- 1ANDROFERT, Andrology and Human Reproduction Clinic, Campinas, Brazil
- 2Department of Surgery, University of Campinas (UNICAMP), Campinas, Brazil
- 3Faculty of Health, Aarhus University, Aarhus, Denmark
- 4ORIGEN, Center for Reproductive Medicine, Rio de Janeiro, Brazil
- 5Division of Reproductive Medicine, Department of Gynecology and Obstetrics, University of São Paulo, Ribeirão Preto, Brazil
- 6Department of Neuroscience, Reproductive Science and Odontostomatology, University of Naples Federico II, Naples, Italy
- 7Fertility Clinic Skive Regional Hospital, Skive, Denmark
Women with impaired ovarian reserve or poor ovarian response (POR) to exogenous gonadotropin stimulation present a challenge for reproductive specialists. The primary reasons relate to the still limited knowledge about the POR pathophysiology and the lack of practical solutions for the management of these conditions. Indeed, clinical trials using the current standards to define POR failed to show evidence in favor of a particular treatment modality. Furthermore, critical factors for reproductive success, such as the age-dependent embryo aneuploidy rates and the intrinsic ovarian resistance to gonadotropin stimulation, are not taken into consideration by the current POR criteria. As a result, the accepted definitions for POR have been criticized for their inadequacy concerning the proper patient characterization and for not providing clinicians a guide for therapeutic management. A novel system to classify infertility patients with “expected” or “unexpected” inappropriate ovarian response to exogenous gonadotropins—the POSEIDON criteria—was developed to provide a more nuanced picture of POR and to guide physicians in the management of such patients. The new standards are provoking as they challenge the current terminology of POR in favor of the newly defined concept of “low prognosis.” This article provides readers a critical appraisal of the existing criteria that standardize the definition of POR and explains the primary reasons for the development of the POSEIDON criteria.
Introduction
The primary goal of assisted reproductive technology (ART) is the birth of a healthy child. This outcome depends on a multitude of non-mutual independent factors, including female age and the effect of ovarian stimulation (OS) (1, 2). Nowadays, clinicians rely on patient characteristics, ovarian reserve markers, and treatment history—if available—for clinical decision-making concerning OS strategy, aiming at securing the shortest time to live birth as well as the lowest risk of complications (3, 4).
The number of oocytes retrieved after OS represents a critical cornerstone of ART since it is an independent predictor of the likelihood of pregnancy (5–7). Although the ideal number of oocytes collected after ovum pickup has been a matter of debate in recent years, it seems reasonable to define a typical ovarian response as the retrieval of 10–15 oocytes after conventional OS (5). However, a significant proportion of patients who undergo OS has either a poor (<4 oocytes) or suboptimal (4–9 oocytes) number of oocytes retrieved (3–9). As a consequence, the number of resulting embryos available for transfer or cryopreservation is reduced, thus jeopardizing treatment success (3, 4, 10–12). The cost of in vitro fertilization (IVF) tends to be higher in poor and suboptimal responders than in normal responders because different strategies or repeat treatment cycles might be required. Altogether, these factors cause emotional, physical, and financial distress for the couple, particularly when multiple treatment cycles are required.
The standards that define poor ovarian response (POR) vary widely as several factors either isolated or in combination are used for identification of such patients (13). Not surprisingly, the reported prevalence of POR fluctuates markedly between 5.6 and 35.1% (14, 15). Regardless of the chosen definition, it is clear that the POR population accounts for a substantial subset of women treated in IVF clinics nowadays (16). Driven by socioeconomic and other issues, many women are currently postponing motherhood which results in a higher number of patients seeking ART treatments in their late thirties and early forties. Women in this age range are more likely to have a diminished ovarian response due to natural aging of the ovaries, highlighting the need for particular attention to this group of women undergoing ART (17).
The central element in the pathophysiology of low ovarian response is the presence of a reduced number of follicles responsive to FSH. This phenomenon is most often found in women of advanced maternal age, mainly because of reduced ovarian reserve caused by accelerated follicular loss (18). In some cases, however, a low ovarian response might be seen in good ovarian reserve patients caused by a suboptimal gonadotropin dosage used for OS, for example in obese women (19), or due to the presence of genetic polymorphisms affecting endogenous gonadotrophins or their receptors (20–22). Both conditions ultimately alter the response of recruitable follicles to exogenous gonadotrophins (23–25). It is, therefore, clear that the so-called POR does not have a single cause. Indeed, the population with a diminished ovarian response is heterogeneous and sometimes difficult to characterize (14).
Most women diagnosed as poor responders are less likely to conceive or might even have their IVF cycle canceled due to lack of embryos for transfer (26). Nonetheless, some studies evaluating this patient population report reasonable cumulative pregnancy rates, ranging from 6 to 47% after three cycles, according to patient's age (27). Moreover, up to 40% of women who respond poorly in their first IVF cycle, as defined by the number of oocytes collected, have been reported to end up as normal responders in the second cycle (11, 16, 26). These figures indicate that not all women diagnosed with low ovarian response are similar regarding the likelihood of pregnancy. The optimal portrayal of this group of women with a low ovarian response is essential for proper counseling regarding the chances of pregnancy and the use of individualized strategies to increase IVF success (3, 4). Nevertheless, the current definitions for POR have been criticized for their inadequacy concerning a proper characterization of the POR population and for not providing clinicians a guide for therapeutic management (3, 4, 9, 14, 15). In this review, we provide an overview of existing criteria utilized to define the POR population, along with their advantages and shortcomings. Subsequently, we discuss the issues of ovarian resistance to gonadotropin stimulation and the importance of balancing quantity and quality with regard to oocytes retrieved. Lastly, we explain why a novel system for the identification and classification of low prognosis patients undergoing ART—the so-called POSEIDON criteria—was developed.
