- 1Department of Cardiovascular Surgery of North Sichuan Medical College Hospital, Nanchong, Sichuan, China
- 2Nanchong Health Vocational School, Nanchong, Sichuan, China
- 3School of Nursing, North Sichuan Medical College, Nanchong, Sichuan, China
- 4Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China
Objective: To explore the risk factors for prolonged acute ventilation time after Stanford type A aortic dissection and to construct a nomogram prediction model.
Methods: A total of 178 patients with Stanford type A aortic dissection admitted to the Department of Cardiac and Vascular Surgery of the Affiliated Hospital of North Sichuan Medical College from 2020 to 2024 were retrospectively enrolled. The patients were randomly divided into a modeling group (124 cases) and a validation group (54 cases) at a 7:3 ratio. Risk factors for prolonged mechanical ventilation time after surgery were analyzed using univariate and multivariate logistic regression analysis, and a risk prediction model was constructed based on the results of multivariate logistic regression analysis.
Results: Multivariate logistic regression analysis showed that age, body mass index, preoperative oxygenation index, cardiopulmonary bypass time, and postoperative serum creatinine were risk factors for prolonged mechanical ventilation time after Stanford type A aortic dissection (p < 0.05).A risk prediction model was constructed based on these findings. The area under the ROC curve was 0.91 (95% CI: 0.86–0.97), with an accuracy of 0.88 (95% CI: 0.81–0.93), sensitivity of 0.92 (95%CI: 0.86–0.98), specificity of 0.82 (95%CI: 0.71–0.92), and an optimal cut-off value of 0.527. The results of model validation showed that the area under the ROC curve was 0.79 (95% CI: 0.66–0.92), with an accuracy of 0.72 (95%CI: 0.58–0.84), sensitivity of 0.77 (95%CI: 0.64–0.90), specificity of 0.6 (95%CI: 0.35–0.85).
Conclusion: The prediction model for prolonged mechanical ventilation time in patients with Stanford type A aortic dissection has a good prediction effect and is convenient for clinical use, providing a reference for medical workers to take preventive treatment.
1 Introduction
Aortic dissection (AD) is a pathological condition in which blood seeps into the middle layer of the blood vessel wall after the endocardium of the aorta is torn (1). Clinically, the disease is usually classified according to the initial location and extension of the lesion using Stanford classification and Debakey classification. Among them, Stanford type A aortic dissection (TAAD) is an extremely critical type with acute onset and rapid progression (2). Surgical intervention is the main treatment for TAAD patients, but due to the complexity and technical difficulty of the operation, patients often have a variety of postoperative complications, among which prolonged mechanical ventilation time is one of the most common, with an incidence rate of 28.9%–73.3% (3, 4). The prolonged duration of mechanical ventilation will not only reduce the defense capacity of the respiratory system, but also cause diaphragm injury, which may induce lung injury problems such as pulmonary infection and atelectasis, accelerate the deterioration process of the primary disease, and even increase the risk of death during hospitalization by 2.3%–17.0% (5). Based on this, this study aims to construct a prediction model of prolonged postoperative mechanical ventilation time in Stanford type A aortic dissection patients, hoping to provide reference for medical staff to identify high-risk patients early and implement preventive treatment.
2 Research subjects and methods
2.1 Research subjects
This study retrospectively selected patients with Stanford Type A aortic dissection admitted to the Cardiovascular and Vascular Surgery Department of North Sichuan Medical College Affiliated Hospital from January 2020 to December 2024. The inclusion and exclusion criteria were as follows: Inclusion criteria: (1) Clinically diagnosed as Stanford Type A aortic dissection; (2) surgery for aortic dissection under cardiopulmonary bypass; (3) Complete and reliable medical records. Exclusion criteria: (1) History of mechanical ventilation or pulmonary infection within 14 days prior to surgery; (2) History of organ transplantation, immunosuppressive therapy, or immunodeficiency; (3) Postoperative mortality; (4) Patients discharged without medical supervision; (5) Patients with severe documentation gaps or incomplete clinical data. The study was approved by the Ethics Committee (Approval No.2025ER283-1) and exempted from informed consent. The study adopted the 10EPV rule (6, 7). The study required no fewer than 10 positive events per predictive factor, calculated using the formula N = 10*k/p. After reviewing relevant literature, we determined that 3–6 independent variables would be appropriate for the predictive model. Given that postoperative prolonged mechanical ventilation occurs in 28.9% to 73.3% of Stanford A-type aortic dissection cases, a sample size of 41–208 was required (26). This study ultimately enrolled 178 patients.
2.2 Collection of clinical data
General data: Gender, age, Body Mass Index (BMI), history of hypertension, diabetes, and smoking. Preoperative data: Time from onset to surgery, white blood cell count, preoperative ischemic stroke, lactate levels, troponin T, D-dimer, serum creatinine, albumin, blood gas oxygenation index, carbon dioxide partial pressure, superimposed C-reactive protein, neutrophil count, pericardial effusion. Intraoperative data: Circulatory time, deep hypothermic circulatory arrest temperature (DHCA temperature), deep hypothermic circulatory arrest time(DHCA time), red blood cell transfusion volume, surgical duration, aortic cross-clamping time, intraoperative blood loss, fresh-frozen plasma transfusion. Postoperative data: Serum creatinine levels, postoperative lactate levels, and postoperative stroke incidence.
