- 1Department of Internal Medicine, ESIC Medical College and Hospital, Chennai, Tamilnadu, India
- 2Department of Internal Medicine, Fudan University, Shanghai, China
- 3Department of Internal Medicine, Northeast Georgia Medical Centre, Gainesville, GA, United States
- 4Department of Internal Medicine, Saint Vincent Hospital, Worcester, MA, United States
- 5Department of Internal Medicine, University of Illinois College of Medicine, Peoria, IL, United States
- 6Department of Geriatric Medicine, University of Texas Health Science Center, San Antonio, TX, United States
- 7Hospital Medicine Division, Cheshire Medical Center/Dartmouth Hitchcock, Keene, NH, United States
Introduction: Augmented reality (AugR) is becoming a widely recognized and innovative platform in global healthcare. AugR has revolutionized cardiology by enhancing the understanding of cardiac structure and function. This review highlights its applications in diagnosis, surgical planning, cardiac procedures, training, rehabilitation, and the future impact of AugR-related technology.
Methods: This review compiles original research and review articles on AugR in cardiology from PubMed till 2024.
Results: Advancements in visualization and image processing techniques facilitate the development of AugR tools using holographic displays, enhancing diagnostic accuracy and pre-surgical planning. Current AugR tools offer 3D heart imaging for diagnostic procedures, such as assessing Left Ventricular Ejection Fraction (LVEF). AugR enables real-time visualization for congenital and structural heart diseases, aiding in catheter navigation, transcatheter valve procedures, and arrhythmia treatments. Its effectiveness extends to cardiac resynchronization therapy, ventricular tachycardia ablation, and ultrasound-guided catheterization. AugR surpasses standard 2D fluoroscopy in surgical interventions by optimizing fluoroscopic angles, improving pacemaker placement, reducing X-ray exposure, and increasing procedural accuracy. It also enhances medical training by providing immersive experiences for residents and fellows, improving emergency response training. User-friendly AugR technologies effectively engage patients, promote physical activity, and enhance outcomes in cardiac rehabilitation. Further testing of AugR could serve as a pivotal surgical navigation tool in cardiac transplantology. Mixed reality enhances procedural planning and intraoperative navigation in cardiac electrophysiology by providing real-time 3D visualization and spatial orientation. Holographic visualization techniques combined with 3D and 4D printing hold future potential in cardiac care, particularly for designing patient-specific prosthetics. However, widespread clinical adoption of AugR in many healthcare institutions is limited by technical challenges and high costs related to specialized hardware, software, and maintenance.
Conclusion: AugR holds great promise in transforming cardiac care, but its clinical integration depends on rigorous trials to validate its effectiveness. While much research remains theoretical, increased human testing is essential for real-world applications. Advancing AugR, alongside technologies like 3D/4D printing and holography, could pave the way for a safer and more precise future in cardiology.
Introduction
Immersive digital experiences such as Augmented Reality (AugR), Virtual Reality (VR), and Mixed Reality (MR) are gaining popularity in the medical field today (Flavián et al., 2019; Zuo et al., 2024; Hu et al., 2019). AugR overlays digital objects onto the physical world (Al-Ansi et al., 2023). VR involves a fully computer-generated artificial environment where the real world is completely blocked out (Al-Ansi et al., 2023). MR is a combination of VR and AugR, where digital objects interact dynamically with reality (Speicher et al., 2019). In medicine, AugR integrates real objects (such as patients) and virtual objects (like 3D images derived from anatomical data) through spatial alignment, instrument tracking, and visualization; MR goes further by anchoring and enabling interaction with these virtual objects in the physical world, while VR creates fully virtual environments where clinicians can practice procedures in digital simulations (Zuo et al., 2024; Hu et al., 2019; Goo et al., 2020). Combining these elements allows clinicians to focus on procedures without distraction (Goo et al., 2020). All three realities use advanced processing of medical images for enhanced visualization (Goo et al., 2020). From head-mounted displays (HMDs) to the current models that project blueprints onto surfaces, AugR has found its way into the medical field (Sutherland, 1968; Thomas and Mizell, 1992). Numerous AugR tools are now available to diagnose various pathologies, enhance medical education and training, and calculate and visualize subtle parameters for clinical procedures. Custom AugR applications are designed with specific medical objectives in mind and are subsequently installed on AugR devices such as HMDs, headphones, holographic displays, and smart glasses (Uppot et al., 2019). In 2018, the FDA approved the first use of Microsoft’s HoloLens for pre-operative surgical planning in the United States (FDA, 2024). Interestingly, newer technologies like holography do not require an HMD; instead, they project 3D images using a light source, enabling real-time depth perception. Virtual and augmented reality have become important and innovative tools, especially across cardiovascular subspecialties, including interventional adult, pediatric, and congenital cardiology, as well as structural heart disease and heart failure (Annabestani et al., 2024). In cardiac electrophysiology, 3D reconstruction modeling of the patient’s heart, vascular, and valvular structures enhances understanding of anatomy, physiology, and pathology, leading to improved diagnostic accuracy and outcomes. Additionally, interactive virtual modeling of cardiac structure and function aids comprehension of complex pathologies, contributing to safer interventions and surgeries (Minga et al., 2024). This narrative review summarizes the latest advancements and applications of AugR in cardiology. Furthermore, it explores future research areas, including MR and 4D printing, and examines their potential applications in the field.
Methods
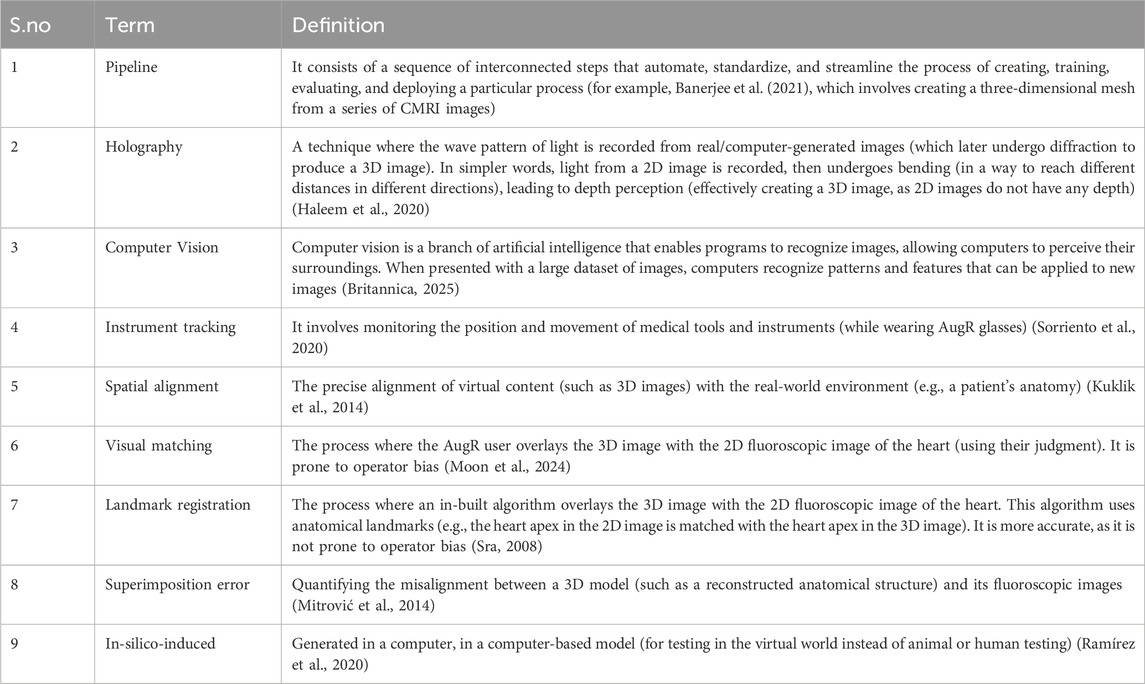
This narrative review article synthesizes findings from studies published on PubMed till December 2024, regarding the applications of augmented reality (AugR) in cardiology. A comprehensive search was conducted using the keywords “augmented reality,” “cardiology,” “cardiac imaging,” “surgical planning,” “interventional cardiology,” and “medical education” to identify relevant literature. Over 250 articles screened and included in this review were published in peer-reviewed journals and focused on using AugR in various aspects of cardiology, including diagnostic imaging, surgical navigation, interventional procedures, and medical education. Both original research articles and review papers were considered. Studies that neither explicitly involved AugR nor focused on cardiology were excluded from the review. To ensure the review was focused on relevant studies, we applied specific inclusion and exclusion criteria. These are outlined below in Table 1. Studies published in languages other than English were also excluded; however, the number of such studies was minimal and unlikely to impact the representativeness of this review. Data extraction focused on the AugR technology used, its clinical applications, and reported outcomes or benefits. Articles were selected for inclusion based on relevance, study design, and quality of evidence. Table 2 shows the definitions of some technical terms used across the article.