Criteria for the Definition of Poor Ovarian Response to Ovarian Stimulation
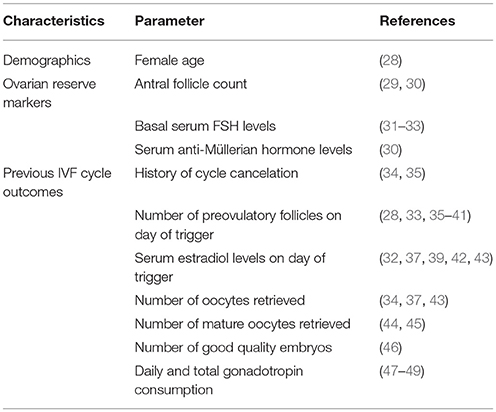
Several standards have been developed for the definition of POR. Parameters related to patient demographics, ovarian reserve tests, and outcomes of previous IVF cycles—alone or combined—are used to define the POR population (Table 1) (28–49). The numerous existing definitions differ concerning the parameters utilized and the threshold values established for each criterion. In a 2011 systematic review of 47 randomized clinical trials involving women with POR, 41 different definitions were used to define this group of patients (13). Notably, different definitions were used even in trials by the same group of researchers and no more than three trials use the same definition. In this review, the authors observed that the age criterion—considered essential by some investigators for the description of POR—was used in only 9% of studies (13). The disparity in POR definition renders the interpretation of trial results challenging. At the very least, conclusions about the different interventions tested must be interpreted with caution as regards their application in clinical practice.
Various terminologies utilized to define this group of patients further reflect the discrepancy of the definition of the POR patient. Researchers and clinicians often use ambiguous terms as POR, low ovarian response (47, 50, 51), hypo-response (20, 21), and diminished ovarian reserve (52–54). According to a 2015 survey study among reproductive specialists, the most used criterion to define POR was “the number of follicles produced” (14), unlike the POR criteria used in research studies. To complicate matters further, a not-for-profit patient organization dedicated to providing education to couples suffering from infertility (https://resolve.org/) defines POR as those women who require large doses of medication and who make less than an optimal number of oocytes, meaning that patients themselves have introduced a new element into the already complicated POR equation, namely, the suboptimal response to ovarian stimulation.
The Bologna Criteria
In 2011, the European Society of Human Reproduction and Embryology (ESHRE) carried out the first systematic effort to define women with inadequate response to OS (55). This consensus definition—known as the Bologna criteria—was initially introduced with the primary objective of standardizing the definition of the POR patient based on oocyte quantity for use in research studies. The authors made specific recommendations for investigators to avoid use of random definitions in prospective clinical trials or conduct meta-analyses including studies with distinct POR definitions (55).
According to Bologna criteria, at least two of the following three criteria must be present to classify a patient as poor responder, namely, (i) Advanced maternal age, (ii) Previous POR after OS, and (iii) Abnormal ovarian reserve tests (Table 2). The age of 40 years and retrieval of three or fewer oocytes were adopted as the cutoffs to discriminate women with and without POR. Ovarian reserve tests, namely antral follicle count (AFC) and anti-Müllerian hormone (AMH) levels were also included, with variable ranges of <5–7 follicles or <0.5–1.1 ng/ml, respectively.
The Bologna criteria were partially successful in its intended primary goal. Among 51 POR interventional trials registered in clinicaltrials.gov from July 2011 to March 2017, 23 (45%) adopted the Bologna criteria. The number of subjects enrolled in such trials varied markedly from 23 to 939, but the vast majority of trials were not powered to detect differences in pregnancy rates. In fact, a sample size of ~1,000 subjects would be required in binary outcome superiority trials to have a 90% chance of detecting, as significant at the level of 5%, a 20% increase in pregnancy rates between the control group and experimental group (https://www.sealedenvelope.com/power/binary-superiority/). Among the published trials with an adequate sample size to avoid a type II error (https://clinicaltrials.gov), only two reported a potential benefit of a given intervention with regard to pregnancy (56, 57).
A few retrospective cohort studies were also published using the Bologna criteria. On average, a live birth rate (LBR) of 10% or less was observed in women diagnosed with POR (58–60), therefore, suggesting that the Bologna criteria might be able to select a homogeneous population with poorer reproductive outcomes during ART. The correct identification of the subset of women with poor prognosis in IVF, apart from its usefulness in terms of clinical management and counseling, would be necessary from a public health perspective, particularly in countries with governmental treatment reimbursement (58).
Limitations of the Existing POR Criteria
A review from 2016 accumulating the evidence of interventional clinical trials in POR revealed that over 90% trials were unable to detect meaningful differences in pregnancy rates (61). These disappointing results might be caused by the fact that the available studies used various POR definitions and suboptimal study designs, thus, making it difficult to draw valid conclusions for any given treatment strategy (62, 63).
Patient heterogeneity is deemed to be a significant shortcoming in studies evaluating strategies for POR, including those in which the Bologna criteria were applied (64). In a 2013 study, different LBRs were reported for Bologna POR aged ≤35 (12%), 36–39 (8%), and ≥40 (6%) (59). Likewise, Hu et al. retrospectively evaluated 592 IVF cycles in Bologna criteria PORs and reported that pregnancy outcomes varied according to age group (65). The authors showed that implantation rates ranged from 15.3 to 29.4% in patients under 35 years. By contrast, it ranged from 6.3 to 24.1% in patients ≥35 years. Along the same lines, Cohen and colleagues retrospectively assessed live birth rates in a large Bologna POR patient cohort aged 40 years or greater (16). The live birth per cycle was 3.3 times higher (11.61 vs. 3.54%, P < 0.001) in patients aged 40–43 with more than three oocytes compared to counterparts with less than three oocytes. Furthermore, a 2017 RCT evaluating the use of recombinant LH supplementation in Bologna criteria POR showed that—in a post-hoc analysis—the subset of patients classified as moderate or severe poor responders who received LH supplementation had higher LBR and lower pregnancy loss than the general population of POR patients (57).
Although the ESHRE consensus established the minimum criteria for the definition of POR, numerous patient categories with potentially different prognosis might be generated by using the criteria mentioned above (Table 3). Notably, studies explicitly evaluating pregnancy outcomes according to these subgroups of patients yielded conflicting results (58, 66, 67) (Table 4). Whereas reproductive success was similar among Bologna subgroups in the studies of Busnelli et al. (58) and La Marca et al. (66), the results differed according to the subset evaluated in the series of Bozdag et al. (67). In the latter study, which to our knowledge included the largest retrospective analysis of POR patients undergoing ART to date, the likelihood of pregnancy varied significantly according to the subgroups of POR evaluated (Table 4).
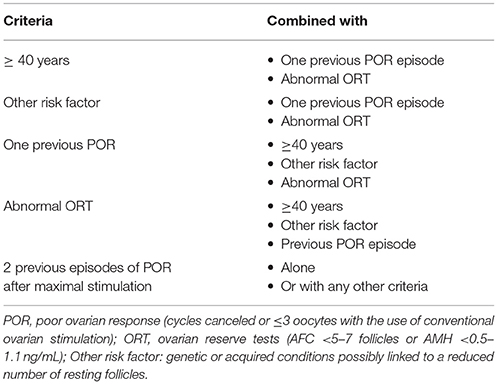
Table 3. Different patient categories generated by combining the parameters used to define the poor ovarian response patient according to Bologna criteria.
