- 1Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States
- 2IdeaPlayground.org, Philadelphia, PA, United States
- 3Sentiar, Principal Human Factors and Design, St. Louis, MO, United States
The use of virtual, augmented, and/or mixed reality in applications targeting medical indications is rapidly growing. A main driver has been the rapid progress in wearable computing, specifically headsets. Yet, most of the headsets currently used for medical applications were not designed specifically for use in medicine, potentially accounting for undesired outcomes and limited adoption. Human Factors (HF) engineering is a design philosophy and methodology which seeks to inform technological development through understanding of human capabilities and limitations. We believe a HF engineering approach is essential when designing for a high stakes environment such as medicine to achieve a useful, and usable extended reality (XR) headset. In this paper, we will briefly review the history of XR headsets and outline the key components of HF engineering. Next, we present a series of real-world experiences depicting utilization of XR headsets in the clinical workflow to illustrate the potential and shortcomings of the current technology. Finally, we introduce medicalextendedreality.com as a forum for gathering user requirements which can be used in the design of future headsets intended for medical use.
1 Introduction
The use of virtual, augmented, and/or mixed reality (which we collectively term extended reality [XR]) in applications targeting medical indications is rapidly growing. There were 4,153 articles indexed by the National Library of Medicine in 2023 with the keyword “augmented reality” OR “virtual reality” OR “mixed reality”, up from just 708 in 2013, an almost 6-fold increase over 10 years. In addition, in the last few years there have been scoping reviews of augmented reality in medical education (Tang et al., 2020), medicine (Eckert et al., 2019; Andrews et al., 2019), and surgery (Zhang et al., 2023; Magalhaes et al., 2024) as well as reviews covering subspecialities such as interventional radiology (Park et al., 2020) and emergency medicine (Munzer et al., 2019). As of September 2024, the FDA cleared 69 medical devices that incorporate XR (Augmented Reality and Virtual Reality in Medical Devices, 2025) and 367 clinical trials have been listed in the National Library of Medicine clinical trials database with the keyword “mixed reality” (Clinical trials.gov: Mixed reality, 2025).
A main driver has been the rapid progress in wearable computing, specifically headsets, which have added functionality and brought down cost. With relatively powerful yet inexpensive headsets in hand, physicians, healthcare workers and entrepreneurs have sought to move into the medical space to investigate how this technology can be integrated in the clinical workflow. However, to date, the development of these headsets has been driven by the gaming industry, as evidenced by the major stakeholders developing the hardware, and which has led to design choices that may not be ideal for a medical application. Human Factors (HF) engineering is the science of understanding human-technology interactions (Fermin et al., 2024; Marshall and Touzell, 2020). Moving forward, we believe it is imperative that HF engineering be integrated earlier and more intentionally, if XR is to find a meaningful place within the clinical workflow.
1.1 Extended reality headsets: a brief history
The history of XR likely begins with stereoscopic viewers, invented in the 1820s, however, the first true head mounted display (HMD) was not until the 1960s (Sutherland, 1968). Subsequent progress led to early virtual reality devices such as the Large Expanse, Extra Perspective (LEEP) (Howlett, 1990) and the EyePhone (Faisal, 2017). These designs are notable for their size, weight, and cost given limitations in CPU power, battery technology, and miniaturization at the time. By the mid-1990s however, cost and size reduction led to commercial products such as the Nintendo Virtual Boy. Around this time, XR also started to enter the medical field (Edwards et al., 1995; Stetten and Chib, 2001). By the early 2000s, the technology had advanced to enable the development of the first usable headsets for medical applications (Birkfellner et al., 2002; Das et al., 2006). Unfortunately, these headsets never made it into routine practice and the field was quiet until the 2010s when there was a rapid proliferation of commercial headsets including the Oculus in 2012, Google Glass in 2013, the Microsoft HoloLens 1 in 2016, and Magic Leap in 2018. Finally, advances in optical and video pass-through technology in the 2020s brought about devices such as the Microsoft HoloLens 2, Apple Vision pro and the Meta Quest 3, which have blurred the distinctions between virtual, augmented and mixed reality and can be truly considered XR devices.