Currently available AugR tools
Currently available AugR tools are used by both healthcare professionals and patients. Clinicians use them to learn complex concepts, prepare for procedures, and perform specialized interventions. For patients, typical applications include cardiovascular education, distraction during procedures, and post-intervention rehabilitation. In cardiology, devices like Google Glass and HoloLens support immersive experiences that help patients understand conditions such as myocardial infarction, arrhythmias, and structural heart defects, along with their treatment options (Hilt et al., 2020). A variety of MxR technologies rely on HMDs to create immersive environments and showcase digital visuals. Some commonly used examples are Google Glass, Microsoft HoloLens, and Magic Leap (Silva et al., 2018). The Microsoft® HoloLens is an HMD that enables users to interact with digital holograms overlaid on their physical environment, facilitating an immersive and interactive experience (Palumbo, 2022). Another widely preferred AugR headset among surgeons is the Magic Leap 1, valued for its ease of use and superior visual quality (Zari et al., 2023). Nreal Air AugR glasses are lightweight devices that connect to smartphones to display data like photos or videos on the lenses using AugR technology, enabling real-time visualization (Choi et al., 2024). EchoPixel True3D is a 3D software platform that comprises EchoPixel’s software, a desktop monitor-style display, special glasses for 3D visualization, and a stylus used for pre-surgical planning (LaunchSquad, 2017). Companies like SentiAR enhance visibility and precision during minimally invasive procedures such as catheter ablation (Sentiar, 2025). SentiAR’s software, when integrated with Microsoft HoloLens, forms the Enhanced Electrophysiology Visualization and Interaction System (ĒLVIS). By utilizing data from electroanatomic mapping systems, ĒLVIS displays cardiac structures, electrical activity maps, and catheter positions directly within the clinician’s field of view. This hands-free, sterile interface allows interaction through eye gaze and gestures, enhancing visualization and precision during electrophysiology procedures (Avari et al., 2020). Figure 1 illustrates the various applications of AugR in cardiology.
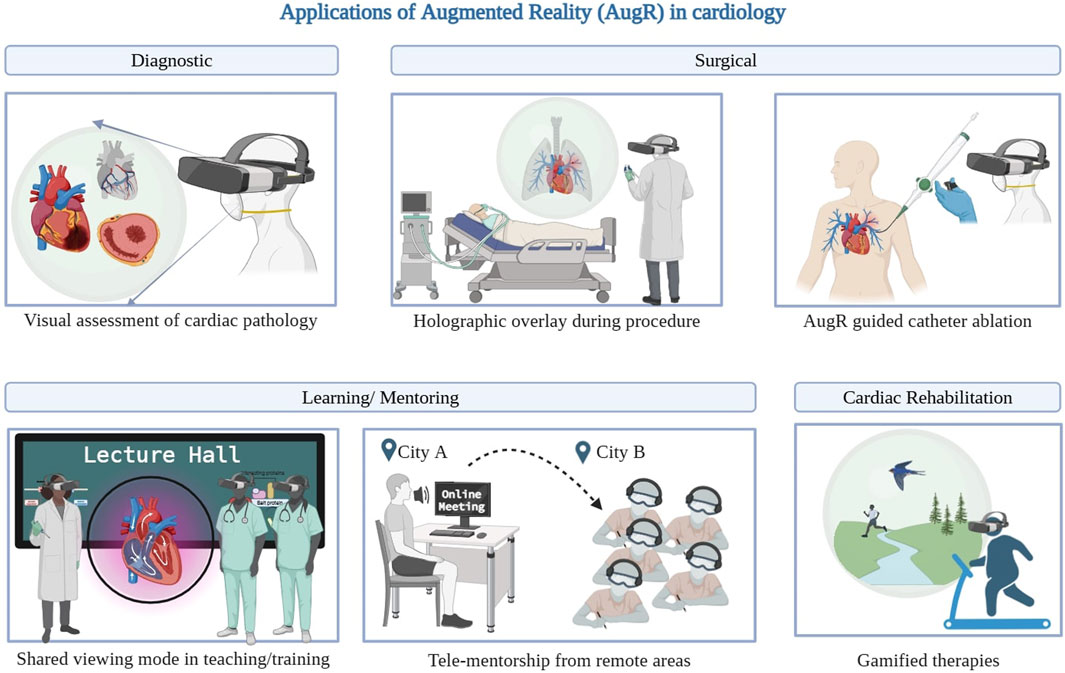
Figure 1. Applications of AugR in cardiology. *Created on BioRender.
Diagnostic applications
AugR offers significant potential in enhancing cardiac diagnostics through the integration of advanced 3D modeling and imaging technologies. By constructing precise 3D models of the heart, clinicians can gain deeper insight into its electrophysiology, allowing for the early detection of cardiac diseases that are not visible in 2D imaging (Banerjee et al., 2021; Smeragliuolo et al., 2016). This predictive capability is further enhanced by gesture recognition systems, which analyze heart motion and deformation patterns to detect coronary artery disease (Smeragliuolo et al., 2016). Experimental studies have demonstrated its effectiveness and reliability in improving diagnosis (Zou et al., 2017). The increasing availability of free and open-source datasets now enables the generation of AugR projections from cardiac MRI and CT scans, making this technology more accessible (Bindschadler et al., 2022). AugR can diagnose cardiac pathologies like scars, edema, or lack of perfusion to heart muscles (Pinto-Coelho, 2023). For instance, Medical Extended Reality (MxR) helps in generating 3D models of the heart’s ventricles from cardiac MRI holograms. Through holography, light from 2D MRI slices is bent to create depth perception. Computer vision selects only relevant holographic images to construct an accurate model, and a statistical shape model reduces misalignment errors from 1.82 mm to 0.72 mm (Shah et al., 2009). In addition, Nreal Air glasses are used for visually assessing left ventricular ejection fraction (LVEF), which may benefit medical students who may struggle with traditional methods like interpreting apical 2D images via Point Of Care Ultrasound (Choi et al., 2024). Using AugR over an echocardiogram to estimate LVEF has proven to be more beneficial in heart failure patients, as their echocardiography often has suboptimal imaging quality (Weidemann et al., 2015). Figure 2 provides a schematic representation of the setup and functioning of the lens used for LVEF estimation.
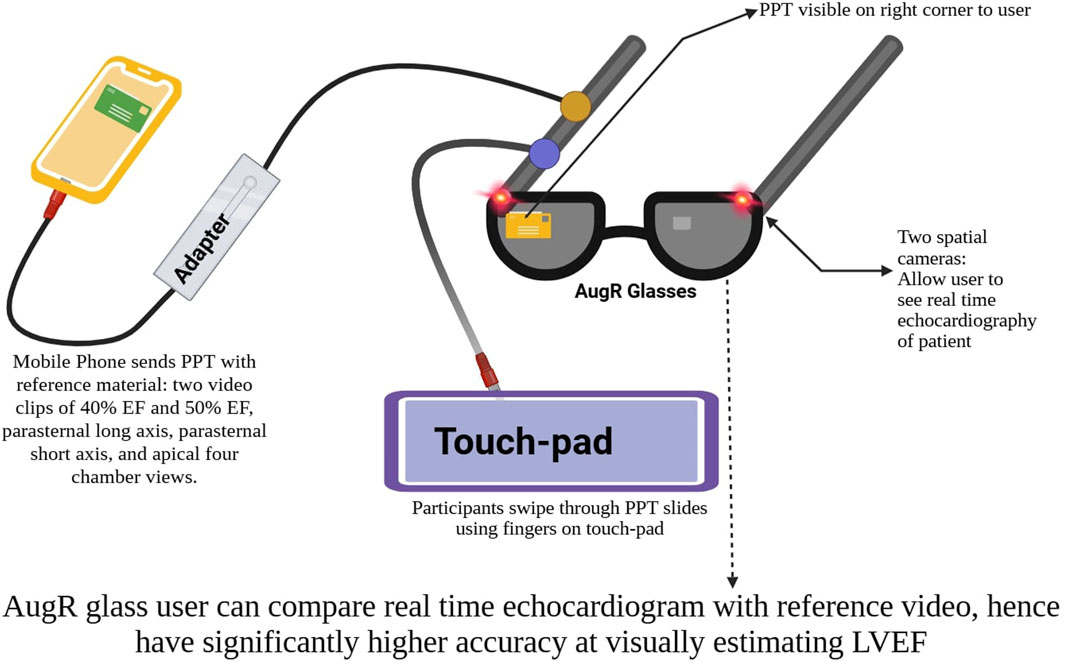
Figure 2. Schematic representation of LVEF estimation using AugR lenses. *Created on BioRender.
