- 1Department of Rehabilitation, Kitasato University Hospital, Sagamihara, Japan
- 2School of Allied Health Sciences, Kitasato University, Sagamihara, Kanagawa, Japan
- 3Department of Rehabilitation, Makita General Hospital, Ota-ku, Tokyo, Japan
- 4Department of Rehabilitation, Showa University Fujigaoka Hospital, Yokohama, Kanagawa, Japan
- 5Department of Rehabilitation, Yokohama Minami Kyosai Hospital, Yokohama, Kanagawa, Japan
- 6Graduate School of Health Sciences, Showa University, Kanagawa, Japan
Background and purpose: Mixed reality (MR) rehabilitation therapy tries to overcome the limitations of virtual reality therapy by integration of real, vivid images into the existing world. The aims of this study were to investigate the effect of MR therapy on finger movement function in hemiplegic stroke patients and to identify issues related to the practical use of MR system.
Methods: The study included five hemiplegic stroke patients with Brunnström stage V or lower in the upper limb or fingers. In this MR-based intervention, using HoloLens® and Leap Motion®, the unaffected hand was projected as a mirrored virtual hand onto the affected side. Patients were instructed to clench their fist on the paralyzed side ten times. The distance traveled by each fingertip between the clenched and open positions was measured using a 3D motion capture system. Additionally, a questionnaire regarding the MR therapy was administered to the patients.
Results: Wearing the MR system increased the distance traveled by the tip of the index finger by a mean of 1.75 cm between the first and tenth fist-clenching movements (p < 0.05). There were no significant differences in the fingertip movement distances of any of the other fingers. The subjects gave positive reactions to most of the questions in the questionnaire.
Conclusion: MR therapy may help improve finger movement function in patients with hemiplegic stroke.
1 Introduction
Stroke remains a leading cause of long-term disability worldwide, with over 13 million new cases reported each year. Notably, 55%–75% of stroke survivors experience persistent upper limb motor impairment, underscoring the importance of hand function recovery as a central goal of post-stroke rehabilitation. In response, various effective therapeutic approaches have been proposed and implemented. (Yavuzer et al., 2008; Katan and Luft, 2018). In particular, therapies utilizing visual illusions can cause kinesthetic illusions and induce excitation of the cerebral motor cortex. The effect on brain activity of visual illusions in mirror therapy has been widely investigated, and improvements in Functional Independence Measure (FIM) scores and Brunnström Stage (BRS) have been reported (Garry et al., 2005). The use of mirrors makes it easy to imagine movements of the paralyzed arm, heightening the excitation of the cerebral cortex. In a previous study, it was reported that the details of how movements are observed, and the direction of movement affect the ease with which these movements can be imagined (Celnik et al., 2008). Another study utilizing virtual reality (VR) found that movements of the paralyzed arm on VR both dispelled patients’ feelings of dissonance with their self-image and increased motor-evoked potentials (Aoyama and Kaneko, 2011). Furthermore, previous study supports that upper extremity training using VR technology has the potential to contribute to the improvement of upper extremity function.
Although the concept of mixed reality (MR) has existed since the 1990s, the years since 2016 have seen the advent of MR as a cutting-edge technology that overcomes the limitations of VR by integrating real, vivid images into the existing world (Tamura H, 1998; Silva A, 2010). Despite the reported efficacy of upper extremity training using VR, studies demonstrating the effectiveness of upper extremity training using MR are still limited. However, MR-based upper extremity training may allow movements of the paralyzed side without dissonance, potentially enhancing its therapeutic effect.
While VR has been widely studied in neurorehabilitation, MR offers a more immersive environment by integrating digital feedback into the real world. Recent studies have suggested that MR can reduce self-image dissonance and more effectively facilitate the integration of visual and proprioceptive inputs (Huang Y and Lee, 2022). Furthermore, interventions using MR technology have shown potential in enhancing sensorimotor integration and supporting the recovery of fine motor function during rehabilitation.
In this study, we investigated the effect of the use of MR therapy on finger movements by hemiplegic stroke patients. We also identified issues facing the practical use of MR.
2 Materials and methods
2.1 Study design
This study was designed as a proof-of-concept exploratory trial.
