- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Changhua Christian Hospital, Changhua, Taiwan
- 2Division of Cardiology, Department of Internal Medicine, Changhua Christian Hospital, Changhua, Taiwan
- 3Department of Radiology, Taichung Veterans General Hospital, Taichung, Taiwan
- 4Department of Accounting and Information Systems, National Taichung University of Science and Technology, Taichung, Taiwan
Background: Heart failure is a frequent complication of type 2 diabetes mellitus (T2DM). Plasma cholesterol, particularly the proatherogenic low-density lipoprotein (LDL) cholesterol, impairs heart function by promoting atheroma formation and ventricular dysfunction. Considering the established effect of cholesterol on the cardiovascular system, we hypothesized that plasma LDL cholesterol may influence left ventricular function in individuals with T2DM.
Methods: This cross-sectional study was conducted at a tertiary care hospital in Taiwan. Enrollment criteria were patients exceeding 21 years of age with T2DM who received antidiabetic and cholesterol-lowering medications. Candidates were excluded if they had heart failure, acute cardiovascular events, or familial hypercholesterolemia. Participants received blood sampling for plasma lipids after a 12-h fast, followed by transthoracic echocardiography in the cardiology clinic.
Results: The study enrolled 118 participants who were divided into two groups according to their plasma LDL cholesterol levels. Demographic characteristics including age (69.7 vs. 66.9 years, P = 0.159), body mass index (26.2 vs. 25.9 kg/m2, P = 0.66), diabetes duration (5.4 vs. 5.1 years, P = 0.48), hemoglobin A1c (7.2 vs. 7.5%, P = 0.225), and systolic blood pressure (129 vs. 130 mm Hg, P = 0.735) were similar between these groups. Moreover, all participants received similar antihypertensive medications. Participants with lower plasma LDL cholesterol levels had better heart function, as measured by the left ventricular ejection fraction (LVEF), than patients with higher LDL cholesterol levels (58.0 vs. 50.5%, P = 0.022). Multivariate regression analysis also showed an inverse correlation between plasma LDL cholesterol and left ventricular function (β coefficient: −0.110, P = 0.024).
Conclusion: This study observed an inverse correlation between plasma LDL cholesterol and heart function in individuals with T2DM. Patients with higher levels of plasma LDL cholesterol had worse left ventricular function. Therefore, plasma LDL cholesterol may be a modifiable risk factor of heart failure in diabetes, but prospective studies are necessary to confirm this finding.
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disease that affects a considerable number of patients worldwide (1). Among diabetic individuals, cardiovascular disease (CVD) is the leading cause of morbidity and mortality (2). Chronic hyperglycemia is associated with vascular dysfunction (3), oxidative stress (4), and proinflammatory cytokines that impair organ function (5). Moreover, patients with T2DM often have concomitant insulin resistance, which aggravates the severity of CVD (6).
The sympathoadrenal system has been implicated in the development of insulin resistance, which has detrimental effects on the cardiovascular system (7–9). Insulin resistance in diabetes is related to both glucose intolerance and impaired fatty acid metabolism (10). Indeed, the intimate relationship between fatty acid and glucose in the Randle cycle has strong implications for its role in the development of diabetes (11). Once insulin resistance has developed, it affects heart function through the activation of protein kinase C (12). Overall, hyperglycemia, dyslipidemia, and hypertensive cardiovascular disease contribute to heart failure in diabetic patients (13).
In the context of dyslipidemia, the low-density lipoprotein (LDL) cholesterol may impair heart function through several mechanisms. Besides its role as a proatherogenic lipoprotein, LDL cholesterol triggers the release of proinflammatory cytokines (14). Oxidized LDL cholesterol also promotes arterial intimal thickening to reduce myocardial blood flow (15). Although a link between plasma cholesterol and clinical outcome in heart failure has been reported (16), there is currently insufficient information about the influence of plasma LDL cholesterol on heart function.
Considering the link between plasma cholesterol and cardiovascular outcome, we hypothesized that plasma LDL cholesterol may influence heart function in diabetic patients. This study investigated the relationship between plasma LDL cholesterol levels and left ventricular function in individuals with T2DM. The influence of other plasma lipids such as triglycerides (TG) and high-density lipoprotein (HDL) cholesterol on heart function will also be evaluated.