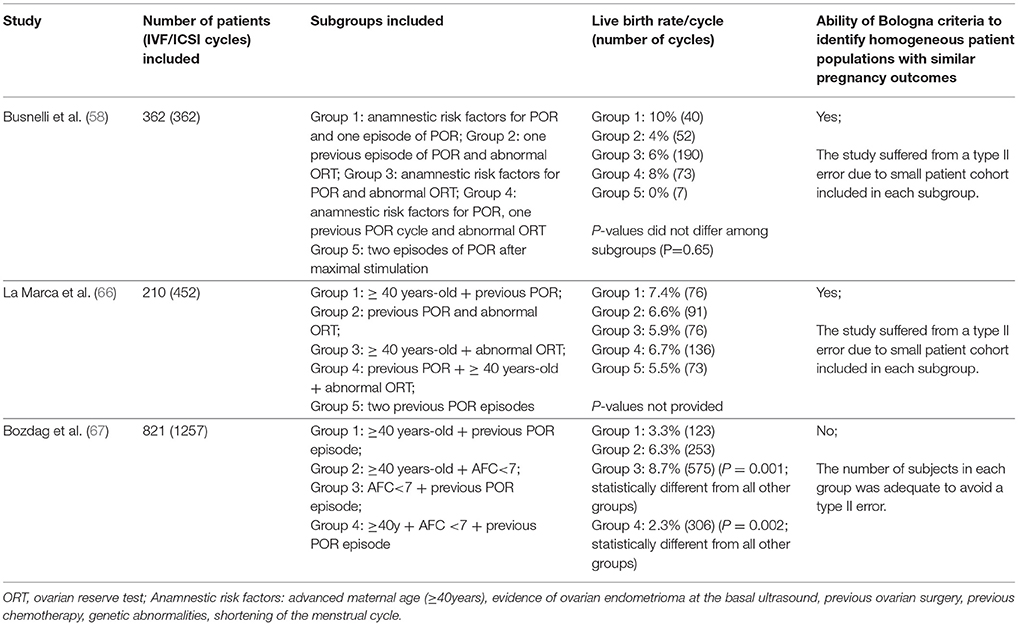
Table 4. Clinical studies evaluating IVF outcomes in different subgroups of poor ovarian responders according to the Bologna criteria.
Lastly, another limitation of the Bologna criteria relates to the biomarkers cut-offs used to classify POR patients. The ranges of 5–7 for AFC and, more importantly, 0.5–1.1 ng/ml for AMH seems quite wide. In fact, little information was provided by the authors of the ESHRE consensus about the accuracy of such ranges in predicting POR (55). Since the attributed importance of ovarian biomarkers is high, technical and performance characteristics should be considered when applying cut-off ranges, in particular, the lack of standardized methods for the assessment of ovarian reserve markers among centers (68).
Ovarian Resistance to Exogenous Gonadotropins: a Previously Neglected Aspect
Ovarian stimulation is a crucial element of most IVF programs. The use of GnRH analogs in association with exogenous gonadotropins promote adequate follicular growth and steroidogenesis in the majority of normogonadotropic women who undergo ART. In the modern ART era, ovarian biomarkers, including AFC, and AMH have been used with fair accuracy to predict ovarian response to gonadotropin stimulation, thus, allowing clinicians to individualize OS (69). However, AFC and AMH cannot predict an unexpectedly poor or suboptimal response to gonadotropin therapy in women with adequate pre-stimulation parameters. Indeed, patients with adequate ovarian reserve might show hypo-responsiveness to gonadotropin stimulation (70, 71). The reasons for ovarian resistance to gonadotropin stimulation are not entirely understood. However, increasing evidence indicates that women with the so-called “hypo-response” to OS might harbor genetic mutations or single nucleotide polymorphisms (SNPs) of gonadotropins and their receptors that influence ovarian sensitivity to gonadotropin stimulation despite an apparently good prognosis (21, 25, 72–74).
Despite broadly categorized as PORs, the fate of women with hypo-response to OS differs from the classic POR patient. The results of a 2014 meta-analysis compiling 1129 IVF/ICSI cycles in POR patients supplemented or not with recombinant human LH (rec-hLH) illustrate this phenomenon (27). In this aforementioned review, the definition of POR to gonadotropin stimulation was based on the criteria utilized by each included study. It was noted that significantly more oocytes were retrieved in rec-LH supplemented cycles than in recombinant human FSH (rec-hFSH) monotherapy cycles (12 studies, n = 1077; weighted mean difference +0.75 oocytes; 95 % CI 0.14–1.36). The use of rec-hLH supplementation also improved clinical pregnancy rates by 30% overall (14 studies, n = 1179; relative risk [RR] 1.30; 95 % confidence interval [CI] 1.01–1.67; intention-to-treat population [ITT] population). Nevertheless, a careful examination of the included studies reveals that the beneficial effect of rec-hLH was more pronounced in studies involving hypo-responders rather than in those with classic POR. The inclusion of studies involving hypo-responders in that review explains the overall favorable results observed with rec-LH supplementation in the POR patient. Indeed, a 2018 systematic review carried out by the International Collaborative Group for the Study of rec-hLH (iCOS-LH) showed that a clear distinction between hypo-responders and classic PORs is paramount since the clinical relevance of adding rec-LH to OS was only evident in hypo-responders (75). Researchers have rightfully argued that critical methodological issues like the one discussed above should be taken into account when designing studies on poor responders (64, 76, 77).
From a clinical perspective, hypo-responders represent a patient category that differs from both normal responders and the classic POR. The hypo-responder is a patient with a normal ovarian reserve who ends up having an unexpected suboptimal or poor response to OS, usually manifested by a low follicular output rate (FORT), use of increased total dosages of gonadotropin, or lower than expected number of oocytes retrieved (9, 21, 25, 72). Management of hypo-responders might be associated with increased treatment costs, decreased cumulative live birth rates, and increased time to live birth. Until now, however, none of the POR criteria have taken into account this group of hypo-responders to ovarian stimulation.