2.2 Subjects
The study subjects were hemiplegic stroke patients admitted to Showa University Fujigaoka Rehabilitation Hospital between October 2019 and 31 March 2021 whose arms or fingers were Brunnström stage V or below. The following patients were excluded: those with a diagnosis of dementia, those who were unable to perform the required movements with their arm on the unaffected side by themselves after these had been explained (for any reason, including decreased mobility of the unaffected arm, diminished balance function when seated, or cognitive functional decline), previous fracture of the paralyzed arm, or peripheral neuropathy. Five patients met these criteria. This study was an exploratory investigation aimed at evaluating the potential effects of mixed MR therapy. The study was approved by the Institutional Review Board of Kitasato University School of Medicine (Approval number: C19-183). Written informed consent was obtained from all participants, including consent for participation and for the use of video recordings in presentations and publications. A patent application for the MR device used has been submitted (Applicant: The Kitasato Institute; Inventors: Shuichi Sasaki, Naonobu Takahira, and four others; Application Number: 2019–118957).
3 Methods
3.1 System
The MR intervention system consisted of HoloLens® (Microsoft Corporation) for visual output and Leap Motion® (Ultraleap Inc.) for three-dimensional motion tracking. Participants observed a virtual mirrored image of their unaffected hand superimposed on the paretic hand, designed to enhance motor imagery. The images were analyzed by the 3D motion capture platform for arm movement analysis and played back through the HMD (Figures 1, 2).

Figure 2. Image projected on the HMD(Image of the subject seen). (A) Virtual hand (B) Normal upper limb.
3.2 Parameters evaluated
3.2.1 Patient data
Information on sex, age, disease, Brunnström stage, and affected side was obtained from Fujigaoka Rehabilitation Hospital medical records.
3.2.2 Fingertip movement distances
Each participant, while seated and wearing the MR system, was instructed to perform ten repetitions of fist-clenching movements with the paretic hand. Fingertip travel distances were measured using Leap Motion® and analyzed as Euclidean displacement from full flexion to full extension.
Fingertip travel distance was selected as the primary outcome measure because improvements in finger motor function have been reported to be particularly challenging within the spectrum of upper limb recovery. Specifically, fine motor tasks such as finger flexion and extension are considered key indicators of recovery progression and are central targets in post-stroke rehabilitation interventions (Hijikata et al., 2020).
3.2.3 Subjective evaluation using a custom questionnaire
Following the intervention, participants completed a custom five-item questionnaire assessing usability, comfort, sense of immersion, and motivation, using a five-point Likert scale. The small sample size reflects the exploratory nature of this study and is consistent with the scope of pilot research in MR-based rehabilitation (Leon et al., 2011).
4 Study protocol
The nature of the study was explained to the subjects and their consent was obtained. After this, patient data were obtained before the subjects were fitted with the MR system and asked to clench their fists 10 times. Finally, the questionnaire was administered.
5 Statistical analysis
The distances traveled by each fingertip during the first and tenth fist-clenching movements while the patient was fitted with the MR system were compared using a t-test, with p < 0.05 regarded as statistically significant. Statistical analyses were performed using a paired t-test to compare the fingertip travel distances between the first and tenth fist-clenching movements. The test statistic (t-value) and p-value were calculated to assess the significance of the observed changes. A p-value of less than 0.05 was considered statistically significant. All analyses were conducted using software IBM SPSS Statistics Ver16.
6 Results
6.1 Subject profiles
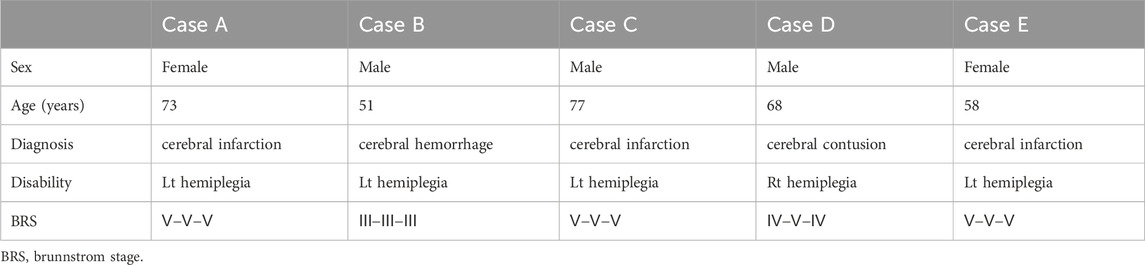
The subject profiles are shown in Table 1. Subjects A, B, C, and E were paralyzed on the left, and subject D on the right.