Surgical applications
Minimizing intraoperative trauma and accelerating postoperative recovery have long been priorities in cardiac surgery, making the application of AugR an area of growing interest due to its potential to enhance precision and reduce surgical invasiveness (Doughty et al., 2022; Iribarne et al., 2011). Various cardiovascular surgeries now use AugR worldwide, often with translucent displays or VR-like headsets providing stereoscopic views via front-facing cameras (Rad et al., 2022; Jung et al., 2022).
AugR visualization of 3D angiography was found to be useful in patients with congenital heart diseases (CHD) for cardiac catheterisation. Intraprocedural visualization of heart anatomy was found to be superior to existing computer models by the operators (Salavitabar et al., 2023). In many cases, the use of AugR during procedures is limited by the need for a handheld control device. However, this study demonstrates a fully hands-free approach to holographic visualization of 3D rotational angiography, utilizing voice commands and hand gestures to manipulate the 3D model (Salavitabar et al., 2023). A case of transposition of the great arteries with dextrocardia was treated using leadless transcatheter pacemaker implantation, guided by 3D CTA reconstruction and a voice-controlled system. Perioperative 3D images were superimposed onto the patient’s skin via Google Glass, enabling optimal fluoroscopic angle selection and precise pacemaker placement. This approach proved more effective than standard 2D fluoroscopy and helped reduce unnecessary X-ray exposure (Opolski et al., 2018).
Another case demonstrated AugR use during percutaneous Pulmonary Vein Isolation for paroxysmal atrial fibrillation. Pre-procedure CT data were converted into 3D holograms, allowing detailed anatomical visualization via an HMD. The system enabled real-time, voice-controlled interaction and overlaid patient vitals and procedural data (Lodziński et al., 2018). This approach reduced fluoroscopy time and radiation exposure, enhancing procedural safety. However, it requires pre-procedure CT scanning, depends on specialized hardware and software, and its clinical efficacy and cost-effectiveness still require further evaluation (Lodziński et al., 2018).
True3D technology of EchoPixel has been useful in pre-procedural planning, by allowing surgeons to have a run-through of the procedure in the virtual world. Surgeons can wear an AugR headset and use the stylus to interact with the surgical site—cutting, rotating, and measuring, in an immersive environment (EchoPixel and Inc, 2025). This was reliable for showing 3D TEE images and accurate in measuring the size of the MV annulus and height of valve prolapse (Ballocca et al., 2019). Figure 3 provides a schematic representation of the workflow of the EchoPixel True 3D system.
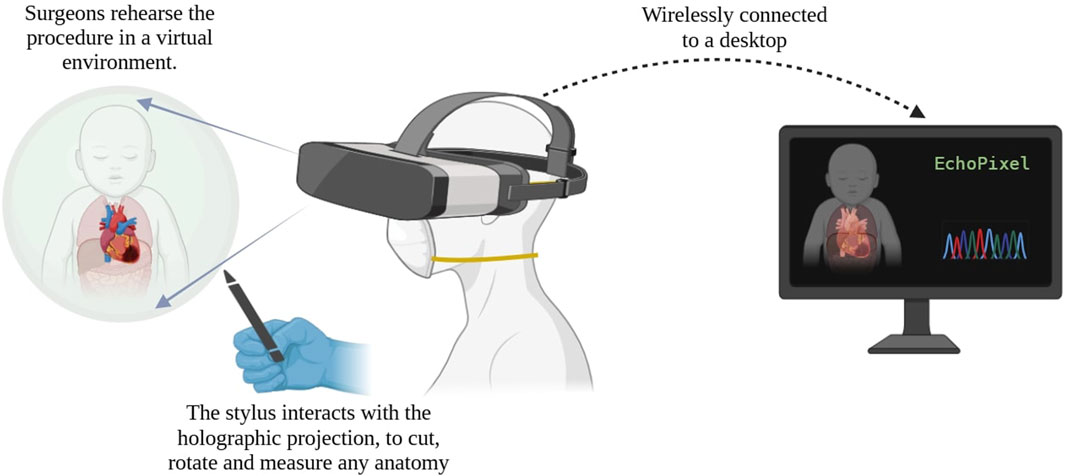
Figure 3. Schematic representation of the EchoPixel True 3D system. *Created on BioRender.
The HoloLens is being utilized in Cardiac Resynchronization Therapy (CRT) by applying AugR to CTA reconstructions of the coronary sinus. Virtual hand gestures visualize and scale the sinus in AugR, aiding optimal placement in the operating room. Witkowski et al. reported that HoloLens use did not impair operators’ vision, and AugR-guided 3D reconstructions enhanced CRT implantation effectiveness, reducing procedure times and fluoroscopy doses, while also providing better visualization of tortuous and kinky veins. The accuracy of CT scans limits AugR reconstruction, but combining it with other visualization methods may enhance anatomical assessment of the coronary sinus (Witkowski and Lodziński, 2019). In another study done at Harvard and Munich universities, a framework was created for 3D holographic visualization of myocardial scars using HoloLens. The scar was imaged using late gadolinium enhancement in the context of ventricular tachycardia (VT) treatment. HoloLens 3D LGE provided an immersive and interactive 3D perception of complex scar architecture, which can potentially facilitate AugR-guided VT ablation (Jang et al., 2018). However, this study was conducted in an animal model and did not use HoloLens 3D LGE for real-time guidance during ablation procedures, but rather to demonstrate the feasibility of image visualization (Jang et al., 2018).
Transcatheter Valve Implantation, a standard practice around the world, was developed years ago to treat cardiac valve dysfunction to avoid open-heart surgeries (Masson et al., 2009). AugR has demonstrated a promising application for assisting in these valve replacement surgeries, enhancing outcomes and patient-centered care (Nanchahal et al., 2024; Currie et al., 2016). Another procedure where AugR has made major strides forward is the Valve-in-valve (ViV) procedures, which implant a new valve within a previously implanted bioprosthetic valve to treat valve degeneration. Procedures like these require precise navigation to avoid major complications like coronary ostia obstruction (Bernardi et al., 2019). Belhaj Soulami et al. evaluated the superimposition error of the 3D model onto the fluoroscopic images for five aortic ViV procedures for validation and then prospectively carried out two successful aortic ViV procedures (Belhaj et al., 2016). In 2005, De Buck et al. introduced custom AugR systems to create patient-specific 3D heart models from MRIs, integrating them with real-time fluoroscopic images during cardiac catheter ablation. Models were aligned using visual matching or landmark-based registration, the latter employing mathematical algorithms for greater accuracy and reduced operator bias. Tested on 11 patients, this method enhanced coronary sinus mapping accuracy, surpassing existing solutions reliant on costly hardware and catheters (De Buck et al., 2005). These advancements underscore the longstanding efforts in AugR research. Furthermore, the AugR platform has the potential to significantly improve the accuracy of percutaneous epicardial access for the mapping and ablation of cardiac arrhythmias (Bamps et al., 2024).
The potential of AugR in improving catheter navigation accuracy during in-silico-induced ventricular tachycardia (VT) ablations has been demonstrated. MRI-based virtual hearts with in-silico-induced VT models were used to test the termination of VTs through catheter ablation. Initial tests showed AugR improved ease of use, but repeated runs were inconclusive, with success depending on the number of inputs. While AugR-guided VT termination outperforms manual procedures, more training is needed to ensure consistency. Further research is required to understand AugR’s interaction with different substrates during VT ablation (Prakosa et al., 2021; Liu et al., 2019). In another study, the use of smart glasses during ultrasound-guided radial artery catheterization was found to improve first-attempt success, ergonomic satisfaction, as well as reduce both procedure time and complication rates in small pediatric patients. It was suggested that applying a similar approach to adult patients undergoing left or right heart catheterization could offer comparable benefits (Jang et al., 2021). A recent study highlighted the benefits of VR/AugR for patients, including improved understanding of diagnoses, procedural steps, and rehabilitation, as well as providing distraction during procedures. VR/AugR also aids healthcare providers in learning and refining skills for procedures like transcatheter ventricular ablation and coronary angiography, while enhancing outcomes through better visualization of complex cardiac and vascular structures (Jung et al., 2022).