The availability of mass produced, commercially available hardware at a reasonable price led to a renaissance of investigations into how this technology could be incorporated into medicine; however, this also marked a turning point. Previously, headsets were developed in a laboratory or research setting. The hardware could be tailored to the application and there was freedom to iterate over the hardware design. With the shift to commercially available devices, the paradigm changed from building hardware to fit a need to finding a need within the limitations of specific hardware. The majority of current XR medical devices and research investigations now utilize a commercial headset (Gsaxner et al., 2023; Palumbo, 2022; Avari et al., 2020) with only a small minority using either in house developed (Harel et al., 2022) or OEM headsets (Lee et al., 2021). Finally, while the devices are described as hardware, commercial offerings represent the combination of hardware and software. Similar to other mobile technologies, the trend is to develop hardware and software together to maximize capabilities, both performance and efficiency, but also further limiting customizability through pre-specified or fixed firmware. As a result, the line between software and hardware is blurred. Decisions about one directly affect the other. The type of sensor installed will determine feasible software interaction methods and supported programming languages will determine the necessary CPU architecture. As a result, device issues should be seen as having both hardware and software components and software solutions may require hardware updates.
2 Best practices for medical device development: Human Factors
XR has a lot of potential to improve the clinical workflow, including solutions in education, workflow efficiency, or communication; the possibilities are endless. But the plausibilities must be thought through. Medicine is complex and pushes the limits of human capabilities, knowledge, patience, teamwork, and endurance. It is hard enough for a new hardware to insert itself into the high stakes field of medicine and made that much harder when the hardware is not designed for the intended use. Headsets designed for gaming are unlikely to satisfy the needs and demands of the medical community. People who use XR devices for games have the freedom to take breaks whereas a surgeon may need to keep working, leading to new ergonomic problems even with the same hardware. However, simply “fitting” new technology to specific people or demand is not trivial.
Managing the above problem has become the domain of “Human Factors,” (HF) the empirical scientific discipline concerned with the interactions between humans and other elements of a system (Privitera, 2019). HF research strives to understand human capabilities and limitations during the product design process, including sensing, perception, cognition and action (anthropometry and movements) (Privitera, 2019) (Figure 1).
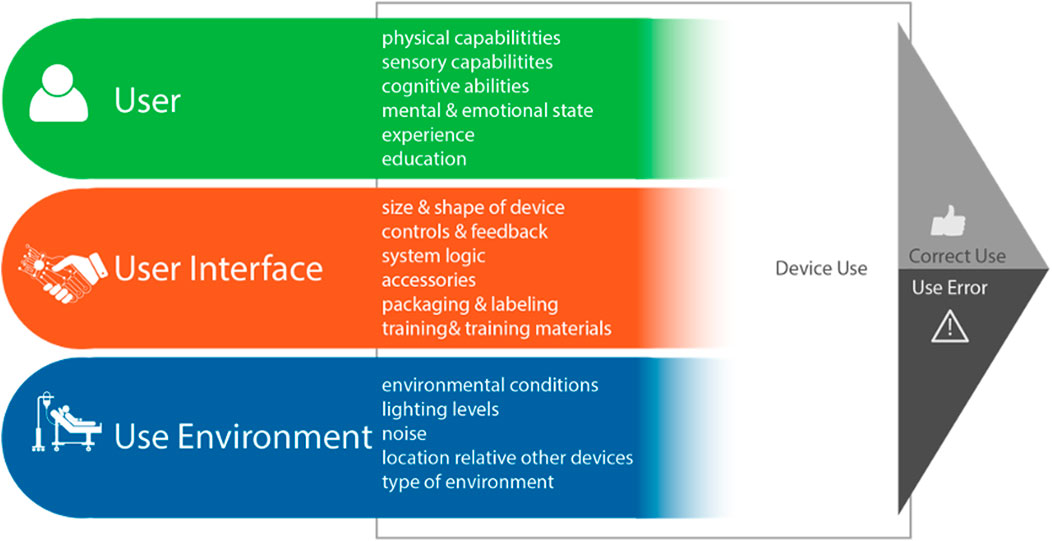
Figure 1. Major Human Factors (HF) components of a device-user system interactions which result in device use outcomes [image used with permission from (Privitera, 2019)].
