- 1AppliedVR, Inc., Van Nuys, CA, United States
- 2Neptune Medical, Burlingame, CA, United States
- 3Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University School of Medicine, Palo Alto, CA, United States
Chronic lower back pain (cLBP) is the most prevalent pain condition globally. Pain education and cognitive behavior therapy (CBT) are one of many recommended front-line treatments, but access is poor due to barriers such as few trained and available local therapists, health insurance limits, and burdens associated with travel and treatment time. Immersive therapeutics, such as virtual reality-delivered therapy, might provide an effective, low-risk, and accessible cLBP treatment. This manuscript describes the path followed to develop, obtain FDA-authorization for, and commercially launch a Virtual Reality-Delivered Skills-Based therapy for cLBP, called RelieVRx®. We detail the iterative path to design, develop and validate this immersive therapeutic medical device and the process followed to obtain FDA-authorization. We briefly summarize the results from over 30 publications that empirically test iterations of the VR-delivered therapy. Key lessons for translating innovation from the laboratory into the commercial market are identified, including commercial launch, reimbursement strategy, and clinical implementation in the home.
Clinical Trial Registration: ClinicalTrials.gov, identifier NCT05263037.
Introduction
Chronic lower back pain (cLBP) affects over 600 million adults around the world and is the most prevalent pain condition worldwide (Institute of Medicine US, 2011). With opioid prescribing decreasing, the US Centers for Disease Control and Prevention (CDC) and US Centers for Medicare and Medicaid Services (CMS) (Dowell et al., 2022; Singh et al., 2019; Traylor, 2019) are calling for low-risk, accessible, and long-term effective nonpharmacologic behavioral interventions to help clinicians treat cLBP. Pain education, cognitive behavior therapy (CBT) and other behavioral interventions are one of many recommended front-line therapies (Foster et al., 2018; Williams et al., 2020; Monticone et al., 2015; Cherkin et al., 2016), but access is poor due to barriers such as few trained and available local therapists, health insurance limits, and burdens associated with travel and treatment time (Darnall et al., 2016; Day et al., 2023).
Immersive therapeutics, such as virtual reality (VR)-delivered therapy, can provide therapeutic content in a consistent, quality-controlled manner that is effective, low-risk, and accessible in the home (Maddox et al., 2023a; Brennan, 2020). Unlike other digital technologies (e.g., smartphone mobile applications), VR is immersive and thus delivers therapeutic content in a way that broadly engages multiple centers in the brain in synchrony (Maddox et al., 2023b; Maddox and Fitzpatrick, 2019). Although a detailed discussion is beyond the scope of this manuscript, as reviewed by Martucci and Mackey (Martucci and MacKey, 2018), pain processing can be represented by four neural networks (Institute of Medicine US, 2011): sensory/motor/multisensory (e.g., somatosensory and motor cortices) (Dowell et al., 2022), pain affect/cognitive control (e.g., prefrontal cortex, anterior cingulate) (Singh et al., 2019), emotion/behavior (e.g., nucleus accumbens, putamen, anterior cingulate, insular cortex), and (Traylor, 2019) descending modulation (e.g., locus coeruleus, medulla). VR-delivered therapeutic content can target these pain processing regions in the brain known to be involved in cLBP (Hoffman et al., 2004; Hoffman et al., 2007; Deng et al., 2022; Lee et al., 2022; Fu et al., 2021; Tran et al., 2022),.For example, CBT has been shown to normalize structural and functional connectivity in many of these brain regions (Bao et al., 2022; Seminowicz et al., 2013; Shpaner et al., 2014; Yoshino et al., 2018). Therefore, VR can deliver therapeutic programs that teach patients coping skills and improve self-regulation to manage cLBP long-term when outside of VR.
In keeping with the theme of this Frontiers Research Collection Topic: “Enabling the Medical Extended Reality Ecosystem - Advancements in Technology, Applications and Regulatory Science”, this manuscript details the journey of AppliedVR Inc, an immersive therapeutics healthcare technology company, to develop, obtain FDA-authorization for, and commercially launch a Virtual Reality-Delivered Skills-Based therapy for cLBP, called RelieVRx®. We share the iterative path to design, develop and validate this immersive therapeutic medical device, the process followed to obtain FDA-authorization for the device1, as well as establishing key lessons for translating innovation from the laboratory into the commercial market, including commercial launch, reimbursement strategy, and clinical implementation in the home.
Organization of the manuscript
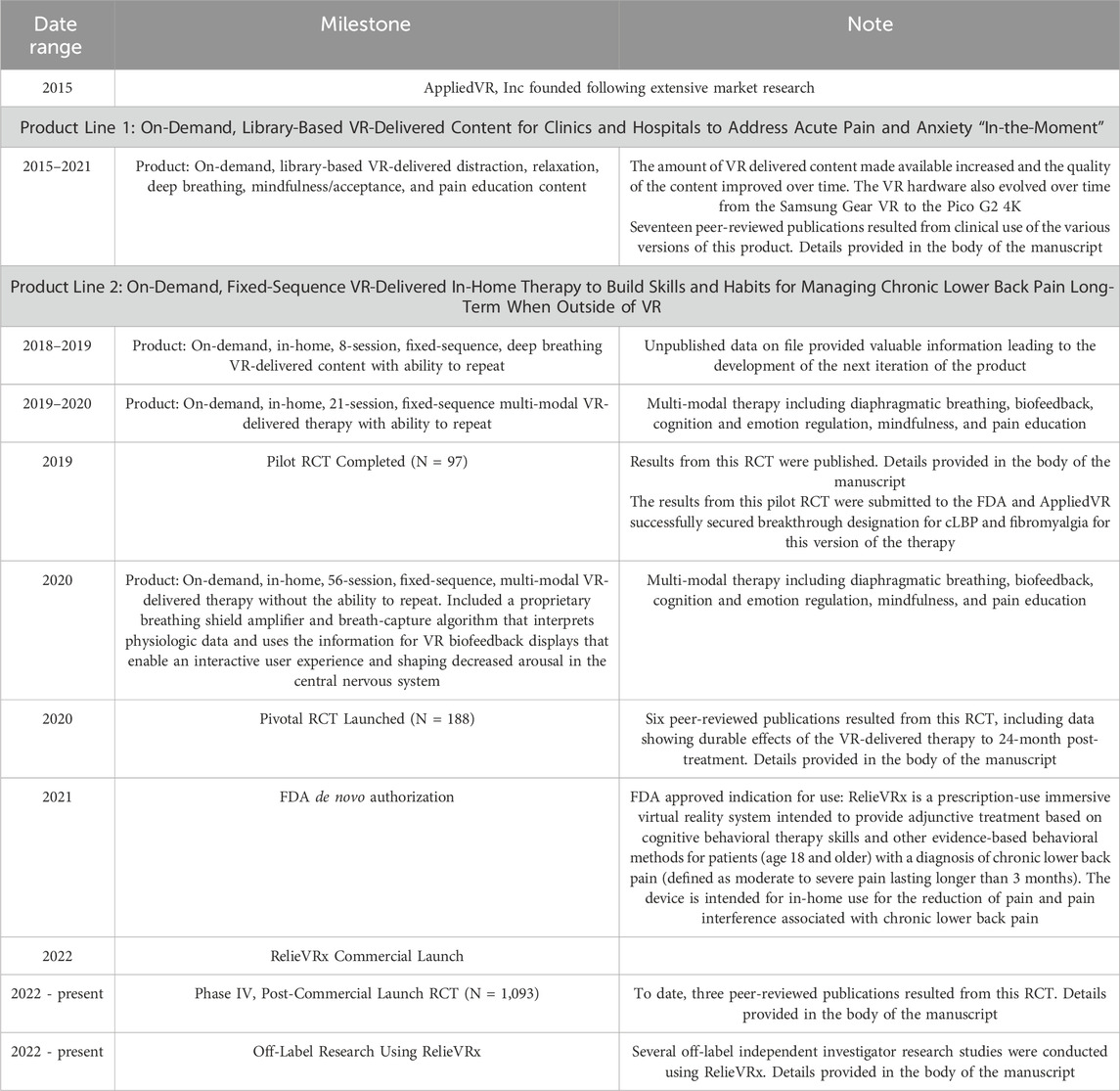
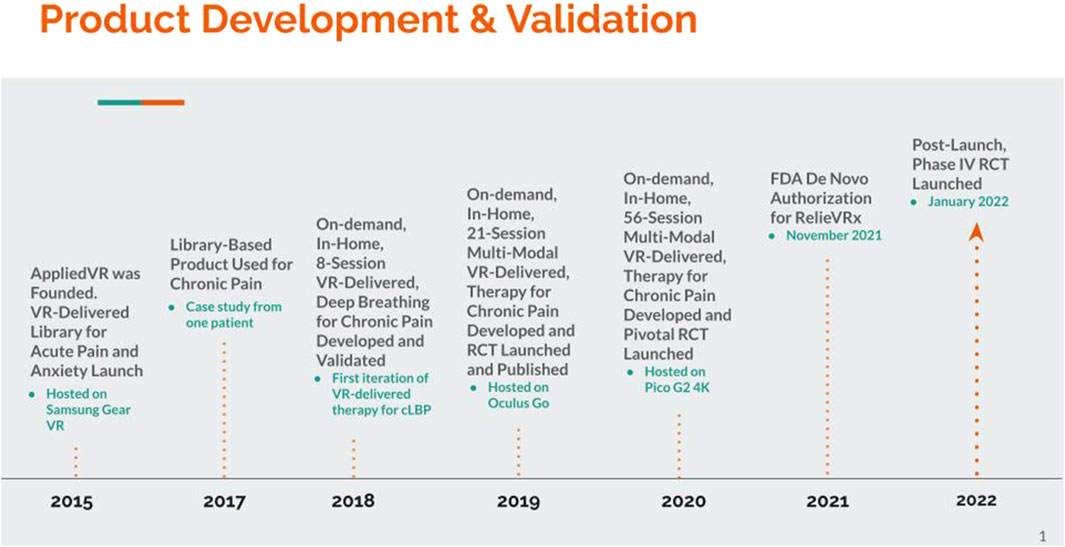
As with many commercial endeavors, the path toward FDA-authorization and commercialization of RelieVRx was not straightforward. Rather, AppliedVR developed and validated two distinct (albeit partially overlapping) product lines before fully committing to RelieVRx. A timeline of the evidence generated by the company and major milestones across these two product lines is summarized in Table 1; see also Figure 1 We begin with a brief description of the Founding Vision for the company, then describe the initial product line (active from 2015–2021) focused on the development and commercial implementation of an on-demand, library-based VR-delivered content platform to be used in clinics and hospitals to help patients cope with acute pain and anxiety “in-the-moment”. Initially content was delivered with the Samsung Gear VR that required technical expertise and training for setup, thus restricting access to clinics and hospitals where personnel could be trained. The lessons learned regarding content development, product user experience, and operational processes for deploying in hospitals and clinics were invaluable. Several research studies were conducted using the on-demand, library-based platform with many resulting in peer-reviewed publications that are summarized in Table 2 and discussed below.
Next, we describe the second product line (active from 2018 - present) focused on the development and commercial implementation of an on-demand, in-home, fixed-sequence VR-delivered therapy to build skills and habits, and to improve self-regulation for managing cLBP long-term and outside of VR (aka RelieVRx). By 2018, easy to use, stand-alone VR headsets were available making it possible to reach patients in their home.