Although the high cost of implementing AugR, particularly in precision-demanding fields like cardiac surgery, has been a barrier, advances in affordable, high-performance technology over the past decade have significantly reduced this challenge (Rad et al., 2022; Parham et al., 2019). However, technical challenges still exist. Integrating different imaging systems in real time within AugR/VR platforms is complex, as it requires smooth data flow and precise image alignment, which are not yet fully optimized (Jung et al., 2022). Moreover, the effectiveness of image overlays can be compromised by restricted movement and narrow peripheral vision caused by HMDs, along with insufficient patient tracking, which further reduces accuracy (Jung et al., 2022; Caluya et al., 2022). Other concerns include battery life, as AugR/VR headsets must function wirelessly and sustain power throughout lengthy surgical procedures without interruption. Performance issues like latency and low frame rates can lead to image lag and distortion, which may cause cybersickness and distract the surgeon. Additionally, ergonomic problems such as uncomfortable earpieces, unstable visual fixation during head movement, and camera misalignment have been reported, all of which can negatively impact surgical performance (Jung et al., 2022; Arslan and Gerckens, 2021). Continued technological advancements and user-centered design are crucial to addressing these limitations, enabling AugR to become a reliable, comfortable, and effective tool that enhances surgical precision and improves patient outcomes.
Learning and mentoring using AugR
AugR is transforming medical training and mentoring globally, with a new application enhancing cardiology education. Research explores innovative uses, including gamified therapies and immersive patient-specific experiences for diagnosis, treatment, and patient engagement (Cheng and Ebrahimi, 2023). It has demonstrated significant potential in alleviating pre-procedural anxiety among cardiac patients by familiarizing them with the surgical environment and clearly outlining what to expect on the day of the procedure (Rizzo et al., 2023). Tools like VR, AugR and gamified learning have demonstrated effectiveness in enhancing students’ understanding of anatomy and serve as viable alternatives to cadavers and mannequins, especially in regions where access to traditional resources is limited (Karbasi and Niakan Kalhori, 2020). A study on AugR-based simulation training for cardiac anesthesiology was conducted, which was positively perceived by members at the institution (Tsai et al., 2023). However, there is a need to evaluate the long-term impact of these interventions on patient outcomes, including health-related quality of life, treatment adherence, and clinical outcomes.
The usability of AugR in telementorship was evaluated by providing remote guidance for managing conditions such as acute coronary syndrome and acute myocardial infarction, utilizing laptops and AugR headsets. The study received high ratings from both mentors and mentees, indicating the potential of AugR in training students for real-life clinical scenarios (Bui et al., 2023). However, it also highlighted challenges, including cognitive load on users and a limited visual field for mentors, which restricted their ability to observe mentees during tasks. Additionally, the small sample size limits the generalizability of the findings (Bui et al., 2023). Another qualitative study utilized the Chariot Augmented Reality Medical simulator to deliver a remote advanced cardiovascular life support (ACLS) training scenario. Participants noted that the realistic AugR patient models and real-time vital sign feedback significantly enhanced their learning compared to other remote education methods (Hess et al., 2022). Similarly, AugR shows promise in Cardiopulmonary Resuscitation (CPR) and Basic Life Support training. These applications have been effective for both real-time assisted resuscitation and self-directed learning (Hou et al., 2022; Ingrassia et al., 2020). However, the absence of nuanced non-verbal cues and body language in the avatars limited the realism of emergency interaction training (Hess et al., 2022).
AugR in cardiac rehabilitation
AugR enhances cardiac rehabilitation by integrating interactive gaming with traditional exercise programs to stimulate physiological responses similar to conventional methods, demonstrating that affordable, user-friendly AugR technologies can effectively engage patients, promote physical activity, and improve outcomes (Wiederhold et al., 2019; Ghlichi Moghaddam et al., 2023). Patients with chronic heart diseases often face challenges in adopting healthier lifestyles. While hospital-based rehabilitation programs improve quality of life and exercise capacity, many are unable to participate due to frailty or distance. Telerehabilitation, particularly when enhanced with technologies like AugR, offers a promising alternative (Mocan et al., 2022). A user-centered AugR platform tailored to cardiac patients’ preferences overcomes barriers to use and compliance, improving empowerment in future research (Cerdán de Las Heras et al., 2022). Certain rehabilitation tools used in both physical and cardiac rehabilitation, across inpatient and outpatient settings, combine a standard monitor with motion-tracking cameras, enabling patients to control a virtual avatar in a 3D environment. AugR enhances this experience by amplifying movement feedback, thereby improving the effectiveness of the rehabilitation process (Southworth et al., 2020a). However, technical issues, such as motion tracking accuracy and internet connectivity, may further affect usability and effectiveness.
Mixed reality and beyond
Mixed reality (MR) creates interactive 3D projections that not only overlay but also anchor virtual objects in the real world, unlike AugR, which simply overlays them (Smith et al., 2020). Compared to AR, it demands a higher level of integration between physical and digital elements, with virtual components making up a larger portion of the environment (Hu et al., 2019). This immersive technology has been shown to improve medication knowledge, making it a practical tool for enhancing patient education after myocardial infarction (Hilt et al., 2020). In cardiac electrophysiology, certain MR systems offer real-time 3D visualization of cardiac geometry and catheter positioning, supporting precision and workflow (Bloom et al., 2023). MR has also proven effective in CT-based pre-procedural planning for interventions such as Left Atrial Appendage Occlusion (LAAO), where enhanced 3D perception improves accuracy and efficiency during the planning phase (Pasquali et al., 2022). MR, validated through technical, preclinical, and clinical assessments conducted by interventional cardiologists, has successfully demonstrated precision (Lippert et al., 2024) and user-friendliness (Xander et al., 2024). Additionally, MR HMD are safe and effective in reducing procedure time in the cardiac catheterization laboratory (Chahi et al., 2022). Verifying the clinical reliability of off-the-shelf hardware may further accelerate MR adoption in medical practice (Southworth et al., 2020b).
AugR in 3D/4D printing
Technologies like 4D/3D printing and AugR/MR are transforming healthcare into highly digital and data-driven, marking a shift to intelligent medicine (Gao and Ye, 2021). Holographic visualization techniques in 3D/4D printing are areas of future potential in cardiac management (D’Aiello et al., 2023). AugR can be used for designing patient-specific prosthetics, which are later 4D printed, and predicting how these would behave in a beating heart before implantation (Buyck et al., 2024). By providing a dynamic, interactive view of anatomical structures and their motion throughout the cardiac cycle, AugR allows for precise assessment of shape, orientation, and functional behavior (Torab-Miandoab et al., 2023). This detailed anatomical mapping supports the development of 4D-printed devices that not only conform to the patient’s unique cardiac geometry but also adapt to physiological conditions over time (Buyck et al., 2024). These include valves that replicate natural motion, stents responsive to vessel dynamics, and bioengineered patches that replace infarcted myocardium and integrate with native tissue (Torab-Miandoab et al., 2023). Holographic projections enable accurate virtual positioning of these devices before implantation, supporting pre-surgical planning and personalized customization (Torab-Miandoab et al., 2023). This made it possible to create a translucent 3D-printed heart model for the treatment of sinus venosus atrial septal defect as well as CHD, which allowed for in vitro testing of the procedure and the prediction of postoperative effects (Chessa et al., 2022; Butera et al., 2019).
Other potential applications of AugR
Another potential application of AugR has been demonstrated in cardiac transplantology. By scanning both the donor and recipient at a specific stage of the transplantation process, a physician could use AugR to align the two in a unique 3D reconstruction. This would allow the physician to assess whether the donor’s heart and lungs are appropriately sized to fit the recipient’s body. This could potentially be used for other organ transplants as well (Kosieradzki et al., 2020). Accuracy of procedures such as balloon pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension and stent implantation for pulmonary artery stenosis, respectively, can also be enhanced using AugR (Witowski et al., 2019). One promising area of investigation is the application of AugR to address persistent ischemia in patients following percutaneous coronary intervention (PCI). In particular, Quantitative Flow Ratio (QFR)-based virtual PCI utilizes AugR to simulate post-procedural outcomes by integrating pre-procedural angiograms with computational coronary physiology. This approach enables interventionalists to preselect optimal vessels, segments, and stents, potentially enhancing physiological outcomes and reducing residual ischemia (Xu and Zhang, 2023). Traditional simulation labs have limited ability to visually represent real-time mental, respiratory, and perfusion changes, a gap that AugR-enhanced training can effectively address (Zackoff et al., 2021).
Discussion and limitations
Despite the promising applications of AugR in cardiology, several limitations hinder its widespread adoption in clinical settings. The high implementation costs, including expenses for specialized hardware, software licensing, and ongoing maintenance, pose a significant financial barrier for many healthcare institutions (Rad et al., 2022; Murali et al., 2021). Additionally, AugR systems often require high-speed wireless internet connections to stream digital information, which may not be available in all healthcare settings (FDA, 2025). Existing healthcare technologies are often not implemented in resource-limited countries due to financial constraints (Torab-Miandoab et al., 2023). As a result, many low- and middle-income regions continue to rely on 2D flat-screen visualizations, which lack the realistic depth and spatial understanding of the three-dimensional relationship between anatomy and pathology (Shrestha et al., 2024). As technology advances, more affordable and effective solutions will hopefully make AugR widely accessible and practical in clinical settings (Rad et al., 2022).