HF engineering provides systematic processes for anticipating usability issues early in the product design process, rather than only running into such issues near the end of design or after products are marketed. While a comprehensive review of HF is beyond the scope of this perspective, one key tool is contextual inquiry (CI). CI is promoted as a best practice by the US Food and Drug Administration (FDA) (Applying Human Factors and Usability, 2016) and AAMI TIR 51 (AAMI TIR51, 2014) and cited in the International IEC standard for Usability Engineering for Medical Devices, 62,366-1 (IEC 62366-1, 2015) and the United Kingdom Medicines and Healthcare products Regulation Agency (MHRA) HF Guidance statement (MRHA, 2016). The process of CI involves immersing a small research team at the site of clinical care, often using video recordings to identify key elements that the design of the novel device must take into account to improve usability and overall acceptance. The results are analyzed by HF specialists or device designers with the intent to inform new product development (Figure 2).
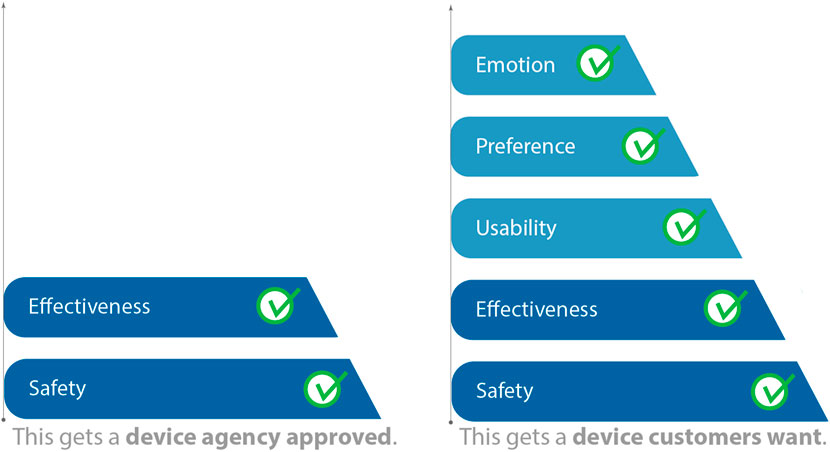
Figure 2. Although Human Factors (HF) is part of device approval as part of safety evaluation, the HF processes such as contextual inquiry (CI) are used to create a desirable product rather than simply an approved product. [Adapted from (Story, 2017) in (Privitera, 2019), used with permission].
As an example of the benefit of a robust CI, consider an XR system developed for use in a cardiac catheterization lab. Many XR systems enable several input methods: gaze, gaze-dwell, and gesture. Rather than selecting a method a priori, the development team decided to conduct a thorough CI study along with formative usability tests to understand the user input methods. During the investigation, it became clear that during intraprocedural use, clinicians can “look” but they cannot “touch” as their hands are busy manipulating catheters. As a result, gaze-dwell was found to be the most convenient method of user interaction. This type of information and development direction can only come from both strong clinical involvement as well as detailed observation by the development team.
HF engineering has defined design frameworks for understanding and identifying potential flaws and failures (Cafazzo and St-Cyr, 2012; Halloran et al., 2009; Sanders and Stappers, 2012). Many medical devices and technologies have benefited from this framework, most notably intravenous infusion pumps which initially were a large source of adverse events prompting the FDA to issue new guidelines which included HF reporting. This resulted in updated physical designs to ensure appropriate insertion of tubing and user interface to prevent incorrect dosing (Smith and Gray, 2020). Additional examples include the redesign of medicine vials so similar sounding medications have a different physical look and the development of retractable needles which eliminate both the urge and the need to recap used needles. Utilization of this framework is a key consideration for medical XR to prevent a premature jump from “this is cool” to “let’s use it for surgery.” The headsets that have impressed people for decades and inspire brainstorming were not built on the basis of research into the lives, practices, and customs of working medical professionals. Not that this is necessarily an insurmountable barrier. After all, cathode-ray tubes and LCD screens were not designed for medicine, yet screens of all sorts have made their way into healthcare without any fuss.
2.1 Real world experiences
A key component of HF engineering is understanding the user. While interviews and opinion are important, evaluating the user in their actual environment can expose issues that may not have been considered but need to be addressed. To that end, below we briefly describe a few of own personal observations of some key failures experienced when trying to deploy XR technology at a single large urban hospital. This data represents direct observation and informal interviews by one of the authors (AD) and was not a formal study. Individual use cases of the XR technology within clinical practice was cleared (approved or exempt) by the local institutional review board. While our observations are limited, we believe they are representative of broader categories. Italic text following each category represents authors opinions on possible solutions.
