for 6 h. Patients who developed a puncture site hematoma did not receive any specific additional intervention. For the patient diagnosed with a pseudoaneurysm, follow-up color Doppler ultrasound performed 24 h postoperatively revealed a small aneurysm sac diameter, and thus no specific treatment was administered.
Research indicates that the optimal femoral artery puncture site is located at the midline level of the femoral head, which serves as a key bony landmark to indicate femoral artery course. The central plane of the femoral head closely corresponds to the anatomical course of the femoral artery. Schnyder et al. (8, 9) found that the femoral artery bifurcation is located at or slightly below the level of the femoral head midline in 98% of patients. Puncture at the level of the femoral head midline can avoid retroperitoneal bleeding caused by high-level puncture into the external iliac artery (10). Ahn et al. found that the safe distance between the puncture point and the femoral artery bifurcation is the largest and the risk of complications is the lowest when the puncture point is at the level of the femoral head midline. Moreover, femoral artery calcification is typically concentrated at the arterial bifurcation, whereas the vascular wall at the level of the femoral head midline generally exhibits minimal calcification (10). Puncturing calcified vessels often results in needle tip deviation or incomplete vessel entry, increasing the risk of repeated puncture attempts and subsequent hematoma formation (11). However, it is difficult to monitor the long blood vessels on the same interface due to the probe scanning range limitations. The femoral head provides a firm bony support for post-procedural compression at the vascular puncture site, facilitating effective vessel closure through compression devices or manual pressure, thereby significantly reducing time to hemostasis and minimizing vascular displacement (7, 12–14). In addition, the distance between the femoral nerve and the blood vessels at this level is the largest (1–2 cm lateral), which can prevent nerve damage (15).
Long-term hyperglycemia exacerbates vascular lesions through multiple pathological mechanisms, including inducing endothelial cell dysfunction, increasing vascular permeability, and promoting low-density lipoprotein cholesterol (LDL-C) to penetrate the intima, oxidize, and form early atherosclerotic lesions (16). The hyperglycemic environment produces excessive reactive oxygen species, accelerating LDL-C oxidation and further promoting atherosclerosis progression (17). In addition, persistent hyperglycemia enhances the accumulation of advanced glycation end products (AGEs) by activating the Receptor for Advanced Glycation End Products (RAGE) receptor pathway. This condition increases collagen cross-linking and reduces elastic fiber content in the vascular wall, promoting vascular sclerosis (18–20). It simultaneously stimulates the proliferation and migration of vascular smooth muscle cells, thereby accelerating vascular calcification (20). The formation of calcified plaques significantly increases vascular fragility and the risk of vascular tearing or rupture during puncture operations (21, 22), weakens the vascular contraction and hemostasis ability, and reduces the efficiency of vascular closure devices (VCDs).
AGEs also impair the function of endothelial progenitor cells, delaying vascular repair and resulting in prolonged hemostasis and extended compression time. This, in turn, increases the risk of a hematoma and pseudoaneurysm formation at the puncture site. Additionally, hyperglycemia can directly activate platelets, stimulate thromboxane A2 release, and enhance platelet aggregation and adhesion (23–25). Studies have shown that platelet reactivity in diabetic patients is significantly higher than that in non-diabetic patients (26). The accompanying endothelial damage reduces the secretion of nitric oxide and prostacyclin and induces abnormalities in the morphology and function of red blood cells and platelets, leading to a hypercoagulable state of blood (27–29). In this context, local hemodynamic changes after femoral artery puncture can easily induce thrombosis at the puncture site, thereby causing lower limb arterial embolism and circulatory disorders. It is worth noting that the peripheral nerve and microvascular lesions associated with diabetes can cause local tissue perfusion insufficiency and nutrient deficiency, significantly delaying the wound healing process and increasing the risk of complications, such as wound dehiscence and infection.