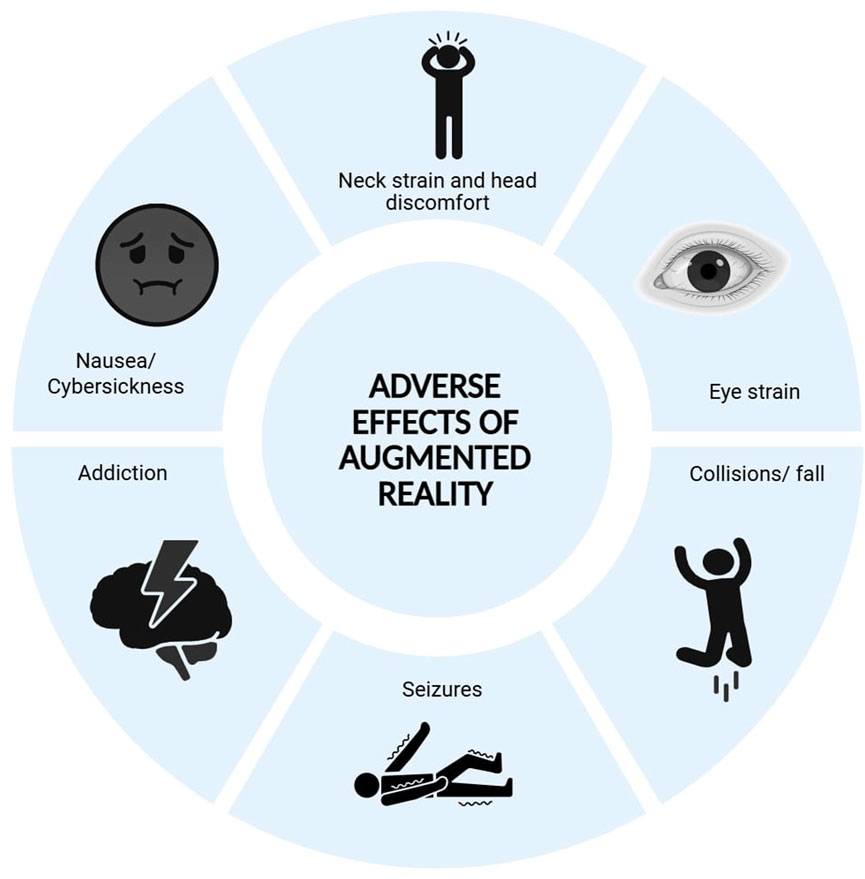
Concerns around AugR use in healthcare include data security, regulatory compliance, and the absence of standardized frameworks (Warren et al., 2022; Keramati et al., 2024). Interoperability issues with existing hospital information systems may limit seamless integration into routine workflows (Keramati et al., 2024). These devices pose unique privacy risks by tracking detailed user behavior through cameras and sensors, making data breaches potentially serious (Lehman et al., 2022). Training medical and technical staff involves a significant learning curve, requiring considerable time and resources, which may temporarily impact clinical efficiency during the early stages of implementation (Ramsay et al., 2001). Furthermore, AugR raises complex legal challenges involving privacy, intellectual property, liability, and user safety, underscoring the need for clear legal and ethical frameworks to ensure user protection and responsible deployment (Tyagi, 2025). Ergonomic issues with heavy headsets and handheld controllers can cause neck strain, eye fatigue, and repetitive stress injuries, which could pose challenges in clinical settings (Jang et al., 2018; Wiederhold, 2022). The immersive nature of AugR can also reduce situational awareness, increasing the likelihood of collisions or falls, while cybersickness, marked by nausea, dizziness, and fatigue, disproportionately affects vulnerable groups such as women, children, and older adults (FDA, 2025). AugR-based gamified therapies may become habit-forming, potentially leading patients to neglect physical activity and self-care. Additionally, individuals with photosensitive epilepsy are at risk of seizures due to the full-field flickering effects of some headsets (FDA, 2025). Moreover, current research on AugR in cardiology is largely confined to in silico experiments, simulated environments, or isolated case studies, which are susceptible to biased reporting and lack generalizability (Prakosa et al., 2021; Bui et al., 2023). Figure 4 shows the adverse effects associated with the use of AugR. These limitations underscore the need for large-scale, multicenter clinical trials and standardized implementation frameworks to validate and support the broader use of AugR in cardiovascular care. Because this technology has only recently become more widely adopted, other unknown risks may emerge over time (FDA, 2025). Despite these limitations, we believe that AugR should not replace human-human interaction, serving as an assistive tool to enhance efficient, high-quality care.
Conclusion
In summary, augmented reality technology has the potential to revolutionize cardiology by enhancing diagnostic accuracy, improving patient education and cardiac rehabilitation, and advancing cardiac surgery through increased precision, reduced invasiveness, and improved procedural planning and outcomes. However, widespread clinical adoption remains limited by technical challenges, high costs, and the need for rigorous validation through clinical trials. Additionally, we encourage further exploration of technologies such as 3D/4D printing and holography, which together hold promise for creating a safer, more precise, and more efficient future in cardiac care.
Author contributions
II: Conceptualization, Investigation, Resources, Visualization, Writing – original draft. DR: Writing – review and editing, Data curation, Visualization. SJ: Conceptualization, Resources, Writing – original draft. BS: Conceptualization, Resources, Visualization, Writing – original draft. RG: Conceptualization, Data curation, Writing – original draft. YG: Writing – original draft. UT: Writing – original draft. RS: Writing – original draft. SY: Supervision, Writing – review and editing. VS: Supervision, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Ansi, A. M., Jaboob, M., Garad, A., and Al-Ansi, A. (2023). Analyzing augmented reality (AR) and virtual reality (VR) recent development in education. Soc. Sci. Humanit Open 8 (1), 100532. doi:10.1016/j.ssaho.2023.100532
Annabestani, M., Olyanasab, A., and Mosadegh, B. (2024). Application of mixed/augmented reality in interventional cardiology. J. Clin. Med. 13 (15), 4368. doi:10.3390/jcm13154368
Arslan, F., and Gerckens, U. (2021). Virtual support for remote proctoring in TAVR during COVID-19. Catheter Cardiovasc Interv. 98 (5), E733–E736. doi:10.1002/ccd.29504
Avari, S. J. N., Southworth, M. K., Blume, W. M., Andrews, C., Van Hare, G. F., Dalal, A. S., et al. (2020). First-in-human use of a mixed reality display during cardiac ablation procedures. JACC Clin. Electrophysiol. 6 (8), 1023–1025. doi:10.1016/j.jacep.2020.04.036
Ballocca, F., Meier, L. M., Ladha, K., Qua Hiansen, J., Horlick, E. M., and Meineri, M. (2019). Validation of quantitative 3-dimensional transesophageal echocardiography mitral valve analysis using stereoscopic display. J. Cardiothorac. Vasc. Anesth. 33 (3), 732–741. doi:10.1053/j.jvca.2018.08.013
Bamps, K., Bertels, J., Minten, L., Puvrez, A., Coudyzer, W., De Buck, S., et al. (2024). Phantom study of augmented reality framework to assist epicardial punctures. J. Med. Imaging (Bellingham) 11 (3), 035002. doi:10.1117/1.jmi.11.3.035002
Banerjee, A., Camps, J., Zacur, E., Andrews, C. M., Rudy, Y., Choudhury, R. P., et al. (2021). A completely automated pipeline for 3D reconstruction of human heart from 2D cine magnetic resonance slices. Philos. Trans. A Math. Phys. Eng. Sci. 379 (2212), 20200257. doi:10.1098/rsta.2020.0257
Belhaj, S. R., Verhoye, J. P., Nguyen Duc, H., Castro, M., Auffret, V., Anselmi, A., et al. (2016). Computer-assisted transcatheter heart valve implantation in Valve-in-Valve procedures. Innovations 11 (3), 193–200. doi:10.1177/155698451601100307
Bernardi, F. L. M., Dvir, D., Rodes-Cabau, J., and Ribeiro, H. B. (2019). Valve-in-Valve challenges: how to avoid coronary obstruction. Front. Cardiovasc Med. 6, 120. doi:10.3389/fcvm.2019.00120
Bindschadler, M., Buddhe, S., Ferguson, M. R., Jones, T., Friedman, S. D., and Otto, R. K. (2022). HEARTBEAT4D: an open-source toolbox for turning 4D cardiac CT into VR/AR. J. Digit. Imaging 35 (6), 1759–1767. doi:10.1007/s10278-022-00659-y
Bloom, D., Catherall, D., Miller, N., Southworth, M. K., Glatz, A. C., Silva, J. R., et al. (2023). Use of a mixed reality system for navigational mapping during cardiac electrophysiological testing does not prolong case duration: a subanalysis from the cardiac augmented REality study. Cardiovasc Digit. Health J. 4 (4), 111–117. doi:10.1016/j.cvdhj.2023.06.003
Britannica (2025). “The editors of encyclopedia britannica. computer vision,” in Encyclopedia britannica. Available online at: https://www.britannica.com/technology/computer-vision.