2.1.1 “I can’t look stupid in front of my patients”
Patients want to know that their providers are experts, that they know what they are doing and have a wealth of experience. Providers want to give off an aura of knowledge and expertise to put the patients at ease. The first time most providers use the headset they fumbled through the interface. Voice commands did not work properly, or the user appeared to be frantically grabbing at the air in front of them. Providers felt this made them look unprofessional and inexperienced and were worried that patients would not have faith in their skills. As a result, providers were hesitant to use the technology.
To address these issues, XR hardware developers might consider further research regarding the selection of input modes that better match the clinical situation; headsets that require little training and/or provide refreshers that can be readily accessed in the case of delayed use between the time of training and the time of clinical use.
2.1.2 Eye obscuration
Non-verbal cues, such as facial expressions, are an important part of human communication, with eye contact being one of the most important. Both optical and video pass through devices occlude the eyes to some degree. Providers were concerned about the effect of losing this eye contact both within their team and with patients. Providers were concerned that the headset would act as a barrier between the patient-provider relationship or that critical non-verbal communication may be missed during an urgent procedure or surgery. In addition, eye contact is one of the many cues used to identify if the listener is paying attention to the speaker. Although optical passthrough provide translucent lens, issues like “cyber eye,” where the optical waveguides cause the lens to be bright when a screen is open, were found to be distracting and prevented true eye contact.
2.1.3 The prescription problem
Many providers need corrective lens and most providers wear glasses rather than contacts. Two solutions currently exist, the headset can be designed 1) to use with custom prescription inserts, or 2) to accommodate the user’s own glasses. Providers took issue with both the solutions. First, the headset is a shared resource making custom inserts cumbersome due to the need to install and uninstall the insert each time as well as keep track of the insert between uses. Second, many users had glasses frames that did not comfortably fit within the headset eye box. This resulted in unfocused or incorrectly colored virtual objects. For both cases, this was difficult to troubleshoot as the issue was specific to the user and difficult to reproduce by a second user.
XR systems inherently rely on a user’s visual capabilities. As such, they may not be appropriate for all users. For future hardware development, the ability to make eye contact is a critical feature in any clinical case where a patient is awake and aware. Further, even in instances where the patient is anesthetized, clinicians often need to identify and approve various disposable devices throughout the case. Future hardware developers might consider materials and means to increase the transparency of the lens while maintaining XR functionality and responsiveness. Additionally, prescription inserts solve the issue of accommodation however lack a storage solution that encases all inserts for a clinical group along with the headset. A simple solution could be to include a set of prescription insert cavities with identification in the headset storage.
2.1.4 Protection problem
Personal protective equipment is a key component of keeping healthcare providers healthy. After needle sticks, eye splashes are the second most common route of infectious spread in a hospital. In addition, separate protection is needed for users performing fluoroscopy and who need eye protection from radiation. Many providers were concerned about the protective status of the headset as little information is known about the splash protection or radiation protection for these devices. As a result, users sought to place barrier shields in front of the headset or directly in front of their eyes. However, this often would cause issues with headset sensors, limiting the functionality of the device. For example, surgeons often wear special face masks that cover the mouth and include a clear plastic shield to cover their eyes. Devices such as the Microsoft Hololens have a gap between the lens and the face. The user must now decide if the shield should go over the lens, affecting the sensors, or under potentially obscuring the lens.
Safety of the provider is paramount. XR systems designed to be deployed in medicine need to be rigorously tested to the appropriate protection standards for their intended environment such as ISO 22609:2004 Clothing for protection against infectious agents—Medical face masks and ISO 15382:2015 Radiological protection—Procedures for monitoring the dose to the lens of the eye.
2.1.5 Hair
Many of the medical XR applications are intended for deployment during sterile procedures during which providers much wear a hair covering. Providers with long hair put their hair in a bun under a surgical cap. This configuration resulted in a larger head diameter and many providers were simply unable to place the headset on their head.
2.1.6 It’s a pain in the neck
Pilot data (unpublished) evaluating where providers are looking while wearing the headset found that to look down, providers would keep their head straight and just move their eyes down as this was more ergonomic and reduced neck motion and excessive flexion. Unfortunately, this puts the eye path outside the headset field of view. Therefore, for the provider to see a virtual object, they had to bend their neck down. When done repeatedly, this resulted in neck pain, made worse by the extra weight at the front of the head from the headset.