2.3 Grouping
The definition of prolonged mechanical ventilation time after Stanford type A operation was not clearly stated by many domestic and foreign scholars (8, 9). Based on clinical practice and review of the relevant literature (10, 11), the postoperative mechanical ventilation time of Stanford type A is defined as prolonged if it exceeds 48 h. We defined the postoperative mechanical ventilation time of Stanford type A as prolonged group if it exceeds 48 h and as non-prolonged group if it is less than 48 h.
2.4 Statistical methods
The data were processed using SPSS 27.0 statistical software. Count data were expressed as relative numbers, with inter-group comparisons using the χ2-test. Normal-distributed quantitative data were represented by (x ± s), with paired t-tests for group comparisons. Non-normal distributed quantitative data were presented as M (25,75), with Mann–Whitney U-tests for group comparisons. A multi-factor Logistic regression analysis was conducted to explore risk factors for prolonged postoperative mechanical ventilation in acute Stanford A-type aortic dissection patients, followed by model development. The diagnostic efficacy of the model was evaluated through receiver operating characteristic (ROC) curves, while decision curves (DCA curves) were used to assess clinical utility. Significant differences were defined as P < 0.05. Categorical variables were coded as follows: gender (1 = female, 2 = male), hypertension (1 = no, 2 = Yes), diabetes (1 = No, 2 = Yes), smoking (1 = No, 2 = Yes), ischemic stroke (1 = No, 2 = Yes), pericardial effusion (1 = No, 2 = Yes), postoperative stroke (1 = No, 2 = Yes).
3 Results
3.1 Comparison of baseline data
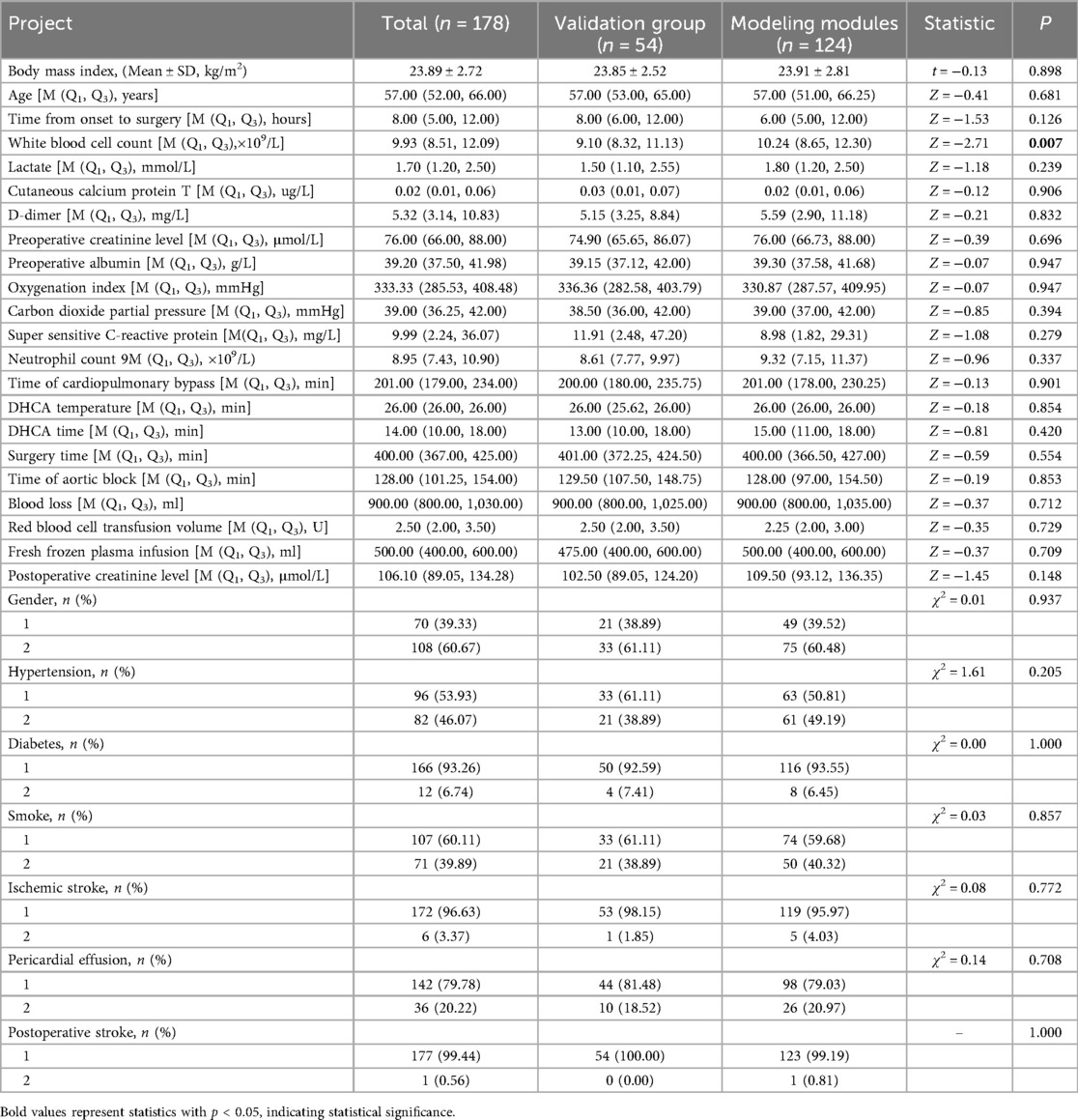
According to the inclusion and exclusion criteria, a total of 178 patients were enrolled in the study, including 108 males and 70 females. Among these patients, 64 cases (35.9%) required prolonged mechanical ventilation duration. The patients were randomly divided into a modeling group (124 cases) and a validation group (54 cases) at a 7:3 ratio. There were no statistically significant difference between the two groups. As shown in Table 1.