6.2 Fingertip movement distances
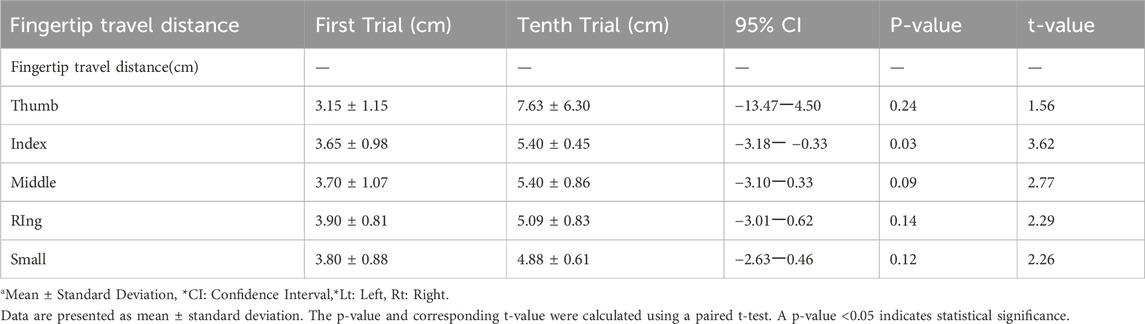
Statistical analysis was performed using paired t-tests to compare finger-tip travel distances between the first and tenth fist-clenching repetitions (Table 2). A significance level of p < 0.05 was applied, consistent with thresholds used in clinical exploratory studies (Leon et al., 2011). Among all fingers, only the index finger showed statistically significant improvement (mean increase: 1.75 cm, p = 0.03). The remaining fingers demonstrated non-significant trends toward improvement.
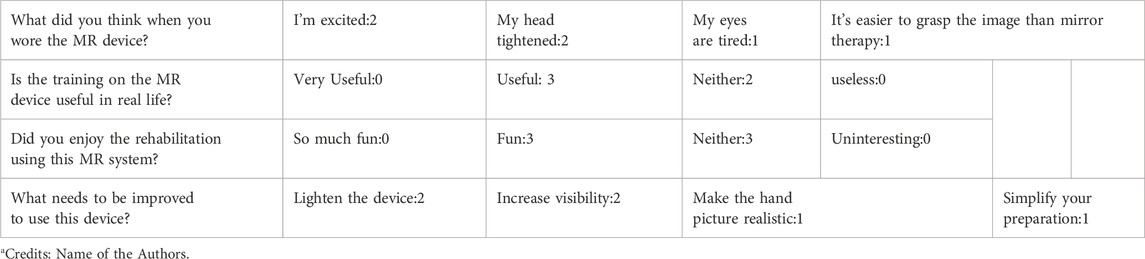
6.3 Subjective evaluation using a custom questionnaire
Questionnaire results indicated that most participants rated the MR experience as realistic and motivating, although two reported minor discomfort with the head-mounted display. A summary of the questionnaire responses is provided in Table 3.
7 Discussion
The findings of this preliminary study suggest that MR-based therapy using a HoloLens and Leap Motion system may enhance fine motor control in hemiplegic stroke patientsWe found that MR therapy significantly increased the distance traveled by the tip of the index finger between the first and tenth fist-clenching movements. In a previous study, movement image training using a mirror box was shown to increase wrist extension and joint range of motion in forearm pronation and supination (Stevens and Stoykov, 2003). Visual stimulation by means of MR therapy may make movement imaging easier, activating the cerebral motor cortex. MR therapy may thus have the potential to improve the finger movements of hemiplegic stroke patients.
7.1 Visual stimulation and sensorimotor integration
MR therapy promotes sensorimotor integration through visual stimulation. Garry et al. (2005) reported that mirror therapy enhances the excitability of the primary motor cortex (M1) via visual feedback (Garry et al., 2005; Stevens and Stoykov, 2003). Compared with VR, MR technology provides a more natural and realistic visual experience, which may facilitate motor imagery. This could explain why MR therapy was especially effective for the index finger, which has a large sensory cortical representation.
In this study, the only significant improvement was in the distance traveled by the tip of the index finger. In addition to the primary motor cortex, activation of the primary sensory cortex is also believed to contribute to fist-clenching movements (Tsuda and Oku, 2005), suggesting that there is a complex interaction between the motor and sensory cortexes during movement. Role of Sensory Cortical Representation.