Materials and Methods
Participant Selection
This cross-sectional study was conducted at Changhua Christian Hospital, a tertiary care hospital in Taiwan. Patients visiting the cardiology clinic between January 2016 and December 2017 were assessed for eligibility. Enrollment criteria were diabetic patients over 21 years of age who received antidiabetic and cholesterol-lowering medications. Candidates must be able to comply with transthoracic echocardiography for enrollment. Patients were excluded if they had previously been diagnosed with heart failure. Moreover, individuals with acute cardiovascular events, congenital heart disease, familial hypercholesterolemia, or chronic kidney disease were ineligible.
Ethics Approval
This study was carried out in accordance with the World Medical Association's Declaration of Helsinki. The study was approved by the Institutional Review Board of Changhua Christian Hospital (CCH IRB No. 181103). All participants provided written informed consent to receive blood tests and transthoracic echocardiography in accordance with the Declaration of Helsinki.
Laboratory Evaluation
Participants received blood tests for plasma TG, LDL cholesterol, HDL cholesterol, and glycated hemoglobin A1c (HbA1c) after a 12-h fast. Blood samples were sent to the central laboratory within 1 h and assayed by Beckman Coulter UniCel DxC 800 Synchron™ Clinical Systems. Specifically, plasma LDL cholesterol was measured by the timed-endpoint method using a commercial polyanion solution. Analytical precision was within 1.7, 3.0, and 7.5 ml/dL for HDL cholesterol, LDL cholesterol and TG, respectively.
Echocardiographic Assessment
Sonographers at the cardiology clinic performed transthoracic echocardiography using Canon Medical Systems' Aplio™ 300 CV Platinum system. Echocardiographic measurements were performed according to current guidelines (17). Specifically, the left atrial diameter, left ventricular end systolic diameter (LVESD) and end diastolic diameter (LVEDD) were measured in the parasternal long axis view. The right ventricular diameter was measured in the apical four-chamber view. E and A velocities from mitral valve inflow were used to calculate the E/A ratio. The left ventricular ejection fraction (LVEF) was derived from the modified Quinones equation (18).
Statistical Analysis
Participants were divided into two groups according to their plasma LDL cholesterol levels. Power analysis indicated that a sample size of 48 participants per group was necessary to detect a significant difference in left ventricular function with 80% statistical power. The Kolmogorov-Smirnov test was used to confirm the normal distribution of clinical variables in this study (D = 0.11807, P = 0.06859). Demographic characteristics between groups were compared using Student's independent t-test for continuous variables and Pearson's χ2-test for categorical variables. Echocardiographic parameters were compared using Student's independent t-test. Moreover, multivariate regression analysis was used to examine the relationship between left ventricular function and plasma lipids. Statistical analysis was performed using IBM SPSS version 22.0 (IBM SPSS Statistics for Windows. Armonk, NY, USA) with a two-tailed P < 0.05 indicating statistical significance.
Results
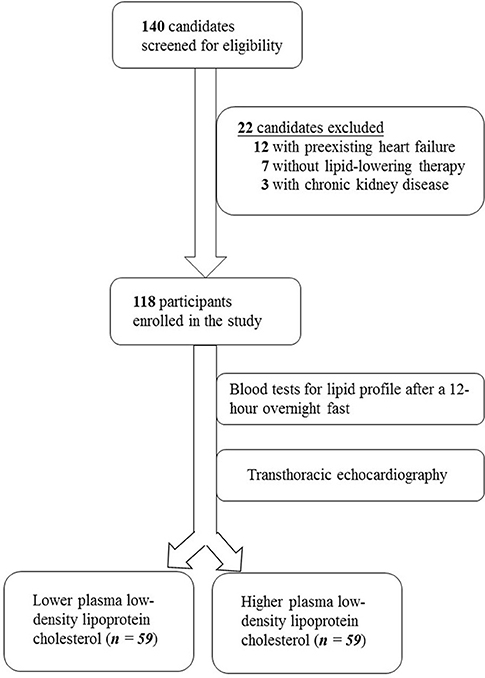
The study screened 140 individuals for eligibility. Twelve individuals were excluded due to heart failure, seven patients did not receive cholesterol-lowering medications and were thus ineligible, and three candidates with chronic kidney disease were excluded. The enrollment process is illustrated in Figure 1.