We summarize the evolution of the second product line from an 8-session diaphragmatic breathing only therapy to an in-home, 21-session fixed sequence, multi-modal VR-delivered skills-based program that included aspects of CBT, as well as relaxation and mindfulness training, to an in-home, 56-session, fixed sequence, multi-modal VR-delivered therapy that integrated diaphragmatic breathing, biofeedback, cognition and emotion regulation, mindfulness, and pain education. Critically, this latter version of the therapy included a patented breathing shield that interprets physiologic data and uses the information in VR biofeedback displays that enable an interactive user experience that decreases arousal in the central nervous system. The 21-session version of the therapy was subjected to a small pilot randomized controlled trial (RCT) that yielded strong clinical efficacy and resulted in a successful submission to the FDA that secured breakthrough designation for cLBP and fibromyalgia (Darnall et al., 2020). The 56-session version was subjected to a “pivotal” randomized controlled trial that tested the clinical efficacy of the Skills-Based VR-Delivered therapy that was included in a De Novo submission to the FDA to obtain FDA-Authorization for this software-in-a-medical-device (SiMD). A summary of the clinical evidence is followed by a description of the FDA De Novo authorization process from pre-submission communications with the FDA to submission of the full FDA Authorization request to final approval of the medical device in November 2021.
Next, we discuss and summarize the results from a Phase IV randomized controlled trial conducted post-launch to test clinical effectiveness of RelieVRx in a large community-based sample of demographically diverse and clinically severe cLBP individuals. The large sample size allowed several secondary analyses to be conducted that are briefly summarized as well. We also summarize several off-label research projects using RelieVRx conducted by independent investigators. Finally, we discuss clinical implementation of the RelieVRx-delivered therapy at point-of-care in the home, as well as the successes, the challenges and the directions for the future (prescribing, billing), including the commercialization and the reimbursement pathway (payor adoption), the importance of these data, as well as the need to consider real world evidence.
The founding vision
AppliedVR was founded in 2015 and grounded in the belief that VR had the long-term potential to revolutionize healthcare by providing a vehicle for delivering healthcare-relevant content to patients in their homes at scale. Critically, this immersive healthcare-relevant content must be high quality, consistent, on-demand, and consist of evidence-based treatments that yield clinical meaningful benefits. Although the empirical data was more limited in 2015 than it is today, there was a 20-year body of peer-reviewed research showing that VR-delivered content had strong positive impacts on acute pain, post-traumatic stress disorder, anxiety, mental health, and many other healthcare applications (Maddox et al., 2023b; Hoffman et al., 2004; Hoffman et al., 2007; Trost et al., 2015; Botella et al., 2015; Fodor et al., 2018; Li et al., 2023; Riva et al., 2007). Even so, all this work was conducted in research laboratories using expensive VR hardware that required technical expertise to administer. Thus, although strong empirical data existed to support the healthcare value of VR-delivered content, the hardware and technical expertise requirements were too restrictive to bring this technology to patients at scale and in a cost-effective manner.
In late 2015, Samsung released the Samsung Gear VR. The Gear VR was designed to work with Samsung phones, using the phone’s display and processing power to deliver VR content. Although operation of the Gear VR was often technically challenging and required some training to use effectively, it did allow staff to deliver healthcare-relevant VR content to patients in hospitals and clinics with only modest training. Given the speed of technological advancements, it was very likely that within a few years VR headsets would be developed that could deliver VR content directly to patients in their homes. This was the ultimate goal.
On-demand, library-based VR-delivered content for clinics and hospitals to address acute pain and anxiety “in-the-moment”
In 2015, the initial product offering deployed the Samsung Gear VR in hospitals and clinics across the country to address acute pain and anxiety “in-the-moment” by delivering immersive VR distraction, relaxation, mindfulness and escape content to patients. Figure 2 displays the Samsung Gear VR device (Figure 2A), along with screenshots of other sample content (Figures 2B–D). Distraction content included dynamic, immersive VR-based distraction games where patients explored a captivating, fully interactive environment calibrated to the player’s skill level enabling consistently engaging game play (Figure 2B). Relaxation was addressed with dynamic, immersive VR environments with soothing narratives that promoted relaxation and taught the value of breath, mindfulness and acceptance (Figure 2C). Patients could escape boredom or negative moods with 360 travel content that allowed users to travel to foreign lands, wilderness landscapes and the ocean (Figure 2D).
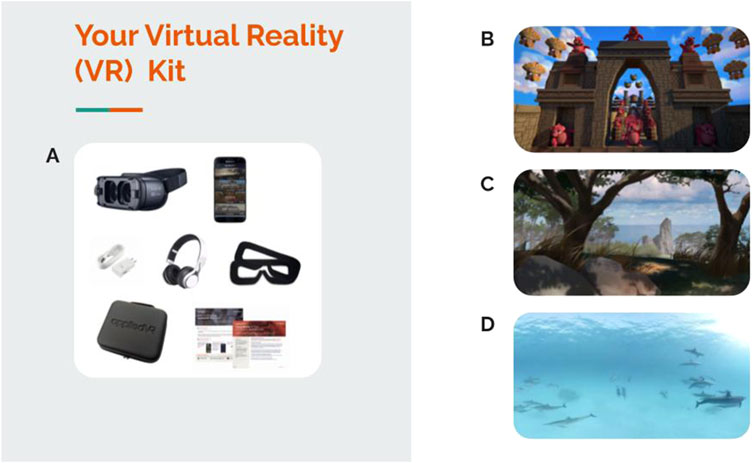
Figure 2. On-demand, library-based VR-delivered content for clinics and hospitals to address acute pain and anxiety “in-the-moment”. (A) Samsung Gear VR kit, (B) screenshot from distraction content, (C) screenshot from relaxation content, and (D) screenshot from escape content.
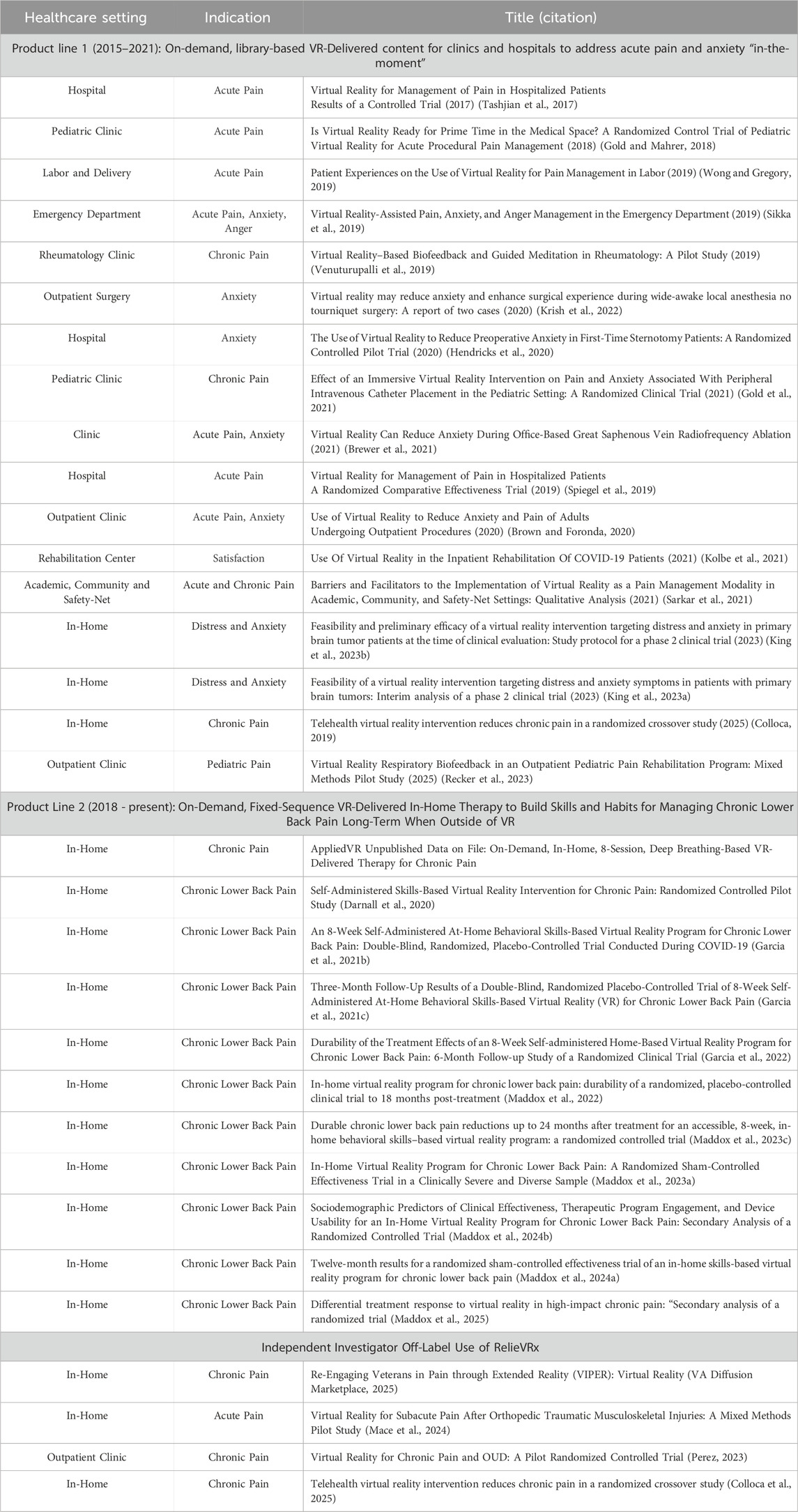
As the amount of high-quality VR content increased and headset technology advanced, the on-demand, library-based product evolved to include more high-resolution content, more content types, and migrated from the less user-friendly Samsung Gear VR to the Pico G2 4K in 2020. This enabled a gaze-based, user-friendly navigation experience, a high resolution 4K screen, and an easily wipeable face pad. By 2020, the on-demand library-based VR solution had been deployed in over 250 hospitals and clinics, with a focus on academic medical centers. Several healthcare professionals conducted empirical research with the various versions of the VR solution and to date a total of 17 peer-reviewed publications exist. (See Table 2 for the healthcare setting, indication and title of the published work). Published studies include those showing that the VR solution was successful in managing acute pain, anxiety, anger, and chronic pain pre-, peri-, and post-operatively in hospitals and clinics, in adult, pediatric, and primary brain tumor patients (Tashjian et al., 2017; Gold and Mahrer, 2018; Wong and Gregory, 2019; Krish et al., 2022; Sikka et al., 2019; Gold et al., 2021; Hendricks et al., 2020; Venuturupalli et al., 2019; Brewer et al., 2021; Spiegel et al., 2019; Brown and Foronda, 2020; Kolbe et al., 2021; King et al., 2023a; Colloca et al., 2025; Sarkar et al., 2021; King et al., 2023b; Recker et al., 2023).
Toward an FDA-authorized, on-demand, in-home, fixed-sequence VR-delivered therapy for chronic lower back pain: an iterative approach combining product development and empirical validation testing
Development and empirical testing of an on-demand, in-home, 8-session, deep breathing-based VR-delivered therapy for chronic pain
In 2017, while deploying the on-demand, library-based VR-delivered content to clinics and hospitals, the company received a request to help a patient experiencing severe chronic pain after being involved in a traumatic accident. The patient was sent the Samsung Gear VR kit, was trained to use the device, and was informed that the VR content could be used to help cope with pain flare ups, enhance relaxation and reduce stress in the moment. Interestingly, periodic follow-up with the patient revealed that they were not just using the device for temporary pain relief but rather were using the device to learn and retain pain management skills that persisted beyond the VR sessions (Follow this link or this link for more details regarding this patient).
This fortuitous event made clear the potential opportunity to target chronic pain with in-home VR-delivered therapy. To explore this possibility, the first step was to bring prototype modules into pain clinics and to conduct usability testing with patients and providers. Briefly, the prototype device included several diaphragmatic breathing, mindfulness and distraction modules. Two critical learnings from this user testing stand out. First, simplified content was found to be superior. Simple breathing-based biofeedback was valuable, but extensively gamified interaction was overly complex and unnecessary. Second, the value of therapeutic content repetition, spaced over time, was clear, in particular, when it came to diaphragmatic breathing and the development of long-term pain management skills. This led to the development of an 8-session VR-delivered therapy focused on helping patients develop diaphragmatic breathing techniques for managing chronic pain when outside of VR (see Figure 3).