3.2 Single factor analysis of prolonged mechanical ventilation time in Stanford A type aortic dissection patients after surgery
Univariate analysis of the modeling module revealed statistically significant differences (p < 0.05) between the prolonged mechanical ventilation group and the non-prolonged group in age, hypertension, body mass index (BMI), white blood cell count, preoperative lactate levels, D-dimer, preoperative oxygenation index, Time of cardiopulmonary bypass, DHCA temperature, DHCA time, Surgery time, fresh frozen plasma infusion, and postoperative creatinine levels. As shown in Table 2.
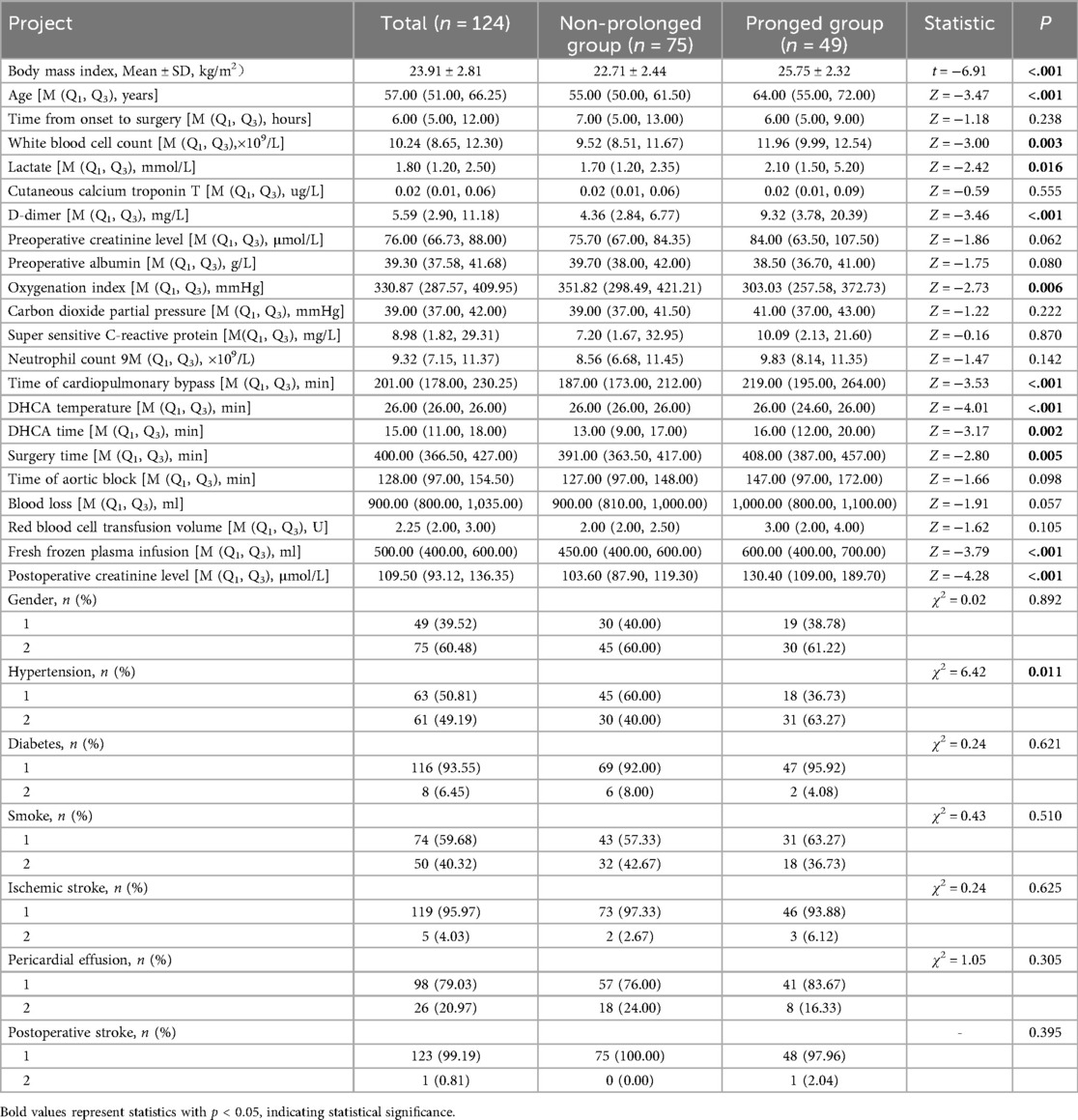
Table 2. Single factor analysis results of prolonged mechanical ventilation time after surgery in Stanford A type aortic dissection patients.
