- 1Academy of Chinese Medical Sciences, Henan University of Chinese Medicine, Zhengzhou, China
- 2College of Animal Science and Veterinary Medicine, Henan Agricultural University, Zhengzhou, China
- 3College of Animal Science, Tarim University, Alar, China
Cyclosporiasis is caused by the coccidian parasite Cyclospora cayetanensis and is associated with large and complex food-borne outbreaks worldwide. Associated symptoms include severe watery diarrhea, particularly in infants, and immune dysfunction. With the globalization of human food supply, the occurrence of cyclosporiasis has been increasing in both food growing and importing countries. As well as being a burden on the health of individual humans, cyclosporiasis is a global public health concern. Currently, no vaccine is available but early detection and treatment could result in a favorable clinical outcome. Clinical diagnosis is based on cardinal clinical symptoms and conventional laboratory methods, which usually involve microscopic examination of wet smears, staining tests, fluorescence microscopy, serological testing, or DNA testing for oocysts in the stool. Detection in the vehicle of infection, which can be fresh produce, water, or soil is helpful for case-linkage and source-tracking during cyclosporiasis outbreaks. Treatment with trimethoprim-sulfamethoxazole (TMP-SMX) can evidently cure C. cayetanensis infection. However, TMP-SMX is not suitable for patients having sulfonamide intolerance. In such case ciprofloxacin, although less effective than TMP-SMX, is a good option. Another drug of choice is nitazoxanide that can be used in the cases of sulfonamide intolerance and ciprofloxacin resistance. More epidemiological research investigating cyclosporiasis in humans should be conducted worldwide, to achieve a better understanding of its characteristics in this regard. It is also necessary to establish in vitro and/or in vivo protocols for cultivating C. cayetanensis, to facilitate the development of rapid, convenient, precise, and economical detection methods for diagnosis, as well as more effective tracing methods. This review focuses on the advances in clinical features, diagnosis, and therapeutic intervention of cyclosporiasis.
Introduction
Cyclosporiasis is caused by Cyclospora cayetanensis, and in humans it typically induces periodic profuse watery diarrhea (Shields and Olson, 2003a; Ortega and Sanchez, 2010; Almeria et al., 2019; Giangaspero and Gasser, 2019). Human C. cayetanensis infection has been documented in over 56 countries worldwide, and 13 of these have recorded cyclosporiasis outbreaks (Li et al., 2019a). The latest large scale cyclosporiasis outbreaks occurred in 2013 and 2018 in multiple states of the US (Abanyie et al., 2015; Casillas et al., 2018).
As of today, 22 Cyclospora species are identified in humans and various animals, including vipers, moles, myriapodes, rodents, and monkeys (Li et al., 2015a, 2019a; McAllister et al., 2018). C. cayetanensis is the only documented Cyclospora species known to infect humans (Ortega and Sanchez, 2010; Li et al., 2019a). The overall prevalence of C. cayetanensis was 3.6% in humans worldwide (Li et al., 2019a). Of the other species, C. papionis was detected in 17.9% of the captured baboons in Kenya (Li et al., 2011) and C. macacae in 6.8% of the rhesus monkeys in China (Li et al., 2015b). In humans, most of these infections are contracted via the fecal-oral route, and water, berries, basil, cilantro, and other food produce can be a vehicle for Cyclospora transmission (Almeria et al., 2019). The Cyclospora infection is evidenced to be linked with consumption of contaminated food and water or contact with transmission vehicles of oocysts (Li et al., 2019a).
Although large outbreaks of cyclosporiasis have been documented in developed countries, C. cayetanensis infections are most commonly reported in developing countries or in endemic areas (Li et al., 2019a). In susceptible individuals, cyclosporiasis is reported to be most prevalent in immunocompetent diarrheic patients (Li et al., 2019a). There are notable seasonal distributions of C. cayetanensis infections that commonly occur in rainy or summer season (Zhou et al., 2011; Kaminsky et al., 2016). Cyclosporiasis causes significant health problem to the people traveling or expatriating to the under developed or developing countries having poor sanitation and high population density (Fryauff et al., 1999; Mansfield and Gajadhar, 2004; Pandey et al., 2011; Kłudkowska et al., 2017).
The entire genome of C. cayetanensis had been sequenced (Liu et al., 2016; Qvarnstrom et al., 2018), and there have been recent improvements in detection methods and therapeutic interventions for cyclosporiasis. This review presents an update on aspects of the clinical features, detection methods, therapy, and prevention of cyclosporiasis.
Clinical Features
Intestinal Infection Features
Clinical Symptoms
Frequently the cyclosporiasis patient is an immunocompetent traveler living in an industrialized country, returning from a tropical and/or developing country such as the Dominican Republic, Mexico, Guatemala, Haiti, Peru, or Nepal, among others. Typical symptoms on presentation include watery diarrhea, abdominal cramps, vomiting, anorexia, weight loss, and severe fatigue. Less frequently patients also report flu-like symptoms (Marques et al., 2017; Giangaspero and Gasser, 2019). Cyclosporiasis patients with immune dysfunction can experience severe, protracted, or chronic watery diarrhea along with nausea, abdominal pain, mild fever, lethargy, and emaciation (Field, 2002; Shields and Olson, 2003a; Mansfield and Gajadhar, 2004). The condition can be particularly challenging in organ transplant recipients undergoing immunosuppressive treatment (Giangaspero and Gasser, 2019).
Human cyclosporiasis can be asymptomatic, or range from mild to severe in endemic countries, such as Guatemala, Haiti, Peru, and Nepal (Mansfield and Gajadhar, 2004; Giangaspero and Gasser, 2019). The clinical outcomes of cyclosporiasis are related to the age and immune status of the host, endemicity in a specific area and some other unknown factors (Almeria et al., 2019). Infants and the elderly tend to exhibit more severe clinical symptoms, whereas milder or asymptomatic infections typically occur in older children and non-elderly adults (Giangaspero and Gasser, 2019).
The incubation period of C. cayetanensis infection ranges from 2 to 11 days, and the median incubation period is ~7 days (Almeria et al., 2019). The clinical symptoms usually resolve with the treatment by specific drugs. However, persistent infection is seen in untreated patients that can last for a few days to a month or even longer (Thapa and Basnyat, 2017). The mean duration of diarrhea caused by cyclosporiasis is longer in AIDS patients (199 days) compared to other patients (57 days) (Schubach et al., 1997; Sancak et al., 2006; Ortega and Sanchez, 2010).
Endoscopic Change
In a previous study involving endoscopy of 17 Peruvian cyclosporiasis patients, moderate to marked erythema was observed in the distal duodenum of some cases and mild to moderate inflammation in the intestinal lamina propria of the other cases (Ortega et al., 1997). However, ulcer or hemorrhage like gross abnormalities were absent in both stomach and small intestine in any of the cyclosporiasis patients (Ortega et al., 1997).
Histology Change
Striking intestinal histological changes have been observed in patients with cyclosporiasis. Alteration of the overall architecture of the intestinal mucosa has been reported, with dramatic shortening of the intestinal villi and disruption of the surface epithelium (Ortega et al., 1997; Ortega and Sanchez, 2010). Villous atrophy and crypt hyperplasia in the duodenum and ileum have also been described (Connor et al., 1993; Field, 2002). In the aforementioned 17 Peruvian patients there was reactive hyperemia with vascular dilatation and congestion of villous capillaries (Ortega et al., 1997), and some patients have exhibited variably increased chronic inflammatory cells and intense lymphocytic infiltration in the lamina propria and epithelial tissue (Ortega et al., 1997; Wiwanitkit, 2006). Ortega et al. (1997) also reported extensive infiltration of lymphocytes into the surface epithelium, which was particularly prominent at the tip of the shortened villi. In another report, the C. cayetanensis induced inflammatory reactions were found to be lasted even after clearance of parasitic infection (Connor et al., 1999). Notably however, the pathogenesis underlying these symptoms has not been defined.
Intracellular Changes
Increases in lymphocytes in the intestinal surface epithelium have been reported during oocyst infections, during which parasitophorous vacuoles containing C. cayetanensis at various stages of the sexual and asexual life-cycle were observed in the apical cytoplasm of the enterocytes overlying the tips of the villi (Field, 2002). Via electron microscopy, rounded or more mature elongated fusiform merozoites up to 6 μm in length can be seen stacked in vacuoles within enterocytes, as well as occasional micro-gametocytes and macro-gametocytes (Field, 2002).