Bui, D. T., Barnett, T., Hoang, H., and Chinthammit, W. (2023). Usability of augmented reality technology in situational telementorship for managing clinical scenarios: quasi-experimental study. JMIR Med. Educ. 9, e47228. doi:10.2196/47228
Butera, G., Sturla, F., Pluchinotta, F. R., Caimi, A., and Carminati, M. (2019). Holographic augmented reality and 3D printing for advanced planning of sinus venosus ASD/partial anomalous pulmonary venous return percutaneous management. JACC Cardiovasc Interv. 12 (14), 1389–1391. doi:10.1016/j.jcin.2019.03.020
Buyck, D., Gherciuc, S., Gorbaty, B., Escudero, E. V., Arango, S., Perry, T., et al. (2024). “Virtual and augmented realities for cardiac education and device training,” in Handbook of cardiac anatomy, physiology, and devices (Cham: Springer Nature Switzerland), 967–981.
Caluya, N. R., Plopski, A., Sandor, C., Fujimoto, Y., Kanbara, M., and Kato, H. (2022). Does overlay field of view in head-mounted displays affect spatial memorization? Comput. Graph 102, 554–565. doi:10.1016/j.cag.2021.09.004
Cerdán de Las Heras, J., Tulppo, M., Kiviniemi, A. M., Hilberg, O., Løkke, A., Ekholm, S., et al. (2022). Augmented reality glasses as a new tele-rehabilitation tool for home use: patients’ perception and expectations. Disabil. Rehabil. Assist. Technol. 17 (4), 480–486. doi:10.1080/17483107.2020.1800111
Chahine, J., Mascarenhas, L., George, S. A., Bartos, J., Yannopoulos, D., Raveendran, G., et al. (2022). Effects of a mixed-reality headset on procedural outcomes in the cardiac catheterization laboratory. Cardiovasc Revasc Med. 45, 3–8. doi:10.1016/j.carrev.2022.08.009
Cheng, C., and Ebrahimi, O. V. (2023). Gamification: a novel approach to mental health promotion. Curr. Psychiatry Rep. 25 (11), 577–586. doi:10.1007/s11920-023-01453-5
Chessa, M., Van De Bruaene, A., Farooqi, K., Valverde, I., Jung, C., Votta, E., et al. (2022). Three-dimensional printing, holograms, computational modelling, and artificial intelligence for adult congenital heart disease care: an exciting future. Eur. Heart J. 43 (28), 2672–2684. doi:10.1093/eurheartj/ehac266
Choi, S., Nah, S., Cho, Y. S., Moon, I., Lee, J. W., Ah Lee, C., et al. (2024). Accuracy of visual estimation of ejection fraction in patients with heart failure using augmented reality glasses. Heart 110 (6), 432–440. doi:10.1136/heartjnl-2023-323067
Currie, M. E., McLeod, A. J., Moore, J. T., Chu, M. W. A., Patel, R., Kiaii, B., et al. (2016). Augmented reality system for ultrasound guidance of transcatheter aortic valve implantation. Innov. (Phila) 11 (1), 31–39. doi:10.1177/155698451601100106
D’Aiello, A. F., Bognoni, L., Bevilacqua, F., Negura, D. G., Ferrero, P., Micheletti, A., et al. (2023). Holographic techniques as a novel method for intervention planning: a tertiary centres experience. Curr. Health Sci. J. 49 (4), 584–593. doi:10.12865/CHSJ.49.04.15
De Buck, S., Maes, F., Ector, J., Bogaert, J., Dymarkowski, S., Heidbüchel, H., et al. (2005). An augmented reality system for patient-specific guidance of cardiac catheter ablation procedures. IEEE Trans. Med. Imaging 24 (11), 1512–1524. doi:10.1109/tmi.2005.857661
Doughty, M., Ghugre, N. R., and Wright, G. A. (2022). Augmenting performance: a systematic review of optical see-through head-mounted displays in surgery. J. Imaging 8 (7), 203. doi:10.3390/jimaging8070203
EchoPixel, Inc (2025). EchoPixel, inc. Available online at: https://echopixeltech.com/.
FDA (2024). FDA clears Microsoft’s HoloLens for pre-operative surgical planning. Available online at: https://www.fdanews.com/articles/188966-fda-clears-microsofts-hololens-for-pre-operative-surgical-planning.
FDA (2025). PEAC executive summary–augmented reality (AR) and virtual reality (VR) medical devices. Available online at: https://www.fda.gov/media/159709/download.
Flavián, C., Ibáñez-Sánchez, S., and Orús, C. (2019). The impact of virtual, augmented and mixed reality technologies on the customer experience. J. Bus. Res. 100, 547–560. doi:10.1016/j.jbusres.2018.10.050
Gao, F., and Ye, Z. W. (2021). A brief history of intelligent medicine. Zhonghua Yi Shi Za Zhi 51 (2), 97–102. doi:10.3760/cma.j.cn112155-20201229-00205
Ghlichi Moghaddam, N., Namazinia, M., Hajiabadi, F., and Mazlum, S. R. (2023). The efficacy of phase I cardiac rehabilitation training based on augmented reality on the self-efficacy of patients undergoing coronary artery bypass graft surgery: a randomized clinical trial. BMC Sports Sci. Med. Rehabil. 15 (1), 156. doi:10.1186/s13102-023-00770-9
Goo, H. W., Park, S. J., and Yoo, S. J. (2020). Advanced medical use of three-dimensional imaging in congenital heart disease: augmented reality, mixed reality, virtual reality, and three-dimensional printing. Korean J. Radiol. 21 (2), 133–145. doi:10.3348/kjr.2019.0625
Haleem, A., Javaid, M., and Khan, I. H. (2020). Holography applications toward medical field: an overview. Indian J. Radiol. Imaging 30 (3), 354–361. doi:10.4103/ijri.ijri_39_20
Hess, O., Qian, J., Bruce, J., Wang, E., Rodriguez, S., Haber, N., et al. (2022). Communication skills training using remote augmented reality medical simulation: a feasibility and acceptability qualitative study. Med. Sci. Educ. 32 (5), 1005–1014. doi:10.1007/s40670-022-01598-7
Hilt, A. D., Mamaqi Kapllani, K., Hierck, B. P., Kemp, A. C., Albayrak, A., Melles, M., et al. (2020). Perspectives of patients and professionals on information and education after myocardial infarction with insight for mixed reality implementation: cross-sectional interview study. JMIR Hum. Factors 7 (2), e17147. doi:10.2196/17147
Hou, L., Dong, X., Li, K., Yang, C., Yu, Y., Jin, X., et al. (2022). Comparison of augmented reality-assisted and instructor-assisted cardiopulmonary resuscitation: a simulated randomized controlled pilot trial. Clin. Simul. Nurs. 68, 9–18. doi:10.1016/j.ecns.2022.04.004
Hu, H. Z., Feng, X. B., Shao, Z. W., Xie, M., Xu, S., Wu, X. H., et al. (2019). Application and prospect of mixed reality technology in medical field. Curr. Med. Sci. 39 (1), 1–6. doi:10.1007/s11596-019-1992-8
Ingrassia, P. L., Mormando, G., Giudici, E., Strada, F., Carfagna, F., Lamberti, F., et al. (2020). Augmented reality learning environment for basic life support and defibrillation training: usability study. J. Med. Internet Res. 22 (5), e14910. doi:10.2196/14910
Iribarne, A., Easterwood, R., Chan, E. Y. H., Yang, J., Soni, L., Russo, M. J., et al. (2011). The golden age of minimally invasive cardiothoracic surgery: current and future perspectives. Future Cardiol. 7 (3), 333–346. doi:10.2217/fca.11.23
Jang, J., Tschabrunn, C. M., Barkagan, M., Anter, E., Menze, B., and Nezafat, R. (2018). Three-dimensional holographic visualization of high-resolution myocardial scar on HoloLens. PLoS One 13 (10), e0205188. doi:10.1371/journal.pone.0205188
Jang, Y. E., Cho, S. A., Ji, S. H., Kim, E. H., Lee, J. H., Kim, H. S., et al. (2021). Smart glasses for radial arterial catheterization in pediatric patients: a randomized clinical trial. Anesthesiology 135 (4), 612–620. doi:10.1097/aln.0000000000003914
Jung, C., Wolff, G., Wernly, B., Bruno, R. R., Franz, M., Schulze, P. C., et al. (2022). Virtual and augmented reality in cardiovascular care: state-of-the-art and future perspectives. JACC Cardiovasc Imaging 15 (3), 519–532. doi:10.1016/j.jcmg.2021.08.017
Karbasi, Z., and Niakan Kalhori, S. R. (2020). Application and evaluation of virtual technologies for anatomy education to medical students: a review. Med. J. Islam Repub. Iran. 34, 163. doi:10.47176/mjiri.34.163
Keramati, H., Lu, X., Cabanag, M., Wu, L., Kushwaha, V., and Beier, S. (2024). Applications and advances of immersive technology in cardiology. Curr. Probl. Cardiol. 49 (10), 102762. doi:10.1016/j.cpcardiol.2024.102762
Kosieradzki, M., Lisik, W., Gierwiało, R., and Sitnik, R. (2020). Applicability of augmented reality in an organ transplantation. Ann. Transpl. 25, e923597. doi:10.12659/aot.923597
Kuklik, P., Bidar, E., Gharaviri, A., Maessen, J., and Schotten, U. (2014). Application of phase coherence in assessment of spatial alignment of electrodes during simultaneous endocardial-epicardial direct contact mapping of atrial fibrillation. Europace 16 (Suppl. 4), iv135–iv140. doi:10.1093/europace/euu247
LaunchSquad (2017). EchoPixel announces progress in the clinical adoption of interactive virtual reality for pediatric surgery. Available online at: http://www.marketwired.com/press-release/echopixel-announces-progress-clinical-adoption-interactive-virtual-reality-pediatric-2202796.htm.