Demographic Characteristics of Participants
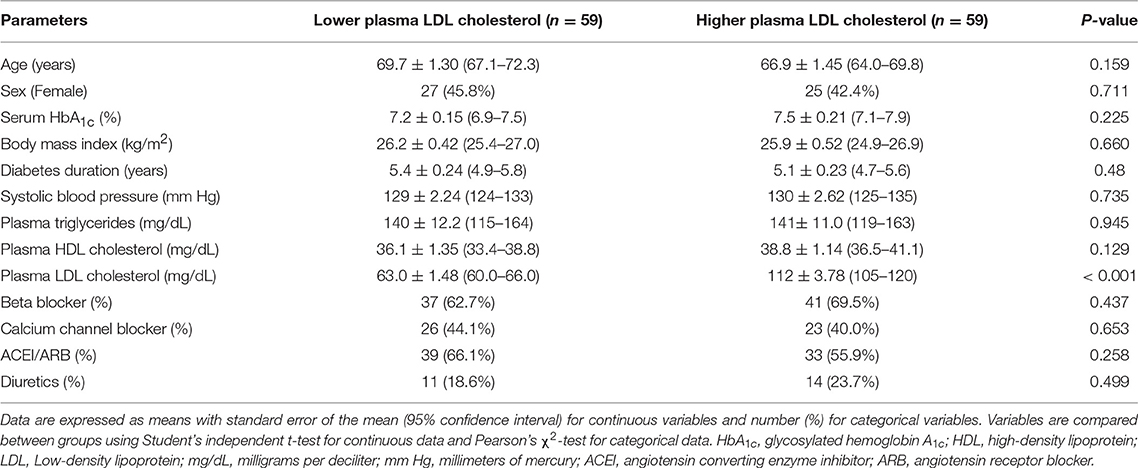
The study enrolled a total of 118 participants who were divided into two groups according to their plasma LDL cholesterol levels. As summarized in Table 1, demographic characteristics including age (69.7 vs. 66.9 years, P = 0.159), female sex (45.8 vs. 42.4%, P = 0.711), body mass index (26.2 vs. 25.9 kg/m2, P = 0.66), diabetes duration (5.4 vs. 5.1 years, P = 0.48), plasma TG level (140 vs. 141 mg/dL, P = 0.945), plasma HDL cholesterol level (36.1 vs. 38.8 mg/dL, P = 0.129), serum HbA1c (7.2 vs. 7.5%, P = 0.225), and systolic blood pressure (129 vs. 130 mm Hg, P = 0.735) were similar between these groups. Comparable proportions of participants in both groups received beta blockers (62.7 vs. 69.5%, P = 0.437), calcium channel blockers (44.1 vs. 40.0%, P = 0.653), angiotensin converting enzyme inhibitors or angiotensin receptor blockers (66.1 vs. 55.9%, P = 0.258), and diuretics (18.6 vs. 23.7%, P = 0.499). All participants received cholesterol-lowering medications. The mean plasma LDL cholesterol level was significantly different between these groups (63.0 vs. 112 mg/dL, P < 0.001), which formed the basis of this clinical investigation.
Correlation Between Plasma LDL Cholesterol and Left Ventricular Function
As shown in Table 2, participants with lower plasma LDL cholesterol levels had better heart function, as measured by the LVEF, than patients with higher LDL cholesterol levels (58.0 vs. 50.5%, P = 0.022). These groups had similar left atrial diameter (43.2 vs. 43.3 mm, P = 0.913), right ventricular diameter (25.3 vs. 25.1 mm, P = 0.81), LVESD (31.5 vs. 33.0 mm, P = 0.43), LVEDD (48.7 vs. 49.7 mm, P = 0.476), and E/A ratio (0.96 vs. 0.93, P = 0.57).