Although this issue partly arises from technological limitations around component weight and battery technology, HFE requires taking into account the product use, as is, in its intended environment. Design of XR headsets with specific attention to weight distribution, head circumference, splash protection, and donning and doffing the headset will be important for any future device. However, if technical limitations cannot be overcome, other mitigations can be employed such as identifying procedures which are of short enough duration that the device remains comfortable, or suggesting alternate form factors with a counter-balance to alleviate strain.
2.1.7 Benefit to setup ratio
In its present form, XR applications are not grab and go. Providers need to set up the device prior to use. This could include connecting to a special network, logging into a specific application, or transferring information to the device. Several users noted that when asked to set up the device, the set-up time took longer than the actual procedure, especially if the procedure was easy, straightforward, or commonly performed. As a result, they were hesitant to start the process of device set-up unless they knew that the case would be difficult enough to warrant the need for the headset. Several providers did acknowledge that there were cases that should have been easy, but become difficult and that headset would have helped; however, by the time it became apparent, it was too late to set it up.
The goal of HF engineering is to understand all the users of the device. Dedicated thought will need to go into designing a user interface (UI) that works best for the healthcare provider. In addition, UI and system setup will also need to be designed for support staff such as technologists who may be preparing the technology prior to a surgery or the Information Technology (IT) staff who need to onboard the device onto the hospital network and help troubleshoot if the technology is not working. Ensuring the concerns of IT and other staff who must support device deployment are addressed will be important to ensure the technology can scale with the enterprise. This may includes incorporating methods to remote into the devices so IT staff and “see” what the issue is or incorporating end user serviceable parts that can be repaired on site.
2.1.8 Demanding to use or use is demanding
Certain technologies have become so integral into the clinical workflow that providers will not proceed unless it is available. A prime example is ultrasound for guiding catheter placement. Providers will wait for an ultrasound machine to become available before starting the procedure. They demand to use it. Other technologies may be available but because of various limitations or constraints rarely or never get used. Either providers are not aware of it, are not trained on it, or do not find it useful. When discussing XR technology, most providers currently consider XR to be in the second category. The find the use of XR demanding. They never ask for the technology themselves, but only use it if someone else has asked them to try it.
This list is by no means comprehensive, but rather highlights some of the user factors that should be considered in future XR technology development. Additional technical aspects which also have a HF component include virtual object contrast in varying light environments and background textures, battery life, spatial resolution, or frame rate. In addition, cyber sickness, or feelings of nausea and vertigo when using XR, remains a real issue with a certain percentage of providers who will likely never be able to use the technology.
3 Next steps
Medical XR requires understanding the whole person, as well as the people around the user. Put on a headset, and now you cannot make eye contact the way the team is used to. The user’s peripheral vision might be blocked, and this might affect what they see and what others expect them to be able to see. The “R” in XR reminds us that something, at least metaphorically, changes about reality. Whereas designers often try to take the end-users' perspective, XR systems transform the user’s perspective. In some ways XR is more like a performance-enhancing drug than it is like a scalpel, in the degree to which it affects something about the users themselves.
Given this effect on the user, the high stakes of medicine, and the cost of hardware development, we believe there is a need for publicly available user feedback to help articulate and close the gap between gaming hardware and medical hardware. We have set up the website MedicalExtendedReality.com to provide a forum for healthcare stakeholders to provide information about their environments, about themselves, and about their own personal expectations and desires with respect to XR products. Utilization of this site is entirely voluntary, data will be public, and any collected data will be made available for further research and analysis.
MedicalExtendedRealty.com is intended to illustrate as well as begin a bottom-up data-gathering process for medical XR devices, as if the hardware did not already exist. This bottom-up process can lead to design inputs for future devices, whether new hardware or software, to address gaps between gaming hardware and medical needs.
Unlike consumer products intended for use in the home, healthcare stakeholders work in a myriad of locations within a hospital or clinic, each having their own set of expected conditions and behaviors. By detailing situations where XR applications would or would not work and the complexities involved, it is the hope of this survey to serve as a starting place for further conversation. Ideally, developers can identify those practitioners with a keen interest in applying medical XR to their practice in order to enable a full CI program, which is the recommended first step in a robust HF design program.