In high-magnification light microscopic examination, the parasite was seen at the luminal surface and the glandular clefts (Ortega et al., 1997) with having two completely developed asexual forms, Type I and II meronts. About 8–12 fully mature merozoites (~0.5 × 3–4 μm) were observed in Type I meront while 4 fully differentiated merozoites (~0.7–0.8 × 12–15 μm) were in Type II meront (Ortega et al., 1997). The parasite was also observed to have sexual forms, such as gametocytes. As typically seen in other coccidia, the merozoites of both Type I and II meronts contained rhoptries, micronemes, and nuclei. Meanwhile, the characteristic wall-forming body types I and II and polysaccharide granules were observed in macro-gametocytes of the parasite (Ortega et al., 1997). However, the gastric antral biopsy could not detect the Cyclospora parasite (Ortega et al., 1997). The findings of light microscopic examination were all substantiated by the transmission electron microscopy of the both asexual and sexual forms of the parasite.
External Features of Infection
Cyclospora cayetanensis oocysts were evidenced to infect extraintestinal tissue such as biliary tract (Sifuentes-Osornio et al., 1995), resulting in acalculous cholecystitis in an AIDS patient (Zar et al., 2001). The pathogenesis of biliary infections is unknown. Presumably sporozoites from the intestinal lumen travel to bile ducts and initiate the development of Cyclospora there (Almeria et al., 2019).
Although C. cayetanensis infection was not reported in the respiratory tract, oocysts were detected in the nasal secretion of two patients suffering from tuberculosis (Di Gliullo et al., 2000; Hussein et al., 2005). Additionally, C. cayetanensis infections were found to be linked with some other diseases, including Reiter syndrome (reactive arthritis syndrome) (Connor et al., 2001) and Guillain-Barre syndrome (Richardson et al., 1998).
Detection in Stool
Oocyst Morphology Detection
Wet Smears
Wet smears and conventional microscopy methods have been widely used to detect C. cayetanensis oocysts in clinical stool samples (Ortega and Sanchez, 2010). The smears can be made directly from fresh stools, or concentrated samples obtained from formalin-ether sedimentation or sucrose flotation techniques that enhance detection efficiency of small amount of Cyclospora oocysts in stool samples (Becker et al., 2013). When observed, Cyclospora oocysts in stool are easily identified as spherical and refractile entities that are 8–10 μm in size and have a central morula (Li et al., 2019a). Since the Cyclospora oocysts shed discontinously, manifold fecal samples (each at 2–3 days interval) should be collected in a week or more from a patient for accurate detection of oocysts (Yang et al., 2014). Wet smear examination is a simple, direct, rapid, and reliable method for visual detection of parasites, but it is costly and laborious, and it requires specific expertise. Given the size similarity to some other microorganisms such as Cryptosporidium (4–6 μm), C. cayetanensis should be further differentiated by staining on wet or dry mounts (Almeria et al., 2019).
Staining Tests
The oocyst walls of Cyclospora, Cryptosporidium, and Cystoisospora parasites have acid-fast lipids that help their detection by acid-fast staining (Garcia et al., 2017). Although the modified acid-fast stain test can be useful for identifying Cyclospora oocysts, variable levels of dye uptake may result in ghost oocysts, or pink-stained or poorly stained oocysts, or oocysts that are not stained at all and appear as non-refractile glassy spheres against the blue-green background, along with well-stained oocysts (deep red with a mottled appearance) (Ortega and Sanchez, 2010; Garcia et al., 2017; Almeria et al., 2019).
Modified acid-fast staining with further minor modifications are developed to improve Cyclospora detection, one of which is the use of 1% H2SO4 as a decolorizer (Garcia et al., 2017). Another modification is devised with addition of dimethyl sulfoxide to the phenol-basic fuchsine, and the inclusion of acetic acid with malachite green as a combined decolorizer counter-stain to achieve better penetration and thus enhanced visualization of the internal structures of oocysts (Garcia et al., 2017). Apart from this, other modified acid-fast staining methods, such as Ziehl-Neelsen acid-fast stain (Brennan et al., 1996; Clarke and McIntyre, 1996), modified Kinyoun's acid-fast stain (Gonçalves et al., 2005; Behera et al., 2008; Dillingham et al., 2009; Bhandari et al., 2015), and modified Kinyoun's carbolfuchsin stain (Alakpa et al., 2002; Chacín-Bonilla et al., 2007) are commonly used. Some other staining methods, including modified safranin stain (Visvesvara et al., 1997), trichrome stain (Turgay et al., 2007), and lacto-phenol cotton blue stain (Parija et al., 2003) were also found to be highly sensitive to detect Cyclospora oocysts in fecal smears.
Even with the aid of conventional staining methods for Cyclospora oocysts microscopic detection can be challenging (McHardy et al., 2014), but it remains the recommended diagnostic method. Of the various available stains, the modified Ziehl-Neelsen stain technique has been recommended for detecting Cyclospora oocysts in clinical samples (Brennan et al., 1996; Khanna et al., 2014).
Fluorescence Tests
Cyclospora oocysts' strong autofluorescence properties render fluorescence microscopy useful for identification (Garcia et al., 2017). On 365- and 450–490-nm ultraviolet light exposures, the oocysts appear blue and green, respectively (Ortega and Sanchez, 2010; McHardy et al., 2014). In epifluorescence microscopic examination using a 330–380-nm ultraviolet filter, C. cayetanensis oocysts were observed to be easily visible in clinical samples (Eberhard et al., 1997), which enhances detection at least 2-fold over direct wet mounts, particularly in cases where the mounts or stained slides contain few oocysts (Berlin et al., 1998). The autofluorescence technique is reportedly markedly superior to wet smears and staining procedures for Cyclospora oocyst detection (Berlin et al., 1998).
Flow Cytometry
On the basis of morphological and autofluorescence properties of oocysts, a flow cytometry detection assay was developed for C. cayetanensis (Dixon et al., 2005). While the sample preparation time for flow cytometry is slightly longer than that for microscopy, the actual analysis time is much shorter. Furthermore, the flow cytometry is mostly automated that omits the problems of technical experience and tiresomeness of other manual analyses which may affect the results of detection (Li et al., 2014). A comparative study for the detection and quantification of Cyclospora oocysts observed no significant differences between flow cytometry and quantitative real-time PCR assays (Hussein et al., 2007).
To sum up, among the various available detection methods of Cyclospora oocyst based on morphology, the modified Ziehl-Neelsen staining and autofluorescence techniques have been recommended for the detection of oocysts in the clinical samples (Berlin et al., 1998; Khanna et al., 2014).
Serological Tests
Serological screening for Cyclospora would facilitate epidemiological studies, especially in outbreak investigations (Ortega and Sanchez, 2010), but currently commercial serological assays to identify human exposure to Cyclospora are not available (Almeria et al., 2019). There have been attempts to characterize human serological immune responses to cyclosporiasis, involving specific IgG and IgM antibodies being tested via enzyme-linked immunosorbent assays (Wang et al., 2002), but specific diagnosis of infection at the individual patient level has not been achieved (Giangaspero and Gasser, 2019). The main constrain is the unavailability of suitable Cyclospora culture method that can be used for the propagation of oocysts and also the in-depth study of various aspects of the parasite (Eberhard et al., 2000; Cinar et al., 2015).