Oocyte Quantity Versus Quality
The decline in fertility with aging is caused by both a progressive reduction in the primordial follicle number across the woman's lifespan as well as an increased rate of oocyte chromosomal abnormalities and cytoplasmic dysfunctions (18). These phenomena ultimately result in a reduction of oocyte quantity and quality, thus, explaining the poorer IVF outcomes in older women when compared to younger counterparts.
Data from large databases unequivocally show that IVF success depends on both the number of oocytes retrieved and the women's age (5, 6). The critical role of female age on oocyte quality is easily illustrated by comparing delivery rates according to age in women with similar oocyte yield (5, 6); in this scenario, the older the patient the lower the delivery rates. This effect is noted not only in the general infertile population, but also in poor responders (15).
Despite the overall notion that the prognosis of a patient undergoing IVF can be measured by the number of oocytes retrieved, a valid critique of Bologna criteria and other classification systems for POR is that these standards fail to identify young women with expected POR due to abnormal ovarian biomarkers; i.e., women below 35 years-old who have not undergone OS (78, 79). Preimplantation genetic studies using microarray-based comparative genomic hybridization and next-generation sequencing (NGS) show that embryo euploidy rates are markedly higher in women younger than 35 years of age than older counterparts (80, 81). In fact, embryo ploidy is probably the leading factor explaining the differences in success rates between younger and older women who undergo IVF (82).
The probability of achieving at least one euploid blastocyst for transfer in patients undergoing IVF increases as a function of blastocyst cohort size in all age categories (80, 81). Since blastocyst euploidy rates are independent of cohort sizes, the higher the number of oocytes retrieved the higher the probability of having an embryo cohort with at least one euploid embryo (80, 81). Therefore, oocyte quantity and the age-related embryo euploidy rate are essential aspects to consider for both counseling purposes and treatment planning in women with POR. Failure to include these aspects in clinical studies might result in stratification of women with distinct biological characteristics, a bias that could dilute the magnitude of the effect concerning the intervention studied.
A Plea for a More Optimal Definition and Stratification of the Low Responder Patient Undergoing ART: the Poseidon Criteria
Despite the advancement toward a better definition of the POR patient with the publication of the Bologna criteria in 2011 (55), little has been achieved in terms of clinical guidance concerning management. To date, clinicians remain without evidence-based guidance for therapeutic management of the POR patient and often rely on personal experience or anecdotal facts to handle such patients (14). Thus, development of criteria aiming at identifying and stratifying patients with low prognosis in ART is of utmost importance for clinical management. A correct stratification of homogeneous groups of low prognosis women could also help researchers identify treatment strategies best suited for each patient category.
The recently established POSEIDON (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number) Group, a collaborative effort among clinicians and researchers with a particular interest in reproductive endocrinology and ART, proposes a new and more detailed stratification of low prognosis patients who undergo OS for IVF (83, 84). A series of articles within this research topic of Frontiers in Endocrinology will discuss in great detail the newly launched POSEIDON criteria. In brief, this new system aims to introduce a fine-tuning of POR, using clinically relevant criteria to guide the physician (Figure 1). Essentially, the POSEIDON group proposes a change in the definition of POR from quite heterogeneous criteria to the concept of low prognosis, which better reflects the reproductive potential of these patients.

Figure 1. The new Poseidon criteria to identify and stratify infertility patients with “expected” or “unexpected” impaired ovarian response to exogenous gonadotropins undergoing ART. Four distinct groups of low prognosis patients can be established based on quantitative and qualitative parameters, namely: 1. The age of the patient and the expected embryo aneuploidy rate; 2. Ovarian biomarkers [antral follicle count [AFC] and/or anti-Müllerian hormone [AMH]], and 3. The ovarian response of the patient in terms of oocyte quantity provided a previous cycle of stimulation was carried out. Art drawing by Chloé Xilinas, EXCEMED, Rome, Italy.
“Low Prognosis” seems to be the ideal terminology because it allows not only to identify patients who have a reduced probability of pregnancy in ART, but also to stratify the low prognosis patients into distinct categories based on quantitative and qualitative parameters, namely: (i) The age of the patient and the expected embryo aneuploidy rate; (ii) Ovarian biomarkers, and (iii) The ovarian response of the patient provided a previous cycle of stimulation was carried out (83). In addition to providing a system for the identification and classification of low prognosis patients undergoing ART, the group introduced a new measure of clinical success, namely, the ability to retrieve the number of oocytes needed to obtain at least one euploid blastocyst for transfer in each patient (84).
Notably, the POSEIDON group does not advocate trial-and-error to identify patients classified as groups 1 and 2. Other published algorithms might be considered as a means to optimize oocytes yield on the first cycle (85). However, the information from a previous cycle should be used wisely, whenever available, to most optimally plan the next ovarian stimulation strategy.
The POSEIDON criteria allow the clinician to first of all classify patients who have low prognosis in ART and secondly to prepare a stimulation plan aiming at reaching the number of oocytes needed to obtain at least one euploid blastocyst for transfer (4, 86). It is anticipated that the new concept of low prognosis will help improve the management of patients undergoing ART, promote a tailored approach to patient handling, and identify more homogeneous populations for clinical trials, thereby, providing better tools with which to maximize IVF success rates.
Conclusions
Management of patients with an impaired ovarian reserve or POR to exogenous gonadotropin stimulation has challenged reproductive specialists for several decades. Apart from our limited understanding of its pathophysiology, wide heterogeneity exists in the definition of POR. A critical shortcoming of the existing POR criteria, which is largely based on ovarian biomarkers and numbers of oocytes retrieved after OS, is that they group women with distinct clinically relevant characteristics. This could explain the lack of scientific evidence to support any effective intervention for POR patients. As a result, practitioners have utilized different strategies in clinical management—often not evidence-based—since none of the existing POR criteria provide a clear path for management. In practical terms, counting the number of oocytes retrieved or estimating such numbers using ovarian biomarkers is not enough for clinical management. Equally important is the ability to determine the ovarian sensitivity to gonadotropins, which is modulated by genetic factors involving both gonadotropins and their receptors, and the age-related decrease in oocyte quality which largely depends on chromosomal abnormalities occurring before meiosis II.