Vascular calcification can be classified into atherosclerosis-related intimal calcification and non-occlusive medial calcification based on its anatomical location, the latter primarily contributing to increased vascular stiffness and reduced compliance. Clinical studies have confirmed that severe vascular calcification can significantly impact VCD effectiveness and is an independent risk factor limiting successful VCD application (30). Additionally, calcified plaques create irregularities in the vessel wall, increasing the risk of intimal tearing and plaque rupture, which may lead to thrombosis or lipid embolism. The associated luminal stenosis further contributes to thrombus formation by disrupting normal hemodynamics (31, 32). Studies have shown that ultrasound guidance can increase the success rate of vascular closure device (VCD) deployment and reduce the incidence of complications (33). Calcified plaques can interfere with the accurate positioning of blood vessels using ultrasound (34) and cause the course of blood vessels to be relatively fixed, increasing the difficulty of the operation. Severe calcification in specific areas, such as the aorta and dialysis access vessels, is more likely to cause ischemia or even fatal complications. These risks are further heightened in the presence of hypertension or coagulation disorders (35, 36). Therefore, calcification of the anterior femoral artery wall has been reported as an independent risk factor for adverse events, such as vascular puncture complications and vascular suture device failure (37).
Therefore, appropriate perioperative preventive measures can be implemented, including establishing and strictly adhering to a standardized operating procedure for femoral artery puncture, improving preoperative evaluations, planning surgical and puncture procedures based on patient CT and three-dimensional reconstruction images, maintaining adequate glycemic control, enhancing theoretical knowledge and technical skills training, and standardizing postoperative monitoring, so as to minimize surgical trauma and reduce the incidence of severe postoperative complications.
The present study had several limitations. As a single-center retrospective analysis investigation, it was subject to inherent biases. Additionally, the low incidence of complications and limited sample size underscore the need for further studies with larger cohorts. The absence of long-term follow-up data and the omission of certain potential risk factors may render the conclusions somewhat limited and subjective. The sample size of the constructed model was relatively small, and only internal validation methods were used for verification. Therefore, future large-sample, multicenter prospective studies are needed to further revise and optimize the model to improve its generalization ability and better meet the clinical needs.
5 Conclusions
Platelet count, vascular calcification, positional relationship between the puncture site and the femoral head, and diabetes are the independent risk factors for femoral artery access complications. The nomogram prediction model developed based on these risk factors demonstrated a strong predictive value.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: National Whole Population Health Protection Information Platform—Medical Research Registration and Record Filing Information System.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Yongchuan Hospital, Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
ZF: Writing – original draft, Writing – review & editing. JD: Writing – review & editing. ZL: Project administration, Resources, Writing – review & editing. ST: Project administration, Resources, Writing – review & editing. CX: Investigation, Validation, Writing – review & editing. DL: Investigation, Validation, Writing – review & editing. DF: Investigation, Validation, Writing – review & editing. GZ: Data curation, Formal analysis, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Chongqing Technology Innovation and Application Development Special Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sherev DA, Shaw RE, Brent BN. Angiographic predictors of femoral access site complications: implication for planned percutaneous coronary intervention. Catheter Cardiovasc Interv. (2005) 65(2):196–202. doi: 10.1002/ccd.20354