Molecular Detection
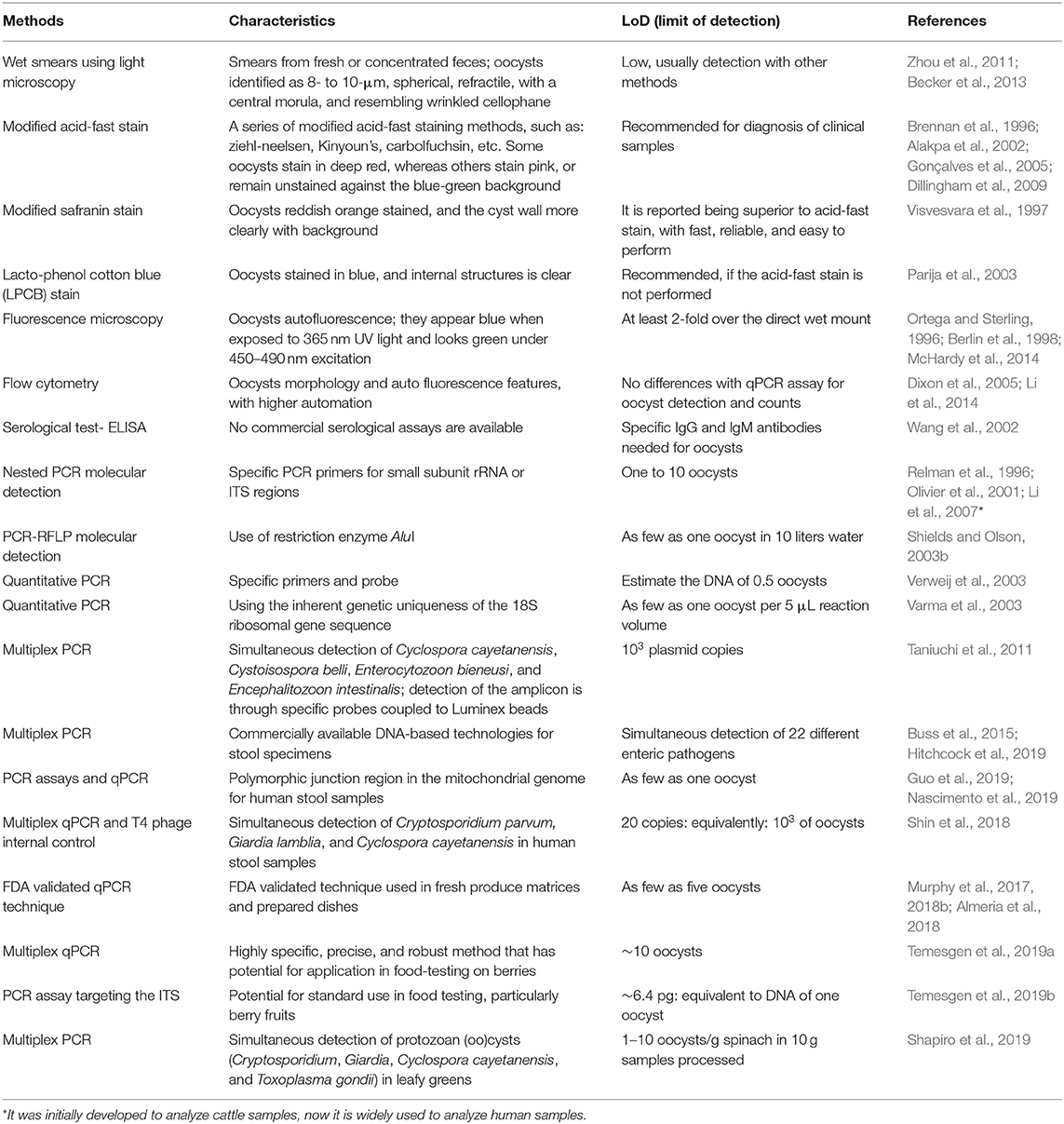
Over the few decades, several conventional, nested, and quantitative PCR (qPCR) as well as multiplex PCR assays (together with other parasites) are developed for the identification of Cyclospora (Relman et al., 1996; Varma et al., 2003; Taniuchi et al., 2011; Li et al., 2015a, 2019a; Almeria et al., 2019) (Table 1). There is one commercially available fully automated system involving high-order multiplex PCR reactions that is capable of detecting C. cayetanensis with high sensitivity and specificity (Buss et al., 2015). The multiplex Biofire (Salt Lake City, UT, USA) FilmArray Gastrointestinal Panel is a commercially available DNA-based technology for the detection of C. cayetanensis (Ryan et al., 2017; Hitchcock et al., 2019).
Recently, a molecular diagnostic method employing multiplex real-time PCR and a T4 phage internal control has been devised for the simultaneous detection of Cryptosporidium parvum, Giardia lamblia, and C. cayetanensis in human stools (Shin et al., 2018). The QIAstat gastrointestinal panel can detect a large range of acute gastroenteritis pathogens with a high sensitivity, including C. cayetanensis (Hannet et al., 2019). Molecular-based detection methods have the capacity to screen a number of organisms at a time with using multiplex platforms, and to detect them rapidly with high sensitivity (even detection of a single oocyst), thus overcoming some of the limitations of microscopy-based diagnosis. Now-a-days, the molecular techniques have been widely used in laboratory testing or verification of suspected clinical samples.
Case-Linking and Tracking
Numerous genotyping methods for C. cayetanensis have been developed, and successfully used for epidemiological trace-back investigations of cyclosporiasis. Recently, a multilocus sequence typing (MLST) tool involving five microsatellite loci has been established and used for epidemiological source tracking of C. cayetanensis (Guo et al., 2016). There have many other potential uses of this MLST tool (Hofstetter et al., 2019). It has been used to investigate the population genetics of C. cayetanensis (Li et al., 2017; Guo et al., 2018). More recently, qPCR (Guo et al., 2019) and standard PCR assays (Nascimento et al., 2019) have been evolved for the genotyping of C. cayetanensis that use the polymorphic region of parasitic mitochondrial genome. Relationships have been corroborated by a significant number of epidemiological linkages, suggesting the usefulness of the technique for aiding epidemiological trace-back, case-linkage, source-tracking, and distinct case cluster investigations (Barratt et al., 2019; Guo et al., 2019; Nascimento et al., 2019), especially during cyclosporiasis outbreaks.
Detection in Vehicles
Fresh Produces
Usually only low numbers of protozoan oocysts exist in naturally contaminated produce. A very important step in the isolation process is the productive gaining of oocysts after a careful washing of the fresh produce (Shields et al., 2012; Li et al., 2019b). Different washing solutions have been used for the recovery of C. cayetanensis oocysts (Shields et al., 2012; Chandra et al., 2014; Lalonde and Gajadhar, 2016; Li et al., 2019b). A filter bag (BagPage®, Interscience Lab. Inc., Boston, MA) along with a commercial laboratory detergent (Alconox®, White Plains, NY) (Shields et al., 2012) is used as a valid wash protocol for the successful recovery of C. cayetanensis oocysts from fresh produce and thereby DNA extraction and a specific qPCR are followed for the detection of the parasite (Murphy et al., 2018a).
In recent years, several molecular techniques have been developed for the detection of C. cayetanensis in fresh produce, including qPCR and various multiplex qPCR methods (Steele et al., 2003; Lalonde and Gajadhar, 2011, 2016; Murphy et al., 2017; Shapiro et al., 2019) (Table 1). qPCR can steadily identify a few oocysts in the fresh produce, such as three oocysts in a gram of fruit, or five oocysts in a gram of herbs or green onions (Lalonde and Gajadhar, 2016). In other reports as few as five oocysts were detected in samples of raspberries, cilantro, parsley, basil, and carrots via a qPCR technique (Murphy et al., 2017, 2018b; Almeria et al., 2018). Other methods for C. cayetanensis detection in fresh produce have been developed, including a new multiplex qPCR technique that is highly specific, precise, and robust and has potential for application in food-testing laboratories (Temesgen et al., 2019a). The limit of detection of that technique was estimated to be 10 oocysts for Cyclospora organisms. Another qPCR assay targeting the internal transcribed spacer 1 region was developed for the detection of C. cayetanensis in berries, and proved to be an effective approach that may be a suitable option for use in food-testing laboratories (Temesgen et al., 2019b). A multiplex PCR assay has been developed for simultaneous detection of four protozoan oocysts via a rapid, inexpensive, and simple protocol (Shapiro et al., 2019).
Robertson et al. (2000) validated the use of lectin-coated paramagnetic beads for the isolation of Cyclospora oocysts from fruits and vegetables. As reported for the detection of Cryptosporidium parvum, specific antibody-coated beads can be used to isolate and concentrate the C. cayetanensis oocysts, but antibodies are not yet commercially available (Almeria et al., 2019).
Water or Soil
There are many documented reports of Cyclospora oocysts contaminating water and soil derived from multiple countries (Sturbaum et al., 1998; Sherchand and Cross, 2001; Tram et al., 2008; Giangaspero et al., 2015; Bilung et al., 2017), and various techniques have been developed for the isolation and identification of Cyclospora oocysts from the environmental samples, such as water and soil (Quintero-Betancourt et al., 2002; Steele et al., 2003; Murphy et al., 2018b). As Cryptosporidium oocysts detection is performed by filtration or purification via immunomagnetic separation, followed by the labeling of oocysts with a specific fluorochrome and differential interference microscopy detection (Giangaspero and Gasser, 2019), Cyclospora oocysts can also potentially be detected. However, due to the lack of a specific antibody for C. cayetanensis oocysts, the probability can not be tested.
Viability and Infectivity Tests
Determination of parasite viability and infectivity is important in clinical settings. Since there is no accurate assay for the evaluation of viability or infectivity of C. cayetanensis, it can be assessed via analysis of the sporulation rates of oocysts. It has been reported that C. cayetanensis oocysts complete sporulated in 2.5% potassium dichromate within 7–13 days at 25 or 32°C (Ortega et al., 1993, 1998). Unsporulated oocysts carry developing sporocysts, while sporulated oocysts carry two ovoid sporocysts, each of which has two sporozoites. The excystation of oocysts occurs on the exposure to trypsin (0.5%) and sodium taurocholate (1.5%) in phosphate-buffered saline, followed by mechanical disruption (Ortega et al., 1993). Based on the morphology and physicochemical properties of oocysts, electrorotation technique was developed for observing the changes in the oocysts (Dalton et al., 2001, 2004), but the technique is not handy due to procedural complexity and only can be used in research settings.