Figure 3. On-demand, 8-session, deep breathing-based VR-delivered therapy for chronic pain. (A) Samsung Gear VR headset and brief description of deep breathing content for each of the 8 sessions, (B) screenshot of breathing content with inset of patient experiencing content.
Although the Samsung Gear VR posed technical challenges for in-home use, in 2018 a small-scale in-home feasibility study was conducted with the 8-session deep breathing-based VR-delivered VR therapy in 35 chronic pain sufferers. Patients were shipped a device, received training over the phone on use of the device along with a technical support number to call should problems arise, and used the device for 8-day with the ability to repeat experiences if desired. Patients were asked to rate their pain, anxiety, mood and sleep at baseline and at the end of the 8 days with the therapy. On average, pain was reduced by 1.3 points, anxiety was reduced by 1.4 points, mood was enhanced by 2.0 points and sleep was enhanced by 1.3 points (all on 0–10-point scales).
Development and empirical validation testing of an on-demand, in-home, 21-session multi-modal VR-Delivered therapy for chronic lower back pain
In 2019, while developing and testing the 8-session VR-delivered breathing therapy, AppliedVR was introduced to Dr. Beth Darnall, a pain psychologist at Stanford University School of Medicine. Dr. Darnall saw the potential of VR-delivered therapy for chronic pain and entered an advisory role with the company to develop the next iteration of the VR-delivered therapy. Dr. Darnall advocated for a multi-modal therapeutic approach that integrated diaphragmatic breathing, biofeedback, cognition and emotion regulation, mindfulness, and pain education. Like in-office pain treatments with standardization facilitating treatment testing, Dr. Darnall also advocated for a fixed therapeutic sequence of VR-delivered content that followed a rigorous therapeutic journey as opposed to a user-selected approach. The result was a 21-session, multi-modal, fixed sequence VR-delivered skills-based therapy for cLBP. The skills-based program involved a range of sessions lasting between two and 15 min that guided participants through pain self-management skills that form a part of CBT programs (e.g., adaptive regulation of pain-related cognition and emotions), as well as relaxation training (diaphragmatic breathing exercises to enhance parasympathetic nervous system function), and mindfulness.
While the 21-session VR-delivered program was being developed, the Oculus Go VR headset was also gaining traction in the market. The Oculus Go VR headset was notable because it was the first commercially available standalone headset that did not require connecting a phone into a holder or using a head mounted display tethered to a computer. With the 21-session program deployed on the Oculus Go headset, a small-scale in-home pilot randomized controlled trial was launched. A total of 97 participants who were living with cLBP or fibromyalgia for at least 6 months were consented into the study and were assigned to either the 21-session skills-based VR program or an audio-only version of the VR program (Darnall et al., 2020) to be completed at home. A sample headset and VR content are displayed in Figure 4.
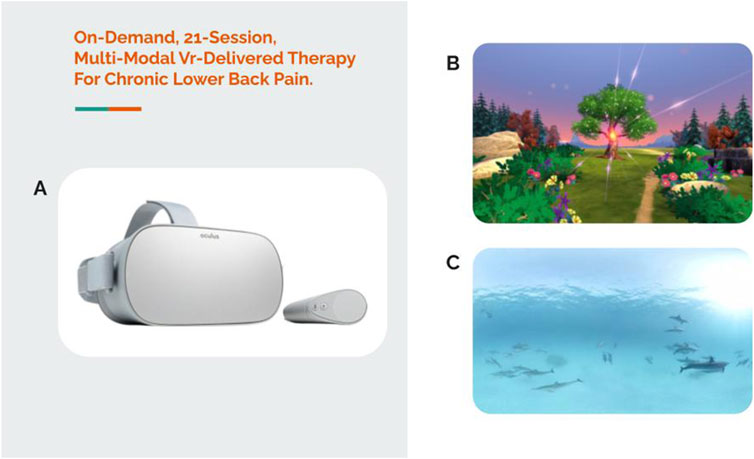
Figure 4. On-demand, 21-session, multi-modal VR-delivered therapy for chronic lower back pain. (A) Oculus Go VR headset, (B) screenshot of breathing content, and (C) screenshot of relaxation/escape content.
Both groups saw decreases in pain intensity and pain interference over the course of the 21-session intervention, although the average effects were statistically larger for the VR-delivered therapy group compared to the audio-only group. Baseline pain intensity and the four pain interference measures were moderate, ranging from 4.6 to 5.4 on a 0–10-point scale. The study revealed a 1.4-point reduction in pain intensity, and a 1.8-, 2.1-, 2.7-, and 2.6-point reduction in pain interference with activity, sleep, mood and stress, respectively. The pain intensity and composite pain interference reductions for the 21-session VR-delivered therapy are presented in Table 3. (Table 3 includes pain reductions, VR therapeutic engagement and system usability scores across several AppliedVR conducted studies). Engagement with the VR-delivered therapy was strong. Participants were given a 21-session therapy but were allowed to repeat experiences at any time and utilized this option, completing an average of 34.4 experiences. Critically, these data were included in a successful submission to the FDA that secured breakthrough designation for the 21-session VR-delivered therapy for cLBP and fibromyalgia.
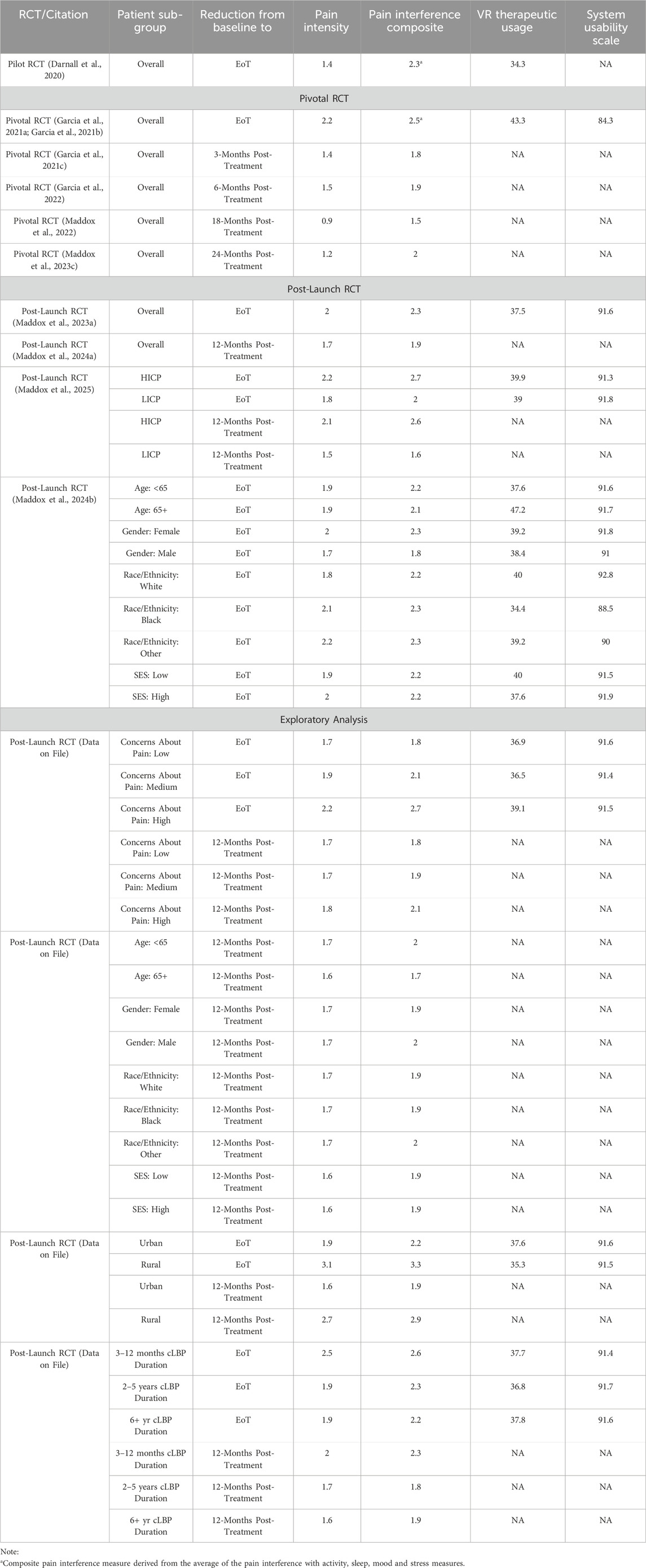
Table 3. Pain reductions, VR therapeutic engagement and system usability scores across all AppliedVR conducted studies.
Development and empirical testing of an on-demand, in-home, 56-session multi-modal VR-delivered therapy for chronic lower back pain
A decision was made to migrate from the Oculus Go to the Pico G2 4K for the next version of the VR-delivered therapy. The Pico G2 4K was already being used successfully for the on-demand, library-based product. It was gaze-based, with a user-friendly navigation experience, a high resolution 4K screen, and an easily wipeable face pad. For the VR-delivered therapy, a proprietary breathing shield amplifier and breath-capture algorithm was added to help visualize the patient’s breathing pattern and to use their breathing pattern to interactively modify the ongoing VR experience. The therapy was also expanded from a 21-session to a 56-session, fixed sequence VR-delivered therapy with more varied and higher resolution VR content. (Several possibilities for further refinement of the VR-delivered therapy are discussed in the Future Directions). The 56 sessions were organized into 8 weekly themes, such as “Breath and Pain”, “Attention and Distraction, and “Shaping the Nervous System Toward Relief” to name a few. Each week users experienced a range of session content. This included pain education that provided a medical and scientific rationale for the VR experiences, relaxation/interoception scenes that progressively change from busy/active to calm to train users to understand the benefits of progressive relaxation, 360-degree mindful escape videos designed to maximize the relaxation response and participant engagement, interactive pain distraction content to train the skill of shifting focus away from pain, and dynamic breathing to support self-regulation and relaxation with modules becoming increasingly challenging as users increase their skill with diaphragmatic breathing and parasympathetic control. Crucially, the 56-session treatment included interoceptive modules that were designed for more rapid entrainment of CNS downregulation via vivid and frequent enhanced biofeedback experiences. The 56-session skills-based VR-delivered therapy was constructed with daily brief exposures to pain coping skills experiences to improve self-regulation and long-term skills acquisition that could be applied in daily life after they had completed the therapy and returned the VR headset. Although the 56-session, fixed-sequence VR delivered-therapy was designed to build the skills and habits needed to mitigate and cope with chronic pain. Clearly this “one size fits all” approach can be improved upon in this era of precision medicine. This is briefly discussed in the Future Directions.
A “pivotal” RCT was launched in 2020 whose data would be used to support a De Novo submission to the FDA (ClinicalTrials.gov NCT04415177). A total of 188 community-based cLBP individuals were consented and randomized 1:1 to either the 56-session skills-based VR program for cLBP (at the time referred to as EaseVRx) or 56-session Sham VR program that included 2D nature content (Garcia et al., 2021a; Garcia LM. et al., 2021). Both groups received an identical VR headset that was delivered directly to their home. Across both groups, each session ranged in duration from 2-15 min with an average of 6 min per session. Figure 5 summarizes the 56-session sequential structure of the RelieVRx program. Also shown is the Pico G2 4K VR device and patented breathing shield. The study protocol proposed to follow participants from pre-treatment baseline to 6 months post-treatment.