4 Conclusion
An “amazing” and commercially successful gaming headset can translate poorly into the operating room. A person wearing an XR headset to play a game might not mind how they look and can take a break if their neck gets sore. As a result, a gaming device can be commercially successful even if it leaves some potential users out. However, in medicine, lives are on the line when groups of people are unable to use a medical device safely and effectively. We have personally observed XR failing to find a place within a hospital due to numerous usability issues–and we are disappointed because we see the potential. MedicalExtendedReality.com might help point the field towards a bottom-up approach to future hardware that is better designed to suit specific real-world medical applications.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AD: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review and editing. ME: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review and editing. MP: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors wish to acknowledge John Rice for early ideas and stimulation of the writing effort.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andrews, C., Southworth, M. K., Silva, J. N. A., and Silva, J. R. (2019). Extended reality in medical practice. Curr. Treat. Options Cardiovasc Med. 21 (4), 18. doi:10.1007/s11936-019-0722-7
Applying human factors and usability engineering to medical devices: guidance for industry and Food and drug administration staff In: U.S. Department of health and human services, Food and drug administration, center for devices and radiological health, evaluation OoD, editors (2016).
Augmented Reality and Virtual Reality in Medical Devices (2025). Available online at: https://www.fda.gov/medical-devices/digital-health-center-excellence/augmented-reality-and-virtual-reality-medical-devices#list.
Avari, S. J. N., Southworth, M. K., Blume, W. M., Andrews, C., Van Hare, G. F., Dalal, A. S., et al. (2020). First-in-human use of a mixed reality display during cardiac ablation procedures. JACC Clin. Electrophysiol. 6 (8), 1023–1025. doi:10.1016/j.jacep.2020.04.036
Birkfellner, W., Figl, M., Huber, K., Watzinger, F., Wanschitz, F., Hummel, J., et al. (2002). A head-mounted operating binocular for augmented reality visualization in medicine--design and initial evaluation. IEEE Trans. Med. Imaging 21 (8), 991–997. doi:10.1109/tmi.2002.803099
Cafazzo, J. A., and St-Cyr, O. (2012). From discovery to design: the evolution of human factors in healthcare. Healthc. Q. 15, 24–29. doi:10.12927/hcq.2012.22845
Clinical trials.gov: Mixed reality (2025). Available online at: https://clinicaltrials.gov/search?term=mixed%20reality.
Das, M., Sauer, F., Schoepf, U. J., Khamene, A., Vogt, S. K., Schaller, S., et al. (2006). Augmented reality visualization for CT-guided interventions: system description, feasibility, and initial evaluation in an abdominal phantom. Radiology 240 (1), 230–235. doi:10.1148/radiol.2401040018
Eckert, M., Volmerg, J. S., and Friedrich, C. M. (2019). Augmented reality in medicine: systematic and bibliographic review. JMIR Mhealth Uhealth 7 (4), e10967. doi:10.2196/10967
Edwards, P. J., Hawkes, D. J., Hill, D. L. G., Jewell, D., Spink, R., Strong, A., et al. (1995). Augmentation of reality using an operating microscope for otolaryngology and neurosurgical guidance. J. Image Guid. Surg. 1 (3), 172–178. doi:10.1002/(sici)1522-712x(1995)1:3<172::aid-igs7>3.3.co;2-u
Faisal, A. (2017). Computer science: visionary of virtual reality. Nature 551 (7680), 298–299. doi:10.1038/551298a
Fermin, L., Lobaugh, L., Parr, K. G., and Currie, M. (2024). The role of human factors engineering in patient safety. Curr. Opin. Anaesthesiol. 37 (6), 683–688. doi:10.1097/aco.0000000000001437
Gsaxner, C., Li, J., Pepe, A., Jin, Y., Kleesiek, J., Schmalstieg, D., et al. (2023). The HoloLens in medicine: a systematic review and taxonomy. Med. Image Anal. 85, 102757. doi:10.1016/j.media.2023.102757
Halloran, J., Hornecker, E., Stringer, M., Harris, E., and Fitzpatrick, G. (2009). The value of values: resourcing co-design of ubiquitous computing. CoDesign 5 (4), 245–273. doi:10.1080/15710880902920960
Harel, R., Anekstein, Y., Raichel, M., Molina, C. A., Ruiz-Cardozo, M. A., Orru, E., et al. (2022). The XVS system during open spinal fixation procedures in patients requiring pedicle screw placement in the lumbosacral spine. World Neurosurg. 164, e1226–e1232. doi:10.1016/j.wneu.2022.05.134
Howlett, E. M. (1990). “Wide-angle orthostereo,” in Proc. SPIE 1256, Stereoscopic Displays and Applications. doi:10.1117/12.19915
IEC 62366-1 (2015). ANSI/AAMI/IEC 62366-1. ISO. Available online at: https://www.iso.org/standard/63179.html.