The POSEIDON (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number) group—founded in 2015- introduced a new system to stratify infertility patients with “expected” or “unexpected” impaired ovarian response to exogenous gonadotropins. Furthermore, the group proposed a new measure for successful ART treatment, namely, the ability to retrieve the number of oocytes necessary to obtain at least one euploid embryo for transfer in each patient. This new stratification aims at providing a more nuanced picture of POR using clinically relevant criteria to guide the physician in the management of this increasing group of patients. Thus, the POSEIDON group proposes a change in the definition of POR, with sub-grouping, resulting in more homogenous populations. Hopefully, this new classification system will prove to be of daily help for clinicians as well as for patients, ultimately facilitating treatment and resulting in a shorter time to pregnancy and live birth.
Author Contributions
SE designed the manuscript. All authors contributed to drafting and critical discussions. GB and MR scrutinized the literature and developed the Tables. All authors contributed to revised and accepted the final manuscript.
Conflict of Interest Statement
SE received honoraria for lectures from Merck, Besins, and Lilly. MR received honoraria for lectures from Merck. GB and AC have nothing to disclose. PH received unrestricted research grants from MSD, Merck, and Ferring as well as honoraria for lectures from MSD, Merck and IBSA. CA received honoraria for lectures from Merck.
The reviewer NP declared a past co-authorship with several of the authors to the handling Editor.
References
1. Malchau SS, Henningsen AA, Loft A, Rasmussen S, Forman J, Nyboe Andersen A, et al. The long-term prognosis for live birth in couples initiating fertility treatments. Hum Reprod. (2017) 32:1439–49. doi: 10.1093/humrep/dex096
2. Haahr T, Roque M, Esteves SC, Humaidan P. GnRH agonist trigger and LH activity luteal phase support versus hCG trigger and conventional luteal phase support in fresh embryo transfer IVF/ICSI cycles-a systematic PRISMA review and meta-analysis. Front Endocrinol. (2017) 8:116. doi: 10.3389/fendo.2017.00116
3. Haahr T, Esteves SC, Humaidan P. Poor definition of poor-ovarian response results in misleading clinical recommendations. Hum Reprod. (2018) 33:979–80. doi: 10.1093/humrep/dey059
4. Haahr T, Esteves SC, Humaidan P. Individualized controlled ovarian stimulation in expected poor-responders: an update. Reprod Biol Endocrinol. (2018) 16:20. doi: 10.1186/s12958-018-0342-1
5. Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. (2011) 26:1768–74. doi: 10.1093/humrep/der106
6. De Geyter C, Fehr P, Moffat R, Gruber IM, von Wolff M. Twenty years' experience with the Swiss data registry for assisted reproductive medicine: outcomes, key trends and recommendations for improved practice. Swiss Med Wkly (2015) 145:w14087. doi: 10.4414/smw.2015.14087
7. Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H, et al. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod (2016) 31:370–6. doi: 10.1093/humrep/dev316
8. Verberg MF, Eijkemans MJ, Macklon NS, Heijnen EM, Baart EB, Hohmann FP, et al. The clinical significance of the retrieval of a low number of oocytes following mild ovarian stimulation for IVF: a meta-analysis. Hum Reprod Update (2009) 15:5–12. doi: 10.1093/humupd/dmn053
9. Polyzos NP, Sunkara SK. Sub-optimal responders following controlled ovarian stimulation: an overlooked group? Hum Reprod. (2015) 30:2005–8. doi: 10.1093/humrep/dev149
10. Zhen XM, Qiao J, Li R, Wang LN, Liu P. The clinical analysis of poor ovarian response in in-vitro-fertilization embryo-transfer among Chinese couples. J Assist Reprod Genet. (2008) 25:17–22. doi: 10.1007/s10815-007-9187-9
11. Hendriks DJ, te Velde ER, Looman CW, Bancsi LF, Broekmans FJ. Expected poor ovarian response in predicting cumulative pregnancy rates: a powerful tool. Reprod Biomed Online (2008) 17:727–36. doi: 10.1016/S1472-6483(10)60323-9
12. Baka S, Makrakis E, Tzanakaki D, Konidaris S, Hassiakos D, Moustakarias T, et al. Poor responders in IVF: cancellation of a first cycle is not predictive of a subsequent failure. Ann N Y Acad Sci. (2006) 1092:418–25. doi: 10.1196/annals.1365.040
13. Polyzos NP, Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil Steril (2011) 96:1058–61 e7. doi: 10.1016/j.fertnstert.2011.09.048
14. Patrizio P, Vaiarelli A, Setti L, Tobler KJ, Shoham G, Leong M, et al. How to define, diagnose and treat poor responders? Responses from a worldwide survey of IVF clinics. Reprod Biomed Online (2015) 30:581–92. doi: 10.1016/j.rbmo.2015.03.002T
15. Oudendijk JF, Yarde F, Eijkemans MJ, Broekmans FJ, Broer SL. The poor responder in IVF: is the prognosis always poor?: a systematic review. Hum Reprod Update (2012) 18:1–11. doi: 10.1093/humupd/dmr037
16. Cohen Y, Tannus S, Alzawawi N, Son WY, Dahan M, Buckett W. Poor ovarian response as a predictor for live birth in older women undergoing IVF. Reprod Biomed Online (2018) 36:435–41. doi: 10.1016/j.rbmo.2018.01.008
17. Kocourkova J, Burcin B, Kucera T. Demographic relevancy of increased use of assisted reproduction in European countries. Reprod Health (2014) 11:37. doi: 10.1186/1742-4755-11-37
18. Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS ONE (2010) 5:e8772. doi: 10.1371/journal.pone.0008772
19. Sampo AV, Palena C, Ganzer L, Maccari V, Estofan G, Hernandez M. The adverse effect of overweight in assisted reproduction treatment outcomes. JBRA Assist Reprod. (2017) 21:212–6. doi: 10.5935/1518-0557.20170041
20. Alviggi C, Clarizia R, Pettersson K, Mollo A, Humaidan P, Strina I, et al. Suboptimal response to GnRHa long protocol is associated with a common LH polymorphism. Reprod BioMed Online (2011) 22:S67–72. doi: 10.1016/S1472-6483(11)60011-4