2. Huggins CE, Gillespie MJ, Tan WA, Laundon RC, Costello FM, Darrah SB, et al. A prospective randomized clinical trial of the use of fluoroscopy in obtaining femoral arterial access. J Invasive Cardiol. (2009) 21(3):105–9. PMID: 19258640
3. Panagrosso M, Cavallo E, Bracale UM, Peluso A, Silvestri O, Intrieri F, et al. Collagen-based vascular closure device multicenter Italian experience in endovascular aortic aneurysm repair compared with suture-mediated closure vascular device. J Endovasc Ther. (2024):15266028241275804. doi: 10.1177/15266028241275804
4. Chen Y, Du H, Wei BH, Chang XN, Dong CM. Development and validation of risk-stratification delirium prediction model for critically ill patients: a prospective, observational, single-center study. Medicine (Baltimore). (2017) 96(29):e7543. doi: 10.1097/MD.0000000000007543
5. Hong W, Earnest A, Sultana P, Koh Z, Shahidah N, Ong MEH. How accurate are vital signs in predicting clinical outcomes in critically ill emergency department patients. Eur J Emerg Med. (2013) 20(1):27–32. doi: 10.1097/MEJ.0b013e32834fdcf3
6. Trimarchi S, Smith DE, Share D, Jani SM, O'Donnell M, McNamara R, et al. Retroperitoneal hematoma after percutaneous coronary intervention: prevalence, risk factors, management, outcomes, and predictors of mortality: a report from the BMC2 (blue cross blue shield of Michigan cardiovascular consortium) registry. JACC Cardiovasc Interv. (2010) 3(8):845–50. doi: 10.1016/j.jcin.2010.05.013
7. Chandrasekar B, Doucet S, Bilodeau L, Crepeau J, deGuise P, Gregoire J, et al. Complications of cardiac catheterization in the current era: a single-center experience. Catheter Cardiovasc Interv. (2001) 52(3):289–95. doi: 10.1002/ccd.1067
8. Chen HZ, Liang WS, Yao WF, Liu TX. Compression methods after femoral artery puncture: a protocol for systematic review and network meta-analysis. Medicine (Baltimore). (2021) 100(4):e24506. doi: 10.1097/MD.0000000000024506
9. Noori VJ, Eldrup-Jørgensen J. A systematic review of vascular closure devices for femoral artery puncture sites. J Vasc Surg. (2018) 68(3):887–99. doi: 10.1016/j.jvs.2018.05.019
10. Ahn HY, Lee HJ, Lee HJ, Yang JH, Yi JS, Lee IW. Assessment of the optimal site of femoral artery puncture and angiographic anatomical study of the common femoral artery. J Korean Neurosurg Soc. (2014) 56(2):91–7. doi: 10.3340/jkns.2014.56.2.91
11. Li J, Cao Z, Zhang T, Zhao K, Zhao J, Yang Y, et al. Meta-analysis of ultrasound-guided and traditional femoral artery puncture. Front Cardiovasc Med. (2023) 10:1161834. doi: 10.3389/fcvm.2023.1161834
12. Belli AM, Cumberland DC, Knox AM, Procter AE, Welsh CL. The complication rate of percutaneous peripheral balloon angioplasty. Clin Radiol. (1990) 41(6):380–3. doi: 10.1016/S0009-9260(05)80595-1
13. Schnyder G, Sawhney N, Whisenant B, Tsimikas S, Turi ZG. Common femoral artery anatomy is influenced by demographics and comorbidity: implications for cardiac and peripheral invasive studies. Catheter Cardiovasc Interv. (2001) 53(3):289–95. doi: 10.1002/ccd.1169
14. Yi H, Peng G, Xiao Yang N, Bing W, Yue W, Ying W, et al. A novel femoral artery compression device (butterfly compress) versus manual compression for hemostasis after femoral artery puncture: a randomized comparison. Minim Invasive Ther Allied Technol. (2022) 31(1):50–7. doi: 10.1080/13645706.2020.1773856
15. Rao SS, Agasthi P. Femoral Vascular Closure Devices After Catheterization Procedure [M]. Treasure Island, FL: StatPearls. (2025). ineligible companies. Disclosure: Pradyumna Agasthi declares no relevant financial relationships with ineligible companies.; StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC.
16. Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. (2020) 21(5):1835. doi: 10.3390/ijms21051835
17. Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. (2013) 19(32):5695–703. doi: 10.2174/1381612811319320005
18. London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. (2003) 18(9):1731–40. doi: 10.1093/ndt/gfg414
19. Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Mönckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. (1999) 100(21):2168–76. doi: 10.1161/01.CIR.100.21.2168
20. Shu M, Cheng W, Jia X, Bai X, Zhao Y, Lu Y, et al. AGEs promote atherosclerosis by increasing LDL transcytosis across endothelial cells via RAGE/NF-κB/caveolin-1 pathway. Mol Med. (2023) 29(1):113. doi: 10.1186/s10020-023-00715-5