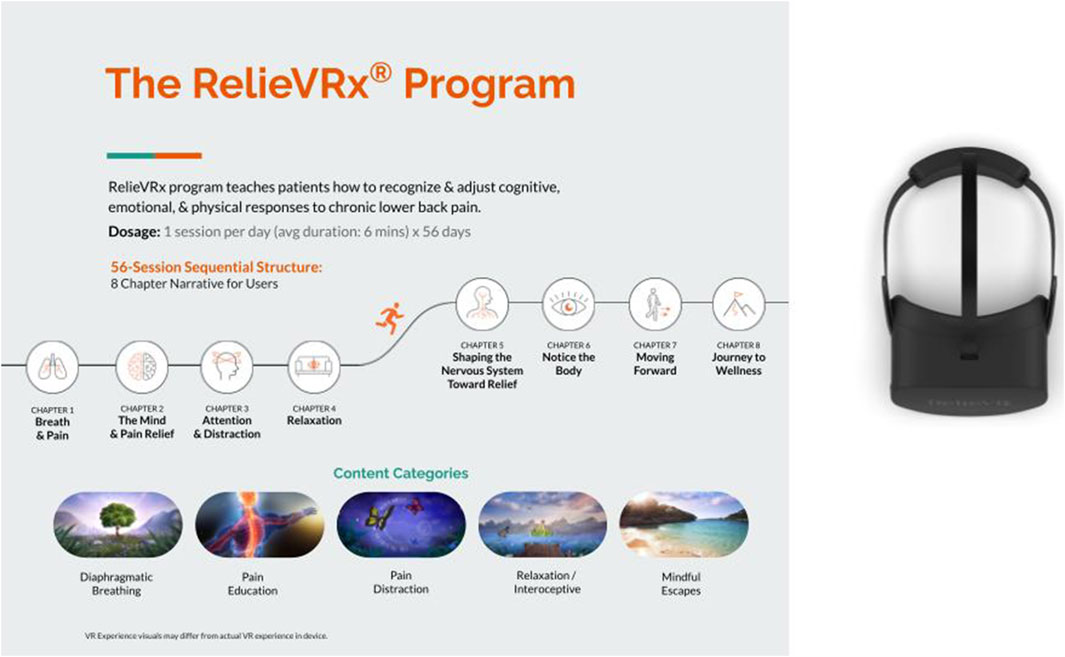
Figure 5. On-demand, 56-session, multi-modal VR-delivered therapy for chronic lower back pain (RelieVRx). Pico G2 4K VR device and patented breathing shield also shown.
Detailed descriptions of the statistical tests as well as patient-reported outcome measures for Sham VR are presented in the original published manuscript. Here we focus our discussion only on the VR-delivered therapy. Pain intensity and pain interference decreased significantly over the course of the 56-session intervention, and this reduction was statistically larger for the VR-delivered therapy group than for the Sham VR group. Baseline pain intensity and the four pain interference measures were moderate, ranging from 4.5 to 5.3 on a 0–10-point scale. Across all five primary endpoints, clinically meaningful 2+-point reductions were observed at end-of-treatment, with a 2.2-point average reduction in pain intensity, and a 2.6-, 2.5-, 2.5-, and 2.5-point average reduction in pain interference with activity, sleep, mood and stress, respectively (Farrar et al., 2001). The pain intensity and composite pain interference reductions for the 56-session VR-delivered therapy are presented in Table 3. Engagement with the VR-delivered therapy was strong with participants completing an average of 43.3 of the 56 experiences. The System Usability Scale (Brooke, 1996) was also collected and the VR device obtained an average rating of 84.3 on a 0–100 point scale that represents an A+ system usability rating. The device was also safe and tolerable. Although 9.7% of participants reported at least one episode of nausea or motion sickness, in all cases the condition resolved quickly, and no serious adverse events were reported.
To test the durability of pain intensity and pain interference reductions, participants were asked to report their pain levels at 1-, 2-, and 3-month post-treatment (Garcia et al., 2021c) and at 6-month post-treatment (Garcia et al., 2022). The 6-month post-treatment results were promising (see Table 3) so the study protocol was modified to include 18-month post-treatment (Maddox et al., 2022), and 24-month post-treatment surveys (Maddox et al., 2023c). At 24-month post-treatment, the pain intensity reduction was 1.2 points, and the composite pain interference reduction was 2.0 points for the VR-delivered therapy (see Table 3), and both were statistically larger than for Sham VR, suggesting that the VR-delivered therapy was providing the necessary long-term skill development that patients needed to manage their pain. The next goal was to utilize these data in an FDA De Novo Authorization application.
The path toward FDA-authorization for RelieVRx
The path toward FDA-authorization is a long and complex process but it is an important one when one’s goal is to provide patients with evidence-based therapies that have been vetted by independent, objective bodies such as the Food and Drug Administration [for an interesting discussion of the FDA process see (Courtier and Beberman, 2025). Before designing and conducting the Pivotal randomized controlled trial summarized above, and before submitting the 56-session Skills-Based VR-Delivered Therapy for FDA authorization, AppliedVR had to decide whether to pursue the software-as-a-medical-device (SaMD) or software-in-a-medical-device classification (SiMD) pathway. The FDA defines SaMD as software intended for medical purposes that operates independently of a physical medical device—such as apps that diagnose conditions or recommend treatments. In contrast, SiMD refers to software that is part of a medical device’s internal system, such as the code that controls a pacemaker or an MRI machine. The key difference lies in whether the software functions on its own (SaMD) or is embedded in and dependent on a physical device (SiMD). Whether to pursue SaMD or SiMD has implications on the appropriate FDA pathway and on how payors assess reimbursement eligibility and regulatory pathways.
Once the decision was made to pursue the SiMD pathway, AppliedVR engaged in multiple pre-submission discussions with the FDA to align on regulatory expectations and ensure a smooth approval process. These interactions helped refine the clinical and technical approach to meet the FDA’s requirements. For example, FDA guidance was sought on whether the VR-delivered therapy would qualify for the De Novo classification or require a different regulatory pathway (US Food and Drug Administration, 2018). Briefly, the FDA De Novo classification process provides a regulatory pathway for novel medical devices that have no prior predicate but are considered low to moderate risk. Instead of being automatically classified as Class III, which requires premarket approval (PMA), the De Novo pathway allows these devices to be classified as Class II with special controls to ensure safety and effectiveness. The process begins with the submission of a De Novo request, where manufacturers provide data demonstrating the device’s safety and effectiveness. The FDA then evaluates whether general and special controls are sufficient to regulate the device. If approved, the device is officially classified as Class II, establishing a new regulatory category that future similar devices can use for 510(k) authorization, thereby streamlining the approval process. The FDA agreed that the De Novo pathway was appropriate.
Discussions were also focused on the design, endpoints, and methodology of the pivotal randomized controlled trial to ensure it would generate sufficient evidence for safety and effectiveness. It was through these discussions that the decision was made to develop a rigorous Sham VR control, something rarely used in VR healthcare research (Persky and Colloca, 2023). The company also provided specifications for the Pico G2 4K hardware and software, addressing potential risks, cybersecurity concerns, and mitigations to comply with medical device regulations. Discussions around labeling and the Indications for Use were also undertaken. The company worked with the FDA to determine the necessary evidence to support De Novo approval and identify any special controls needed for post-market safety monitoring. Five special controls were applied to the authorization concerning clinical performance, biocompatibility, software verification, electromagnetic compatibility and electrical, mechanical, and thermal safety, and product labeling. All of these pre-submission interactions were crucial in ensuring that the De Novo submission met regulatory expectations.
With the pre-submission discussions concluded, a structured process was undertaken to complete the necessary tasks. First, the pivotal randomized controlled clinical trial was conducted to demonstrate the safety and effectiveness of RelieVRx in reducing chronic lower back pain and improving patients’ quality of life. With this supporting data, a De Novo request was submitted to the FDA, providing both clinical and technical evidence to show that the device met the agency’s safety and effectiveness requirements. The FDA then reviewed the submission, assessing whether general and special controls were sufficient for classification. In November 2021, the FDA granted De Novo authorization, classifying RelieVRx as a Class II medical device. This milestone made RelieVRx the first VR-based digital therapeutic for chronic pain management, establishing a new product category (US Food and Drug Administration, 2025) and paving the way for future VR-based pain management solutions to seek regulatory approval through the 510(k) pathway.
The FDA authorized the following indication for use: “RelieVRx is a prescription-use immersive virtual reality system intended to provide adjunctive treatment based on cognitive behavioral therapy skills and other evidence-based behavioral methods for patients (age 18 and older) with a diagnosis of chronic lower back pain (defined as moderate to severe pain lasting longer than 3 months). The device is intended for in-home use for the reduction of pain and pain interference associated with chronic lower back pain.”
Commercial launch of RelieVRx and post-launch effectiveness RCT
Once FDA-Authorized, RelieVRx was launched commercially as a prescription-based immersive therapeutic in 2022. Progress on the commercialization and reimbursement front, as well as clinical implementation in the home are summarized in the next major section of the manuscript. Here we briefly summarize the results from a Phase IV, Post-Launch randomized controlled trial conducted with RelieVRx that explores the clinical effectiveness of RelieVRx in a large, community-based, diverse sample of cLBP participants (much larger and more diverse than were included in the Pivotal trial) from pre-treatment baseline to 24-month post-treatment (clinicaltrials.gov NCT05263037). Detailed statistical analyses and comparison with Sham can be found in the original articles. Here we briefly summarize the results from the VR-delivered therapy.
A total of 1,093 cLBP participants were consented and randomized 1:1 to the RelieVRx or Sham VR groups (Maddox et al., 2023a) with both groups receiving their device by mail at their home. Pain intensity and pain interference decreased significantly over the course of the 56-session VR-delivered therapy, and the reductions were statistically larger in the RelieVRx group than in the Sham VR group. The results for RelieVRx are presented in Table 3. Whereas baseline pain intensity and interference levels were clinically moderate in the pivotal trial (ranging from 4.5 to 5.3 on a 0–10-point scale), they were clinically severe in the Post-Launch RCT (ranging from 6.2 to 6.6 on a 0–10 point scale). Clinically meaningful 2+ point reductions from baseline to end of treatment were observed for pain intensity (2.0) and pain interference (2.3) in the RelieVRx group. Several secondary endpoints were also examined. These included the PROMIS anxiety, sleep disturbance, and depression scales along with the Oswestry Disability Scale. Statistically significant reductions in sleep disturbance, depression and physical disability were observed for the RelieVRx group from baseline to end-of-treatment. Engagement with RelieVRx was strong with participants completing an average of 37.6 of the 56 experiences. The System Usability Scale (Brooke, 1996) was also collected and the VR device obtained an average rating of 91.6 on a 0–100 point scale that represents an A+ system usability rating. The device was also safe and tolerable. Although 4.9% of participants reported at least one episode of nausea or motion sickness, in all cases the condition resolved quickly, and no serious adverse events were reported.
As with the Pivotal RCT, we followed participants at 1, 2, 3, 6, and 12-month post-treatment. At 12 months post-treatment (see Table 3), the RelieVRx group revealed a 1.7-point pain intensity reduction and a 1.9-point pain interference reduction, both of which were statistically larger than those observed in the Sham VR group (Maddox et al., 2024a). Data collection and analysis at 24-month post-treatment is ongoing.
Post-launch RCT sub-group analyses
The large sample size in the RelieVRx group facilitated several important sub-group analyses. Importantly, many of these sub-group analyses were motivated from discussion with payors, CMS, and healthcare professionals. Two separate sub-group analyses focused on Amtmann et al., 2019 high impact chronic pain and (Dowell et al., 2022) sociodemographic factors are published (Maddox et al., 2024b; Maddox et al., 2025). Detailed statistical analyses can be found in the original publications. Three additional distinct sub-group analyses focused on Amtmann et al., 2019 pain catastrophizing (Dowell et al., 2022), rural/urban setting, and (Singh et al., 2019) duration of cLBP are currently exploratory and await detailed statistical analyses (e.g., MMRM). We briefly review the findings from all 5 sub-group analyses.