3.3 Multivariate regression analysis of prolonged mechanical ventilation in Stanford A-type aortic dissection patients after surgery
We performed a multivariate logistic regression analysis using 13 risk factors that were statistically significant in the univariate analysis as independent variables. The dependent variable was the duration of postoperative mechanical ventilation in patients with Stanford Type A aortic dissection. The model included the following independent variables: age (actual measurement), hypertension (no = 1, yes = 2), body mass index (actual measurement), white blood cell count (actual measurement), preoperative lactate levels (actual measurement), D-dimer (actual measurement), preoperative oxygenation index (actual measurement), Time of cardiopulmonary bypass (actual measurement), DHCA temperature(actual measurement), DHCA time (actual measurement), Surgery time (actual measurement), fresh frozen plasma transfusion (actual measurement), and postoperative creatinine level (actual measurement). The results showed that age, body mass index, preoperative oxygenation index, Time of cardiopulmonary bypass, and postoperative creatinine level were significant predictors of prolonged mechanical ventilation after Stanford Type A aortic dissection (p < 0.05), as detailed in Table 3.
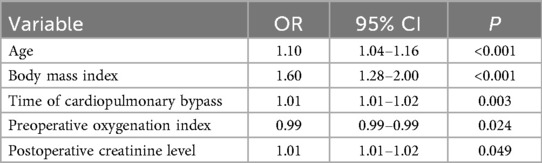
Table 3. Multivariate regression analysis of prolonged mechanical ventilation time in patients with Stanford A-type aortic dissection after surgery.
3.4 Effect analysis of risk prediction scoring model for prolonged mechanical ventilation time in Stanford A-type aortic dissection patients
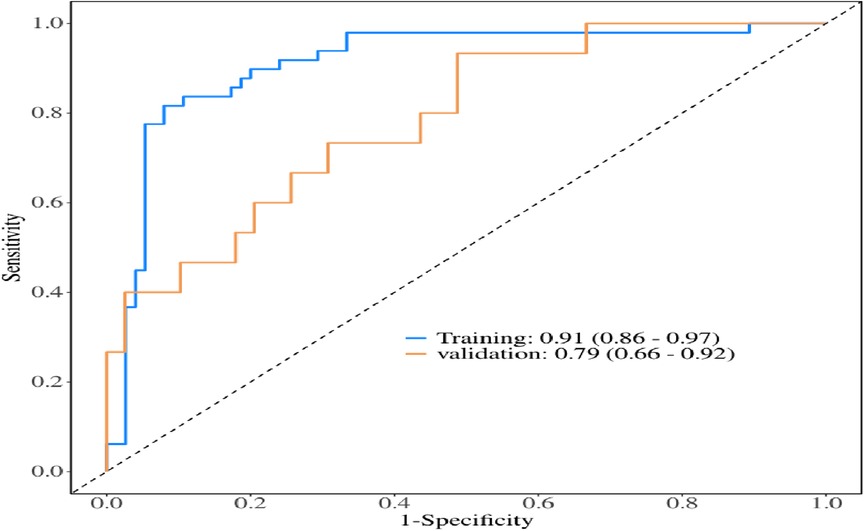
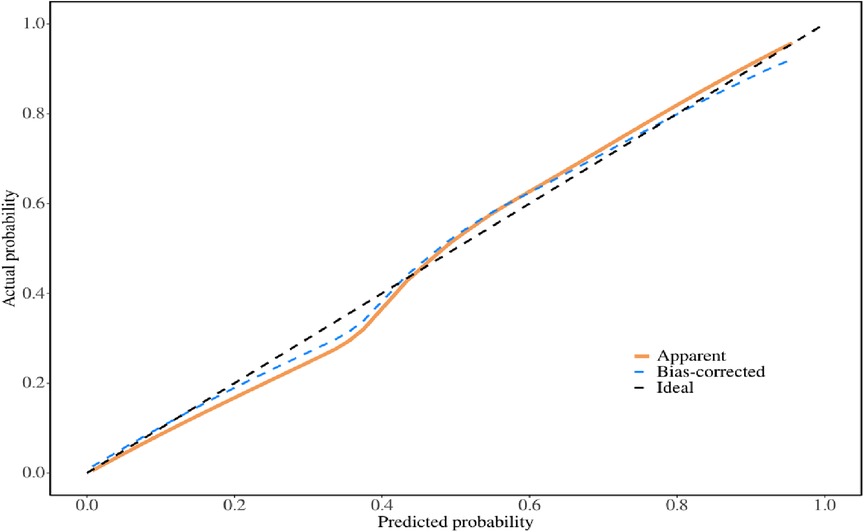
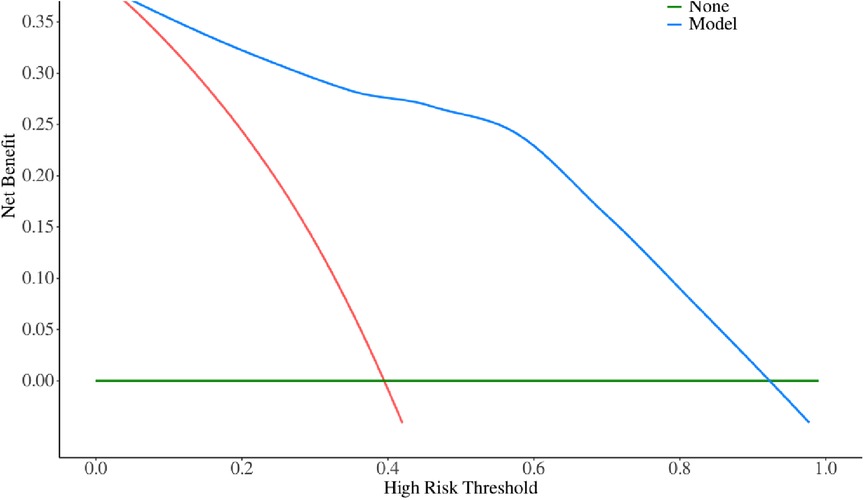