Oocyst sporulation and infectivity testing in animal model is an ideal method for evaluating the viability and infectivity of the oocysts (Giangaspero and Gasser, 2019). However, due to the unavailability of effective in vitro culture methods and in vivo animal models for C. cayetanensis, sporulation in 2.5% potassium dichromate is currently regarded as the only indicator for oocyst viability (Eberhard et al., 2000).
Therapy
No vaccine is available for cyclosporiasis (Giangaspero and Gasser, 2019) but early detection and treatment can yield a favorable clinical outcome. Expectant treatment and chemotherapeutic treatment is crucial in human cyclosporiasis, particularly in immunodeficient individuals. Although case fatality due to cyclosporiasis is rare in humans, long lasting diarrhea sometimes results in dehydration or malnutrition, and occasionally may cause severe dehydration and death in infants (Behera et al., 2008; Bednarska et al., 2015).
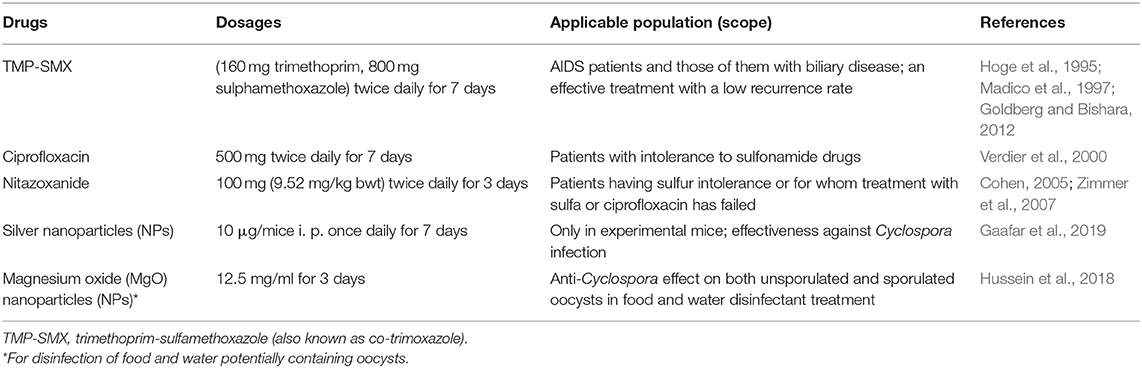
Chemotherapy including treatment with 160 mg trimethoprim and 800 mg sulfamethoxazole (TMP-SMX, also known as co-trimoxazole) twice daily for 7 days can reportedly cure human cyclosporiasis (Hoge et al., 1995; Escobedo et al., 2009). TMP-SMX is considered as an effective drug, with many studies reporting low recurrence rates (Hoge et al., 1995; Madico et al., 1997; Goldberg and Bishara, 2012). It is also an effective chemotherapeutic treatment for C. cayetanensis infection in AIDS patients (Pape et al., 1994; Verdier et al., 2000) and those of them with biliary disease (Sifuentes-Osornio et al., 1995).
In some patients, TMP-SMX creates intolerance and allergy. In such cases, ciprofloxacin antibiotic with having less effectivity than TMP-SMX is a suitable treatment option for cyclosporiasis in human (Verdier et al., 2000). Nitazoxanide is another drug that can also be used in the cases of sulfonamide intolerance and ciprofloxacin resistance (Diaz et al., 2003; Cohen, 2005; Zimmer et al., 2007). Nitazoxanide has been used to treat mixed parasite infection with intestinal protozoa (including C. cayetanensis) and helminths (Diaz et al., 2003). The efficacy of nitazoxanide for cyclosporiasis was reported to be ranging from 71 to 87%. The tolerance level of the drug was found to be very high with having no serious adverse effects (Table 2). Conversely, norfloxacin, metronidazole, tinidazole, and quinacrine have proven ineffective in some studies of human cyclosporiasis (Escobedo et al., 2009; Almeria et al., 2019). In a more recent study in mice silver nanoparticles were effective against Cyclospora infection (Gaafar et al., 2019). This will draw attention to its potential for use as an alternative to the standard therapy in both immunocompetent and immunosuppressed hosts.
Prevention
Cyclospora cayetanensis is contracted via a fecal-oral transmission cycle, and direct person-to-person transmission seems unlikely. In developed nations, C. cayetanensis infections can be common in people who travel to endemic areas of underdeveloped and developing countries and consume the contaminated food, specially fresh produce imported from that regions (Almeria et al., 2019). Cyclospora cayetanensis is mainly transmitted via feces contaminated food, water, and soil (Almeria et al., 2019). Therefore, improvement of personal hygiene and sanitary conditions can be a suitable preventive approach for C. cayetanensis infections because it obviously cuts off the fecal-oral route of transmission of the parasite in the endemic areas. The practice of not consuming of raw fresh produce, especially those supplied from endemic areas can avert the problem of cyclosporiasis in humans. Regular boiling and filtering of water necessary for drinking, food preparation, and washing of fresh produce can also prevent the infection (Almeria et al., 2019).
While usual sanitizers and disinfectants can not destroy C. cayetanensis and coccidia in general, some exploratory methods for removing or inactivating C. cayetanensis oocysts in fresh fruits and raw vegetables have been investigated (El Zawawy et al., 2010; Butot et al., 2018; Hussein et al., 2018). In one study magnesium oxide nanoparticles had a significant anti-Cyclospora effect on both unsporulated and sporulated oocysts, prompting speculation that it may be useful as a preventive agent in food and water disinfection treatment (Hussein et al., 2018).
Care should be taken to keep the fresh produce out of contamination at the field and packaging unit, and also from the farm workers to efferently prevent the C. cayetanensis infection in endemic areas. The practice of toilet use, hand washing after toilet use and before meal and proper disposal and treatment of human excreta are also important for prevention of cyclosporiasis. Any worker bearing the gastrointestinal diseases should not handle the vegetables or other produce. Other coccidiosis control measures can also be applied for the prevention and control of C. cayetanensis infections.
Conclusions
In conclusion, there are some advances in clinical features, diagnosis and therapeutic intervention of cyclosporiasis, which primarily diagnosed by the important clinical symptoms of watery diarrhea, abdominal cramps, and bloating. Conventional and laboratory diagnosis usually involves microscopy examination of wet smears, staining tests (typically the modified acid-fast stain), fluorescence microscopy, serological testing, or advanced molecular testing for oocyst DNA in the human stool. No vaccine is available for cyclosporiasis, but early detection and treatment can yield a favorable clinical outcome. Detection in a transmission vehicle such as fresh produce, water, or soil is helpful for case-linkage and source-tracking during cyclosporiasis outbreaks. The sensitivity of Cyclospora detection can be increased by the concentration of oocysts obtained from clinical or biological samples. Treatment with TMP-SMX has proven effective for cyclosporiasis. Ciprofloxacin, although less effective than TMP-SMX, can suitably be used in patients having sulfur drug intolerance. Nitazoxanide is an alternative drug can be used in the cases of sulfur intolerance and ciprofloxacin resistance. The water- and food-borne parasite Cyclospora has epidemiologically been investigated only in few underprivileged communities and developed nations. More epidemiological research on cyclosporiasis in humans should be conducted at various locations around the world, to achieve a better understanding of its characteristics in this regard. Attempts should also be made to establish in vitro or in vivo methods for cultivating C. cayetanensis. Rapid, convenient, precise, and economical detection methods for diagnosis, as well as effective tracing methods should be developed to monitor the transmission of C. cayetanensis infection.
Author Contributions
LZ provided the ideas. JL and ZC wrote the draft manuscript. MQ participated in the modification of manuscript. All the authors had read the final manuscript.