21. Alviggi C, Conforti A, Caprio F, Gizzo S, Noventa M, Strina I, et al. Is estimated good prognosis patients could unexpected “hyporesponse” to controlled ovarian stimulation be related to genetic polymorphisms of FSH receptors? Reprod Sci. (2016) 23:1103–8. doi: 10.1177/1933719116630419
22. La Marca A, Papaleo E, Alviggi C, Ruvolo G, De Placido G, Candiani M, et al. The combination of genetic variants of the FSHB and FSHR genes affects serum FSH in women of reproductive age. Hum Reprod. (2013) 28:1369–74. doi: 10.1093/humrep/det061
23. La Marca A, Sighinolfi G, Argento C, Grisendi V, Casarini L, Volpe A, et al. Polymorphisms in gonadotropin and gonadotropin receptor genes as markers of ovarian reserve and response in vitro fertilization. Fertil Steril (2013) 99:970–8.e1. doi: 10.1016/j.fertnstert.2013.01.086
24. Alviggi C, Mollo A, Clarizia R, De Placido G. Exploiting LH in ovarian stimulation. Reprod Biomed Online (2006) 12:221–33. doi: 10.1016/S1472-6483(10)60865-6
25. Alviggi C, Pettersson K, Longobardi S, Andersen CY, Conforti A, De Rosa P, et al. A common polymorphic allele of the LH beta-subunit gene is associated with higher exogenous FSH consumption during controlled ovarian stimulation for assisted reproductive technology. Reprod Biol Endocrinol. (2013) 11:51. doi: 10.1186/1477-7827-11-51
26. Klinkert ER, Broekmans FJ, Looman CW, Te Velde ER. A poor response in the first in vitro fertilization cycle is not necessarily related to a poor prognosis in subsequent cycles. Fertil Steril (2004) 81:1247–53. doi: 10.1016/j.fertnstert.2003.10.030
27. Lehert P, Kolibianakis EM, Venetis CA, Schertz J, Saunders H, Arriagada P, et al. Recombinant human follicle-stimulatin hormone (r-hFSH) plus recombinant luteinizing hormone versus r-hFSH alone for ovarian stimulation during assisted reproductive technology: systematic review and meta-analysis. Reprod Biol Endocrinol. (2014) 20:17. doi: 10.1186/1477-7827-12-17
28. Goswami SK, Das T, Chattopadhyay R, Sawhney V, Kumar J, Chaudhury K, et al. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum Reprod. (2004) 19:2031–5. doi: 10.1093/humrep/deh359
29. van Tilborg TC, Torrance HL, Oudshoorn SC, Eijkemans MJC, Koks CAM, Verhoeve HR, et al. OPTIMIST study group. Individualized versus standard FSH dosing in women starting IVF/ICSI: an RCT. Part 1: The predicted poor responder. Hum Reprod. (2017) 32:2496–505. doi: 10.1093/humrep/dex318
30. Narkwichean A, Maalouf W, Baumgarten M, Polanski L, Raine-Fenning N, Campbell B, et al. Efficacy of dehydroepiandrosterone (DHEA) to overcome the effect of ovarian ageing (DITTO): a proof of principle double blinded randomized placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. (2017) 218:39–48. doi: 10.1016/j.ejogrb.2017.09.006
31. Cedrin-Durnerin I, Bständig B, Hervé F, Wolf J, Uzan M, Hugues J. A comparative study of high fixed-dose and decremental-dose regimens of gonadotropins in a minidose gonadotropin-releasing hormone agonist flare protocol for poor responders. Fertil Steril (2000) 73:11055–6. doi: 10.1016/S0015-0282(00)00471-4
32. Akman MA, Erden HF, Tosun SB, Bayazit N, Aksoy E, Bahcece M. Comparison of agonistic flare-up-protocol and antagonistic multiple dose protocol in ovarian stimulation of poor responders: results of a prospective randomized trial. Hum Reprod. (2001) 16:868–70. doi: 10.1093/humrep/16.5.868
33. Lok IH, Yip SK, Cheung LP, Yin Leung PH, Haines CJ. Adjuvant low-dose aspirin therapy in poor responders undergoing in vitro fertilization: a prospective, randomized, double-blind, placebo-controlled trial. Fertil Steril (2004) 81:556–61. doi: 10.1016/j.fertnstert.2003.07.033
34. Malmusi S, La Marca A, Giulini S, Xella S, Tagliasacchi D, Marsella T, et al. Comparison of a gonadotropin-releasing hormone (GnRH) antagonist and GnRH agonist flare-up regimen in poor responders undergoing ovarian stimulation. Fertil Steril (2005) 84:402–6. doi: 10.1016/j.fertnstert.2005.01.139
35. Morgia F, Sbracia M, Schimberni M, Giallonardo A, Piscitelli C, Giannini P, et al. A controlled trial of matural cycle versus microdose gonadotropin-releasing hormone analog flare cycles in poor responders undergoing in vitro fertilization. Fertil Steril (2004) 81:1542–7. doi: 10.1016/j.fertnstert.2003.11.031
36. Garcia-Velasco JA, Isaza V, Requena A, Martínez-Salazar FJ, Landazábal A, Remohí J, et al. High doses of gonadotrophins combined with stop versus non-stop protocol of GnRH analogue administration in low responder IVF patients: a prospective, randomized, controlled trial. Hum Reprod. (2000) 15:2292–6. doi: 10.1093/humrep/15.11.2292
37. Weissman A, Farhi J, Royburt M, Nahum H, Glezerman M, Levran D. Prospective evaluation of two stimulation protocols for low responders who were undergoing in vitro fertilization-embryo transfer. Fertil Steril (2003) 79:886–92. doi: 10.1016/S0015-0282(02)04928-2
38. Cheung LP, Lam PM, Lok IH, Chiu TT, Yeung SY, Tjer CC, et al. GnRH antagonist versus long GnRH agonist protocol in poor responders undergoing IVF: a randomized controlled trial. Hum Reprod. (2005) 20:616–21. doi: 10.1093/humrep/deh668
39. Schimidt DW, Bremner T, Orris JJ, Maier DB, Benadiva CA, Nulsen JC. A randomized prospective study of microdose leuprolide versus ganirelix in vitro fertilisation cycles for poor responders. Fertil Steril (2005) 83:1568–71. doi: 10.1016/j.fertnstert.2004.10.053
40. Mohamed KA, Davies WA, Lashen H. Effect of gonadotropin-releasing hormone agonist and antagonist on steroidogenesis of low responders undergoing in vitro fertilization. Gynecol Endocrinol. (2006) 22:57–62. doi: 10.1080/09513590500519260