The multivariate logistic regression analysis revealed that constructing a predictive model for prolonged mechanical ventilation duration in patients with Stanford Type A aortic dissection, using patient age, body mass index (BMI), preoperative oxygenation index, cardiopulmonary bypass time, and postoperative serum creatinine levels yielded the following results (Figure 1): The model showed an area under the ROC curve (AUC) of 0.91(95% CI: 0.86–0.97 (Figure 2), with accuracy of 0.88 (95%CI: 0.81–0.93), sensitivity of 0.92 (95% CI: 0.86–0.98), and specificity of 0.82 (95% CI: 0.71–0.92). The optimal cutoff value was 0.527 (Table 4). The calibration curve demonstrated a high degree of fit (Figure 3). Model validation results indicated an AUC of 0.79 (95% CI: 0.66–0.92, accuracy of 0.72 (95% CI: 0.58–0.84), sensitivity of 0.77 (95% CI: 0.64–0.90), and specificity of 0.6 (95% CI: 0.35–0.85) (Table 4). Decision curve analysis (DCA) confirmed the models good clinical utility, as illustrated in Figure 4.
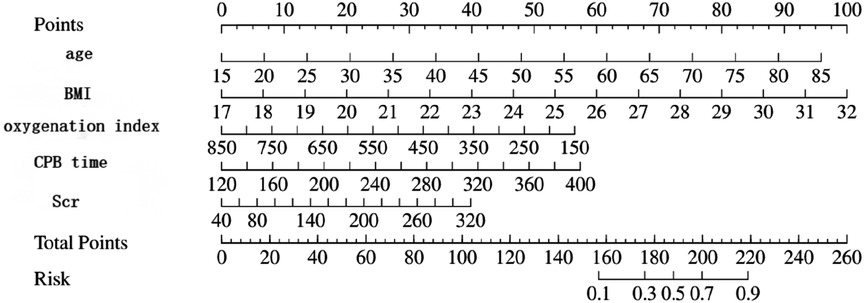
Figure 1. Shows the linear prediction of prolonged mechanical ventilation time after Stanford type A aortic dissection based on patient age, body mass index, preoperative oxygenation index, cardiopulmonary bypass time, and postoperative serum creatinine.
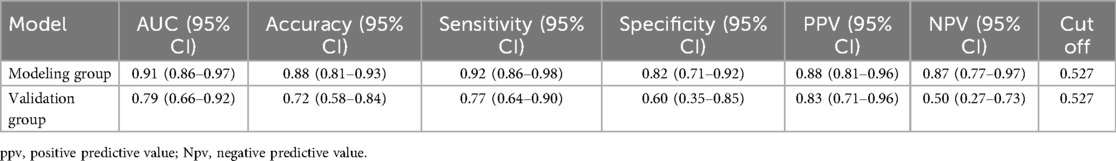
Table 4. Accuracy, sensitivity, specificity and best cutoff values of the modeling group and the control group.
4 Discussion
Prolonged postoperative mechanical ventilation duration in patients with Stanford A-type aortic dissection significantly extends hospital stays, exacerbates medical resource consumption, and poses potential threats to both quality of life and patient safety. By developing a predictive model for prolonged postoperative mechanical ventilation, healthcare providers can identify high-risk individuals early and implement targeted management strategies. This approach not only shortens mechanical ventilation duration but also improves surgical outcomes and enhances patients quality of life.