High impact chronic pain (HICP) patients benefit more from RelieVRx
High-impact chronic pain (HICP) is defined as pain that persists for at least 3 months with at least one major activity restriction (Şentürk et al., 2023; Dahlhamer et al., 2018; Pitcher et al., 2019; Vaegter et al., 2023), and affecting 8.5% of the population (Pitcher et al., 2019; Lucas and Sohi, 2024). HICP have higher healthcare utilization and costs, and greater likelihood for opioid prescription than lower-impact chronic pain patients (LICP) (Herman et al., 2019) (Pitcher et al., 2019; Herman et al., 2019). A sub-group analysis of the Post-Launch RCT was conducted to examine HICP vs. LICP treatment responses to RelieVRx at end-of-treatment and at 12-month post-treatment for pain intensity and pain interference (Maddox et al., 2025). RelieVRx participants were classified as HICP vs. LICP using their baseline Brief Pain Inventory Pain Interference score (HICP: BPI pain interference ratings ≥7) (Cook et al., 2024). As shown in Table 3, clinically meaningful, and significantly larger reductions in pain intensity and interference were observed for HICP vs. LICP at end-of-treatment and 12-month post-treatment. End-of-treatment reduction in pain interference among HICP reclassified 70% of them as LICP, and this improvement held at 12-month post-treatment (67%). No differences were found for HICP vs. LICP for device engagement or usability scores.
RelieVRx clinical effectiveness is invariant to several important sociodemographic factors
Several studies have found heterogeneity in the effectiveness of digital therapeutics as a function of age, gender, race/ethnicity, and socioeconomic status (SES), with patients who are older, females, non-White, or of low SES being disadvantaged (Yao et al., 2022; Seifert and Schlomann, 2021; Seifert et al., 2019; Mis et al., 2022). To explore potential heterogeneities, we conducted a sub-group analysis of the Post-Launch RCT (see Table 3). The clinical effectiveness, therapeutic program engagement, and VR device usability of RelieVRx were high and generally unaffected by age (<65 vs. 65+ years), gender (male vs. female), race/ethnicity (White vs. Black vs. other), and SES (education and income), with a few exceptions (age difference for therapeutic program engagement; race/ethnicity difference for device usability) (Maddox et al., 2024b). Follow up analyses at 12-month post-treatment (unpublished data on file) suggest that clinical effectiveness remains invariant across these important sociodemographic factors.
Patients with high levels of pain catastrophizing benefit more from RelieVRx
Clinicians must make patient-centric treatment choices daily and one factor that has received much attention is patients’ perceptions about their pain; in particular pain catastrophizing (Quartana et al., 2009). We explored the impact of baseline levels of pain catastrophizing (as measured by the Concerns about Pain measure; Amtmann et al. (2019)) on clinical effectiveness, therapeutic program engagement, and VR device usability of RelieVRx (see Table 3; (Oldstone et al., 2024)). Clinical effectiveness, therapeutic program engagement, and VR device usability of RelieVRx were uniformly high across low, medium and high levels of baseline pain catastrophizing, with an advantage for patients with high levels of pain catastrophizing. Although weakened somewhat, these advantages continued to hold at 12-month post-treatment.
RelieVRx shows consistent effectiveness across rural and urban settings
Research shows that individuals in rural areas experience chronic pain more frequently and with greater intensity compared to those in urban areas (Baker et al., 2024). RelieVRx offers an at-home chronic lower back pain management option requiring no internet connection or travel to a pain clinic that could improve access and outcomes. To determine whether RelieVRx is effective across rural and urban settings, a sub-group analysis of the Post-Launch RCT was conducted (see Table 3; (Maddox, 2025)). Clinical effectiveness, therapeutic program engagement, and VR device usability of RelieVRx were uniformly high across rural and urban participants. In fact, numerically, rural participants showed larger pain intensity and pain interference reductions at end-of-treatment that were sustained at 12-month post-treatment.
RelieVRx shows consistent effectiveness regardless of cLBP duration
Pain specialists agree that early intervention and consistent treatment generally lead to better outcomes for CLBP patients (Norbye et al., 2016). We performed a sub-group analysis of our Post-Launch RCT to determine whether the amount of time that a patient had been dealing with cLBP (3–12 months, 2–5 years, or 11+ years) impacted clinical effectiveness, therapeutic program engagement, and VR device usability of RelieVRx (see Table 3 (Maddox, 2025)). Clinical effectiveness, therapeutic program engagement, and VR device usability of RelieVRx were uniformly high across the three cLBP duration categories. In fact, numerically, participants early in the chronic lower back pain journey showed larger pain intensity and pain interference reductions at end-of-treatment. These were sustained at 12-month post-treatment. Taken together, these sub-group analyses suggest some patient profiles for which RelieVRx works best, and as well as factors for which invariance is observed.
Independent investigator “off-label” research conducted with RelieVRx
Independent investigator research that explores “off-label” uses of RelieVRx are valuable. Several independent investigator studies are ongoing, but to date, four have been completed as described below.
One study examined the effectiveness of RelieVRx combined with telehealth and goal setting in veterans with chronic pain who live in rural areas (VA Diffusion Marketplace, 2025). 20 rural Veterans with chronic pain participated and provided pre-treatment surveys, completed 56 daily sessions with RelieVRx, and provided post-treatment surveys including pain intensity, pain interference with enjoyment of life, general activity, mood, as well as sleep disturbance, opioid use and PT participation. Results showed that Veterans completed on average 5 RelieVRx sessions per week. Clinically meaningful reductions in pain intensity, pain interference and sleep disturbance were observed. No increase in opioid use was observed, and participation in physical therapy increased from 50% to 93%. These findings suggest that in-home use of RelieVRx combined with telehealth and goal setting may provide a powerful therapy for Veterans living in isolated rural settings.
A second study examined the effectiveness of RelieVRx therapy on opioid craving and several measures of pain in chronic pain participants enrolled in a methadone maintenance program (ClinicalTrials, 2025; Perez, 2023). Fourteen participants with chronic pain and Opioid Use Disorder (OUD) were randomized (ClinicalTrials, 2025; Perez, 2023) to RelieVRx (N = 8) or Sham (N = 6) and completed two onsite sessions per week (2 VR experiences per session) across 6 weeks (24 VR experiences max; not the full 56-session RelieVRx therapy) at an Opioid Treatment Program (OTP). RelieVRx reduced opioid craving and several measures of pain at end-of-treatment relative to baseline while also increasing pain acceptance at end-of-treatment relative to baseline). Importantly, these data served as pilot data for a recently funded $3.7 million NIH grant to explore the impact of the full 56-session RelieVRx program on OUD and chronic pain management.
A third study examined the effectiveness of RelieVRx for pain self-management at home following orthopedic injury (Mace et al., 2024). Ten adults from a Level 1 Trauma Clinic within the Mass General Brigham healthcare system with a recent (≤2 months), isolated orthopedic injury who were at risk for persistent pain and functional limitations were recruited. All participants provided pre-treatment surveys, completed 56 daily sessions with RelieVRx, and provided pos-treatment surveys including measures of pain intensity, interference, catastrophizing, anxiety, depression, sleep, physical function, mindfulness, and self-efficacy. Nine of 10 patients completed all 56-sessions. RelieVRx led to statistically significant improvements in all patient reported outcomes. These data suggest that RelieVRx may be effective at treating acute pain and may reduce the prevalence of acute pain evolving into chronic pain.
Finally, a fourth study examined the efficacy of 10 sessions from the RelieVRx therapy compared to the same therapy delivered as audio-only content using a 5-week randomized crossover design in 54 patients with chronic pain due to temporomandibular disorders (Colloca et al., 2025). Immersive VR-delivered therapy significantly reduced pain intensity, anxiety, and pain interference while improving mood and sleep quality. This study provides strong methodological support for the importance of the immersive quality of VR-delivered therapy in managing chronic pain.
RelieVRx: commercialization, reimbursement, and clinical implementation in-home
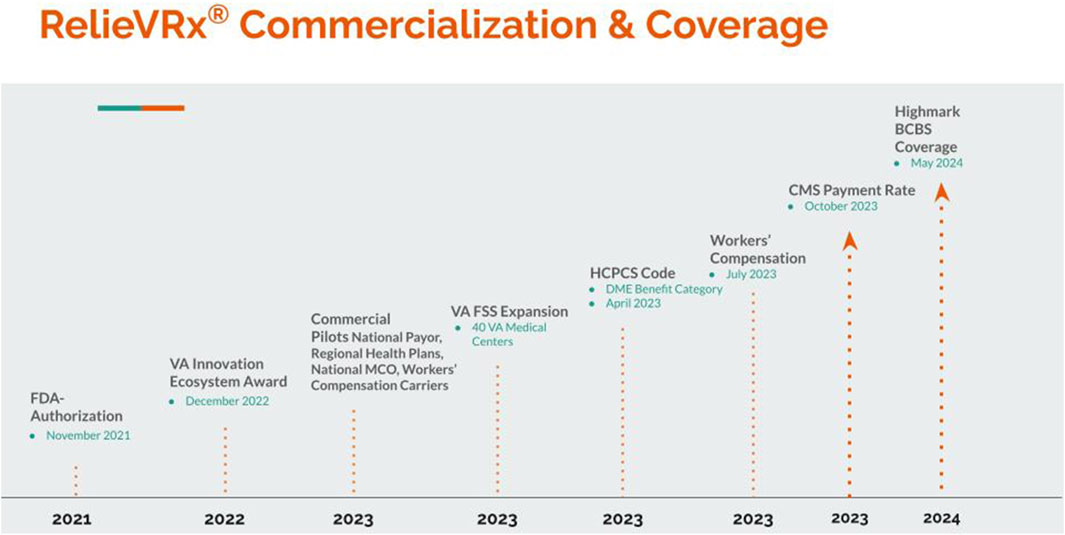
As summarized above, RelieVRx received FDA De Novo Authorization as software-in-a-medical-device in November 2021 and was officially launched commercially in 2022. A timeline of the major commercial and coverage milestones for RelieVRx are summarized in Figure 6. In this section we briefly summarize the path taken toward RelieVRx commercialization, reimbursement and clinical implementation in the home.
Commercialization and reimbursement
AppliedVR followed several parallel tracks toward commercialization and reimbursement of RelieVRx. These included launching several commercial payor pilots with national payors, regional health plans, national managed care organizations, and workers’ compensation carriers. Commercial payor pilots allow for validation and demonstration of the product’s effectiveness in real-world settings. This can lead to broader payor coverage and reimbursement opportunities, build trust with insurance companies, drive market adoption, attract other potential users to the product, and position the company and product as leaders in innovative solutions, increasing its credibility in the healthcare sector. As of publication of this manuscript, several commercial pilots have been completed, and several others are underway. This real-world evidence generation has been key when conducting value analyses which is often necessary to secure positive coverage policies.
Commercialization efforts also included direct engagement in target markets. The company was successful in obtaining a 1-year VA Innovation ecosystem award in December 2022 that was extended to a second year of support and treated 750 Veterans across the country with RelieVRx. In parallel there was an expansion of access to RelieVRx in the VA through the VA Federal Supply Schedule (FSS) starting in 2023. As of publication of this manuscript, nearly half of the VA Medical Centers across the country have prescribed RelieVRx, and thousands of Veterans have obtained relief for their chronic pain with RelieVRx.
The path toward reimbursement is best understood as a combination of coding, benefit category and payment, and coverage. At present, many VR-specific healthcare services are not reimbursed by CMS or other insurance payors. In April 2023, and following extensive interaction with CMS, CMS created a VR-specific billing code–HCPCS code E1905 – for RelieVRx (Centers for Med icare and Medicaid Services, 2022). A payment rate was subsequently published in October 2023 (Centers for Med icare and Medicaid Services, 2022). The specifics of how this process unfolded are instructive and are briefly summarized below (for details see Oldstone and Judge (2024)).