41. Diluigi AJ, Engmann L, Schmidt DW, Benadiva CA, Nulsen JC. A randomized trial of microdose leuprolide acetate protocol versus luteal phase ganirelix protocol in predicted poor responders. Fertil Steril (2011) 95:2531–3. doi: 10.1016/j.fertnstert.2011.01.134
42. Marci R, Caserta D, Dolo V, Tatone C, Pavan A, Moscarini M. GnRH antagonist in IVF poor-responder patients: results of a randomized trial. Reprod Biomed Online (2005) 11:189–93. doi: 10.1016/S1472-6483(10)60957-1
43. Massin N, Cedrin-Durnerin I, Coussieu C, Galey-Fontaine J, Wolf JP, Hughes JN. Effects of transdermal testosterone application on the ovarian response to FSH in poor responders undergoing assisted reproduction technique – a prospective, randomized, double-blind study. Hum Reprod. (2006) 21:1204–11. doi: 10.1093/humrep/dei481
44. Kahraman K, Berker B, Atabekoglu CS, Sonmezer M, Cetinkaya E, Aytac R, et al. Microdose gonadotropin-releasing hormone agonist flare-up protocol versus multiple dose gonadotropin-releasing hormone antagonist protocol in poor responders undergoing intracytoplasmic sperm injection-embryo transfer cycle. Fertil Steril (2009) 91:2437–44. doi: 10.1016/j.fertnstert.2008.03.057
45. Tazegül A, Görkemli H, Ozdemir S, Aktan TM. Comparison of multiple dose GnRH antagonista and mini-dose long agonist protocols in poor responders indergoing in vitro fertilization: a randomized controlled trial. Arch Gynecol Obstet. (2008) 278:467–72. doi: 10.1007/s00404-008-0620-9
46. Shahine LK, Milki AA, Westphal LM, Baker VL, Behr B, Lathi RB. Day 2 versus day 3 embryo transfer in poor responders: a prospective randomized trial. Fertil Steril (2011) 95:330–2. doi: 10.1016/j.fertnstert.2010.06.093
47. Kim CH, Howles CM, Lee HA. The effect of transdermal testosterone gel pretreatment on controlled ovarian stimulation and IVF outcome in low responders. Fertil Steril (2011) 95:679–83. doi: 10.1016/j.fertnstert.2010.07.1077
48. Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, Shulman A. Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Hum Reprod (2010) 25:2496–500. doi: 10.1093/humrep/deq220
49. Lainas TG, Sfontouris IA, Papanikolaou EG, Zorzovilis JZ, Petsas GK, Lainas GT, et al. Flexible GnRH antagonist versus flare-up GnRH agonist protocol in poor responders treated by IVF: a randomized controlled trial. Hum Reprod (2008) 23:1355–8. doi: 10.1093/humrep/den107
50. Tarlatzis BC, Zepiridis L, Grimbizis G, Bontis J. Clinical management of low ovarian response to stimulation for IVF: a systematic review. Hum Reprod Update (2003) 9:61–76. doi: 10.1093/humupd/dmg007
51. Feigenberg T, Simon A, Ben-Meir A, Gielchinsky Y, Laufer N. Role of androgens in the treatment of patients with low ovarian response. Reprod Biomed Online (2009) 19:888–98. doi: 10.1016/j.rbmo.2009.09.012
52. Levi AJ, Raynault MF, Bergh PA, Drews MR, Miller BT, Scott RT Jr. Reproductive outcome in patients with diminished ovarian reserve. Fertil Steril (2001) 76:666–9. doi: 10.1016/S0015-0282(01)02017-9
53. Devine K, Mumford SL, Wu M, DeCherney AH, Hill MJ, Propst A. Diminished ovarian reserve in the United States assisted reproductive technology population: diagnostic trends among 181,536 cycles from the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Fertil Steril (2015) 104:612–19. doi: 10.1016/j.fertnstert.2015.05.017
54. Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril (2017) 108:980–7. doi: 10.1016/j.fertnstert.2017.09.011
55. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod (2011) 26:1616–24. doi: 10.1093/humrep/der092
56. Xu B, Li Z, Yue J, Jin L, Li Y, Ai J, Zhang H, Zhu G. Effect of dehydroepiandrosterone administration in patients with poor ovarian response according to the Bologna criteria. PLoS ONE (2014) 9:e99858. doi: 10.1371/journal.pone.0099858
57. Humaidan P, Chin W, Rogoff D, D'Hooghe T, Longobardi S, Hubbard J, et al. Efficacy and safety of follitropin alfa/lutropin alfa in ART: a randomized controlled trial in poor ovarian responders. Hum Reprod. (2017) 32:544–55. doi: 10.1093/humrep/dew360
58. Busnelli A, Papaleo E, Del Prato D, La Vecchia I, Iachini E, Paffoni A, et al. A retrospective evaluation of prognosis and cost-effectiveness of IVF in poor responders according to the Bologna criteria. Hum Reprod (2015) 30:315–22. doi: 10.1093/humrep/deu319
59. Ke H, Chen X, Liu YD, Ye DS, He YX, Chen SL. Cumulative live birth rate after three ovarian stimulation IVF cycles for poor ovarian responders according to the bologna criteria. J Huazhong Univ Sci Technolog Med Sci. (2013) 33:418–22. doi: 10.1007/s11596-013-1134-7
60. Polyzos NP, Nwoye M, Corona R, Blockeel C, Stoop D, Haentjens P, et al. Live birth rates in Bologna poor responders treated with ovarian stimulation for IVF/ICSI. Reprod Biomed Online (2014) 28:469–74. doi: 10.1016/j.rbmo.2013.11.010
61. Papathanasiou A, Searle BJ, King NM, Bhattacharya S. Trends in ‘poor responder’ research: lessons learned from RCTs in assisted conception. Hum Reprod Update (2016) 22:306–19. doi: 10.1093/humupd/dmw001
62. Boza A, Oguz SY, Misirlioglu S, Yakin K, Urman B. Utilization of the Bologna criteria: a promise unfulfilled? A review of published and unpublished/ongoing trials. Fertil Steril (2018) 109:104–9. doi: 10.1016/j.fertnstert.2017.09.024
63. Venetis C. The Bologna criteria for poor ovarian response: the good, the bad and the way forward. Hum Reprod (2014) 29:1839–41. doi: 10.1093/humrep/deu138
64. Papathanasiou A. Implementing the ESHRE ‘poor responder’ criteria in research studies: methodological implications. Hum Reprod (2014) 29:1835–8. doi: 10.1093/humrep/deu135
65. Hu L, Bu Z, Guo Y, Su Y, Zhai J, Sun Y. Comparison of different ovarian hyperstimulation protocols efficacy in poor ovarian responders according to the Bologna criteria. Int J Clin Exp Med. (2014) 7:1128–34.