21. Zhang W, Sun Y, Yang Y, Chen Y. Impaired intracellular calcium homeostasis enhances protein O-GlcNAcylation and promotes vascular calcification and stiffness in diabetes. Redox Biol. (2023) 63:102720. doi: 10.1016/j.redox.2023.102720
22. Chen Y, Zhao X, Wu H. Arterial stiffness: a focus on vascular calcification and its link to bone mineralization. Arterioscler Thromb Vasc Biol. (2020) 40(5):1078–93. doi: 10.1161/ATVBAHA.120.313131
23. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. (2003) 26(12):3333–41. doi: 10.2337/diacare.26.12.3333 PMID: 14633825
24. Johansson M, Eriksson AC, Östgren CJ, Whiss PA. Platelet adhesion in type 2 diabetes: impact of plasma albumin and mean platelet volume. Thromb J. (2021) 19(1):40. doi: 10.1186/s12959-021-00291-w
25. Li X, Weber NC, Cohn DM, Hollmann MW, DeVries JH, Hermanides J, et al. Effects of hyperglycemia and diabetes Mellitus on coagulation and hemostasis. J Clin Med. (2021) 10(11):2419. doi: 10.3390/jcm10112419
26. Schneider DJ. Factors contributing to increased platelet reactivity in people with diabetes. Diabetes Care. (2009) 32(4):525–7. doi: 10.2337/dc08-1865
27. Li Z, Zhang J, Ma Z, Zhao G, He X, Yu X, et al. Endothelial YAP mediates hyperglycemia-induced platelet hyperactivity and arterial thrombosis. Arterioscler Thromb Vasc Biol. (2024) 44(1):254–70. doi: 10.1161/ATVBAHA.123.319835
28. Essawi K, Dobie G, Shaabi MF, Hakami W, Saboor M, Madkhali AM, et al. Comparative analysis of red blood cells, white blood cells, platelet count, and indices in type 2 diabetes Mellitus patients and normal controls: association and clinical implications. Diabetes Metab Syndr Obes. (2023) 16:3123–32. doi: 10.2147/DMSO.S422373
29. Vazzana N, Ranalli P, Cuccurullo C, Davì G. Diabetes mellitus and thrombosis. Thromb Res. (2012) 129(3):371–7. doi: 10.1016/j.thromres.2011.11.052
30. Alesiani F, Zenunaj G, Cosacco AM, Baldazzi G, Acciarri P, Fargion AT. Predictors of hemostasis failure with angio-seal device in a real-world setting for infrainguinal revascularization procedures. Ann Vasc Surg. (2025) 121:120–7. doi: 10.1016/j.avsg.2025.05.040
31. Pan W, Jie W, Huang H. Vascular calcification: molecular mechanisms and therapeutic interventions. MedComm (2020). (2023) 4(1):e200. doi: 10.1002/mco2.200
32. Langer S, Paulus N, Koeppel TA, Greiner A, Buhl A, Krombach GA, et al. Cardiovascular remodeling during arteriovenous fistula maturation in a rodent uremia model. J Vasc Access. (2011) 12(3):215–23. doi: 10.5301/JVA.2010.6066
33. Oddi FM, Fresilli M, Pennetta FF, Micali R, Ascoli Marchetti A, Ippoliti A. Ultrasound-guided femoseal® vascular closure device in antegrade common femoral artery puncture. J Vasc Access. (2025):11297298251342871. doi: 10.1177/11297298251342871
34. Wang Y, Osborne MT, Tung B, Li M, Li Y. Imaging cardiovascular calcification. J Am Heart Assoc. (2018) 7(13):e008564. doi: 10.1161/JAHA.118.008564
35. Kalra SS, Shanahan CM. Vascular calcification and hypertension: cause and effect. Ann Med. (2012) 44(Suppl 1):S85–92. doi: 10.3109/07853890.2012.660498
36. Elango K, Javaid A, Khetarpal BK, Ramalingam S, Kolandaivel KP, Gunasekaran K, et al. The effects of warfarin and direct oral anticoagulants on systemic vascular calcification: a review. Cells. (2021) 10(4):773. doi: 10.3390/cells10040773
37. Staudacher DL, Braxmeier K, Stachon P, Hilgendorf I, Schlett C, Zehender M, et al. Ventral calcification in the common femoral artery: a risk factor for major transcatheter aortic valve intervention access site complications. Catheter Cardiovasc Interv. (2021) 98(6):E947–53. doi: 10.1002/ccd.29885
Keywords: femoral artery, interventional access, postoperative complications, nomogram, risk factors
Citation: Feng Z, Ding J, Liu Z, Tan S, Xia C, Li D, Fu D and Zhang G (2025) Construction and validation of a prediction model for complications of femoral artery access. Front. Surg. 12:1689625. doi: 10.3389/fsurg.2025.1689625
Received: 20 August 2025; Accepted: 16 September 2025;
Published: 21 October 2025.
Edited by:
Alberto Settembrini, Saint Camillus International University of Health and Medical Sciences, ItalyReviewed by:
Eugenio Martelli, University of Rome Tor Vergata, ItalyGladiol Zenunaj, University Hospital of Ferrara, Italy
Fabio Massimo Oddi, University of Rome Tor Vergata, Italy
Copyright: © 2025 Feng, Ding, Liu, Tan, Xia, Li, Fu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guowu Zhang, emd3MTI3OEAxNjMuY29t
 Zhifei Feng
Zhifei Feng Jiali Ding1,2
Jiali Ding1,2 Sen Tan
Sen Tan