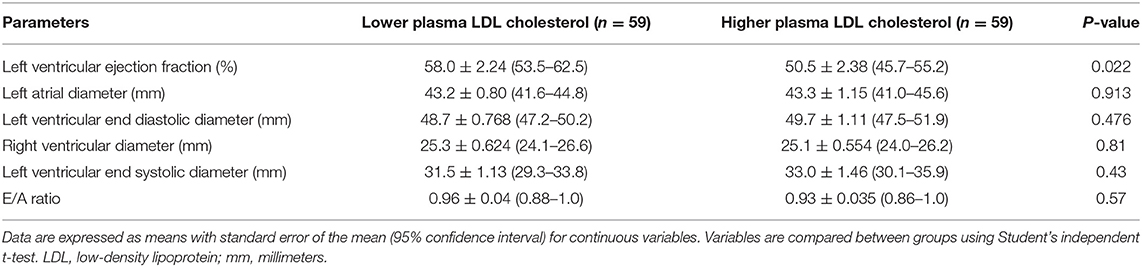
Table 2. Association between plasma low-density lipoprotein cholesterol and left ventricular function.
Multivariate Regression Analysis of LVEF Determinants
In Table 3, multivariate regression analysis revealed that plasma LDL cholesterol was inversely correlated with left ventricular function (β coefficient: −0.110, P = 0.024). In contrast, other plasma lipids such as TG (β coefficient: 0.026, P = 0.142) and HDL cholesterol (β coefficient: 0.182, P = 0.278) had limited correlation with LVEF. The structural parameter LVEDD was shown to be correlated with left ventricular function (β coefficient: −1.085, P = 0.001).
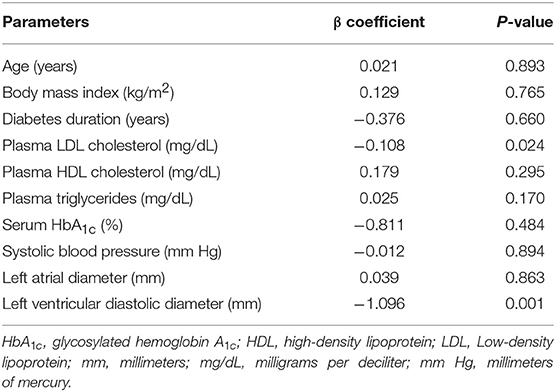
Table 3. Multivariate regression analysis of parameters associated with left ventricular ejection fraction.
Discussion
This study revealed an inverse relationship between plasma LDL cholesterol levels and heart function in patients with T2DM. Specifically, patients with lower plasma LDL cholesterol levels had better LVEF than participants with higher LDL cholesterol levels. Moreover, multivariate regression analysis showed a link between left ventricular function and plasma LDL cholesterol. To our knowledge, this is the first study to investigate the association between plasma LDL cholesterol and heart function in patients with diabetes.
Several physiologic mechanisms may explain the link between plasma LDL cholesterol and heart function. First, LDL cholesterol is a recognized risk factor of atherosclerotic heart disease and heart failure mortality (19, 20). Furthermore, investigators have demonstrated that insulin resistance can decrease myocardial glucose uptake to impair heart function (21). Excessive lipid metabolites also lead to structural abnormalities such as myocardial fibrosis (22). As mentioned previously, a synergistic effect between hyperglycemia, dyslipidemia, and hypertensive cardiovascular disease leads to heart failure in patients with T2DM.
This study excluded candidates with heart failure to avoid the cholesterol paradox. Individuals with terminal heart failure paradoxically fared better with higher plasma cholesterol levels (23). This phenomenon may be attributable to heart failure induced cachexia, which reduces plasma cholesterol levels (24). Researchers also suggest that the presence of CVD can modify the relationship between plasma cholesterol and heart function (16).
The inverse relationship between plasma LDL cholesterol levels and heart function has clinical implications. Clinical trials have shown that cholesterol-lowering therapy in diabetic patients can reduce CVD mortality (25). Our study suggests that cholesterol-lowering intervention may also preserve heart function in individuals with T2DM. Indeed, a recent study suggests that the lipid-lowering effect of insulin can improve heart function in elderly individuals with diabetes (26). In addition, medical nutrition therapy that targets LDL cholesterol may attenuate the development of heart failure.
This study provides novel information about the relationship between plasma LDL cholesterol and left ventricular function. Echocardiographic measurements were verified by three cardiologists to lessen the inter-operator variability (F = 2.1, P = 0.22). Participants received blood tests at the same medical center, which helps to reduce variability in laboratory techniques. Participants in both groups also received similar antihypertensive medications to reduce the potential confounding effects of these medications on heart function.