Regulatory strategy is central to the path toward reimbursement. Because RelieVRx is SiMD and is FDA-Authorized for in-home use independent of clinician involvement, it fits best in the existing CMS benefit category of durable medical equipment (DME). The function of any piece of DME is best represented by its CMS Healthcare Common Procedure Coding System (HCPCS) code. At the time of FDA-Authorization for RelieVRx, an appropriate HCPCS code did not exist so AppliedVR petitioned CMS to create a new HCPCS code, and this request was granted with the establishment of the new HCPCS Level II code E1905, “Virtual reality cognitive behavioral therapy device (CBT), including pre-programmed therapy software” to describe RelieVRx. While AppliedVR initiated the application that led to this code, it can potentially apply to other VR-delivered therapies meeting its criteria.
This coding decision positioned the RelieVRx program as the first immersive or digital therapeutic to be integrated into an existing benefit category, offering a clear path to Medicare coverage eligibility, thereby influencing wider commercial coverage. The final step in the HCPCS process is determination of the payment rate (for details see Oldstone and Judge (2024)). In October 2023, RelieVRx E1905 was classified by CMS as a 13-month rental DME item with established monthly rental rates. The full course of therapy typically requires a 3-month episode of care with the total expected reimbursement for the standard treatment duration to be at $1,888.98, based on CMS’s published fee schedule.
Coverage relies on the product or service being deemed “reasonable and necessary” for each patient, once the code, benefit category, and payment rate are determined. This may be determined on a case-by-case basis by either the Medicare Administrative Contractors (MACs) or the insurance company’s medical team for new products, like RelieVRx. Coverage policy can be written over time to clearly define the conditions for coverage. This may include diagnostic or procedure codes or specific patient characteristics that must be documented for the technology to be covered and paid for.
Commercial coverage of RelieVRx continues to expand. AppliedVR strategically introduced RelieVRx into the workers’ compensation sector in July 2023, aiming to provide injured workers with a non-pharmacologic pain management option. Also in May 2024, Highmark BCBS, a Pittsburgh-based health insurer, became the first commercial payor to cover RelieVRx, extending access to over four million members.
Clinical implementation in-home: patient experience
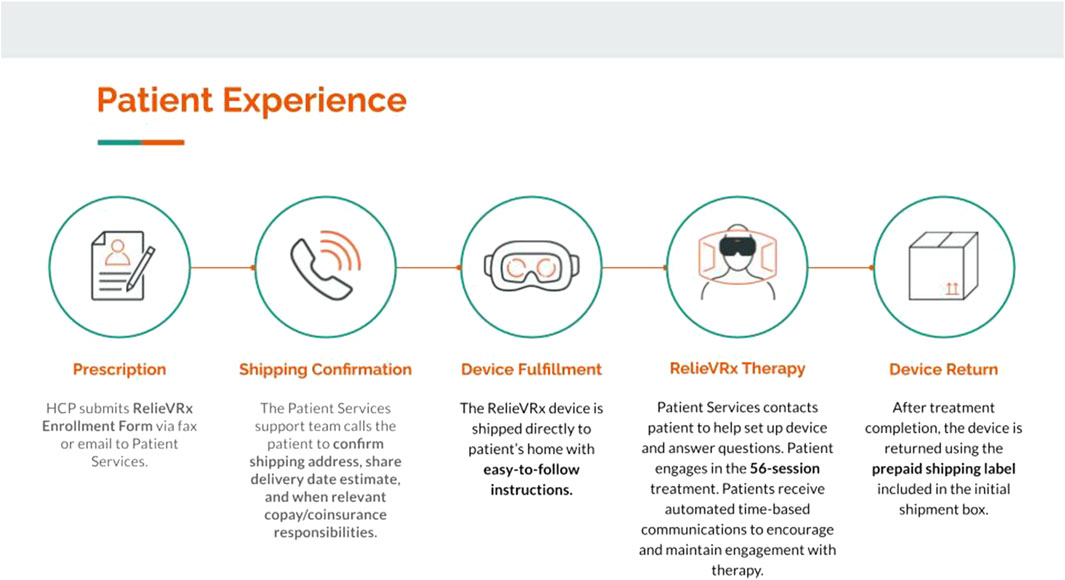
AppliedVR is a traditional, vertically integrated DME, serving as both manufacturer and distributor of RelieVRx to patients’ homes. A typical episode of care lasts 3 months with RelieVRx following a “rent and return” model. Figure 7 summarizes the flow of the patient’s experience. The healthcare professional prescribes RelieVRx to the patient and the device is shipped directly to the patient’s home along with easy-to-follow instructions. The shipping contents are displayed in Figure 8. Once the device is received, the patient can begin the therapy or wait for a member of the patient services team to contact the patient to help set up the device and answer any questions. Although wi-fi connectivity is not required, it is encouraged as it allows the patient services team to track engagement with the device and to use this information to guide periodic check-ins to facilitate therapeutic engagement. Patients receive automated email and text messages at specific times throughout their treatment to encourage and maintain usage. Once the episode of treatment is complete, the device can be placed in the initial shipping box along with the included prepaid shipping label and can be dropped off (free of charge) at a local FedEx facility, or a pickup can be scheduled. Patients have several methods of contacting the patient services team (phone, text, email) should any problems arise with the device or if any questions need to be addressed.
General discussion
In line with the focus of this Frontiers Research Collection Topic: “Enabling the Medical Extended Reality Ecosystem - Advancements in Technology, Applications and Regulatory Science”, this manuscript outlined the journey of AppliedVR Inc, an immersive therapeutics healthcare technology company, to develop, obtain FDA-authorization for, and commercially launch a Virtual Reality-Delivered Skills-Based therapy for cLBP, called RelieVRx. We described the product development path for two distinct product lines, one library-based for clinic and hospital use and one with a fixed-therapeutic sequence for use in the home. We emphasize the importance of evidence-based iteration and sharing the empirical findings through peer-reviewed scientific outlets. This has the dual advantage of allowing outside experts to vet the quality of the product and shares that knowledge broadly with the scientific and entrepreneurial community. To date, over 30 scientific articles have been published that test usability, engagement, feasibility, and/or efficacy of one of these products (Maddox et al., 2023a; Maddox et al., 2023b; Darnall et al., 2020; Tashjian et al., 2017; Gold and Mahrer, 2018; Wong and Gregory, 2019; Krish et al., 2022; Sikka et al., 2019; Gold et al., 2021; Hendricks et al., 2020; Venuturupalli et al., 2019; Brewer et al., 2021; Spiegel et al., 2019; Brown and Foronda, 2020; Kolbe et al., 2021; King et al., 2023a; Colloca et al., 2025; Sarkar et al., 2021; King et al., 2023b; Recker et al., 2023; Garcia et al., 2021a; Garcia LM. et al., 2021; Garcia et al., 2021c; Garcia et al., 2022; Maddox et al., 2022; Maddox et al., 2023c; Maddox et al., 2024a; Maddox et al., 2024b; Maddox et al., 2025; Mace et al., 2024). We highlight the importance of technological advances in both software and hardware that ultimately led to the development and validation of the FDA-authorized RelieVRx product for in-home use. We briefly summarize the FDA authorization process as well as the path toward commercialization, reimbursement and clinical implementation in the home. All of which are critical for helping cLBP patients manage and cope with their pain at home and at scale.
Future directions for RelieVRx and for immersive technology in healthcare
A primary goal at AppliedVR is wider adoption and insurance coverage for RelieVRx. As VR-delivered therapy gains recognition as a cost-effective adjunctive and/or alternative to opioids and traditional treatments for cLBP, more healthcare providers and insurers will support its use. Another goal is to expand RelieVRx’s label beyond cLBP to other pain conditions, and to expand the label to other indications such as anxiety, depression and stress disorders to name a few. All of this will involve extensive engagement with the FDA.
Future advances in healthcare for immersive technology, like VR, are more difficult to predict. Even so, many current trends are suggestive. One major trend is going to be toward personalized, precision healthcare. One simple step toward personalization would be to offer an on-demand, library-based version of RelieVRx after the patient has completed the RelieVRx therapy. Individual differences in skills development and skill decay are well established. By offering an on-demand, library of content to patients following therapy, patients in need of additions skills training could receive that and periodic boosters could be initiated by the patient to mitigate skills-based decay. Of course, more sophisticated personalization is also likely that will involve AI-driven adaptive therapy, where machine learning algorithms analyze patient responses in real time, adjusting VR content, difficulty levels, and therapeutic exercises to maximize effectiveness. The breathing-based algorithms in RelieVRx are a step in this direction, but applications will be much more sophisticated soon.
Biometrics will also be leveraged. Biofeedback integration will become more sophisticated, utilizing wearable sensors to track physiological indicators like heart rate, muscle tension, and breathing patterns. Advanced biofeedback will allow patients to interact with therapy in real time, reinforcing relaxation and pain management techniques more effectively. In the early Samsung Gear VR days, AppliedVR explored integration of heart rate into the therapy, but the technical hurdles were too great. Those hurdles will be much smaller and the value-add much larger soon.
Experiences will also become multisensory and may incorporate haptic feedback, temperature control, and even scent-based stimulation to create deeply immersive environments that enhance relaxation and cognitive distraction from pain. Hybrid therapies that include VR-delivered therapies like RelieVRx that are combined with social experiences that allow group support and social interactions designed to address isolation and leverage group therapy dynamics will be explored. Although an exciting avenue for the future, FDA-authorization in precision medicine is going to require rethinking on the regulatory front as the current approaches focus on one-size-fits-all therapies that are easier to validate empirically.
Limitations
There are several limitations to consider when evaluating the empirical data summarized in this article. First and foremost, one must always objectively evaluate the quality of the control condition used in any randomized controlled trial. Both randomized controlled trials summarized here used an active Sham VR control that displayed 2D nature scenes. Although on the surface this control appears strong, unintended placebo effects and issues of blinding are always possible (for an excellent discussion of VR control group selection see (Persky and Colloca, 2023). Second, the sample size for the initial randomized controlled trial was relatively small, as were the sample sizes for all the studies conducted on the first product line. Third, cLBP was self-reported and was not confirmed by healthcare professionals. Finally, more informative experimental designs and other indications were not explored.
Author contributions
TM: Writing – original draft, Writing – review and editing, Methodology, Formal Analysis, Project administration, Conceptualization. JS: Conceptualization, Writing – review and editing, Writing – original draft. MS: Writing – original draft, Writing – review and editing. MC: Writing – review and editing, Writing – original draft. EJ: Writing – original draft, Writing – review and editing. RR: Writing – original draft, Writing – review and editing. JD: Writing – original draft, Writing – review and editing. RM: Writing – original draft, Writing – review and editing. BD: Writing – original draft, Conceptualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Special thanks to Liesl Oldstone, PhD and Charisse Sparks, MD for their guidance and contributions to many of the milestones summarized in this manuscript. Special thanks to Tracie Kim for her graphical support with the figures in this article.
Conflict of interest
Authors TM, JS, MS, EJ, RR, JD, RM were employed by AppliedVR, Inc. Author MC was employed by Neptune Medical.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1During the FDA-authorization process, the product was referred to as EaseVRx. At product launch it was rebranded as RelieVRx®, and for consistency, will be referred to as RelieVRx throughout this manuscript.
References
Amtmann, D., Jensen, M. P., Turk, D., Bamer, A. M., and Liljenquist, K. S. (2019). University of Washington concerns about pain (UW-CAP) users guide. Available online at: https://uwcorr.washington.edu/wp-content/uploads/2019/05/uw-cap-userguide.pdf.