Lee, K. W., Choi, H. S., Chun, H. J., Lee, J. M., Kim, E. S., Keum, B., et al. (2021). Feasibility of wearable display glasses for medical students in the endoscopy room. Clin. Endosc. 54 (5), 694–700. doi:10.5946/ce.2020.246
MRHA (2016). UK notified bodies for medical devices - GOV.UK. Available online at: https://www.gov.uk/government/publications/medical-devices-uk-notified-bodies/uk-notified-bodies-for-medical-devices.
Magalhaes, R., Oliveira, A., Terroso, D., Vilaca, A., Veloso, R., Marques, A., et al. (2024). Mixed reality in the operating room: a systematic review. J. Med. Syst. 48 (1), 76. doi:10.1007/s10916-024-02095-7
Marshall, S. D., and Touzell, A. (2020). Human factors and the safety of surgical and anaesthetic care. Anaesthesia 75 (Suppl. 1), e34–e38. doi:10.1111/anae.14830
Munzer, B. W., Khan, M. M., Shipman, B., and Mahajan, P. (2019). Augmented reality in emergency medicine: a scoping review. J. Med. Internet Res. 21 (4), e12368. doi:10.2196/12368
Palumbo, A. (2022). Microsoft HoloLens 2 in medical and healthcare context: state of the art and future prospects. Sensors (Basel) 22 (20), 7709. doi:10.3390/s22207709
Park, B. J., Hunt, S. J., Martin, C., Nadolski, G. J., Wood, B. J., and Gade, T. P. (2020). Augmented and mixed reality: technologies for enhancing the future of IR. J. Vasc. Interv. Radiol. 31 (7), 1074–1082. doi:10.1016/j.jvir.2019.09.020
Privitera, M. B. (2019). “Association for the advancement of medical I,” in Applied human factors in medical device design. London: Elsevier/Academic Press.
Sanders, E. B. N., and Stappers, P. J. (2012). Convivial toolbox: generative research for the front end of design. Amsterdam, Netherlands: BIS Publishers.
Smith, E. A., and Gray, G. (2020). Developing a smart infusion pump dedicated to infusion safety. Ergonomics Des. Q. Hum. Factors Appl. 30 (2), 4–12. doi:10.1177/1064804620944760
Stetten, G. D., and Chib, V. S. (2001). Overlaying ultrasonographic images on direct vision. J. Ultrasound Med. 20 (3), 235–240. doi:10.7863/jum.2001.20.3.235
Story, M. (2017). The Human Touch: develop a patient-centric injection device. Available online at: https://www.wesrch.com/medical/paper-details/pdf-ME1XXF000KSCW-the-human-touch-a-patient-centric-injection-device.
Sutherland, I. E. (1968). “A head-mounted three dimensional display,” in Proceedings of the December 9-11, 1968, fall joint computer conference, part I. San Francisco, California: Association for Computing Machinery, 757–764.
Tang, K. S., Cheng, D. L., Mi, E., and Greenberg, P. B. (2020). Augmented reality in medical education: a systematic review. Can. Med. Educ. J. 11 (1), e81–e96. doi:10.36834/cmej.61705
Keywords: Human factors engineering (HFE), human centered design (HCD), extended reality (VR/AR/MR), medical extended reality (MXR), image guided intervention
Citation: Dhanaliwala AH, Egeth M and Privitera MB (2025) Surgery is not a game: the importance of human centered design in medical extended reality device development. Front. Virtual Real. 6:1580467. doi: 10.3389/frvir.2025.1580467
Received: 20 February 2025; Accepted: 30 June 2025;
Published: 16 July 2025.
Edited by:
Ryan Beams, United States Food and Drug Administration, United StatesReviewed by:
Rishindra Reddy, University of Michigan, United StatesCopyright © 2025 Dhanaliwala, Egeth and Privitera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali H. Dhanaliwala, YWxpLmRoYW5hbGl3YWxhQHBlbm5tZWRpY2luZS51cGVuLmVkdQ==
 Ali H. Dhanaliwala
Ali H. Dhanaliwala Marc Egeth
Marc Egeth Mary Beth Privitera3
Mary Beth Privitera3