Funding
This study was supported by the Doctoral Startup Foundation of Henan University of Chinese Medicine (00104311-2019-31), and the National Key Research and Development Program of China (Grant Nos. 2017YFD0500405 and 2017YFD0501305).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Owen Proudfoot from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
References
Abanyie, F., Harvey, R. R., Harris, J. R., Wiegand, R. E., Gaul, L., Desvignes-Kendrick, M., et al. (2015). 2013 multistate outbreaks of Cyclospora cayetanensis infections associated with fresh produce: focus on the Texas investigations. Epidemiol. Infect. 143, 3451–3458. doi: 10.1017/S0950268815000370
Alakpa, G., Fagbenro-Beyioku, A. F., and Clarke, S. C. (2002). Cyclospora cayetanensis in stools submitted to hospitals in Lagos, Nigeria. Int. J. Infect. Dis. 6, 314–318. doi: 10.1016/S1201-9712(02)90167-0
Almeria, S., Cinar, H. N., and Dubey, J. P. (2019). Cyclospora cayetanensis and cyclosporiasis: an update. Microorganisms 7:E317. doi: 10.3390/microorganisms7090317
Almeria, S., da Silva, A. J., Blessington, T., Cloyd, T. C., Cinar, H. N., Durigan, M., et al. (2018). Evaluation of the U.S. Food and Drug Administration validated method for detection of Cyclospora cayetanensis in high-risk fresh produce matrices and a method modification for a prepared dish. Food Microbiol. 76, 497–503. doi: 10.1016/j.fm.2018.07.013
Barratt, J. L. N., Park, S., Nascimento, F. S., Hofstetter, J., Plucinski, M., Casillas, S., et al. (2019). Genotyping genetically heterogeneous Cyclospora cayetanensis infections to complement epidemiological case linkage. Parasitology 146, 1275–1283. doi: 10.1017/S0031182019000581
Becker, S. L., Vogt, J., Knopp, S., Panning, M., Warhurst, D. C., Polman, K., et al. (2013). Persistent digestive disorders in the tropics: causative infectious pathogens and reference diagnostic tests. BMC Infect. Dis. 13:37. doi: 10.1186/1471-2334-13-37
Bednarska, M., Bajer, A., Welc-Faleciak, R., and Pawelas, A. (2015). Cyclospora cayetanensis infection in transplant traveller: a case report of outbreak. Parasit. Vectors 8:411. doi: 10.1186/s13071-015-1026-8
Behera, B., Mirdha, B. R., Makharia, G. K., Bhatnagar, S., Dattagupta, S., and Samantaray, J. C. (2008). Parasites in patients with malabsorption syndrome: a clinical study in children and adults. Dig. Dis. Sci. 53, 672–679. doi: 10.1007/s10620-007-9927-9
Berlin, O. G., Peter, J. B., Gagne, C., Conteas, C. N., and Ash, L. R. (1998). Autofluorescence and the detection of cyclospora oocysts. Emerg. Infect. Dis. 4, 127–128. doi: 10.3201/eid0401.980121
Bhandari, D., Tandukar, S., Parajuli, H., Thapa, P., Chaudhary, P., Shrestha, D., et al. (2015). Cyclospora infection among school children in Kathmandu, Nepal: prevalence and associated risk factors. Trop. Med. Health 43, 211–216. doi: 10.2149/tmh.2015-25
Bilung, L. M., Tahar, A. S., Yunos, N. E., Apun, K., Lim, Y. A., Nillian, E., et al. (2017). Detection of Cryptosporidium and Cyclospora oocysts from environmental water for drinking and recreational activities in Sarawak, Malaysia. Biomed Res. Int. 2017:4636420. doi: 10.1155/2017/4636420
Brennan, M. K., MacPherson, D. W., Palmer, J., and Keystone, J. S. (1996). Cyclosporiasis: a new cause of diarrhea. CMAJ 155, 1293–1296.
Buss, S. N., Leber, A., Chapin, K., Fey, P. D., Bankowski, M. J., Jones, M. K., et al. (2015). Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J. Clin. Microbiol. 53, 915–925. doi: 10.1128/JCM.02674-14
Butot, S., Cantergiani, F., Moser, M., Jean, J., Lima, A., Michot, L., et al. (2018). UV-C inactivation of foodborne bacterial and viral pathogens and surrogates on fresh and frozenberries. Int. J. Food Microbiol. 275, 8–16. doi: 10.1016/j.ijfoodmicro.2018.03.016
Casillas, S. M., Bennett, C., and Straily, A. (2018). Notes from the field: multiple cyclosporiasis outbreaks - United States, 2018. MMWR Morb. Mortal. Wkly. Rep. 67, 1101–1102. doi: 10.15585/mmwr.mm6739a6
Chacín-Bonilla, L., Barrios, F., and Sanchez, Y. (2007). Epidemiology of Cyclospora cayetanensis infection in San Carlos Island, Venezuela: strong association between socio-economic status and infection. Trans. R. Soc. Trop. Med. Hyg. 101, 1018–1024. doi: 10.1016/j.trstmh.2007.05.008
Chandra, V., Torres, M., and Ortega, Y. R. (2014). Efficacy of wash solutions in recovering Cyclospora cayetanensis, Cryptosporidium parvum, and Toxoplasma gondii from basil. J. Food Prot. 77, 1348–1354. doi: 10.4315/0362-028X.JFP-13-381
Cinar, H. N., Gopinath, G., Jarvis, K., and Murphy, H. R. (2015). The complete mitochondrial genome of the foodborne parasitic pathogen Cyclospora cayetanensis. PLoS ONE 10:e0128645. doi: 10.1371/journal.pone.0128645
Clarke, S. C., and McIntyre, M. (1996). Modified detergent Ziehl-Neelsen technique for the staining of Cyclospora cayetanensis. J. Clin. Pathol. 49, 511–512. doi: 10.1136/jcp.49.6.511
Cohen, S. A. (2005). Use of nitazoxanide as a new therapeutic option for persistent diarrhea: a pediatric perspective. Curr. Med. Res. Opin. 21, 999–1004. doi: 10.1185/030079905X50534
Connor, B. A., Johnson, E. J., and Soave, R. (2001). Reiter syndrome following protracted symptoms of Cyclospora infection. Emerg. Infect. Dis. 7, 453–454. doi: 10.3201/eid0703.017317
Connor, B. A., Reidy, J., and Soave, R. (1999). Cyclosporiasis: clinical and histopathologic correlates. Clin. Infect. Dis. 28, 1216–1222. doi: 10.1086/514780
Connor, B. A., Shlim, D. R., Scholes, J. V., Rayburn, J. L., Reidy, J., and Rajah, R. (1993). Pathologic changes in the small bowel in 9 patients with diarrhea associated with a coccidia-like body. Ann. Intern. Med. 119, 377–382. doi: 10.7326/0003-4819-119-5-199309010-00005
Dalton, C., Goater, A. D., Burt, J. P., and Smith, H. V. (2004). Analysis of parasites by electrorotation. J. Appl. Microbiol. 96, 24–32. doi: 10.1046/j.1365-2672.2003.02113.x
Dalton, C., Goater, A. D., Pethig, R., and Smith, H. V. (2001). Viability of Giardia intestinalis cysts and viability and sporulation state of Cyclospora cayetanensis oocysts determined by electrorotation. Appl. Environ. Microbiol. 67, 586–590. doi: 10.1128/AEM.67.2.586-590.2001
Di Gliullo, A. B., Cribari, M. S., Bava, A. J., Cicconetti, J. S., and Collazos, R. (2000). Cyclospora cayetanensis in sputum and stool samples. Rev. Inst. Med. Trop. São Paulo 42, 115–117. doi: 10.1590/S0036-46652000000200009
Diaz, E., Mondragon, J., Ramirez, E., and Bernal, R. (2003). Epidemiology and control of intestinal parasites with nitazoxanide in children in Mexico. Am. J. Trop. Med. Hyg. 68, 384–385. doi: 10.4269/ajtmh.2003.68.384
Dillingham, R. A., Pinkerton, R., Leger, P., Severe, P., Guerrant, R. L., Pape, J. W., et al. (2009). High early mortality in patients with chronic acquired immunodeficiency syndrome diarrhea initiating antiretroviral therapy in Haiti: a case-control study. Am. J. Trop. Med. Hyg. 80, 1060–1064. doi: 10.4269/ajtmh.2009.80.1060
Dixon, B. R., Bussey, J. M., Parrington, L. J., and Parenteau, M. (2005). Detection of Cyclospora cayetanensis oocysts in human fecal specimens by flow cytometry. J. Clin. Microbiol. 43, 2375–2379. doi: 10.1128/JCM.43.5.2375-2379.2005
Eberhard, M. L., Ortega, Y. R., Hanes, D. E., Nace, E. K., Do, R. Q., Robl, M. G., et al. (2000). Attempts to establish experimental Cyclospora cayetanensis infection in laboratory animals. J. Parasitol. 86, 577–582. doi: 10.1645/0022-3395(2000)086[0577:ATEECC]2.0.CO;2
Eberhard, M. L., Pieniazek, N. J., and Arrowood, M. J. (1997). Laboratory diagnosis of Cyclospora infections. Arch. Pathol. Lab. Med. 121, 792–797.
El Zawawy, L. A., El-Said, D., Ali, S. M., and Fathy, F. M. (2010). Disinfection efficacy of sodium dichlorois ocyanurate (NADCC) against common food-borne intestinal protozoa. J. Egypt. Soc. Parasitol. 40, 165–185.