Lehman, S. M., Alrumayh, A. S., Kolhe, K., Ling, H., and Tan, C. C. (2022). Hidden in plain sight: exploring privacy risks of mobile augmented reality applications. ACM Trans. Priv. Secur 25 (4), 1–35. doi:10.1145/3524020
Lippert, M., Dumont, K. A., Birkeland, S., Nainamalai, V., Solvin, H., Suther, K. R., et al. (2024). Cardiac anatomic digital twins: findings from a single national centre. Eur. Heart J. Digit. Health 5 (6), 725–734. doi:10.1093/ehjdh/ztae070
Liu, J., Al’Aref, S. J., Singh, G., Caprio, A., Moghadam, A. A. A., Jang, S. J., et al. (2019). An augmented reality system for image guidance of transcatheter procedures for structural heart disease. PLoS One 14 (7), e0219174. doi:10.1371/journal.pone.0219174
Lodziński, P. R., Balsam, P., Peller, M., Kamiński, J., and Opolski, G. (2018). First-in-man percutaneous pulmonary vein isolation enhanced by augmented reality system. Kardiol. Pol. 76 (2), 475. doi:10.5603/kp.2018.0046
Masson, J. B., Kovac, J., Schuler, G., Ye, J., Cheung, A., Kapadia, S., et al. (2009). Transcatheter aortic valve implantation: review of the nature, management, and avoidance of procedural complications. JACC Cardiovasc Interv. 2 (9), 811–820. doi:10.1016/j.jcin.2009.07.005
Minga, I., Al-Ani, M. A., Moharem-Elgamal, S., Md, A. V. H., Md, A. S. A., Masoomi, M., et al. (2024). Use of virtual reality and 3D models in contemporary practice of cardiology. Curr. Cardiol. Rep. 26 (6), 643–650. doi:10.1007/s11886-024-02061-2
Mitrović, U., Špiclin, Ž., Likar, B., and Pernuš, F. (2014). “Automatic detection of misalignment in rigid 3D-2D registration,” in Clinical image-based procedures translational research in medical imaging (Cham: Springer International Publishing), 117–124.
Mocan, B., Mocan, M., Fulea, M., Murar, M., and Feier, H. (2022). Home-based robotic upper limbs cardiac telerehabilitation system. Int. J. Environ. Res. Public Health 19 (18), 11628. doi:10.3390/ijerph191811628
Moon, I. T., Ko, S. K., Kang, S. H., Yoon, C. H., Youn, T. J., and Chae, I. H. (2024). Augmented reality in cardiology: enhancing visualization and precision. Curr. Cardiovasc Risk Rep. 18 (12), 175–186. doi:10.1007/s12170-024-00744-7
Murali, S., Paul, K. D., McGwin, G., and Ponce, B. A. (2021). Updates to the current landscape of augmented reality in medicine. Cureus 13 (5), e15054. doi:10.7759/cureus.15054
Nanchahal, S., Arjomandi Rad, A., Naruka, V., Chacko, J., Liu, G., Afoke, J., et al. (2024). Mitral valve surgery assisted by virtual and augmented reality: cardiac surgery at the front of innovation. Perfusion 39 (2), 244–255. doi:10.1177/02676591221137480
Opolski, M. P., Michałowska, I. M., Borucki, B. A., Nicińska, B., Szumowski, Ł., and Sterliński, M. (2018). Augmented-reality computed tomography-guided transcatheter pacemaker implantation in dextrocardia and congenitally corrected transposition of great arteries. Cardiol. J. 25 (3), 412–413. doi:10.5603/cj.2018.0058
Palumbo, A. (2022). Microsoft HoloLens 2 in medical and healthcare context: state of the art and future prospects. Sensors (Basel) 22 (20), 7709. doi:10.3390/s22207709
Parham, G., Bing, E. G., Cuevas, A., Fisher, B., Skinner, J., Mwanahamuntu, M., et al. (2019). Creating a low-cost virtual reality surgical simulation to increase surgical oncology capacity and capability. Ecancermedicalscience 13, 910. doi:10.3332/ecancer.2019.910
Pasquali, M., Fusini, L., Italiano, G., Maltagliati, A., Tamborini, G., Penso, M., et al. (2022). Feasibility study of a mixed reality tool for real 3D visualization and planning of left atrial appendage occlusion. J. Cardiovasc Comput. Tomogr. 16 (5), 460–462. doi:10.1016/j.jcct.2022.02.010
Pinto-Coelho, L. (2023). How artificial intelligence is shaping medical imaging technology: a survey of innovations and applications. Bioeng. (Basel) 10 (12), 1435. doi:10.3390/bioengineering10121435
Prakosa, A., Southworth, M. K., Avari Silva, J. N., Silva, J. R., and Trayanova, N. A. (2021). Impact of augmented-reality improvement in ablation catheter navigation as assessed by virtual-heart simulations of ventricular tachycardia ablation. Comput. Biol. Med. 133, 104366. doi:10.1016/j.compbiomed.2021.104366
Rad, A. A., Vardanyan, R., Lopuszko, A., Alt, C., Stoffels, I., Schmack, B., et al. (2022). Virtual and augmented reality in cardiac surgery. Braz J. Cardiovasc Surg. 37 (1), 123–127. doi:10.21470/1678-9741-2020-0511
Ramírez, W. A., Gizzi, A., Sack, K. L., Guccione, J. M., and Hurtado, D. E. (2020). In-silico study of the cardiac arrhythmogenic potential of biomaterial injection therapy. Sci. Rep. 10 (1), 12990. doi:10.1038/s41598-020-69900-4
Ramsay, C. R., Grant, A. M., Wallace, S. A., Garthwaite, P. H., Monk, A. F., and Russell, I. T. (2001). Statistical assessment of the learning curves of health technologies. Health Technol. Assess. 5 (12), 1–79. doi:10.3310/hta5120
Rizzo, M. G., Costello, J. P., Luxenburg, D., Cohen, J. L., Alberti, N., and Kaplan, L. D. (2023). Augmented reality for perioperative anxiety in patients undergoing surgery: a randomized clinical trial. JAMA Netw. Open 6 (8), e2329310. doi:10.1001/jamanetworkopen.2023.29310
Salavitabar, A., Whiteside, W., and Zampi, J. D. (2023). Feasibility of intraprocedural augmented reality visualisation of 3D rotational angiography in congenital cardiac catheterisation. Cardiol. Young 33 (3), 476–478. doi:10.1017/s1047951122002153
Sentiar (2025). SentiAR – realtime clinical AR. Available online at: https://sentiar.com/.