However, this study also has limitations. Since sonographic examination is technique-dependent (27), the accuracy of LVEF measurement depends on the sonographer's experience. Moreover, dietary intake of omega-3 polyunsaturated fatty acids can influence left ventricular function (28). Thirdly, antidiabetic medications such as sodium glucose cotransporter 2 inhibitors can modify left ventricular contractility (29). Obesity is also related to heart failure, but abdominal circumference was not included in the analysis. The study is also limited by its non-randomized design, relatively small sample size, and cross-sectional design. Therefore, prospective studies with a larger sample size are required to confirm its findings.
In conclusion, there is an inverse correlation between plasma LDL cholesterol and heart function in people with diabetes. Participants with higher levels of plasma LDL cholesterol had worse LVEF than patients with lower LDL cholesterol levels. Therefore, this study supports the hypothesis that plasma LDL cholesterol may influence heart function in diabetic patients, but prospective studies are necessary to confirm this finding.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
The research dataset is available as Supplementary Table 1.
Ethics Statement
This study was carried out in accordance with the World Medical Association's Declaration of Helsinki. The study was approved by the Institutional Review Board of Changhua Christian Hospital (CCH IRB No. 181103). All participants provided written informed consent to receive blood tests and transthoracic echocardiography in accordance with the Declaration of Helsinki.
Author Contributions
P-CC, S-RH, J-CL, C-PC, S-CC, S-TT, and J-FK conceived and designed the experiments, performed the experiments, contributed reagents, materials, analysis tools, and wrote the manuscript. Y-CC and Y-HL analyzed the data, prepared figures and tables, and wrote the manuscript. Y-HL is a qualified statistician who verified the statistical methods in this study. P-CC and Y-CC contributed equally to the study as first authors. All authors have reviewed and approved of the manuscript to be submitted.
Funding
The authors declare that the research was conducted in the absence of any external source of funding.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank sonographers at the Cardiology clinic for performing echocardiographic evaluation of the participants.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00234/full#supplementary-material
References
1. International Diabetes Federation. IDF diabetes atlas. 8th ed. Brussels: International Diabetes Federation (2017).
2. Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. (2013) 2013: 653789. doi: 10.1155/2013/653789
3. Williams LJ, Nye BG, Wende AR. Diabetes-related cardiac dysfunction. Endocrinol Metab. (2017) 32:171–9. doi: 10.3803/EnM.2017.32.2.171
4. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. (2010) 107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545
5. Hedayat M, Mahmoudi MJ, Rose NR, Rezaei N. Proinflammatory cytokines in heart failure: double-edged swords. Heart Fail Rev. (2010) 15:543–62. doi: 10.1007/s10741-010-9168-4
6. Vella CA, Burgos X, Ellis CJ, Zubia RY, Ontiveros D, Reyes H, et al. Associations of insulin resistance with cardiovascular risk factors and inflammatory cytokines in normal-weight Hispanic women. Diabetes Care. (2013) 36:1377–83. doi: 10.2337/dc12-1550
7. Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities-the role of insulin resistance and the sympathoadrenal system. N Engl J Med. (1996) 334:374–81. doi: 10.1056/NEJM199602083340607
8. Santulli G, Iaccarino G. Adrenergic signaling in heart failure and cardiovascular aging. Maturitas. (2016) 93:65–72. doi: 10.1016/j.maturitas.2016.03.022
9. Straznicky NE, Grima MT, Sari CI, Lambert EA, Phillips SE, Eikelis N, et al. Comparable attenuation of sympathetic nervous system activity in obese subjects with normal glucose tolerance, impaired glucose tolerance, and treatment naïve type 2 diabetes following equivalent weight loss. Front Physiol. (2016) 7:516. doi: 10.3389/fphys.2016.00516
10. McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. (1992) 258:766–70.
11. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. (1963) 1:785–9.
12. Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. (2016) 126:12–22. doi: 10.1172/JCI77812
13. Shu J, Matarese A, Santulli G. Diabetes, body fat, skeletal muscle, and hypertension: The ominous chiasmus? J Clin Hypertens. (2019) 21:239–242. doi: 10.1111/jch.13453
14. Lara-Guzmán OJ, Gil-Izquierdo Á, Medina S, Osorio E, Álvarez-Quintero R, Zuluaga N, et al. Oxidized LDL triggers changes in oxidative stress and inflammatory biomarkers in human macrophages. Redox Biol. (2018) 15:1–11. doi: 10.1016/j.redox.2017.11.017
15. Weingärtner O, Pinsdorf T, Rogacev KS, Blömer L, Grenner Y, Gräber S, et al. The relationships of markers of cholesterol homeostasis with carotid intima-media thickness. PLoS ONE. (2010) 5:e13467. doi: 10.1371/journal.pone.0013467
16. Sakatani T, Shirayama T, Suzaki Y, Yamamoto T, Mani H, Kawasaki T, et al. The association between cholesterol and mortality in heart failure: Comparison between patients with and without coronary artery disease. Int Heart J. (2005) 46:619–29.
17. Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. (2018) 32:1–64. doi: 10.1016/j.echo.2018.06.004
18. Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL Jr, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. (1981) 64:744–53
19. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. Evidence from genetic, epidemiologic, and clinical studies: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. (2017) 38:2459–72. doi: 10.1093/eurheartj/ehx144
20. Lala A, Desai AS. The role of coronary artery disease in heart failure. Heart Fail Clin. (2014) 10:353–65. doi: 10.1016/j.hfc.2013.10.002
21. Zlobine I, Gopal K, Ussher JR. Lipotoxicity in obesity and diabetes-related cardiac dysfunction. Biochim Biophys Acta. (2016) 1861:1555–68. doi: 10.1016/j.bbalip.2016.02.011
22. Ritchie RH, Zerenturk EJ, Prakoso D, Calkin AC. Lipid metabolism and its implications for type 1 diabetes-associated cardiomyopathy. J Mol Endocrinol. (2017) 58: R225–40. doi: 10.1530/JME-16-0249
23. Zhao Q, Li J, Yang J, Li R. Association of total cholesterol and HDL-C levels and outcome in coronary heart disease patients with heart failure. Medicine. (2017) 96:e6094. doi: 10.1097/MD.0000000000006094
24. Velavan P, Huan Loh P, Clark A, Cleland JG. The cholesterol paradox in heart failure. Congest Heart Fail. (2007) 13:336–41. doi: 10.1111/j.1527-5299.2007.07211.x
25. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. (2018) 18:39033–8. doi: 10.1016/j.jacc.2018.11.002
26. Sardu C, Marfella R, Santulli G. Impact of diabetes mellitus on the clinical response to cardiac resynchronization therapy in elderly people. J Cardiovasc Transl Res. (2014) 7:362–8. doi: 10.1007/s12265-014-9545-9
27. Pinto A, Pinto F, Faggian A, Rubini G, Caranci F, Macarini L, et al. Sources of error in emergency ultrasonography. Crit Ultrasound J. (2013) 5(Suppl. 1): S1. doi: 10.1186/2036-7902-5-S1-S1
28. Wang C, Xiong B, Huang J. The role of omega-3 polyunsaturated fatty acids in heart failure: a meta-analysis of randomised controlled trials. Nutrients. (2016) 9:18–34. doi: 10.3390/nu9010018
Keywords: type 2 diabetes mellitus, hyperlipidemia, low-density lipoprotein cholesterol, heart function, left ventricular ejection fraction, heart failure
Citation: Cheng P-C, Hsu S-R, Li J-C, Chen C-P, Chien S-C, Tu S-T, Cheng Y-C, Liu Y-H and Kuo J-F (2019) Plasma Low-Density Lipoprotein Cholesterol Correlates With Heart Function in Individuals With Type 2 Diabetes Mellitus: A Cross-Sectional Study. Front. Endocrinol. 10:234. doi: 10.3389/fendo.2019.00234
Received: 30 December 2018; Accepted: 25 March 2019;
Published: 11 April 2019.
Edited by:
Jan Polák, Charles University, CzechiaReviewed by:
Jose Mario Franco De Oliveira, Universidade Federal Fluminense, BrazilAngela Lombardi, Albert Einstein College of Medicine, United States
Copyright © 2019 Cheng, Hsu, Li, Chen, Chien, Tu, Cheng, Liu and Kuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeng-Fu Kuo, MTAzMzQ4QGNjaC5vcmcudHc=
 Po-Chung Cheng
Po-Chung Cheng Shang-Ren Hsu1
Shang-Ren Hsu1 Shih-Te Tu
Shih-Te Tu