Baker, M. B., Liu, E. C., Bully, M. A., Hsieh, A., Nozari, A., Tuler, M., et al. (2024). Overcoming barriers: a comprehensive review of chronic pain management and accessibility challenges in rural America. Healthcare 12 (17), 1765. doi:10.3390/healthcare12171765
Bao, S., Quao, M., Lu, Y., and Jiant, Y. (2022). Neuroimaging mechanism of cognitive behavioral therapy in pain management. Pain Res. Manag. 2022, 1–8. doi:10.1155/2022/6266619
Botella, C., Serrano, B., Baños, R. M., and Garcia-Palacios, A. (2015). Virtual reality exposure-based therapy for the treatment of post-traumatic stress disorder: a review of its efficacy, the adequacy of the treatment protocol, and its acceptability. Neuropsychiatric Dis. Treat. 11, 2533–2545. doi:10.2147/ndt.s89542
Brennan, S. (2020). VRx: how virtual therapeutics will revolutionize medicine. New York, NY: Hachette Books.
Brewer, M. B., Lau, D. L., Chu, E. A., Millan, A. T., and Lee, J. T. (2021). Virtual reality can reduce anxiety during office-based great saphenous vein radiofrequency ablation. J. Vasc. Surg. Venous Lymphatic Disord. 9, 1222–1225. doi:10.1016/j.jvsv.2020.12.081
Brooke, J. (1996). SUS -A quick and dirty usability scale Usability and context. Usability Eval. Industry 189 (194).
Brown, K., and Foronda, C. (2020). Use of virtual reality to reduce anxiety and pain of adults undergoing outpatient procedures. Informatics 7 (3), 36. doi:10.3390/informatics7030036
Centers for Medicare and Medicaid Services (2022). “Centers for Medicare and Medicaid services” (CMS) healthcare Common procedure coding system (HCPCS) level II final coding,” in Benefit category and payment determinations.
Cherkin, D. C., Sherman, K. J., Balderson, B. H., Cook, A. J., Anderson, M. L., Hawkes, R. J., et al. (2016). Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA J. Am. Med. Assoc. 315 (12), 1240. doi:10.1001/jama.2016.2323
Colloca, L. (2019). The placebo effect in pain therapies. Annu. Rev. Pharmacol. Toxicol. 59 (6), 191–211. doi:10.1146/annurev-pharmtox-010818-021542
Colloca, L., Han, A., Massalee, R., Raghuraman, N., Cundiff-O’Sullivan, R. L., Colloca, G., et al. (2025). Telehealth virtual reality intervention reduces chronic pain in a randomized crossover study. NPJ Digit. Med. 8 (1), 192. doi:10.1038/s41746-025-01553-x
Cook, K. F., Rothrock, N. E., Bocell, F., Stone, A., Veasley, C., Anderson, A., et al. (2024). Threshold-scores for levels of chronic pain impact derived from the perspectives of patients and clinicians based on self-reported pain interference. J. Patient Rep. Outcomes.
Courtier, J., and Beberman, R. (2025). Understanding the FDA process is critical for Young Companies. J. Med. Ext. Real. 2 (1), 81–83. doi:10.1089/jmedxr.2024.0053
Dahlhamer, J., Lucas, J., Zelaya, C., Nahin, R., Mackey, S., DeBar, L., et al. (2018). Prevalence of chronic pain and high-impact chronic pain among adults — United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 67 (36), 1001–1006. doi:10.15585/mmwr.mm6736a2
Darnall, B. D., Krishnamurthy, P., Tsuei, J., and Minor, J. D. (2020). Self-administered skills-based virtual reality intervention for chronic pain: randomized controlled pilot study. JMIR Form. Res. 4 (7), e17293. doi:10.2196/17293
Darnall, B. D., Scheman, J., Davin, S., Burns, J. W., Murphy, J. L., Wilson, A. C., et al. (2016). Pain psychology: a global needs assessment and national call to action. Pain Med. (United States) 17 (2), 250–263. doi:10.1093/pm/pnv095
Day, M. A., Ehde, D. M., Bindicsova, I., and Jensen, M. P. (2023). Understanding the role of therapist quality in accounting for heterogeneity of patient outcomes in psychosocial chronic pain treatments. J. Pain 25, 843–856. doi:10.1016/j.jpain.2023.10.007
Deng, X., Jian, C., Yang, Q., Jiang, N., Huang, Z., and Zhao, S. (2022). The analgesic effect of different interactive modes of virtual reality: a prospective functional near-infrared spectroscopy (fNIRS) study. Front. Neurosci. 16, 1033155. doi:10.3389/fnins.2022.1033155
Dowell, D., Ragan, K. R., Jones, C. M., Baldwin, G. T., and Chou, R. (2022). CDC clinical practice guideline for prescribing opioids for pain - United States, 2022. MMWR Recomm. Rep. 71 (3), 1–95. doi:10.15585/mmwr.rr7103a1
Farrar, J. T., Young, J. P., LaMoreaux, L., Werth, J. L., and Poole, R. M. (2001). Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94 (2), 149–158. doi:10.1016/s0304-3959(01)00349-9
Fodor, L. A., Coteţ, C. D., Cuijpers, P., Szamoskozi, Ş., David, D., and Cristea, I. A. (2018). The effectiveness of virtual reality based interventions for symptoms of anxiety and depression: a meta-Analysis. Sci. Rep. 8 (1), 10323. doi:10.1038/s41598-018-28113-6
Foster, N. E., Anema, J. R., Cherkin, D., Chou, R., Cohen, S. P., Gross, D. P., et al. (2018). Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet 391 (10137), 2368–2383. doi:10.1016/s0140-6736(18)30489-6
Fu, H., Garrett, B., Tao, G., Cordingley, E., Ofoghi, Z., Taverner, T., et al. (2021). Virtual reality–guided meditation for chronic pain in patients with cancer: exploratory analysis of electroencephalograph activity. JMIR Biomed. Eng. 6 (2), e26332. doi:10.2196/26332
Garcia, L., Birckhead, B., Krishnamurthy, P., Mackey, I., Sackman, J., Salmasi, V., et al. (2021c). Three-month follow-up results of a double-blind, randomized placebo-controlled trial of 8-week self-administered at-home behavioral skills-based virtual reality (VR) for chronic low back pain. J. Pain 23, 822–840. doi:10.1016/j.jpain.2021.12.002
Garcia, L., Birckhead, B., Krishnamurthy, P., Mackey, I., Sackman, J., Salmasi, V., et al. (2022). Durability of the treatment effects of an 8-week self-administered home-based virtual reality program for chronic low back pain: 6-month follow-up study of a randomized clinical trial. J. Med. Internet Res. 24 (5), e37480. doi:10.2196/37480
Garcia, L., Darnall, B., Krishnamurthy, P., Mackey, I., Sackman, J., Louis, R., et al. (2021a). Self-administered behavioral skills–Based at-home virtual reality therapy for chronic low back pain: protocol for a randomized controlled trial. JMIR Res. Protoc. 10 (1), e25291. doi:10.2196/25291
Garcia, L. M., Birckhead, B. J., Krishnamurthy, P., Sackman, J., Mackey, I. G., Louis, R. G., et al. (2021b). An 8-week self-administered at-home behavioral skills-based virtual reality program for chronic low back pain: double-blind, randomized, placebo-controlled trial conducted during COVID-19. J. Med. Internet Res. 23 (2), e26292. doi:10.2196/26292
Gold, J. I., and Mahrer, N. E. (2018). Is virtual reality ready for prime time in the medical space? A randomized control trial of pediatric virtual reality for acute procedural pain management. J. Pediatr. Psychol. 43 (3), 266–275. doi:10.1093/jpepsy/jsx129
Gold, J. I., Soohoo, M., Laikin, A. M., Lane, A. S., and Klein, M. J. (2021). Effect of an immersive virtual reality intervention on pain and anxiety associated with peripheral intravenous catheter placement in the pediatric setting: a randomized clinical trial. JAMA Netw. Open 4 (8), e2122569. doi:10.1001/jamanetworkopen.2021.22569
Hendricks, T. M., Gutierrez, C. N., Stulak, J. M., Dearani, J. A., and Miller, J. D. (2020). The use of virtual reality to reduce preoperative anxiety in first-time sternotomy patients: a randomized controlled pilot trial. Mayo Clin. Proc. 95 (6), 1148–1157. doi:10.1016/j.mayocp.2020.02.032
Herman, P. M., Broten, N., Lavelle, T. A., Sorbero, M. E., and Coulter, I. D. (2019). Health care costs and opioid use associated with high-impact chronic spinal pain in the United States. Spine (Phila Pa 1976) 44 (16), 1154–1161. doi:10.1097/brs.0000000000003033
Hoffman, H. G., Richards, T. L., Coda, B., Bills, A. R., Blough, D., Richards, A. L., et al. (2004). Modulation of thermal pain-related brain activity with virtual reality: evidence from fMRI. Neuroreport 15 (8), 1245–1248. doi:10.1097/01.wnr.0000127826.73576.91
Hoffman, H. G., Richards, T. L., Van Oostrom, T., Coda, B. A., Jensen, M. P., Blough, D. K., et al. (2007). The analgesic effects of opioids and immersive virtual reality distraction: evidence from subjective and functional brain imaging assessments. Anesth. Analg. 105 (6), 1776–1783. doi:10.1213/01.ane.0000270205.45146.db
Institute of Medicine (US) (2011). “Committee on Advancing Pain Research C and E. Relieving pain in America: a blueprint for transforming prevention, care, education, and research,” in Relieving pain in America: a blueprint for transforming prevention, care, education, and research.
King, A. L., Acquaye-Mallory, A. A., Vera, E., Mendoza, T., Reyes, J., Stockdill, M. L., et al. (2023b). Feasibility and preliminary efficacy of a virtual reality intervention targeting distress and anxiety in primary brain tumor patients at the time of clinical evaluation: study protocol for a phase 2 clinical trial. BMC Cancer 23 (1), 262. doi:10.1186/s12885-023-10671-2
King, A. L., Roche, K. N., Leeper, H. E., Vera, E., Mendoza, T., Mentges, K., et al. (2023a). Feasibility of a virtual reality intervention targeting distress and anxiety symptoms in patients with primary brain tumors: interim analysis of a phase 2 clinical trial. J. Neurooncol 162 (1), 137–145. doi:10.1007/s11060-023-04271-0
Kolbe, L., Jaywant, A., Gupta, A., Vanderlind, W. M., and Jabbour, G. (2021). Use of virtual reality in the inpatient rehabilitation of COVID-19 patients. Gen. Hosp. Psychiatry 71, 76–81. doi:10.1016/j.genhosppsych.2021.04.008
Krish, G., Immerman, I., and Kinjo, S. (2022). Virtual reality may reduce anxiety and enhance surgical experience during wide-awake local anaesthesia no tourniquet surgery: a report of two cases. J. Perioper. Pract. 32 (6), 136–141. doi:10.1177/1750458920984048
Lee, S. Y., Cha, J. Y., Yoo, J. W., Nazareno, M., Cho, Y. S., Joo, S. Y., et al. (2022). Effect of the application of virtual reality on pain reduction and cerebral blood flow in robot-assisted gait training in burn patients. J. Clin. Med. 11 (13), 3762. doi:10.3390/jcm11133762
Li, P. G., Aquilini, B., Davoli, A., Silvana, G., and Ruini, C. (2023). The use of virtual reality interventions to promote positive mental health: systematic literature review. JMIR Ment. Health 10, e44998. doi:10.2196/44998
Lucas, J. W., and Sohi, I. (2024). Chronic pain and high-impact chronic pain among U.S. Adults, 2023. CDC: Hyattsville, MD.
Mace, R. A., Brewer, J. R., Cohen, J. E., Ly, T. V., Weaver, M. J., and Borsook, D. (2024). Virtual reality for subacute pain after orthopedic traumatic musculoskeletal injuries. Clin. J. Pain 40 (9), 526–541. doi:10.1097/ajp.0000000000001231
Maddox, T. (2025). Who benefits most? Identifying RelieVRx® success factors in chronic low back pain. WHO: Los Angeles.