The study found that patient age was associated with the duration of mechanical ventilation after Stanford Type A aortic dissection, which was consistent with the results of Yu Yangtaos team (12). As age increase, cardiopulmonary function gradually declines, and the tolerance to surgical trauma such as cardiopulmonary bypass, decreases. This leads to an increased incidence of respiratory complications and ultimately prolongs mechanical ventilation (13). Elderly patients often present with atypical clinical symptoms, resulting in a high rate of clinical misdiagnosis and diagnostic delay. This not only increases the risk of surgery and patient mortality but also further prolongs the duration of mechanical ventilation and ICU stay (14). Therefore, for this special population of elderly patients, it is crucial to establish a rapid and accurate diagnostic evaluation system and optimize regional medical transfer mechanisms to ensure timely surgical intervention. In postoperative management, maintaining circulatory stability and actively managing chronic underlying pulmonary diseases are equally important. These measures play a significant role in shortening mechanical ventilation duration and improving clinical outcomes. In conclusion, implementing a multidisciplinary collaborative systematic treatment protocol for elderly patients with aortic dissection is a key approach to enhance therapeutic efficacy and reduce complication risks.
This study demonstrates that body mass index (BMI) is a significant risk factor for prolonged postoperative mechanical ventilation duration. In China, obese individuals predominantly exhibit central obesity, characterized by abnormal fat accumulation in the neck, thoracic, and abdominal regions. This adipose tissue deposition restricts respiratory muscle mobility, increases the workload on respiratory muscles, and raises airway resistance. Concurrently, elevated circulating blood volume induces pulmonary congestion, while the increased functional residual capacity under general anesthesia exacerbates alveolar collapse. These combined pathophysiological mechanisms collectively establish the pathological basis for extended mechanical ventilation duration (15, 16). In addition, obese individuals often have long-term chronic inflammation and oxidative stress responses, which activate a variety of inflammatory mediators and signal transduction pathways. These factors cause persistent damage to the lung parenchyma, delay the recovery of respiratory function, and ultimately prolong the time required for ventilation support (17, 18). Therefore, it is of great clinical value to develop individualized perioperative management plans for obese patients. By strengthening respiratory management measures, postoperative mechanical ventilation time can be effectively shortened.
A decreased preoperative oxygenation index is a significant risk factor for prolonged postoperative mechanical ventilation in patients with Stanford Type A aortic dissection. Pathophysiological analysis indicates that reduced preoperative oxygenation typically suggests potential pulmonary dysfunction, inadequate tissue perfusion, and inflammatory responses. Particularly when the dissection affects pulmonary blood supply, this may lead to impaired pulmonary perfusion and substantially diminished gas exchange capacity (19). Other studies have pointed out that (20). Patients with preoperative oxygenation indices below 200 mmHg require 3–5 days longer mechanical ventilation postoperatively compared to those with normal oxygenation levels. Therefore, clinical healthcare providers should proactively implement interventions to improve patients oxygenation status before surgery and strengthen respiratory management after the operation. These measures help reduce complication risks, ultimately shortening mechanical ventilation duration and improving patient outcomes.
This study revealed that Stanford Type A aortic dissection patients with elevated postoperative serum creatinine levels are more likely to require prolonged mechanical ventilation. According to clinical guidelines issued by the Quality of Care Initiative Working Group, once severe acute kidney injury occurs in critically ill patients, the probability of respiratory dysfunction and secondary pulmonary lesions significantly increases (21). From a pathophysiological perspective, this phenomenon is primarily driven by two factors: inflammatory and non-inflammatory components. On one hand, cardiopulmonary bypass surgery may trigger systemic inflammatory responses, leading to altered microvascular permeability and alveolar edema. The subsequent sodium and water retention caused by renal dysfunction further exacerbates this pathological process. On the other hand, patients face dual challenges: a reduced capacity to clear inflammatory factors while experiencing increased production of these substances. Additionally, renal failure weakens immune defense mechanisms and elevates susceptibility to infections (22, 23). These factors combined make patients more prone to lung parenchymal injury, resulting in prolonged use of ventilators.
The findings of this study indicate that prolonged cardiopulmonary bypass (CPB) duration is a significant risk factor for extended postoperative mechanical ventilation in patients with Stanford A-type aortic dissection. CPB procedures performed under non-physiological circulatory conditions notably impair peripheral blood supply and alter capillary permeability, leading to tissue hypoxia and inadequate perfusion. These pathophysiological changes ultimately contribute to pulmonary complications and prolong the required duration of postoperative respiratory support (24). From a pathophysiological perspective, prolonged extracorporeal circulation triggers inflammatory cascades, including complement activation, thrombin generation, and cytokine release. This process also promotes the production of mediators such as endothelin and endotoxins, which impair the function of neutrophils and macrophages, thereby weakening the body's immune defenses. These pathological changes significantly increase the risk of multiple organ dysfunction syndrome (MODS), with the most pronounced damage occurring in vital organs like the lungs, kidneys, and liver (25). In clinical practice, the duration of cardiopulmonary bypass can be effectively controlled by optimizing the surgical plan and improving the technical level of operation, so as to reduce the risk of related complications.