Escobedo, A. A., Almirall, P., Alfonso, M., Cimerman, S., Rey, S., and Terry, S. L. (2009). Treatment of intestinal protozoan infections in children. Arch. Dis. Child. 94, 478–482. doi: 10.1136/adc.2008.151852
Field, A. S. (2002). Light microscopic and electron microscopic diagnosis of gastrointestinal opportunistic infections in HIV-positive patients. Pathology 34, 21–35. doi: 10.1080/00313020120111230
Fryauff, D. J., Krippner, R., Prodjodipuro, P., Ewald, C., Kawengian, S., Pegelow, K., et al. (1999). Cyclospora cayetanensis among expatriate and indigenous populations of West Java, Indonesia. Emerg. Infect. Dis. 5, 585–588. doi: 10.3201/eid0504.990426
Gaafar, M. R., El-Zawawy, L. A., El-Temsahy, M. M., Shalaby, T. I., and Hassan, A. Y. (2019). Silver nanoparticles as a therapeutic agent in experimental cyclosporiasis. Exp. Parasitol. 207:107772. doi: 10.1016/j.exppara.2019.107772
Garcia, L. S., Arrowood, M., Kokoskin, E., Paltridge, G. P., Pillai, D. R., Procop, G. W., et al. (2017). Laboratory diagnosis of parasites from the gastrointestinal tract. Clin. Microbiol. Rev. 31:e00025–17. doi: 10.1128/CMR.00025-17
Giangaspero, A., and Gasser, R. B. (2019). Human cyclosporiasis. Lancet Infect. Dis. 19, e226–236. doi: 10.1016/S1473-3099(18)30789-8
Giangaspero, A., Marangi, M., Koehler, A. V., Papini, R., Normanno, G., Lacasella, V., et al. (2015). Molecular detection of Cyclospora in water, soil, vegetables and humans in southern Italy signals a need for improved monitoring by health authorities. Int. J. Food Microbiol. 211, 95–100. doi: 10.1016/j.ijfoodmicro.2015.07.002
Goldberg, E., and Bishara, J. (2012). Contemporary unconventional clinical use of co-trimoxazole. Clin. Microbiol. Infect. 18, 8–17. doi: 10.1111/j.1469-0691.2011.03613.x
Gonçalves, E. M., Uemura, I. H., Castilho, V. L., and Corbett, C. E. (2005). Retrospective study of the occurrence of Cyclospora cayetanensis at Clinical Hospital of the University of São Paulo Medical School, SP. Rev. Soc. Bras. Med. Trop. 38, 326–330. doi: 10.1590/S0037-86822005000400009
Guo, Y., Li, N., Ortega, Y. R., Zhang, L., Roellig, D. M., Feng, Y., et al. (2018). Population genetic characterization of Cyclospora cayetanensis from discrete geographical regions. Exp. Parasitol. 184, 121–127. doi: 10.1016/j.exppara.2017.12.006
Guo, Y., Roellig, D. M., Li, N., Tang, K., Frace, M., Ortega, Y., et al. (2016). Multilocus sequence typing tool for Cyclospora cayetanensis. Emerg. Infect. Dis. 22, 1464–1467. doi: 10.3201/eid2208.150696
Guo, Y., Wang, Y., Wang, X., Zhang, L., Ortega, Y., and Feng, Y. (2019). Mitochondrial genome sequence variation as a useful marker for assessing genetic heterogeneity among Cyclospora cayetanensis isolates and source-tracking. Parasit. Vectors 12:47. doi: 10.1186/s13071-019-3294-1
Hannet, I., Engsbro, A. L., Pareja, J., Schneider, U. V., Lisby, J. G., PruŽinec-Popović, B., et al. (2019). Multicenter evaluation of the new QIAstat gastrointestinal panel for the rapid syndromic testing of acute gastroenteritis. Eur. J.Clin. Microbiol. Infect. Dis. 38, 2103–2112. doi: 10.1007/s10096-019-03646-4
Hitchcock, M. M., Hogan, C. A., Budvytiene, I., and Banaei, N. (2019). Reproducibility of positive results for rare pathogens on the FilmArray GI Panel. Diagn. Microbiol. Infect. Dis. 95, 10–14. doi: 10.1016/j.diagmicrobio.2019.03.013
Hofstetter, J. N., Nascimento, F. S., Park, S., Casillas, S., Herwaldt, B. L., Arrowood, M. J., et al. (2019). Evaluation of multilocus sequence typing of Cyclospora cayetanensis based on microsatellite markers. Parasite 26:3. doi: 10.1051/parasite/2019004
Hoge, C. W., Shlim, D. R., Ghimire, M., Rabold, J. G., Pandey, P., Walch, A., et al. (1995). Placebo-controlled trial of co-trimoxazole for Cyclospora infections among travellers and foreign residents in Nepal. Lancet 345, 691–693. doi: 10.1016/S0140-6736(95)90868-4
Hussein, E. M., Abdul-Manaem, A. H., and El-Attary, S. L. (2005). Cyclospora cayetanensis oocysts in sputum of a patient with active pulmonary tuberculosis, case report in Ismailia, Egypt. J. Egypt. Soc. Parasitol. 35, 787–793.
Hussein, E. M., Ahmed, S. A., Mokhtar, A. B., Elzagawy, S. M., Yahi, S. H., Hussein, A. M., et al. (2018). Antiprotozoal activity of magnesium oxide (MgO) nanoparticles against Cyclospora cayetanensis oocysts. Parasitol. Int. 67, 666–674. doi: 10.1016/j.parint.2018.06.009
Hussein, E. M., El-Moamly, A. A., Dawoud, H. A., Fahmy, H., El-Shal, H. E., and Sabek, N. A. (2007). Real-time PCR and flow cytometry in detection of Cyclospora oocysts in fecal samples of symptomatic and asymptomatic pediatrics patients. J. Egypt. Soc. Parasitol. 37, 151–170.
Kaminsky, R. G., Lagos, J., Raudales Santos, G., and Urrutia, S. (2016). Marked seasonality of Cyclospora cayetanensis infections: ten-year observation of hospital cases, Honduras. BMC Infect. Dis. 16:66. doi: 10.1186/s12879-016-1393-6
Khanna, V., Tilak, K., Ghosh, A., and Mukhopadhyay, C. (2014). Modified negative staining of Heine for fast and inexpensive screening of Cryptosporidium, Cyclospora, and Cystoisospora spp. Int. Sch. Res. Notices 2014:165424. doi: 10.1155/2014/165424
Kłudkowska, M., Pielok, Ł., Frackowiak, K., and Paul, M. (2017). Intestinal coccidian parasites as an underestimated cause of travellers' diarrhoea in Polish immunocompetent patients. Acta Parasitol. 62, 630–638. doi: 10.1515/ap-2017-0077
Lalonde, L. F., and Gajadhar, A. A. (2011). Detection and differentiation of coccidian oocysts by real-time PCR and melting curve analysis. J. Parasitol. 97, 725–730. doi: 10.1645/GE-2706.1
Lalonde, L. F., and Gajadhar, A. A. (2016). Optimization and validation of methods for isolation and real-time PCR identification of protozoan oocysts on leafy green vegetables and berry fruits. Food Waterborne Parasitol. 2, 1–7. doi: 10.1016/j.fawpar.2015.12.002
Li, G., Xiao, S., Zhou, R., Li, W., and Wadeh, H. (2007). Molecular characterization of Cyclospora-like organism from dairy cattle. Parasitol. Res. 100, 955–961. doi: 10.1007/s00436-006-0380-z
Li, J., Chang, Y., Shi, K. E., Wang, R., Fu, K., Li, S., et al. (2017). Multilocus sequence typing and clonal population genetic structure of Cyclospora cayetanensis in humans. Parasitology 144, 1890–1897. doi: 10.1017/S0031182017001299
Li, J., Shi, K., Sun, F., Li, T., Wang, R., Zhang, S., et al. (2019b). Identification of human pathogenic Enterocytozoon bieneusi, Cyclospora cayetanensis, and Cryptosporidium parvum on the surfaces of vegetables and fruits in Henan, China. Int. J. Food Microbiol. 307:108292. doi: 10.1016/j.ijfoodmicro.2019.108292
Li, J., Sun, F., Wang, R., and Zhang, L. (2015a). Advances in research on food-borne infection and detection methods of Cyclospora. Food Sci. 36, 261–267. doi: 10.7506/spkx1002-6630-201507048
Li, J., Sun, F., Zhi, Y., Qi, M., Wu, Y., Luo, N., et al. (2014). Establishment of detection method for Cyclospora cayetanensis by flow cytometry. Int. J. Med. Para. Dis. 41, 16–19. doi: 10.3760/cma.j.issn.1673-4122.2014.01.005
Li, J., Wang, R., Chen, Y., Xiao, L., and Zhang, L. (2019a). Cyclospora cayetanensis infection in humans: biological characteristics, clinical features, epidemiology, detection method, and treatment. Parasitology 8, 1–11. doi: 10.1017/S0031182019001471