Maddox, T., and Fitzpatrick, T. (2019). The promise of virtual reality in health-care education and training: it’s all in the neuroscience. Digit. Med. 5 (4), 133–137. doi:10.4103/digm.digm_26_19
Maddox, T., Garcia, H., Ffrench, K., Maddox, R., Garcia, L., Krishnamurthy, P., et al. (2022). In-home virtual reality program for chronic low back pain: durability of a randomized, placebo-controlled clinical trial to 18 months post-treatment. Reg. Anesth. Pain Med. 49, 373–375. doi:10.1136/rapm-2022-104093
Maddox, T., Oldstone, L., Linde-Zwirble, W., Bonakdar, R., Maddox, R., Sackman, J., et al. (2025). Differential treatment response to virtual reality in high-impact chronic pain: secondary analysis of a randomized trial. Sci. Rep. 15 (1), 14430. doi:10.1038/s41598-025-98716-3
Maddox, T., Oldstone, L., Sackman, J., Judge, E., Maddox, R., Adair, T., et al. (2024b). Sociodemographic predictors of clinical effectiveness, therapeutic program engagement, and device usability for an in-home virtual reality program for chronic low back pain: secondary analysis of a randomized controlled trial. J. Med. Ext. Real. 1 (1), 65–72. doi:10.1089/jmxr.2023.0013
Maddox, T., Oldstone, L., Sackman, J., Maddox, R., Adair, T., Ffrench, K., et al. (2024a). Twelve-month results for a randomized sham-controlled effectiveness trial of an in-home skills-based virtual reality program for chronic low back pain. Pain Rep. 9 (5), e1182. doi:10.1097/pr9.0000000000001182
Maddox, T., Oldstone, L., Sparks, C. Y., Sackman, J., Oyao, A., Garcia, L., et al. (2023a). In-home virtual reality program for chronic lower back pain: a randomized sham-controlled effectiveness trial in a clinically severe and diverse sample. Mayo Clin. Proc. Digit. Health 1 (4), 563–573. doi:10.1016/j.mcpdig.2023.09.003
Maddox, T., Sparks, C., Oldstone, L., Chibarro, M., Sackman, J., Judge, E., et al. (2023b). Perspective: the promise of virtual reality as an immersive therapeutic. J. Med. Ext. Real. 1, 13–20. doi:10.1089/jmxr.2023.0003
Maddox, T., Sparks, C., Oldstone, L., Maddox, R., Ffrench, K., Garcia, H., et al. (2023c). Durable chronic low back pain reductions up to 24 months after treatment for an accessible, 8-week, in-home behavioral skills–based virtual reality program: a randomized controlled trial. Pain Med. 24, 1200–1203. doi:10.1093/pm/pnad070
Martucci, K. T., and MacKey, S. C. (2018). Neuroimaging of pain: human evidence and clinical relevance of central nervous system processes and modulation. Anesthesiology. 128, 1241, 1254. doi:10.1097/aln.0000000000002137
Mistry, S. K., Shaw, M., Raffan, F., Johnson, G., Perren, K., Shoko, S., et al. (2022). Inequity in access and delivery of virtual care interventions: a scoping review. Int. J. Environ. Res. Public Health 19, 9411. doi:10.3390/ijerph19159411
Monticone, M., Cedraschi, C., Ambrosini, E., Rocca, B., Fiorentini, R., Restelli, M., et al. (2015). Cognitive-behavioural treatment for subacute and chronic neck pain. Cochrane Database Syst. Rev. 2015, CD010664. doi:10.1002/14651858.cd010664.pub2
Norbye, A. D., Omdal, A. V., Nygaard, M. E., Romild, U., Eldøen, G., and Midgard, R. (2016). Do patients with chronic low back pain benefit from early intervention regarding absence from work? Spine (Phila Pa 1976) 41 (21), E1257–E1264. doi:10.1097/brs.0000000000001878
Oldstone, L., Maddox, T., Garcia, L., Sackman, J., Maddox, R., Ffrench, K., et al. (2024). Psycho-social predictors of treatment effectiveness from skills-based VR-delivered therapy? Secondary analysis of a randomized controlled trial. in Chronic low back pain.
Oldstone, L. M., and Judge, E. (2024). Innovative reimbursement strategy for digital therapeutics. Available online at: https://mdic.org/wp-content/uploads/2024/02/AppliedVR-Case-Study_FINAL.pdf.
Perez, H. (2023). “Virtual reality for chronic pain and OUD,” in A Pilot Randomized Controlled Trial. NIH: Albuquerque.
Persky, S., and Colloca, L. (2023). Medical extended reality trials: building robust comparators, controls, and sham. J. Med. Internet Res. 25, e45821. doi:10.2196/45821
Pitcher, M. H., Von Korff, M., Bushnell, M. C., and Porter, L. (2019). Prevalence and profile of high-impact chronic pain in the United States. J. Pain 20 (2), 146–160. doi:10.1016/j.jpain.2018.07.006
Quartana, P. J., Campbell, C. M., and Edwards, R. R. (2009). Pain catastrophizing a critical review. Expert Rev. Neurother. 9, 745–758. doi:10.1586/ern.09.34
Recker, K., Chmielewska, K., Patel, P., Silliman, J., Santana-Rojas, L., Hildenbrand, A. K., et al. (2023). Piloting virtual reality respiratory biofeedback in an intensive outpatient pediatric pain rehabilitation program. J. Pain 24 (4), 88–89. doi:10.1016/j.jpain.2023.02.254
Riva, G., Mantovani, F., Capideville, C. S., Preziosa, A., Morganti, F., Villani, D., et al. (2007). Affective interactions using virtual reality: the link between presence and emotions. Cyberpsychology Behav. 10 (1), 45–56. doi:10.1089/cpb.2006.9993
Sarkar, U., Lee, J. E., Nguyen, K. H., Lisker, S., and Lyles, C. R. (2021). Barriers and facilitators to the implementation of virtual reality as a pain management modality in academic, community, and safety-net settings: qualitative analysis. J. Med. Internet Res. 23 (9), e26623. doi:10.2196/26623
Seifert, A., Reinwand, D. A., and Schlomann, A. (2019). Designing and using digital mental health interventions for older adults: being aware of digital inequality. Front. Psychiatry 10, 568. doi:10.3389/fpsyt.2019.00568
Seifert, A., and Schlomann, A. (2021). The use of virtual and augmented reality by older adults: potentials and challenges. Front. Virtual Real 2. doi:10.3389/frvir.2021.639718
Seminowicz, D. A., Shpaner, M., Keaser, M. L., Krauthamer, G. M., Mantegna, J., Dumas, J. A., et al. (2013). Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J. Pain 14 (12), 1573–1584. doi:10.1016/j.jpain.2013.07.020
Şentürk, I. A., Şentürk, E., Üstün, I., Gökçedaǧ, A., Yildirim, N. P., and Içen, N. K. (2023). High-impact chronic pain: evaluation of risk factors and predictors. Korean J. Pain 36 (1), 84–97. doi:10.3344/kjp.22357
Shpaner, M., Kelly, C., Lieberman, G., Perelman, H., Davis, M., Keefe, F. J., et al. (2014). Unlearning chronic pain: a randomized controlled trial to investigate changes in intrinsic brain connectivity following Cognitive Behavioral Therapy. Neuroimage Clin. 5, 365–376. doi:10.1016/j.nicl.2014.07.008
Sikka, N., Shu, L., Ritchie, B., Amdur, R. L., and Pourmand, A. (2019). Virtual reality-assisted pain, anxiety, and anger management in the emergency department. Telemedicine e-Health 25 (12), 1207–1215. doi:10.1089/tmj.2018.0273
Singh, V. M., Adkinson, S. M., and Brandow, A. (2019). Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. U. S. Dep. Health Hum. Serv. 11 (2).
Spiegel, B., Fuller, G., Lopez, M., Dupuy, T., Noah, B., Howard, A., et al. (2019). Virtual reality for management of pain in hospitalized patients: a randomized comparative effectiveness trial. PLoS One 14 (8), e0219115. doi:10.1371/journal.pone.0219115
Tashjian, V. C., Mosadeghi, S., Howard, A. R., Lopez, M., Dupuy, T., Reid, M., et al. (2017). Virtual reality for management of pain in hospitalized patients: results of a controlled trial. JMIR Ment. Health 4 (1), e9. doi:10.2196/mental.7387
Tran, Y., Austin, P., Lo, C., Craig, A., Middleton, J. W., Wrigley, P. J., et al. (2022). An exploratory EEG analysis on the effects of virtual reality in people with neuropathic pain following spinal cord injury. Sensors 22 (7), 2629. doi:10.3390/s22072629
Traylor, C. (2019). Medicaid strategies for non-opioid pharmacologic and Non Pharmacologic chronic pain management. CMCS.
Trost, Z., Zielke, M., Guck, A., Nowlin, L., Zakhidov, D., France, C. R., et al. (2015). The promise and challenge of virtual gaming technologies for chronic pain: the case of graded exposure for low back pain. Pain Manag. 5, 197–206. doi:10.2217/pmt.15.6
US Food and Drug Administration (2018). Step 3: pathway to approval. Available online at: https://www.fda.gov/patients/device-development-process/step-3-pathway-approval?utm_source=chatgpt.com.
US Food and Drug Administration (2025). Product classification: external compression device for internal jugular vein compression. Available online at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?id=5458.
VA Diffusion Marketplace (2025). Re-engaging veterans in pain through extended reality (VIPER): virtual reality. Available online at: https://marketplace.va.gov/innovations/re-engaging-veterans-in-pain-through-immersive-technology.
Vaegter, H. B. P., Due Bruun, K. M. P., and Bye-Møller, L. P. (2023). “High-impact chronic pain,” in International association for the study of pain.
Venuturupalli, R. S., Chu, T., Vicari, M., Kumar, A., Fortune, N., and Spielberg, B. (2019). Virtual reality–based biofeedback and guided meditation in rheumatology: a pilot study. ACR Open Rheumatol. 1 (10), 667–675. doi:10.1002/acr2.11092
ClinicalTrials (2025). Virtual reality for chronic pain and opioid use disorder pilot. Available online at: https://clinicaltrials.gov/study/NCT05546749.
Williams, A. C., Fisher, E., Hearn, L., and Eccleston, C. (2020). Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst. Rev. 2020, CD007407. doi:10.1002/14651858.cd007407.pub4
Wong, M. S., and Gregory, K. D. (2019). Patient reported outcomes on the use of virtual reality for pain management in labor [17E]. Obstetrics Gynecol. 133 (1), 55S. doi:10.1097/01.aog.0000559009.58037.52
Yao, R., Zhang, W., Evans, R., Cao, G., Rui, T., and Shen, L. (2022). Inequities in health care services caused by the adoption of digital health technologies: scoping review. J. Med. Internet Res. 24, e34144. doi:10.2196/34144
Keywords: virtual reality, chronic lower back pain, cognitive behavioral therapy, immersive therapeutic, VR
Citation: Maddox T, Sackman J, Stoudt M, Chibbaro M, Judge E, Rothery R, Donini J, Maddox R and Darnall BD (2025) Virtual reality-delivered skills-based therapy for chronic lower back pain: from proof-of-concept to FDA authorization to clinical implementation in-home and beyond. Front. Virtual Real. 6:1613188. doi: 10.3389/frvir.2025.1613188
Received: 16 April 2025; Accepted: 13 June 2025;
Published: 24 June 2025.
Edited by:
Vangelis Lympouridis, University of Southern California, United StatesReviewed by:
Billy Huh, University of Texas MD Anderson Cancer Center, United StatesSabina M. Pinto, Hong Kong Polytechnic University, Hong Kong SAR, China
Binay Biswas, ESIC Medical College and Hospital, India
Copyright © 2025 Maddox, Sackman, Stoudt, Chibbaro, Judge, Rothery, Donini, Maddox and Darnall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Todd Maddox, dG1hZGRveEBhcHBsaWVkdnIuaW8=
 Todd Maddox
Todd Maddox Josh Sackman1
Josh Sackman1 Beth D. Darnall
Beth D. Darnall