5 Summary
This study demonstrates that age, body mass index(BMI), preoperative oxygenation index, cardiopulmonary bypass duration, and postoperative serum creatinine levels are significant predictors of prolonged mechanical ventilation in patients with Stanford Type A aortic dissection. The developed linear model exhibits high predictive accuracy, providing clinical guidance for early identification of high-risk patients requiring extended postoperative ventilation. However, these findings are not consistent with the results of some previous studies. For instance, studies have shown that postoperative blood lactate levels, DHCA time, and elevated preoperative white blood cell counts are associated with prolonged postoperative mechanical ventilation in patients with Stanford Type A aortic dissection, We speculate that it may be related to differences in surgical methods. Our center uses customized stents, which differ from those used in other centers. During the surgery, once the temperature reaches the target, deep hypothermic circulatory arrest with bilateral antegrade cerebral perfusion is performed. The diseased section of the descending aorta is then excised, and the customized intraoperative “elephant trunk” stent is inserted into the descending aorta, immediately restoring circulation. The use of this stent greatly shortens the DHCA time, even reducing it to within 2 min. During the operation, the patient's nasopharyngeal temperature is maintained at 24–28°C. Therefore, in the future, multicenter, large-sample studies are needed to further clarify the related risk factors. Additionally, this model is more suitable for early postoperative risk prediction.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Concerning the patient's right to privacy. Requests to access these datasets should be directed toMTEzODcwMzk3MUBxcS5jb20=.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Affiliated Hospital of North Sichuan Medical College (Approval No. 2025ER283-1). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because The study is a retrospective study of case data and is exempt from informed consent.
Author contributions
YJ: Writing – review & editing, Writing – original draft. LH: Investigation, Conceptualization, Writing – original draft. CT: Methodology, Writing – review & editing, Data curation. WY: Writing – review & editing, Resources, Funding acquisition, Visualization. YW: Methodology, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Nanchong Science and Technology Bureau project (23JCYJPT0045).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liping Z, Yuwei W, Leifang M. Risk factors and risk model construction for ventilator-associated pneumonia after type A aortic dissection. Chin J Hosp Infect. (2022) 32(17):2633–7.
2. Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, et al. Insights from the international registry of acute aortic dissection: a 20-year experience of collaborative clinical research. Circulation. (2018) 137(17):1846–60. doi: 10.1161/CIRCULATIONAHA.117.031264
3. Wei S, Nan L, Xiaolei Y, Lizhong S, Shijie J. Analysis of early complications after surgery for type A aortic dissection. J Cardiovasc Dis. (2011) 30(03):183–6.
4. Cislaghi F, Condemi AM, Corona A. Predictors of prolonged mechanical ventilation in a cohort of 5123 cardiac surgical patients. Eur J Anaesthesiol. (2009) 26(5):396–403. doi: 10.1097/EJA.0b013e3283232c69
5. Wang D, Abuduaini X, Huang X, Wang H, Chen X, Le S, et al. Development and validation of a risk prediction model for postoperative pneumonia in adult patients undergoing Stanford type A acute aortic dissectionsurgery: a case control study. J Cardiothorac Surg. (2022) 17(1):22. doi: 10.1186/s13019-022-01769-y
6. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. (1996) 15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4%3C361::AID-SIM168%3E3.0.CO;2-4
7. Wynants L, Bouwmeester W, Moons KGM, Moerbeek M, Timmerman D, Van Huffel S, et al. A simulation study of sample size demonstrated the importance of the number of events per variable to develop prediction models in clustered data. J Clin Epidemiol. (2015) 68(12):1406–14. doi: 10.1016/j.jclinepi.2015.02.002
8. Li C-N, Chen L, Ge Y-P, Zhu J-M, Liu Y-M, Zheng J, et al. Risk factors for prolonged mechanical ventilation after total aortic arch replacement for acute DeBakey type I aortic dissection. Heart Lung Circ. (2014) 23(9):869–74. doi: 10.1016/j.hlc.2014.03.022
9. Ge M, Wang Z, Chen T, Cheng Y, Ye J, Lu L, et al. Risk factors for and outcomes of prolonged mechanical ventilation in patients received DeBakey type I aortic dissection repairment. J Thorac Dis. (2021) 13(2):735–42. doi: 10.21037/jtd-20-2736
10. Xie L-F, Han X, Xie Y-L, He J, Wu Q-S, Qiu Z-H, et al. A predictive model for prolonged mechanical ventilation after triple-branched stent graft for acute type A aortic dissection. J Surg Res. (2024) 296:66–77. doi: 10.1016/j.jss.2023.12.007
11. Yuanxi L, Li Z, Jiang X, Jiang Y, Wang D, Xue Y. A novel nomogram for predicting prolonged mechanical ventilation after acute type A aortic dissection surgery: a retrospective study investigating the impact of ventilation duration on postoperative outcomes. Ann Med. (2024) 56(1):2392871. doi: 10.1080/07853890.2024.2392871
12. Yangtao Y, Faming H, Ya G, Bolun S, Yanfeng L, Yangyang Y, et al. Analysis of factors influencing extended postoperative mechanical ventilation in acute Stanford A-type aortic dissection patients. J Prac Cardiovasc Dis. (2024) 32(09):11–5.
13. Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Mehta RH, et al. Contemporary results of surgery in acute type A aortic dissection: the international registry of acute aortic dissection experience. J Thorac Cardiovasc Surg. (2005) 129(1):112–22. doi: 10.1016/j.jtcvs.2004.09.005
14. Januzzi JL, Isselbacher EM, Fattori R, Cooper JV, Smith DE, Fang J, et al. Characterizing the young patient with aortic dissection: results from the international registry of aortic dissection (IRAD). J Am Coll Cardiol. (2004) 43(4):665–9. doi: 10.1016/j.jacc.2003.08.054
15. Shumin W, Mingwei W, Bolun S, Xiangbo C, Yanfeng L, Feng Z, et al. The impact of body mass Index on postoperative delayed tube removal in acute Stanford A-type aortic dissection. Chin J Clin Thorac Cardiovasc Surg. (2024) 40(9):559–64.
16. Feifei L, Yanwei Y, Mu J, Weiping C. A retrospective analysis of factors related to delayed extubation after surgery for 375 cases of acute Stanford A-type aortic dissection. J Cardiovasc Dis. (2021) 40(10):1053–7.
17. Ziwei W, Jianping S, Yaling H, Shuang D, Yanhua H. Systematic evaluation and meta-analysis of factors influencing postoperative delayed tube removal in Stanford type A aortic dissection. Chin J Emerg Med. (2024) 33(11):1586–90.
18. Wei S, Tian L, Yifan C, Zhaozhuo N, Long S. Analysis of risk factors for acute kidney injury after surgery for acute stanford type A aortic dissection. Chin J Clin Thorac Cardiovasc Surg. (2019) 35(2):72–5.
19. Yuanling Y, Weifang Z. Clinical analysis of ventilator-related pneumonia in elderly patients after direct ventriloquism. China J Gerontol. (2011) 31(08):1294–5.
20. Yaobang B, Zhenhua W, Yujuan Q, Yunpeng B, Qingliang C. Analysis of risk factors for severe hypoxemia after surgical treatment of aortic dissection in suns patients. China Med Front (Electron Ed). (2025) 17(04):99–103.
21. Joannidis M, Forni LG, Klein SJ, Honore PM, Kashani K, Ostermann M, et al. Lung kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 workgroup. Intensive Care Med. (2020) 46(4):654–72. doi: 10.1007/s00134-019-05869-7
22. Andres-Hernando A, Dursun B, Altmann C, Ahuja N, He Z, Bhargava R, et al. Cytokine production increases and cytokine clearance decreases in mice with bilateral nephrectomy. Nephrol Dial Transpl. (2012) 27(12):4339–47. doi: 10.1093/ndt/gfs256
23. Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol. (2015) 11(2):88–101. doi: 10.1038/nrneph.2014.180
24. Liu N, Zhang W, Ma W, Shang W, Zheng J, Sun L. Risk factors for hypoxemia following surgical repair of acute type A aortic dissection. Interact Cardiovasc Thorac Surg. (2017) 24(2):251–6. doi: 10.1093/icvts/ivw272
25. Zhou W, Wang G, Liu Y, Tao Y, Du Z, Tang Y, et al. Outcomes and risk factors of postoperative hepatic dysfunction in patients undergoing acute type A aortic dissection surgery. J Thorac Dis. (2019) 11(8):3225–33. doi: 10.21037/jtd.2019.08.72
Keywords: aortic dissection, mechanical ventilation, predictive models, risk factors, heart surgery
Citation: Jiajie Y, Hongmei L, Ting C, Yanlin W and Wang Y (2025) Analysis of risk factors and linear prediction model construction for prolonged mechanical ventilation after Stanford A-type aortic dissection. Front. Surg. 12:1697977. doi: 10.3389/fsurg.2025.1697977
Received: 4 September 2025; Accepted: 20 October 2025;
Published: 6 November 2025.
Edited by:
Massimo Bonacchi, University of Florence, ItalyReviewed by:
Mohamed Rahouma, Weill Cornell Medical Center, NewYork-Presbyterian, United StatesBehnam Askari, Urmia University of Medical Sciences, Iran
Copyright: © 2025 Jiajie, Hongmei, Ting, Yanlin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yali Wang, MzkyNDYxNDA0OEBxcS5jb20=
 Yu Jiajie
Yu Jiajie Lian Hongmei1
Lian Hongmei1 Wei Yanlin
Wei Yanlin