The significant improvement observed in the index finger movement could be attributed to its larger sensory cortical representation, as previously illustrated in Penfield’s sensory homunculus (Schott, 1993; Penfield and Bolrey, 1937). This may contribute to the observed improvement, as MR therapy likely stimulates both motor and sensory cortices, facilitating better motor performance of the index finger.
7.2 Comparison with previous VR studies
MR offers advantages over VR by providing realistic, embodied feedback that integrates the user’s own body image, potentially reducing the dissonance observed in VR environments (Aoyama and Kaneko, 2011). Additionally, Garry et al. (2005) showed that mirror therapy enhances M1 excitability through visual stimulation, a mechanism likely engaged in MR as well (Garry et al., 2005). Our results align with recent studies showing that MR can promote neural plasticity and motor recovery by offering more ecologically valid and interactive environments (Huang Y and Lee, 2022). The questionnaire results showed that reactions to MR therapy were mainly positive. However, the accuracy of finger-bending, the difficulty of seeing the simulated arm, and making the HMD less tight around the head were brought up as points for improvement. In this study, we used the first-generation HoloLens, but the use of the HoloLens2, which offers improved operability, wearability, and viewing angles compared with the first-generation HoloLens, may improve its effectiveness.
7.3 Limitations
This study has several limitations. First, the sample size was limited to five participants, as the inclusion criteria required individuals with specific clinical conditions (e.g., hemiplegic patients with Brunnström Stage V or below), and the experimental environment imposed logistical constraints. While this small sample was considered appropriate for an initial proof-of-concept exploratory trial, it limits the statistical power and generalizability of the findings. Second, the absence of a control group precluded direct comparison between MR therapy and standard rehabilitation or other interventions such as mirror therapy. Third, the study utilized a first-generation MR device (HoloLens®), which had limitations in field of view, operability, and comfort.
In contrast to the extensive research on mirror therapy and VR, studies on MR-based interventions remain scarce. However, this study contributes to the emerging body of evidence suggesting that MR therapy may be effective in enhancing motor function, particularly fine motor control. For example, prior work by Stevens and Stoykov (2003) demonstrated that motor imagery training improves motor function, and our findings suggest that MR may amplify these effects through immersive and congruent visual feedback (Stevens and Stoykov, 2003).
To strengthen the evidence base and further elucidate the therapeutic mechanisms of MR therapy, future studies should include randomized controlled trials with larger sample sizes and employ advanced MR hardware such as HoloLens 2, which offers improved wearability, visual clarity, and user experience.
8 Conclusion
The findings of this preliminary study suggest that MR-based therapy using a HoloLens and Leap Motion system may enhance fine motor control in hemiplegic stroke patients. The significant improvement observed in the index finger movement could be attributed to its larger sensory cortical representation, as previously illustrated in Penfield’s sensory homunculus (Penfield and Boldrey, 1937). MR offers advantages over VR by providing realistic, embodied feedback that integrates the user’s own body image, potentially reducing the dissonance observed in VR environments (Aoyama and Kaneko, 2011). Additionally, Garry et al. (2005) showed that mirror therapy enhances M1 excitability through visual stimulation, a mechanism likely engaged in MR as well (Garry et al., 2005). Our results align with recent studies showing that MR can promote neural plasticity and motor recovery by offering more ecologically valid and interactive environments (Huang Y and Lee, 2022). Despite promising results, this study has limitations, including a small sample size, lack of a control group, and use of first-generation MR devices with limited field of view. Future studies should involve randomized controlled trials with larger samples and utilize advanced MR hardware such as HoloLens 2.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Kitasato University School of Medicine and Hospital Ethics Committee (Number: C19-183Number: C19-183). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SS: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing, Data curation, Funding acquisition, Project administration, Resources, Software. NT: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – review and editing, Writing – original draft. CY: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review and editing, Writing – original draft. TW: Formal Analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – review and editing. RN: Formal Analysis, Investigation, Methodology, Software, Writing – review and editing. KA: Conceptualization, Methodology, Project administration, Software, Validation, Writing – review and editing. MM: Conceptualization, Methodology, Project administration, Visualization, Writing – review and editing. SC: Conceptualization, Data curation, Methodology, Validation, Visualization, Writing – original draft, Writing – review and editing. SK: Conceptualization, Data curation, Investigation, Validation, Visualization, Writing – review and editing. AK: Conceptualization, Investigation, Project administration, Software, Visualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aoyama, T., and Kaneko, F. (2011). The effect of motor imagery on gain modulation of the spinal reflex. Brain Res. 1372, 41–48. doi:10.1016/j.brainres.2010.11.023
Celnik, P., Webster, B., Glasser, D. M., and Cohen, L. G. (2008). Effects of action observation on physical training after stroke. Stroke 39 (6), 1814–1820. doi:10.1161/strokeaha.107.508184
Garry, M. I., Loftus, A., and Summers, J. J. (2005). Mirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp. Brain Res. 163 (1), 118–122. doi:10.1007/s00221-005-2226-9
Hijikata, N., Kawakami, M., Ishii, R., Tsuzuki, K., Nakamura, T., Okuyama, K., et al. (2020). Item difficulty of fugl-meyer assessment for upper extremity in persons with chronic stroke with moderate-to-severe upper limb impairment. Front. Neurol. 11, 577855. doi:10.3389/fneur.2020.577855
Huang Y, S. J., and Lee, A. (2022). Enhancing proprioceptive feedback with mixed reality: a pilot study. J. NeuroEngineering Rehabilitation 19 (1), 45. doi:10.1186/s12984-022-00987-3
Katan, M., and Luft, A. (2018). Global burden of stroke. Semin. Neurol. 38 (2), 208–211. doi:10.1055/s-0038-1649503
Leon, A. C., Davis, L. L., and Kraemer, H. C. (2011). The role and interpretation of pilot studies in clinical research. J. Psychiatr. Res. 45 (5), 626–629. doi:10.1016/j.jpsychires.2010.10.008
Microsoft HoloLens (2025). Microsoft HomePage HoloLens2 Available online at: https://www.microsoft.com/en-us/hololens/hardware. (Accessed October 30.2025)
Penfield, W., and Boldrey, E. (1937). Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation1. Brain 60 (4), 389–443. doi:10.1093/brain/60.4.389
Schott, G. D. (1993). Penfield's homunculus: a note on cerebral cartography. J. Neurol. Neurosurg. Psychiatry 56 (4), 329–333. doi:10.1136/jnnp.56.4.329
Silva A, S. D. (2010). Digital cityscapes: merging digital and urban playspaces, XII. New York: Peter Lang Publishing, 372.
Stevens, J. A., and Stoykov, M. E. (2003). Using Motor Imagery in the Rehabilitation of Hemiparesis 11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated. Arch. Phys. Med. Rehabil. 84 (7), 1090–1092. doi:10.1016/s0003-9993(03)00042-x
Tamura H, O. Y. (1998). Media information processing for robotics. Mixed reality: merging real and virtual worlds. J. Robotics Soc. Jpn. 16 (6), 759–762. doi:10.7210/jrsj.16.759
Tsuda, H. A. T., and Oku, N. (2005). Elucidation of functional brain areas involved in lifting a small object using a precision grip with visual guidance: a PET study (in Japanese). J. Jpn. Assoc. Occup. Ther. 24, 40–49.
Keywords: stroke, mixed reality (MR), upper limb, rehabilitation-, occupational thearpy
Citation: Sasaki S, Takahira N, Yoda C, watabe T, Nakanishi R, Aoki K, Miyazaki M, Chiba S, Kurosaki S and Kobayashi A (2025) Effect of mixed-reality therapy on finger movement function of hemiplegic stroke patients. Front. Virtual Real. 6:1559755. doi: 10.3389/frvir.2025.1559755
Received: 13 January 2025; Accepted: 09 May 2025;
Published: 21 May 2025.
Edited by:
Caitlin R Rawlins, United States Department of Veterans Affairs, United StatesReviewed by:
Michael Joseph Dino, Our Lady of Fatima University, PhilippinesDivya Singhal, Veteran Affairs, United States
Copyright © 2025 Sasaki, Takahira, Yoda, watabe, Nakanishi, Aoki, Miyazaki, Chiba, Kurosaki and Kobayashi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuichi Sasaki, c2FzYWtpa2kwODIxQGdtYWlsLmNvbQ==
 Shuichi Sasaki
Shuichi Sasaki Naonobu Takahira2
Naonobu Takahira2 Keiichiro Aoki
Keiichiro Aoki