Shah, S., Chryssos, E. D., and Parker, H. (2009). Magnetic resonance imaging: a wealth of cardiovascular information. Ochsner J. 9 (4), 266–277.
Shrestha, A. B., Taha, A. M., Siddiq, A., Shrestha, S., Thakur, P., Chapagain, S., et al. (2024). Virtual and augmented reality in cardiovascular care in low and middle income country. Curr. Probl. Cardiol. 49 (3), 102380. doi:10.1016/j.cpcardiol.2024.102380
Silva, J. N. A., Southworth, M., Raptis, C., and Silva, J. (2018). Emerging applications of virtual reality in cardiovascular medicine. JACC Basic Transl. Sci. 3 (3), 420–430. doi:10.1016/j.jacbts.2017.11.009
Smeragliuolo, A. H., Hill, N. J., Disla, L., and Putrino, D. (2016). Validation of the leap motion controller using markered motion capture technology. J. Biomech. 49 (9), 1742–1750. doi:10.1016/j.jbiomech.2016.04.006
Smith, R. T., Clarke, T. J., Mayer, W., Cunningham, A., Matthews, B., and Zucco, J. E. (2020). Mixed reality interaction and presentation techniques for medical visualisations. Adv. Exp. Med. Biol. 1260, 123–139. doi:10.1007/978-3-030-47483-6_7
Sorriento, A., Porfido, M. B., Mazzoleni, S., Calvosa, G., Tenucci, M., Ciuti, G., et al. (2020). Optical and electromagnetic tracking systems for biomedical applications: a critical review on potentialities and limitations. IEEE Rev. Biomed. Eng. 13, 212–232. doi:10.1109/rbme.2019.2939091
Southworth, M. K., Silva, J. N. A., Blume, W. M., Van Hare, G. F., Dalal, A. S., and Silva, J. R. (2020b). Performance evaluation of mixed reality display for guidance during transcatheter cardiac mapping and ablation. IEEE J. Transl. Eng. Health Med. 8, 1–10. doi:10.1109/jtehm.2020.3007031
Southworth, M. K., Silva, J. R., and Silva, J. N. A. (2020a). Use of extended realities in cardiology. Trends Cardiovasc Med. 30 (3), 143–148. doi:10.1016/j.tcm.2019.04.005
Speicher, M., Hall, B. D., and Nebeling, M. (2019). “What is mixed reality?,” in Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems, (New York, NY, USA: ACM). doi:10.1145/3290605.3300767
Sutherland, I. E. (1968). “A head-mounted three dimensional display,” in Proceedings of the December 9-11, 1968, fall joint computer conference, part I, (New York, NY, USA: Association for Computing Machinery), 757–764.
Thomas, C., and Mizell, D. (1992). Augmented reality: an application of heads-up display technology to manual manufacturing processes. 659–669.
Torab-Miandoab, A., Samad-Soltani, T., Jodati, A., and Rezaei-Hachesu, P. (2023). Interoperability of heterogeneous health information systems: a systematic literature review. BMC Med. Inf. Decis. Mak. 23 (1), 18. doi:10.1186/s12911-023-02115-5
Tsai, A., Bodmer, N., Hong, T., Frackman, A., Hess, O., Khoury, M., et al. (2023). Participant perceptions of augmented reality simulation for cardiac anesthesiology training: a prospective, mixed-methods study. J. Educ. Perioper. Med. 25 (3), E712. doi:10.46374/volxxv_issue3_Tsai
Tyagi, A. (2025). Regulating the metaverse: legal challenges in virtual worlds and digital assets. SSRN. doi:10.2139/ssrn.5267789
Uppot, R. N., Laguna, B., McCarthy, C. J., De Novi, G., Phelps, A., Siegel, E., et al. (2019). Implementing virtual and augmented reality tools for radiology education and training, communication, and clinical care. Radiology 291 (3), 570–580. doi:10.1148/radiol.2019182210
Warren, A. E., Tham, E., and Abeysekera, J. (2022). Some things change, some things stay the same: trends in Canadian education in paediatric cardiology and the cardiac sciences. CJC Pediatr. Congenit. Heart Dis. 1 (5), 232–240. doi:10.1016/j.cjcpc.2022.08.004
Weidemann, F., Liu, D., Niemann, M., Herrmann, S., Hu, H., Gaudron, P. D., et al. (2015). Assessment of systolic function in patients with poor echogenicity: echocardiographic methods. Herz 40 (2), 240–249. doi:10.1007/s00059-013-3924-x
Wiederhold, B. K. (2022). Virtual experiences causing real injuries: what can be done? Cyberpsychol Behav. Soc. Netw. 25 (4), 211–212. doi:10.1089/cyber.2022.29244.editorial
Wiederhold, M. D., Crisci, M., Patel, V., Nonaka, M., and Wiederhold, B. K. (2019). Physiological monitoring during augmented reality exercise confirms advantages to health and well-being. Cyberpsychol Behav. Soc. Netw. 22 (2), 122–126. doi:10.1089/cyber.2018.0027
Witkowski, M., and Lodziński, P. (2019). Cardiac resynchronization device implantation supported by augmented reality visualization of computed tomography angiography reconstruction of the coronary Polish heart. Available online at: https://journals.viamedica.pl/polish_heart_journal/article/download/82316/61651.
Witowski, J., Darocha, S., Kownacki, Ł., Pietrasik, A., Pietura, R., Banaszkiewicz, M., et al. (2019). Augmented reality and three-dimensional printing in percutaneous interventions on pulmonary arteries. Quant. Imaging Med. Surg. 9 (1), 23–29. doi:10.21037/qims.2018.09.08
Xander, J., Kobe, B., Ruben, M., Christophe, D., Filip, R., Peter, V., et al. (2024). Augmented and virtual reality imaging for collaborative planning of structural cardiovascular interventions: a proof-of-concept and validation study. J. Med. Imaging (Bellingham) 11 (6), 062606. doi:10.1117/1.jmi.11.6.062606
Xu, B., and Zhang, R. (2023). Virtual PCI powered by augmented reality: pave the way to optimal revascularization. JACC Cardiovasc Interv. 16 (7), 795–797. doi:10.1016/j.jcin.2022.11.012
Zackoff, M. W., Cruse, B., Sahay, R. D., Fei, L., Saupe, J., Schwartz, J., et al. (2021). Development and implementation of augmented reality enhanced high-fidelity simulation for recognition of patient decompensation. Simul. Healthc. 16 (3), 221–230. doi:10.1097/sih.0000000000000486
Zari, G., Condino, S., Cutolo, F., and Ferrari, V. (2023). Magic leap 1 versus microsoft HoloLens 2 for the visualization of 3D content obtained from radiological images. Sensors (Basel) 23 (6), 3040. doi:10.3390/s23063040
Zou, Y. B., Chen, Y. M., Gao, M. K., Liu, Q., Jiang, S. Y., Lu, J. H., et al. (2017). Coronary heart disease preoperative gesture interactive diagnostic system based on augmented reality. J. Med. Syst. 41 (8), 126. doi:10.1007/s10916-017-0768-6
Keywords: augmented reality, mixed reality, cardiology, virtual reality, holography, 4D printing
Citation: Ismail Sharieff I, Ravikumar DB, Joshi S, Sivasubramanian BP, Gupta R, Garg Y, Thirupathy U, Saravanabavanandan R, Yarrarapu SN and Samala Venkata VR (2025) Applications of augmented reality in cardiology till 2024: a comprehensive review of innovations and clinical impacts. Front. Virtual Real. 6:1580619. doi: 10.3389/frvir.2025.1580619
Received: 20 February 2025; Accepted: 10 July 2025;
Published: 23 July 2025.
Edited by:
Jennifer N Avari Silva, Washington University in St. Louis, United StatesCopyright © 2025 Ismail Sharieff, Ravikumar, Joshi, Sivasubramanian, Gupta, Garg, Thirupathy, Saravanabavanandan, Yarrarapu and Samala Venkata. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ibthisam Ismail Sharieff, ZHJpYnRoaXNhbUBnbWFpbC5jb20=; Diviya Bharathi Ravikumar, ZGl2aXlhYmhhcmF0aGkyNjEyQGdtYWlsLmNvbQ==
 Ibthisam Ismail Sharieff
Ibthisam Ismail Sharieff Diviya Bharathi Ravikumar
Diviya Bharathi Ravikumar Shashvat Joshi
Shashvat Joshi Barath Prashanth Sivasubramanian
Barath Prashanth Sivasubramanian Rajat Gupta3
Rajat Gupta3 Umabalan Thirupathy
Umabalan Thirupathy Ragavendar Saravanabavanandan
Ragavendar Saravanabavanandan Siva Naga Yarrarapu
Siva Naga Yarrarapu