Li, N., Ye, J., Arrowood, M. J., Ma, J., Wang, L., Xu, H., et al. (2015b). Identification and morphologic and molecular characterization of Cyclospora macacae n. sp. from rhesus monkeys in China. Parasitol. Res. 114, 1811–1816. doi: 10.1007/s00436-015-4367-5
Li, W., Kiulia, N. M., Mwenda, J. M., Nyachieo, A., Taylor, M. B., Zhang, X., et al. (2011). Cyclospora papionis, Cryptosporidium hominis, and human-pathogenic Enterocytozoon bieneusi in captive baboons in Kenya. J. Clin. Microbiol.49, 4326–4329. doi: 10.1128/JCM.05051-11
Liu, S., Wang, L., Zheng, H., Xu, Z., Roellig, D. M., Li, N., et al. (2016). Comparative genomics reveals Cyclospora cayetanensis possesses coccidia-like metabolism and invasion components but unique surface antigens. BMC Genomics 17:316. doi: 10.1186/s12864-016-2632-3
Madico, G., McDonald, J., Gilman, R. H., Cabrera, L., and Sterling, C. R. (1997). Epidemiology and treatment of Cyclospora cayetanensis infection in Peruvian children. Clin. Infect. Dis. 24, 977–981. doi: 10.1093/clinids/24.5.977
Mansfield, L. S., and Gajadhar, A. A. (2004). Cyclospora cayetanensis, a food- and waterborne coccidian parasite. Vet. Parasitol. 126, 73–90. doi: 10.1016/j.vetpar.2004.09.011
Marques, D. F. P., Alexander, C. L., Chalmers, R. M., Elson, R., Freedman, J., Hawkins, G., et al. (2017). Cyclosporiasis in travellers returning to the United Kingdom from Mexico in summer 2017: lessons frsom the recent past to inform the future. Euro. Surveill. 22:30592. doi: 10.2807/1560-7917.ES.2017.22.32.30592
McAllister, C. T., Motriuk-Smith, D., and Kerr, C. M. (2018). Three new coccidians (Cyclospora, Eimeria) from eastern moles, Scalopus aquaticus (Linnaeus) (Mammalia: Soricomorpha: Talpidae) from Arkansas, USA. Syst. Parasitol. 95, 271–279. doi: 10.1007/s11230-018-9782-4
McHardy, I. H., Wu, M., Shimizu-Cohen, R., Couturier, M. R., and Humphries, R. M. (2014). Detection of intestinal protozoa in the clinical laboratory. J. Clin. Microbiol. 52, 712–720. doi: 10.1128/JCM.02877-13
Murphy, H. R., Almeria, S., and da Silva, A. J. (2018a). BAM 19b: Molecular Detection of Cyclospora cayetanensis in Fresh Produce Using Real-Time PCR. Available online at: https://www.fda.gov/food/laboratory-methods-food-safety/bam-19b-molecular-detection-Cyclospora-cayetanensis-fresh-produce-using-real-time-pcr (accessed August 14, 2018).
Murphy, H. R., Cinar, H. N., Gopinath, G., Noe, K. E., Chatman, L. D., Miranda, N. E., et al. (2018b). Interlaboratory validation of an improved method for detection of Cyclospora cayetanensis in produce using a real-time PCR assay. Food Microbiol. 69, 170–178. doi: 10.1016/j.fm.2017.08.008
Murphy, H. R., Lee, S., and da Silva, A. J. (2017). Evaluation of an improved U.S. Food and Drug Administration method for the detection of Cyclospora cayetanensis in produce using real-time PCR. J. Food Prot. 80, 1133–1144. doi: 10.4315/0362-028X.JFP-16-492
Nascimento, F. S., Barta, J. R., Whale, J., Hofstetter, J. N., Casillas, S., Barratt, J., et al. (2019). Mitochondrial junction region as genotyping marker for Cyclospora cayetanensis. Emerg. Infect. Dis. 25, 1314–1319. doi: 10.3201/eid2507.181447
Olivier, C., van de Pas, S., Lepp, P. W., Yoder, K., and Relman, D. A. (2001). Sequence variability in the first internal transcribed spacer region within and among Cyclospora species is consistent with polyparasitism. Int. J. Parasitol. 31, 1475–1487. doi: 10.1016/S0020-7519(01)00283-1
Ortega, Y. R., Nagle, R., Gilman, R. H., Watanabe, J., Miyagui, J., Quispe, H., et al. (1997). Pathologic and clinical findings in patients with cyclosporiasis and a description of intracellular parasite life-cycle stages. J. Infect. Dis. 176, 1584–1589. doi: 10.1086/514158
Ortega, Y. R., and Sanchez, R. (2010). Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin. Microbiol. Rev. 23, 218–234. doi: 10.1128/CMR.00026-09
Ortega, Y. R., and Sterling, C. R. (1996). Cyclospora cayetanensis: Epidemiology and diagnosis. Clin. Microbiol. Newslett. 18, 169–172. doi: 10.1016/0196-4399(96)81655-2
Ortega, Y. R., Sterling, C. R., and Gilman, R. H. (1998). Cyclospora cayetanensis. Adv. Parasitol. 40, 399–418. doi: 10.1016/S0065-308X(08)60128-1
Ortega, Y. R., Sterling, C. R., Gilman, R. H., Cama, V. A., and Diaz, F. (1993). Cyclospora species-a new protozoan pathogen of humans. N. Engl. J. Med. 328, 1308–1312. doi: 10.1056/NEJM199305063281804
Pandey, P., Bodhidatta, L., Lewis, M., Murphy, H., Shlim, D. R., Cave, W., et al. (2011). Travelers' diarrhea in Nepal: an update on the pathogens and antibiotic resistance. J. Travel Med. 18, 102–108. doi: 10.1111/j.1708-8305.2010.00475.x
Pape, J. W., Verdier, R. I., Boncy, M., Boncy, J., and Johnson, W. D. Jr. (1994). Cyclospora infection in adults infected with HIV. Clinical manifestations, treatment, and prophylaxis. Ann. Intern. Med. 121, 654–657. doi: 10.7326/0003-4819-121-9-199411010-00004
Parija, S. C., Shivaprakash, M. R., and Jayakeerthi, S. R. (2003). Evaluation of lacto-phenol cotton blue (LPCB) for detection of Cryptosporidium, Cyclospora and Isospora in the wet mount preparation of stool. Acta Trop. 85, 349–354. doi: 10.1016/S0001-706X(02)00265-6
Quintero-Betancourt, W., Peele, E. R., and Rose, J. B. (2002). Cryptosporidium parvum and Cyclospora cayetanensis: a review of laboratory methods for detection of these waterborne parasites. J. Microbiol. Methods 49, 209–224. doi: 10.1016/S0167-7012(02)00007-6
Qvarnstrom, Y., Wei-Pridgeon, Y., Van Roey, E., Park, S., Srinivasamoorthy, G., Nascimento, F. S., et al. (2018). Purification of Cyclospora cayetanensis oocysts obtained from human stool specimens for whole genome sequencing. Gut Pathog. 10:45. doi: 10.1186/s13099-018-0272-7
Relman, D. A., Schmidt, T. M., Gajadhar, A., Sogin, M., Cross, J., Yoder, K., et al. (1996). Molecular phylogenetic analysis of Cyclospora, the human intestinal pathogen, suggests that it is closely related to Eimeria species. J. Infect. Dis. 173, 440–445. doi: 10.1093/infdis/173.2.440
Richardson, R. F., Remler, B. F. Jr., Katirji, B., and Murad, M. H. (1998). Guillain-Barré syndrome after Cyclospora infection. Muscle Nerve 21, 669–671. doi: 10.1002/(SICI)1097-4598(199805)21:5<669::AID-MUS20>3.0.CO;2-P
Robertson, L. J., Gjerde, B., and Campbell, A. T. (2000). Isolation of Cyclospora oocysts from fruits and vegetables using lectin-coated paramagnetic beads. J. Food Prot. 63, 1410–1414. doi: 10.4315/0362-028X-63.10.1410
Ryan, U., Paparini, A., and Oskam, C. (2017). New technologies for detection of enteric parasites. Trends Parasitol. 33, 532–546. doi: 10.1016/j.pt.2017.03.005
Sancak, B., Akyon, Y., and Ergüven, S. (2006). Cyclospora infection in five immunocompetent patients in a Turkish university hospital. J. Med. Microbiol. 55, 459–462. doi: 10.1099/jmm.0.46279-0
Schubach, T. M., Neves, E. S., Leite, A. C., Araújo, A. Q. C., and de Moura, H. (1997). Cyclospora cayetanensis in an asymptomatic patient infected with HIV and HTLV-1. Trans. R. Soc. Trop. Med. Hyg. 91:175. doi: 10.1016/S0035-9203(97)90212-1
Shapiro, K., Kim, M., Rajal, V. B., Arrowood, M. J., Packham, A., Aguilar, B., et al. (2019). Simultaneous detection of four protozoan parasites on leafy greens using a novel multiplex PCRassay. Food Microbiol. 84:103252. doi: 10.1016/j.fm.2019.103252
Sherchand, J. B., and Cross, J. H. (2001). Emerging pathogen Cyclospora cayetanensis infection in Nepal. Southeast Asian J. Trop. Med. Public Health 32, 143–150.