66. La Marca A, Grisendi V, Giulini S, Sighinolfi G, Tirelli A, Argento C, et al. Live birth rates in the different combinations of the Bologna criteria poor ovarian responders: a validation study. J Assist Reprod Genet. (2015) 32:931–7. doi: 10.1007/s10815-015-0476-4
67. Bozdag G, Polat M, Yarali I, Yarali H. Live birth rates in various subgroups of poor ovarian responders fulfilling the Bologna criteria. Reprod Biomed Online (2017) 34:639–44. doi: 10.1016/j.rbmo.2017.03.009
68. Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Müllerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update (2015) 21:698–710. doi: 10.1093/humupd/dmu062
69. Broer SL, van Disseldorp J, Broeze KA, Dolleman M, Opmeer BC, Bossuyt P, et al. Added value on ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update (2013) 19:26–36. doi: 10.1093/humupd/dms041
70. Genro VK, Grynberg M, Scheffer JB, Roux I, Frydman R, Fanchin R. Serum anti-Müllerian hormone levels are negatively related to Follicular Output RaTe (FORT) in normo-cycling women undergoing controlled ovarian hyperstimulation. Hum Reprod (2011) 26:671–7. doi: 10.1093/humrep/deq361
71. Gallot V, Berwanger da Silva AL, Genro V, Grynberg M, Frydman N, Fanchin R. Antral follicle responsiveness to follicle-stimulating hormone administration assessed by the Follicular Output RaTe (FORT) may predict in vitro fertilization-embryo transfer outcome. Hum Reprod (2012) 27:1066–72. doi: 10.1093/humrep/der479
72. Alviggi C, Conforti A, Esteves SC. Impact of mutations and polymorphisms of gonadotrophins and their receptors on the outcome of controlled ovarian stimulation. In: Ghumman S editor. Principles and Practice of Controlled Ovarian Stimulation in ART 1st Edn. New Delhi: Springer. (2015). p. 147–56.
73. Ramaraju GA, Cheemakurthi R, Prathigudupu K, Balabomma KL, Kalagara M, Thota S, et al. Role of Lh polymorphisms and r-hLh supplementation in GnRh agonist treated ART cycles: a cross sectional study. Eur J Obst Gyn Reprod Biol. (2018) 222:119–125. doi: 10.1016/j.ejogrb.2018.01.025
74. Loutradis D, Vlismas A, Drakakis P, Antsaklis A. Pharmacogenetics in ovarian stimulation – current concepts. Ann N Y Acad Sci. (2008) 1127:10–19. doi: 10.1196/annals.1434.001
75. Alviggi C, Conforti A, Esteves SC, Andersen CY, Bosch E, Bühler K, et al. Recombinant luteinizing hormone supplementation in assisted reproductive technology: a systematic review. Fertil Steril (2018) 109:644–64. doi: 10.1016/j.fertnstert.2018.01.003
76. Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train (2008) 43:215–21. doi: 10.4085/1062-6050-43.2.215
77. Pandian Z, McTavish AR, Aucott L, Hamilton MP, Bhattacharya S. Interventions for ‘poor responders’ to controlled ovarian hyper stimulation (COH) in-vitro fertilisation (IVF). Cochrane Database Syst Rev. (2010) 1:004379. doi: 10.1002/14651858.CD004379
78. Cohen J, Chabbert-Buffet N, Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder - a plea for universal definitions. J Assist Reprod Genet. (2015) 32:1709–12. doi: 10.1007/s10815-015-0595-y
79. Boots CE, Bernardi LA. Bologna criteria: clinically or academically relevant? Fertil Steril (2018) 109:59–60. doi: 10.1016/j.fertnstert.2017.10.022
80. Ata B, Kaplan B, Danzer H, Glassner M, Opsahl M, Tan SL, et al. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod Biomed Online (2012) 24:614–20. doi: 10.1016/j.rbmo.2012.02.009
81. Esteves SC, Carvalho JF, Martinhago CD, Melo AA, Bento FC, Humaidan P, et al. Estimation of age-dependent decrease in blastocyst euploidy by next generation sequencing: development of a novel prediction model. Panminerva Med. (2018). doi: 10.23736/S0031-0808.18.03507-3
82. Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril (2014) 101:656–63. doi: 10.1016/j.fertnstert.2013.11.004
83. Poseidon Group (Patient-Oriented Strategies Encompassing Individualize DOocyte Number), Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril (2016) 105:1452–3. doi: 10.1016/j.fertnstert.2016.02.005
84. Humaidan P, Alviggi C, Fischer R, Esteves SC. The novel POSEIDON stratification of ‘Low prognosis patients in Assisted Reproductive Technology’ and its proposed marker of successful outcome. F1000Res (2016) 5:2911. doi: 10.12688/f1000research.10382.1
85. Yovich JL, Alsbjerg B, Conceicao JL, Hinchliffe PM, Keane KN. PIVET rFSH dosing algorithms for individualized controlled ovarian stimulation enables optimized pregnancy productivity rates and avoidance of ovarian hyperstimulation syndrome. Drug Des Devel Ther. (2016) 10:2561–73. doi: 10.2147/DDDT.S104104
Keywords: assisted reproductive technology, hypo-responder, low responder, ovarian stimulation, poor ovarian response, poor ovarian reserve, POSEIDON criteria
Citation: Esteves SC, Roque M, Bedoschi GM, Conforti A, Humaidan P and Alviggi C (2018) Defining Low Prognosis Patients Undergoing Assisted Reproductive Technology: POSEIDON Criteria—The Why. Front. Endocrinol. 9:461. doi: 10.3389/fendo.2018.00461
Received: 27 April 2018; Accepted: 26 July 2018;
Published: 17 August 2018.
Edited by:
John Lui Yovich, Pivet Medical Center, AustraliaReviewed by:
Nikolaos P. Polyzos, Dexeus University Hospital, SpainKevin Noel Keane, Curtin University, Australia
Copyright © 2018 Esteves, Roque, Bedoschi, Conforti, Humaidan and Alviggi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandro C. Esteves, cy5lc3RldmVzQGFuZHJvZmVydC5jb20uYnI=
 Sandro C. Esteves
Sandro C. Esteves Matheus Roque
Matheus Roque Giuliano M. Bedoschi
Giuliano M. Bedoschi Alessandro Conforti
Alessandro Conforti Peter Humaidan
Peter Humaidan Carlo Alviggi
Carlo Alviggi