Shields, J. M., Lee, M. M., and Murphy, H. R. (2012). Use of a common laboratory glassware detergent improves recovery of Cryptosporidium parvum and Cyclospora cayetanensis from lettuce, herbs and raspberries. Int. J. Food Microbiol. 153, 123–128. doi: 10.1016/j.ijfoodmicro.2011.10.025
Shields, J. M., and Olson, B. H. (2003a). Cyclospora cayetanensis: a review of an emerging parasitic coccidian. Int. J. Parasitol. 33, 371–391. doi: 10.1016/S0020-7519(02)00268-0
Shields, J. M., and Olson, B. H. (2003b). PCR-Restriction fragment length polymorphism method for detection of Cyclospora cayetanensis in environmental waters without microscopic confirmation. Appl. Environ. Microbiol. 69, 4662–4669. doi: 10.1128/AEM.69.8.4662-4669.2003
Shin, J. H., Lee, S. E., Kim, T. S., Ma, D. W., Cho, S. H., Chai, J. Y., et al. (2018). Development of molecular diagnosis using multiplex real-time PCR and T4 phage internal control to simultaneously detect Cryptosporidium parvum, Giardia lamblia, and Cyclospora cayetanensis from human stool samples. Korean J. Parasitol. 56, 419–427. doi: 10.3347/kjp.2018.56.5.419
Sifuentes-Osornio, J., Porras-Cortés, G., Bendall, R. P., Morales-Villarreal, F., Reyes-Terán, G., and Ruiz-Palacios, G. M. (1995). Cyclospora cayetanensis infection in patients with and without AIDS: biliary disease as another clinical manifestation. Clin. Infect. Dis. 21, 1092–1097. doi: 10.1093/clinids/21.5.1092
Steele, M., Unger, S., and Odumeru, J. (2003). Sensitivity of PCR detection of Cyclospora cayetanensis in raspberries, basil, and mesclun lettuce. J. Microbiol. Methods 54, 277–280. doi: 10.1016/S0167-7012(03)00036-8
Sturbaum, G. D., Ortega, Y. R., Gilman, R. H., Sterling, C. R., Cabrera, L., and Klein, D. A. (1998). Detection of Cyclospora cayetanensis in wastewater. Appl. Environ. Microbiol. 64, 2284–2286. doi: 10.1128/AEM.64.6.2284-2286.1998
Taniuchi, M., Verweij, J. J., Sethabutr, O., Bodhidatta, L., Garcia, L., Maro, A., et al. (2011). Multiplex polymerase chain reaction method to detect Cyclospora, Cystoisospora, and Microsporidia in stool samples. Diagn. Microbiol. Infect. Dis. 71, 386–390. doi: 10.1016/j.diagmicrobio.2011.08.012
Temesgen, T. T., Robertson, L. J., and Tysnes, K. R. (2019a). A novel multiplex real-time PCR for the detection of Echinococcus multilocularis, Toxoplasma gondii, and Cyclospora cayetanensis on berries. Food Res. Int. 125:108636. doi: 10.1016/j.foodres.2019.108636
Temesgen, T. T., Tysnes, K. R., and Robertson, L. J. (2019b). A new protocol for molecular detection of Cyclospora cayetanensis as contaminants of berry fruits. Front. Microbiol. 10:1939. doi: 10.3389/fmicb.2019.01939
Thapa, S. S., and Basnyat, B. (2017). Chronic diarrhea in a traveler: cyclosporiasis. Am. J. Med. 130, e535–536. doi: 10.1016/j.amjmed.2017.06.018
Tram, N. T., Hoang, L. M., Cam, P. D., Chung, P. T., Fyfe, M. W., Isaac-Renton, J. L., et al. (2008). Cyclospora spp. in herbs and water samples collected from markets and farms in Hanoi, Vietnam. Trop. Med. Int. Health 13, 1415–1420. doi: 10.1111/j.1365-3156.2008.02158.x
Turgay, N., Yolasigmaz, A., Erdogan, D. D., Zeyrek, F. Y., and Uner, A. (2007). Incidence of cyclosporiasis in patients with gastrointestinal symptoms in western Turkey. Med. Sci. Monit. 13, CR34–39.
Varma, M., Hester, J. D., Schaefer, F. W. 3rd, Ware, M. W., and Lindquist, H. D. (2003). Detection of Cyclospora cayetanensis using a quantitative real-time PCR assay. J. Microbiol. Methods 53, 27–36. doi: 10.1016/S0167-7012(02)00209-9
Verdier, R. I., Fitzgerald, D. W., Johnson, W. D. Jr., and Pape, J. W. (2000). Trimethoprim-sulfamethoxazole compared with ciprofloxacin for treatment and prophylaxis of Isospora belli and Cyclospora cayetanensis infection in HIV-infected patients. A randomized, controlled trial. Ann. Intern. Med. 132, 885–888. doi: 10.7326/0003-4819-132-11-200006060-00006
Verweij, J. J., Laeijendecker, D., Brienen, E. A., van Lieshout, L., and Polderman, A. M. (2003). Detection of Cyclospora cayetanensis in travellers returning from the tropics and subtropics using microscopy and real-time PCR. Int. J. Med. Microbiol. 293, 199–202. doi: 10.1078/1438-4221-00252
Visvesvara, G. S., Moura, H., Kovacs-Nace, E., Wallace, S., and Eberhard, M. L. (1997). Uniform staining of Cyclospora oocysts in fecal smears by a modified safranin technique with microwave heating. J. Clin. Microbiol. 35, 730–733. doi: 10.1128/JCM.35.3.730-733.1997
Wang, K. X., Li, C. P., Wang, J., and Tian, Y. (2002). Cyclospore cayetanensis in Anhui, China. World J. Gastroenterol. 8, 1144–1148. doi: 10.3748/wjg.v8.i6.1144
Wiwanitkit, V. (2006). Intestinal parasite infestation in HIV infected patients. Curr. HIV Res. 4, 87–96. doi: 10.2174/157016206775197682
Yang, R., Jacobson, C., Gardner, G., Carmichael, I., Campbell, A. J. D., and Ryan, U. (2014). Longitudinal prevalence, oocyst shedding and molecular characterisation of Eimeria species in sheep across four states in Australia. Exp. Parasitol. 145, 14–21. doi: 10.1016/j.exppara.2014.06.018
Zar, F. A., El-Bayoumi, E., and Yungbluth, M. M. (2001). Histologic proof of acalculous cholecystitis due to Cyclospora cayetanensis. Clin. Infect. Dis. 33, E140–141. doi: 10.1086/324586
Zhou, Y., Lv, B., Wang, Q., Wang, R., Jian, F., Zhang, L., et al. (2011). Prevalence and molecular characterization of Cyclospora cayetanensis, Henan, China. Emerg. Infect. Dis. 17, 1887–1890. doi: 10.3201/eid1710.101296
Keywords: cyclosporiasis, clinical features, detection methods, prevention, therapy
Citation: Li J, Cui Z, Qi M and Zhang L (2020) Advances in Cyclosporiasis Diagnosis and Therapeutic Intervention. Front. Cell. Infect. Microbiol. 10:43. doi: 10.3389/fcimb.2020.00043
Received: 30 November 2019; Accepted: 22 January 2020;
Published: 11 February 2020.
Edited by:
Wei Cong, Shandong University, Weihai, ChinaReviewed by:
Na Li, South China Agricultural University, ChinaPengtao Gong, Jilin University, China
Copyright © 2020 Li, Cui, Qi and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Longxian Zhang, emhhbmdseDg5OTlAaGVuYXUuZWR1LmNu; emhhbmdseDg5OTlAZ21haWwuY29t
†These authors have contributed equally to this work
 Junqiang Li
Junqiang Li Zhaohui Cui
Zhaohui Cui Meng Qi
Meng Qi Longxian Zhang
Longxian Zhang